Abstract
Wastewater treatment remains a critical issue globally, despite various technological advancements and breakthroughs. The study of different materials and technologies gained new valences in the last years, in order to obtain cheap and efficient processes, to obtain a cleaner environment for future generations. In this context, the present review paper presents the new achievements in the materials domain with highlights on apatitic materials used for decontamination of water loaded with heavy metals. The main goal of this review is to present the adsorptive removal of heavy metals using hydroxyapatite-based adsorbents, offering a general overview regarding the recent progress in this particular area. Developing the current review, an attempt has been made to give appropriate recognition to the most recent data regarding the synthesis methods and targeted pollutants, including important information regarding the synthesis methods and precursors, morphological characteristics of the adsorbent materials and effectiveness of processes.
1. Introduction
To treat and clean contaminated water is a very difficult process, water pollution representing a critical issue of our society. Industrial and agriculture sectors generate huge quantities of chemical compounds that can cause serious problems of environmental damage, heavy metals pollution being one of it, being more persistent than organic contaminants such as pesticides or petroleum byproducts. Heavy metals can produce serious human health problems (including hyperkeratosis, cancers, diabetes, anemia, disorders of the immune nervous and reproductive systems, etc. [1]).
Today, the challenges in finding new materials and technologies for water depollution are directed towards a balance of operating costs (including easy scale-up of the decontamination procedures and materials synthesis) and contaminant removal efficiency. Remediation techniques, such as adsorption, membrane technology, ion exchange, coagulation or electrochemical treatment, are the most versatile applications, which can be comprehensively upgraded for the remediation behavior of heavy metals contaminated environment. Among the depollution strategies, adsorption is well-recognized as a viable and easy-to-apply at industrial level method for the removal of both organic and inorganic pollutants [2,3]. The research into adsorption materials gained new valences from the study of minerals, biopolymers, microalgal and fungal biomass to waste materials/byproducts or nanotechnology products, in order to obtain heavy metals removal efficiency in acidic, neutral or alkaline conditions [4,5,6,7]. Moreover, it is of great interest to use different innovative materials with good biocompatibility, enhanced possibility of biodegradability and bioreactivity [8].
What is important in the environmental practices of depollution is that the tandem “material–technology” has to offer not only economic benefits, but it has to contribute in achieving a sustainable development by being an active part in the global waste management process. These used materials must be removed from the systems in order to not be considered a threat to plants, animals and even humans because of their bioaccumulation, after their role is fully achieved.
In this context, this review reports the recent applications of hydroxyapatite and hydroxyapatite-based materials in the area of heavy metals removal. Due to their chemical characteristics (enhanced thermal and chemical stability, acid-base properties, low solubility, adsorption and ion-exchange ability), these materials can be an important part of the adsorption methods of hazardous contaminants in the field of water and wastewater treatment. Considering those aspects, the main goal of this review is to evaluate and offer a general overview regarding the recent progress on hydroxyapatite-based adsorbents in this particular area. Developing the current review, an attempt has been made to give appropriate recognition to the most recent data regarding the synthesis methods and targeted pollutants, including important information regarding the synthesis methods and precursors, morphological characteristics of the adsorbent materials and effectiveness of process (Scheme 1 depicts a flow diagram of the structure of this review). As the industry is continuously searching for alternative methods to decrease the environmental burden of both the pollutants present and the methods applied for depollution, this review also attempts to present the use of hydroxyapatite with natural origin, along with materials obtained from synthetic precursors, mechanistic aspects, as well as conclusions regarding the current limitations and future perspectives.

Scheme 1.
Flowchart describing the structure of the present review.
In order to select the works presented in the current review, the popular database Scopus was used, applying as keywords “hydroxyapatite”, “adsorption”, “heavy metals” and as search criteria works published after 2019. The works returned were manually checked in order to remove false-positive results, and a decision regarding their insertion in the review was made after carefully reading of the in extenso paper, considering several factors, including the relevancy for the envisaged research area, characterization of the adsorbent materials and the complexity of the study.
2. Adsorption Process Modeling and Mechanism
Evaluation of adsorption processes and adsorbents is based on a thorough modeling using different kinetic models, equilibrium isotherms and thermodynamic data [9]. Together with physicochemical characterization and correlated with operational parameters (temperature, pH, pollutant concentration, adsorbent dose, etc.), these approaches allow a complete image of adsorbent performances and, consequently, process optimization. Several kinetic models [10,11] are primarily used for obtaining relevant insights in adsorption mechanism and rate-controlled steps (mass transport and/or reaction processes, the time to reach equilibrium state, etc.). Generally, for heavy metal adsorption on hydroxyapatite or modified hydroxyapatite, the pseudo-second-order model is more appropriate for lower pollutant concentration while for higher concentration the pseudo-first-order model is in good concordance with experimental data. Adsorption isotherms represent the main approach to assess the optimal adsorption capacity and the interactions between solid and the pollutant from liquid phase and evaluate the distribution between them. There are several mathematical equations [12] applied for fitting the experimental data although the most used are Langmuir and Freundlich in linear or non-linear form, since they satisfactorily cover two general assumptions regarding the sorbate- adsorbent system. For the Langmuir model, the main assumption is that binding sites have similar affinity for heavy metal ions, while the Freundlich model refers to heterogeneous systems where adsorption sites have different affinities for sorbate species. Thermodynamic data are one of the most important methods for characterizing the adsorption process, especially since temperature is an important operational parameter. The Van’t Hoff Equation (1) allows us to determine the thermodynamic functions, such as entropy, enthalpy and free changes in energy. From these data, it can be determined if the process is spontaneous (ΔH0 < 0, ΔS0 > 0, ΔG0 < 0) or non-spontaneous (ΔH0 > 0, ΔS0 < 0, ΔG0 > 0), and, consequently, the performance of the adsorption process can be evaluated.
where Keq = qe/C [11].
Applying different models/equations to experimental data is the first step which is followed by selecting the one that fits the best. This is usually accomplished by comparing the correlation of regression (R2) parameter, which is employed to select the appropriate model. However, there are several error functions which better compensate the experimental data error (Table 1) and can be applied to both kinetical or equilibrium data and are critical for process optimization. Rahman et al. [12] observed that adsorption of Pb2+, Fe2+, and Zn2+ on Kappaphycus sp. followed a Langmuir model according to the R2 parameter while applying another error function, the best fit was for the Temkin model. In addition, the best fit for the Langmuir model was found for Cd(II) adsorption on modified HAP [12] using the sum of square error and hybrid functions.

Table 1.
Several isotherms, kinetic and thermodynamic model equations commonly used in adsorption studies 1.
Several low-cost materials were successfully applied in adsorption studies. Table 2 presents a selection of some materials, as well as their maximum adsorption capacity for comparison purposes.

Table 2.
Maximum adsorption capacity of other low-cost adsorbents.
3. Application of Natural-Derived Hydroxyapatite for the Removal of Heavy Elements
Hydroxyapatite is, first of all, a natural occurring mineral. As such, the use of natural-derived hydroxyapatite (HAP) is encountered in several published works. The natural hydroxyapatite can be obtained by the processing of several resources, including animal and fish bones, coral or egg shells [35]. The natural-derived HAP is mainly used in biomedical applications, due to its intrinsic properties, such as the presence of trace elements or its natural structure. Nevertheless, natural HAP can also be encountered in a series of environmental applications, including the ones targeted by the present review, removal of heavy metals (Table 3). In this category, we also mention the studies presenting the separation of one component (typically CaO) from natural sources (wastes of animal origin or algae), followed by other steps to develop the final hydroxyapatite material.

Table 3.
Application of natural-derived hydroxyapatite for the removal of heavy elements (references presented in chronological order) 1.
Hassan et al. [36] presented the sorption of Sr(II) on a gamma-radiation composite including natural-derived hydroxyapatite and poly(acrylamide-acrylic acid). The authors proposed a complex mechanism for the Sr(II) uptake, including ion exchange (between the Ca and Sr ions) and surface complexation. The equilibrium data presented suggested that the best fitting isotherm models were Langmuir (for 298 K) and, respectively, Freundlich for 318 and 328 K. In addition, the uptake capacity of the composite was superior to other popular sorbents, including carbon nanotubes, kaolinite, or biosorbents, in the studied concentration range (10–50 mg/L).
HAP sorbent also obtained from bovine bones was applied by Caballero et al. [37] for the removal of Pb(II) in a wide range of concentrations (400–1400 mg/L). Their results suggested that the best fitting isotherm for the adsorption process is represented by the Freundlich isotherm, thus supporting an adsorption in a monolayer. The authors also suggested that the optimum adsorbent concentration was 0.7 g/L, for this concentration being reached a removal efficiency of approx. 100%. Adsorption of Pb(II) was also evaluated by Vahdat et al., using both chicken-derived HAP and a magnetic HAP composite, at lower concentrations (1–10 g/L). The obtained results also suggested a pseudo-second-order model kinetic and the Freundlich isotherm being the best model to describe the process. The higher uptake capacity observed can, in our opinion, be assigned on the one hand to the different origin of the HAP, and, on the other hand, to the different lead concentration used.
Removal of Cu(II) was studied by Ngueagni et al. [44], using as sorbent material hydroxyapatite obtained from the core of ox horns. The study involved metal concentration in the range 100–500 mg/L, while the characteristics of the sorbent were determined by the calcination temperature (400–1100 °C), with the Ca/P ratio varying between 1.22 and 1.61, while the specific surface between 130 and 1 m2/g. The copper adsorption process for the sample calcinated at 400 °C was best described by the Langmuir isotherm, reaching a maximum adsorption capacity of 99.98 mg/g, at room temperature and a pH of 5, the phenomenon being governed, according to the authors, by a cation exchange process. The adsorption capacity recorded was superior to other sorbents, such as hazelnut activated carbon or bentonite, but inferior to other more complex materials. The same group utilized the material to study the adsorption of lead and cadmium ions, obtaining similar results, the most promising adsorbent being the sample calcinated at 400 °C, in a process best described by the Langmuir isotherm.
The HAP obtained from bovine femur was applied by Ramdani et al. [47] in sorption studies, using as heavy metals Pb(II) and Cd(II). The obtained results were compared with those resulted for the application of commercial HAP, with superior results for both ions. The natural HAP had superior pore size distribution and pore volume, compared with the commercial sample, and similar specific area. The adsorption processes were found to fit the Langmuir isotherm model for the natural HAP and the Freundlich isotherm model for the commercial sample. This could be explained, in our opinion, by the differences in terms of morphological characteristics between the two samples. A similar approach (with similar results) was applied by the same group [48], who compared the efficiency of natural HAP with the one of commercial HAP for the removal of copper and iron(III) ions. The adsorption process was found to be best fitted by the Langmuir isotherm, and the results were superior for the natural HAP in terms of maximum adsorption capacity.
Fish scales were used by Sricharoen et al. [52] to obtain HAP using an ultrasound-assisted method, with application in the uptake of Hg(II). The material obtained had a high uptake capacity for the targeted ion, superior to several types of complex adsorbents, as presented by the authors, a phenomenon assigned by the authors to the ion exchange with the Ca2+ in HAP structure, as well to the electrostatic interactions between the positively charged Hg2+ and the HAP surface.
An interesting approach was presented by Bi et al. [50]. Using Chlorella powder and a microwave-assisted method, the authors obtained hollow microspheres with multicomponent nanocores, in which the dominant phase was HAP, with the presence of whitmoreite, magnetite and chlorapatite. The material was used for the removal of cadmium ions, with a relatively high adsorption capacity. In addition, the magnetite phase present in the composites allowed the magnetic removal of the composite.
A composite based on natural materials (HAP obtained from bovine cortical bone, chitosan obtained from shrimp shells and snail shell powders) was applied by Bambaeero et al. [59] for the removal of copper and zinc ions. The process obeyed a pseudo-second- order model, while the isotherms that best fitted the experimental data were the Langmuir and Temkin isotherms. The authors reached an ion removal of 90% and 60%, respectively, for 3 mg/L initial ions concentration, 0.02 g of adsorbent and a pH of 5.5.
A particular case regarding the natural-derived hydroxyapatite is represented by the use of natural material to obtain the precursors for the hydroxyapatite synthesis. Typically, the material obtained is CaO (noted in Table 3 as Ca precursor), which is used to obtain, by the addition of phosphoric acid (or other P-containing precursors), HAP. This approach was used by Xia et al. [39], HAP being applied for the adsorption of Sr(II). According to their results, the process is best fitted by the Liu isotherm model, reaching a maximum adsorption capacity of over 45 mg/g. Núñez et al. [40] applied HAP obtained by a similar recipe for the removal of lead, cadmium and copper ions. The process was better fitted by the Langmuir model, but most importantly, the authors also performed a selectivity study, suggesting a competitive effect between different ions. The lead ions were preferably adsorbed by HAP, most probably due to their high electronegativity and ionic radius closer to Ca(II). Elsanafeny et al. [55] applied a similar method for obtaining the calcium precursor from eggshells and applied the synthesized HAP as such or in the form of polymer modified HAP, in order to propose a method for the treatment of wastewater containing radioactive cobalt and strontium. The obtained results showed superior ion uptake capacity for the HAP. Using egg shells as a source of CaCO3, Zeng et al. [43] obtained an anionic/cationic substituted HAP by an ultrasound-assisted procedure. The partial substitution of Ca2+ and PO43− with Na+, SiO44− and CO32− led to a macroporous structure with a relatively high pore volume compared to the other presented data. The material was applied for the adsorption of lead and cadmium ions, with superior registered maximum adsorption capacity, by comparison with HAP previously presented, and the authors described the uptake process as a mixture of ion exchange, precipitation and electrostatic interactions. The stability of the adsorbent was also established by regeneration studies, the materials being effective after four regeneration cycles.
4. Adsorption of Heavy Metals Using Synthesized Hydroxyapatite
The synthesis by various routes of hydroxyapatite can be achieved, including heterogeneous and homogeneous chemical deposition, hydrothermal synthesis, sol-gel methods, and many others [64]. The chemical synthesis method allows the development of materials with tailored morphological properties and composition for envisaged applications [65]. The recent trend in the development of hydroxyapatite-based materials is represented by the development of as-simple-as-possible synthesis methods and with scale-up possibility, in order to achieve their application at industrial scale. Another important aspect in the synthesis of HAP is represented by the necessity of a homogeneous composition of the final material. Generally speaking, in order to achieve this goal, one approach is represented by the used of highly soluble calcium compounds [64]. Another parameter that can be encountered in most of the synthesis methods is represented by the requirement of a high pH value; the replacement of the classical concentrated ammonia with milder alternatives allows the superior control of the process [64]. Other important aspects to be controlled during the synthesis process, especially for environmental applications, are represented by the particle dimensions, specific surface area and porosity, as well as the presence and potential leaching of other elements (in the case of substituted or doped hydroxyapatite). Table 4 presents some of the most recent published works detailing the application of hydroxyapatite for the uptake of heavy metals.

Table 4.
Synthesized hydroxyapatite applied for the removal of heavy metals (references presented in chronological order) 1.
The most encountered synthesis method is represented by co-precipitation. This method can lead to the development of hydroxyapatite that can be used as such or in complex combination with other materials. For example, Ivanets et al. [67] studied the influence of the crystallinity degree and porous structure on the HAP’s adsorption properties of metals from a multi-component solution (Cd2+, Co2+, Cu2+, Fe3+, Ni2+, Pb2+ and Zn2+). The variation in crystallinity and porous structure was achieved by the use of Mg2+ ions and hydroxyethylenediphosphonic acid (HDEP). The authors obtained an almost complete adsorption of Cd2+, Cu2+, Fe3+, Pb2+, Zn2+ within 6 h, at the dose of sorbent 5–10 g/L, when using HAP prepared with Mg2+ ions. On the other hand, HAP prepared with HDEP showed highest efficiency for the removal of Cd2+, Cu2+, Fe3+, Pb2+, Zn2+ ions. The proposed order of adsorbent efficiency was Mg2+-prepared HAP > HAP > HDEP-prepared HAP, while the most probable adsorption mechanism is represented by ion-exchange and dissolution-precipitation. The relatively high adsorption capacity of nano-sized HAP is expected, considering that, according to the study of Zheng and Zhang [99], commercial HAP was proven a superior adsorbent against copper and zinc ions in static and cylinder dynamic experiments, compared with other often used materials, such as medical stone, nano-carbon, and biochar.
Other encountered methods are represented by the hydrothermal synthesis, sol-gel, microwave synthesis or ultrasonication. All these methods usually lead to the development of rod-shaped particles, well in the nanometric range, with a good absorption capacity towards heavy metals.
Calcium deficiency was also proven to have a direct impact on the metal uptake capacity of HAP. This was demonstrated by Van Dat et al. [69] who obtained calcium-deficient HAP, by selecting different Ca/P ratio, inferior to the stoichiometric one. According to the presented results, the calcium-deficient HAP has superior metal uptake capacity, compared with the non-deficient HAP. The authors also hypothesized that at lower metal concentration (under 0.01 mol/L), the ion-exchange mechanism was dominant, while at higher concentrations, additional precipitation also occurred. As such, the calcium-deficiency strategy can be applied for enhancing the HAP metal uptake capacity and to provide superior adsorbent characteristics.
Another important parameter is represented by the synthesis temperature. The influence of this parameter on the HAP synthesis using a hydrothermal route, and on the U(VI) uptake capacity was evaluated by Zheng et al. [105]. As the synthesis temperature influenced the final morphology of HAP (being obtained nanosheets, nanoribbons and, at a working temperature of 180 °C, blocky structures), other important characteristics were also influenced, including the surface area and the pore volume, in a direct dependence with the temperature. The U(VI) uptake capacity was also influenced, ranging from 336.58–403.91 mg/g. The adsorption process obeyed a pseudo-second-order kinetic model and a Freundlich adsorption isotherm model.
By using modern techniques, such as electrospinning, other HAP morphologies can be obtained (such as hollow fibers), which also proved to have a good efficiency in the heavy metal uptake [79]. The variation of HAP morphology was evaluated by Zou et al. [72], as a result of changes in pH, reaction temperature and reactant ratio. The authors obtained a large variety of morphologies, including fluffy spongy deposits, porous spheres, solid spheres, nanotubes and the dimensions varied between few nanometers to hundreds of nanometers. The strongest effect on the final morphology was assigned to the pH, while the reactant ratio had little effect on the morphology, only affecting the HAP yield. The porous nanosphere morphology was chosen for further experiments, exhibiting a good adsorption capacity for individual ions (Table 4), while for the complex matrix, involving the presence of all ions, HAP exhibited an uptake capacity >99%, for Hg2+, Pb2+, Cu2+, Ni2+ and Co2+ ions. The kinetics study, performed using Pb(II) as a model pollutant, revealed that the adsorption process followed a pseudo-second-order kinetic model and a Langmuir isotherm model, with a maximum uptake capacity over 250 mg/g.
The presence of organic pollutants (such as oxytetracycline—OTC) has a different effect on the metal uptake on HAP, depending on the studied metal. As such, the adsorption of copper was found to be greatly increased up to 0.25 mmol/L OTC, the lead uptake to increase up to 0.10 mmol/L OTC, followed by a decrease under the level of single metal presence for 0.25 mmol/L OTC, while the cadmium uptake was not influenced by the OTC presence [74]. The recorded results were explained by the authors through the formation of organic-metallic complexes, with different affinity towards HAP, or the blocking of metal sites by OTC (in the case of lead).
Another important practical aspect is related to the recovery of the adsorbent from the solution. This can be easily achieved by the development of composites based on HAP and a magnetic phase. Several authors presented the incorporation of magnetic phases in HAP (i.e., α, γ-Fe2O3, Fe) and the application of the obtained magnetic composites for the removal of U(VI), Cd(II), or Cu(II) [84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105]. In all cases, high maximum adsorption capacities were obtained in processes obeying the pseudo-second-order model and Langmuir isotherm models.
Rodrigues et al. [92] synthesized HAP from calcium hydroxide and phosphoric acid and used it to develop adsorbent materials with hydrotalcite (HT) and multi-wall carbon nanotubes (MWCNT) by ultrasonic and hydrothermal treatment. The authors applied the composites HAP/HT and HAP/HT/MWCNT (at two carbon nanotubes concentrations) for the removal of Cr(VI), in fixed bed column experiments. The experimental results proved a very good uptake capacity (between approx. 4 g/g and 5.8 g/g), increasing with the increase in MWCNT content. The kinetic studies evidenced a indicated a feasible, spontaneous, and endothermic physisorption, which could be applied for the removal of Cr(VI) from leather industry wastewater. A multi-component adsorbent was also proposed by Hokkanen et al. [93], using HAP, cellulose and bentonite clay, for the removal of As(III). The process was proven to follow a pseudo-first-order model and Langmuir isotherm model. Best results were obtained in the pH range 4–7, with the equilibrium being reached within 5 min.
Synthesized HAP can be found in more complex adsorbent structures, such as composites with cyclodextrin, activated carbon, carbon nanotubes, hydrotalcite, bentonite, alendronate or even coated on ceramic support as filtration membranes, with significant adsorbent activity against a series of heavy metals (Cd2+, Cu2+, Cr6+, As3+, Pb2+) [87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137], over a wide range of pH values, metal and adsorbent concentrations.
5. Mechanistic Aspects and Current Limitations
The development of the proposed adsorbents, either of natural or synthetic origin can be summarized according to Figure 1. The natural route involves two major possible pathways, as previously described.
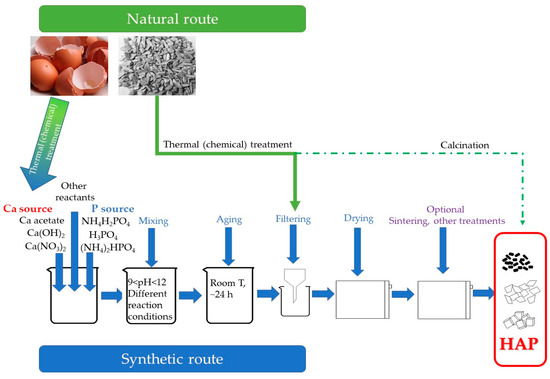
Figure 1.
Development of hydroxyapatite-based adsorbents using natural and synthetic route. Schematic presentation.
One is the recovery of the calcium source and its introduction in the synthetic route (as depicted in Figure 1), while another is the direct recovery of hydroxyapatite, trough mechanical, carbonization (and in some cases calcination) of the raw material (typical animal bones). Regarding the synthetic route, a calcium and a phosphorus source are required, followed by different synthesis methods (typically co-precipitation, but other methods, such as hydrothermal, ultrasonication or sol-gel being also encountered), and post-treatments (drying, vacuum-drying, calcination).
The presence of heavy metals in water effluents can have a negative influence over very long periods of time [138]. Most of the heavy metals have as provenance various industries, such as textile industry, mining, smelting, fertilizer use, sewage discharge, etc. [139,140]. Regardless their origin, in the absence of appropriate decontamination methods, heavy metal ends up in water effluents, having a direct negative effect on flora, fauna, and finally, human health (Figure 2). Development of appropriate decontamination methods (for example, adsorption) cannot only protect us against these effects, but also allows the recovery of heavy metals, thus lowering the pressure on existent resources.
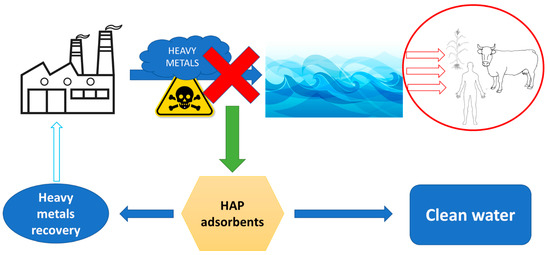
Figure 2.
Fate of heavy metals in the environmental and the application of HAP adsorbents for their removal from ecosystems and recovery/reuse.
At the same time, we must consider that the application of efficient decontamination methods has to be affordable, in order to be ready to accept it and implement it for the industry and general public. In this regard, not only the adsorption process is more economically efficient, compared with other methods, but also has smaller associated costs (including operating costs), while the proposed adsorbents are also low-cost, thus offering a viable depollution alternative [141,142,143]. Hydroxyapatite can be easily produced on a large scale, thus allowing the scale-up of the technologies, at the same time being rapidly regenerated, while the metals can be recovered without any expensive procedures.
Hydroxyapatite was also proven to be easily tunable for particular applications, its extraordinary versatility allowing its use in the hardest working conditions. As can be seen from the examples provided above, HAP adsorbents are not only efficient in the up-take of common pollutants (such as Pb2+, Cu2+, Cd2+, Cr6+, Ni2+, Cd2+, Zn2+, etc.), but can also uptake metals which are very dangerous and hardly removable by other methods (such as As3+) or potential radioactive metals (U6+ serving as a model for such elements) in the conditions encountered in practice (in terms of pH value and temperature). Hydroxyapatites-based adsorbents present multifunctional adsorption capacity resulting from FTIR and XRD studies related to modifications observed on hydroxyl, carbonate, phosphate and Ca2+ by substitution, ion exchange, complexation or precipitation. Thus, substitution process takes place at Ca2+ by metal ions in form of M2+, at phosphate groups by different oxyanions AsO43−, VO43−, etc., and at OH− groups by monovalent anions (F−, Cl−, etc.). In addition, composite HAP/organic structures can be significantly improved in terms of pollutants species than can be eliminated from aqueous solutions.
Regarding the uptake capacity of the proposed materials, the general conclusion that can be drawn from the presented studies is that the capacity increases with the increase in the specific surface area. Even though the natural hydroxyapatite presents relatively good adsorption capacity towards a variety of metals, its capacity is increased when the approach is used as calcium source, as is the case of lead, for example, recording an increase of Qmax from around 250 mg Pb(II)/g for HAP originating from bovine horns to approx. 700 mg/g, for HAP obtained using eggshells as calcium precursor [43,45]. A very good adsorption is achieved using HAP obtained from fish scales for Hg removal (over 200 mg/g) [52]. The adsorption capacity of synthesized HAP is increased not only by comparison with the natural materials, but also with the functionalization of the material or with the incorporation in composites with increased adsorption capacity. For example, the adsorption capacity for esterified HAP reaches approx. 2400 mg Pb(II)/g [81], and is increased up to over 2000 mg U(VI)/g, for the mesoporous HAP, obtained by freeze-drying [136].
Most of the studies concerning the adsorption of the heavy metals on the natural-derived or synthetic HAP suggest that the adsorption process obeys a pseudo-second-order kinetic model, supporting as a main process the chemisorption (with some of the authors also suggesting the presence of the physisorption process, demonstrated by the application of a pseudo-first-order kinetic model), while the most appropriate isotherm to fit the experimental data was the Langmuir isotherm, suggesting a monolayer adsorption (at a fixed number of well-defined sites). The less encountered Freundlich isotherm suggests a different model, presuming that the concentration of the adsorbate on the adsorbent surface increases with its concentration. The Liu isotherm (one of the rarely applied models) is in turn a combination of those two, suggesting that the adsorbate has preferred sites for occupation, but this in turn can be saturated. In our opinion, although less encountered in the literature, this isotherm model should be further studied for the adsorption of heavy metals on HAP adsorbents.
The widely acceptance of the pseudo-second-order kinetic model for the heavy metal uptake by HAP implies that the sorption process is controlled by chemical reactions, such as ion exchange, surface complexation and/or precipitation, and to a lesser extent by the physical absorption (as would be suggested by the pseudo-first-order kinetic model) (Figure 3).
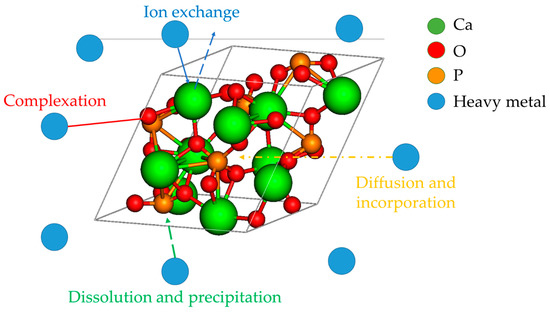
Figure 3.
Possible mechanisms of heavy metal uptake by HAP adsorbents, according to the presented literature data.
An important parameter which governs the adsorption mechanism to a large extent is the pH value correlated with pHPZC which predicts to some extent the solid surface charge and metal speciation. Hence, for some pH intervals, in which the solid particle and the pollutant have opposite charges, the electrostatic attraction occurs, while in other conditions, ionic exchange or precipitation are the main adsorption route.
Regeneration of such adsorbents can be easily achieved using, for example, HNO3 (0.1 M), Ca(NO3)2 (0.5 M, pH = 3), or HCl (1.5 M) solutions at room temperature, as demonstrated by Zeng et al. [43], Ma et al. [119], Shen et al. [125] and Ahmed et al. [127], the adsorbent materials preserving their properties after several cycles. Thus, the regeneration step can be achieved in industrial installation, without the need for very complicated equipment. Once recovered, the heavy metals can be reintroduced in the industrial processes. The application of hydroxyapatite-based adsorbent raises another issue that should be addressed more thoroughly in future studies, namely the presence of competition between metallic species for the binding sites, in the case of real-life multicomponent cases, as observed by some of the cited authors [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123] and also observed in studies regarding other natural adsorbents [144].
Another important aspect is related to data presentation in the published studies. In our opinion, future studies regarding the adsorbent properties of HAP should focus on the establishment of the mechanisms through which the processes are taking place, as well as to working in relevant conditions for real systems.
6. Conclusions and Future Perspectives
Wastewater treatment remains a critical issue globally, despite various technological advancements and breakthroughs. Apatitic materials are a sustainable, safe and clean method for pollutants’ removal from contaminated environments, with a great advantage over other materials that they can be obtained from natural sources and, more importantly, from waste. Due to their chemical and physical characteristics, they can successfully replace expensive materials, and also can be easily regenerated or have the ability to be converted from used materials into value-added products, and thus apply a zero-waste concept. Preparation methods of apatitic materials is an important step which can direct the efficiency of depollution technology. By referring to the presented data, the most promising synthesis method is represented by the synthetic route, for which the final morphology can be more easily controlled to obtain, for example, mesoporous structures with enhanced adsorption capacity. However, the natural route should not be disregarded, especially when discussing the synthesis process from a bio-economical perspective, considering the re-use of natural wastes. In addition, the particular synthesis method should be selected considering multiple factors, including but not limited to the availability of the raw materials, the targeted metals, and their level on the water matrices. These depollution systems are an important progress for environment treatments providing high efficiency without high costs using easy logistics, with a primordial condition of using optimized tailored materials. Future research is needed in order to obtain optimized materials which can be used in real water systems where the matrix is very complex and, from the point of view of heavy metals, there is an abundance of various competing ions in different concentrations, from traces to enhanced amounts. This challenges, related to the scale-up of technologies from lab scale to commercial adsorption processes, must be addressed through economic limitations and excessive use of chemicals. Even though the process of adsorption is predominant and well-established, the development of low-cost and sustainable materials with enhanced selectivity and stability are still primary challenges. Moreover, detailed studies on the adsorption mechanism are still required for more efficient adsorption process and optimized installations design, and toxicity, selectivity, multi-metal adsorption and reusability are some key challenges to be looked for in future years.
Author Contributions
Conceptualization, R.I.B., I.F. and R.C.F.; methodology, R.C.F.; data collection, R.I.B. and S.M.A.; validation, I.F., R.C.F. and R.C.F.; writing—original draft preparation, R.C.F. and I.F.; writing—review and editing, R.C.F. and I.F.; supervision, R.C.F.; project administration, R.C.F.; funding acquisition, R.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Romanian Ministry of Research and Innovation, MCI (Ministry of Research, Innovation and Digitization, MCID) through INCDCP ICECHIM Bucharest 2019–2022 Core Program PN. 19.23–Chem-Ergent, Project No.19.23.03. The authors gratefully acknowledge the support obtained by grants of the Romanian National Authority for Scientific Research and Innovation, CCCDI–UEFISCDI, project number PN-III-P2-2.1-PTE-2019-0222, contract 26PTE/2020, and project number PN-III-P2-2.1-PED-2019-3166, contract 299PED/2020, within PNCDI III. The APC was funded by INCDCP ICECHIM Bucharest 2019-2022 Core Program PN. 19.23–Chem-Ergent, Project No.19.23.03.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Ispas, G.C.; Manea, R.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C.; Raduly, M.F. Iron Oxide/Phosphatic Materials Composites with Potential Applications in Environmental Protection. Materials 2020, 13, 5034. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Garg, V.K.; Kadirvelu, K.; Sillanpää, M. Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons. Int. J. Environ. Sci. Technol. 2015, 13, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Osuna-Martínez, C.C.; Armienta, M.A.; Tiznado, M.E.B.; Páez-Osuna, F. Arsenic in waters, soils, sediments, and biota from Mexico: An environmental review. Sci. Total Environ. 2021, 752, 142062. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total. Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Bano, Z.; Mazari, S.; Saeed, R.Y.; Majeed, M.A.; Xia, M.; Memon, A.Q.; Abro, R.; Wang, F. Water decontamination by 3D graphene based materials: A review. J. Water Process. Eng. 2020, 36, 101404. [Google Scholar] [CrossRef]
- Sun, X.; Guo, P.; Sun, Y.; Cui, Y. Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum. Materials 2021, 14, 2224. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Mongioví, C.; Morin-Crini, N.; Lacalamita, D.; Bradu, C.; Raschetti, M.; Placet, V.; Ribeiro, A.; Ivanovska, A.; Kostić, M.; Crini, G. Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties. Molecules 2021, 26, 4199. [Google Scholar] [CrossRef]
- Zhang, D.; Crini, G.; Lichtfouse, E.; Rhimi, B.; Wang, C. Removal of Mercury Ions from Aqueous Solutions by Crosslinked Chitosan-based Adsorbents: A Mini Review. Chem. Rec. 2020, 20, 1220–1234. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Rahman, S.; Sathasivam, K.V. Heavy Metal Adsorption ontoKappaphycussp. from Aqueous Solutions: The Use of Error Functions for Validation of Isotherm and Kinetics Models. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica AND Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Roginsky, S.Z.; Zeldovich, Y.B. Die Katalische Oxidation von Kohlenmonoxyd Auf Mangandioxyd. Acta Physiochim. URSS 1934, 1, 554–594. [Google Scholar]
- Dubinin, M.M. The Equation of the Characteristic Curve of Activated Charcoal. Proc. USSR Acad. Sci. 1947, 55, 327–329. [Google Scholar]
- Hobson, J.P. Physical adsorption isotherms extending from ultrahigh vacuum to vapor pressure. J. Phys. Chem. 1969, 73, 2720–2727. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar]
- Ho, Y.-S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 1995. [Google Scholar]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, J.W., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Willard Gibbs, J. A method of geometrical representation of the thermodynamic properties of substances by means of surfaces. In Transactions of the Connecticut Academy of Arts and Sciences; Connecticut Academy of Arts and Sciences: New Haven, CT, USA, 1873; Volume 2, pp. 382–404. [Google Scholar]
- Gueu, S.; Yao, B.; Adouby, K.; Ado, G. Kinetics and thermodynamics study of lead adsorption on to activated carbons from coconut and seed hull of the palm tree. Int. J. Environ. Sci. Technol. 2007, 4, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.-H.; Liu, S.-C.; Chen, C.-Y. Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lee, J.Y.; Park, Y.-T.; Baek, K.; Choi, J. Adsorption of As(III), As(V), Cd(II), Cu(II), and Pb(II) from Aqueous Solutions by Natural Muscovite. Sep. Sci. Technol. 2010, 45, 814–823. [Google Scholar] [CrossRef]
- Shahmohammadi-Kalalagh, S. Isotherm and Kinetic Studies on Adsorption of Pb, Zn and Cu by Kaolinite. Casp. J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Lasheen, M.R.; Ammar, N.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Putra, W.P.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies. J. Encapsul. Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Singh, A.; Sharma, A. Studies on the uptake of lead and zinc by lignin obtained from black liquor—A paper industry waste material. Environ. Technol. 1994, 15, 353–361. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Volesky, B.; Prasetyo, I. Cadmium removal in a biosorption column. Biotechnol. Bioeng. 1994, 43, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Tare, V.; Chaudhari, S.; Jawed, M. Comparative Evaluation of Soluble and Insoluble Xanthate Process for Heavy Metal Removal from Wastewaters. Water Sci. Technol. 1992, 26, 237–246. [Google Scholar] [CrossRef]
- Akpan, E.; Dauda, M.; Kuburi, L.; Obada, D.; Dodoo-Arhin, D. A comparative study of the mechanical integrity of natural hydroxyapatite scaffolds prepared from two biogenic sources using a low compaction pressure method. Results Phys. 2020, 17, 103051. [Google Scholar] [CrossRef]
- Hassan, H.S.; El-Kamash, A.M.; Ibrahim, H.A.-S. Evaluation of hydroxyapatite/poly(acrylamide-acrylic acid) for sorptive removal of strontium ions from aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 25641–25655. [Google Scholar] [CrossRef] [PubMed]
- Caballero, N.; Ozuna, P.C.; Monteiro, M. Kinetic Analysis of Lead Removal by Natural Hydroxyapatite from Aqueous Solution in High Concentration. Mater. Res. 2019, 22, 22. [Google Scholar] [CrossRef]
- Vahdat, A.; Ghasemi, B.; Yousefpour, M. Synthesis of hydroxyapatite and hydroxyapatite/Fe3O4 nanocomposite for removal of heavy metals. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100233. [Google Scholar] [CrossRef]
- Xia, X.; Shen, J.; Cao, F.; Wang, C.; Tang, M.; Zhang, Q.; Wei, S. A facile synthesis of hydroxyapatite for effective removal strontium ion. J. Hazard. Mater. 2019, 368, 326–335. [Google Scholar] [CrossRef]
- Núñez, D.; Serrano, J.A.; Mancisidor, A.; Elgueta, E.; Varaprasad, K.; Oyarzún, P.; Cáceres, R.; Ide, W.; Rivas, B.L. Heavy metal removal from aqueous systems using hydroxyapatite nanocrystals derived from clam shells. RSC Adv. 2019, 9, 22883–22890. [Google Scholar] [CrossRef] [Green Version]
- Meski, S.; Tazibt, N.; Khireddine, H.; Ziani, S.; Biba, W.; Yala, S.; Sidane, D.; Boudjouan, F.; Moussaoui, N. Synthesis of hydroxyapatite from mussel shells for effective adsorption of aqueous Cd(II). Water Sci. Technol. 2019, 80, 1226–1237. [Google Scholar] [CrossRef]
- Bernalte, E.; Kamieniak, J.; Randviir, E.P.; Bernalte-García, Á.; Banks, C.E. The preparation of hydroxyapatite from unrefined calcite residues and its application for lead removal from aqueous solutions. RSC Adv. 2019, 9, 4054–4062. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Tang, W.; Ding, C.; Yang, L.; Gong, D.; Kang, Z.; He, Z.; Wu, Y. Preparation of anionic-cationic co-substituted hydroxyapatite for heavy metal removal: Performance and mechanisms. J. Solid State Chem. 2019, 280, 120960. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siéwé, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of Cu(II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Kumar, P.S.; Woumfo, E.D.; Prasanth, S.M. Adsorption of Pb(II) and Cd(II) ions onto modified biogenic slaughterhouse waste: Equilibrium and kinetic analysis. Int. J. Environ. Anal. Chem. 2020, 1–20. [Google Scholar] [CrossRef]
- Omar, S.; Muhamad, M.S.; Chuan, L.T.E.; Rudi, N.N.; Hamidon, N.; Hamid, N.H.A.; Harun, H.; Sunar, N.M.; Ali, R. Effect of Hydroxyapatite (HAp) Adsorbent Dosage towards Lead Removal. Int. J. Emerg. Trends Eng. Res. 2020, 8, 201–205. [Google Scholar] [CrossRef]
- Ramdani, A.; Kadeche, A.; Adjdir, M.; Taleb, Z.; Ikhou, D.; Taleb, S.; Deratani, A. Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Pract. Technol. 2020, 15, 130–141. [Google Scholar] [CrossRef]
- Ramdani, A.; Taleb, Z.; Guendouzi, A.; Kadeche, A.; Herbache, H.; Mostefai, A.; Taleb, S.; Deratani, A. Mechanism study of metal ion adsorption on porous hydroxyapatite: Experiments and modeling. Can. J. Chem. 2020, 98, 79–89. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Bi, L.; Luan, X.; Geng, F.; Xu, X.; Chen, Y.; Zhang, F. Microwave-assisted synthesis of hollow microspheres with multicomponent nanocores for heavy-metal removal and magnetic sensing. ACS Appl. Mater. Interfaces 2020, 12, 46779–46787. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cocoletzi, H.; Salinas, R.A.; Águila-Almanza, E.; Rubio-Rosas, E.; Chai, W.S.; Chew, K.W.; Mariscal-Hernández, C.; Show, P.L. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ. Technol. Innov. 2020, 20, 101109. [Google Scholar] [CrossRef]
- Sricharoen, P.; Limchoowong, N.; Nuengmatcha, P.; Chanthai, S. Ultrasonic-assisted recycling of Nile tilapia fish scale biowaste into low-cost nano-hydroxyapatite: Ultrasonic-assisted adsorption for Hg2+ removal from aqueous solution followed by “turn-off” fluorescent sensor based on Hg2+-graphene quantum dots. Ultrason. Sonochem. 2020, 63, 104966. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, Y.M.; Andoshe, D.; Desissa, T.D. Composite of bentonite/CoFe2O4/hydroxyapatite for adsorption of Pb (II). Mater. Res. Express 2020, 7, 115501. [Google Scholar] [CrossRef]
- Hariani, P.L.; Riyanti, F.; Fatma, F.; Rachmat, A.; Herbanu, A. Removal of Pb(II) using Hydroxyapatite from Golden Snail Shell (Pomacea canaliculata L.) Modified with Silica. Molekul 2020, 15, 130–139. [Google Scholar] [CrossRef]
- Elsanafeny, H.A.; Aly, M.M.A.; Hasan, M.A.; Lasheen, Y.F.; Youssef, M.A. Synthesis and polymeric modification of hydroxyapatite from biogenic raw material for adsorptive removal of Co2+ and Sr2+. J. Radioanal. Nucl. Chem. 2020, 326, 1119–1133. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Ahmadi, A.; Akbari, A.; Farjadfard, S.; Ramavandi, B. Adsorption mercury, cobalt, and nickel with a reclaimable and magnetic composite of hydroxyapatite/Fe3O4/polydopamine. J. Environ. Chem. Eng. 2021, 9, 105709. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A.; Behjat, Y. Invent of a simultaneous adsorption and separation process based on dynamic membrane for treatment Zn(II), Ni(II) and, Co(II) industrial wastewater. Arab. J. Chem. 2021, 14, 103231. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adedapo, A.E.; Oladeji, O.B.; Sodiya, E.F.; Ibadin, F.H.; Zhang, D. Nano-rod hydroxyapatite for the uptake of nickel ions: Effect of sintering behaviour on adsorption parameters. J. Environ. Chem. Eng. 2021, 9, 105931. [Google Scholar] [CrossRef]
- Bambaeero, A.; Bazargan-Lari, R. Simultaneous removal of copper and zinc ions by low cost natural snail shell/hydroxyapatite/chitosan composite. Chin. J. Chem. Eng. 2021, 33, 221–230. [Google Scholar] [CrossRef]
- Ali, M.M.S.; Imam, D.M.; El-Nadi, Y.A. Vanadium(V) removal and recovery by adsorption onto modified activated carbon derived from natural hydroxyapatite. J. Iran. Chem. Soc. 2021, 18, 2771–2784. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hemmati, S.; Ahmadi, A.; Falletta, E.; Ramavandi, B.; Bianchi, C.L. Zn2+ removal from the aqueous environment using a polydopamine/hydroxyapatite/Fe3O4 magnetic composite under ultrasonic waves. RSC Adv. 2021, 11, 27309–27321. [Google Scholar] [CrossRef]
- Wei, W.; Han, X.; Shao, Y.; Xie, W.; Zhang, Y.; Yao, Y.; Zhao, W.; Han, R.; Li, S.; Zheng, C. Comparing the effects of humic acid and oxalic acid on Pb(II) immobilization by a green synthesized nanocrystalline hydroxyapatite. Chemosphere 2021, 285, 131411. [Google Scholar] [CrossRef]
- Ayodele, O.; Olusegun, S.J.; Oluwasina, O.O.; Okoronkwo, E.A.; Olanipekun, E.O.; Mohallem, N.D.; Guimarães, W.G.; de Gomes, B.L.M.; de Souza, G.O.; Duarte, H.A. Experimental and theoretical studies of the adsorption of Cu and Ni ions from wastewater by hydroxyapatite derived from eggshells. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100439. [Google Scholar] [CrossRef]
- Serhiienko, A.; Dontsova, T.; Yanushevska, O.; Lapinskyi, A.; Krymets, G. Synthesis and characterization of hydroxyapatite and composite based on it with collagen/alginate. Chem. Pap. 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Fierascu, I.; Fierascu, R.C.; Somoghi, R.; Ion, R.M.; Moanţă, A.; Avramescu, S.; Damian, C.M.; Ditu, L.M. Tuned apatitic materials: Synthesis, characterization and potential antimicrobial applications. Appl. Surf. Sci. 2018, 438, 127–135. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Guegan, R.; Beuran, M.; Costescu, A.; Predoi, D. Adsorption of Pb (II) Ions onto Hydroxyapatite Nanopowders in Aqueous Solutions. Materials 2018, 11, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanets, A.; Kitikova, N.; Shashkova, I.; Roshchina, M.; Srivastava, V.; Sillanpää, M. Adsorption performance of hydroxyapatite with different crystalline and porous structure towards metal ions in multicomponent solution. J. Water Process. Eng. 2019, 32, 100963. [Google Scholar] [CrossRef]
- Oulguidoum, A.; Bouyarmane, H.; Laghzizil, A.; Nunzi, J.-M.; Saoiabi, A. Development of sulfonate-functionalized hydroxyapatite nanoparticles for cadmium removal from aqueous solutions. Colloid Interface Sci. Commun. 2019, 30, 100178. [Google Scholar] [CrossRef]
- Doan, V.D.; Le, V.T.; Le, H.S.; Ta, D.H.; Nguyen, H.T. Effectiveness of Calcium Deficiency in Nanosized Hydroxyapatite for Removal of Fe(II), Cu(II), Ni(II) and Cr(VI) Ions from Aqueous Solutions. J. Nano Res. 2019, 56, 17–27. [Google Scholar] [CrossRef]
- De Resende, N.S.; Camargo, C.; Reis, P.C.; Perez, C.A.C.; Salim, V.M. Mechanisms of mercury removal from aqueous solution by high-fixation hydroxyapatite sorbents. Int. J. Environ. Sci. Technol. 2019, 16, 7221–7228. [Google Scholar] [CrossRef]
- Ferri, M.; Campisi, S.; Gervasini, A. Nickel and cobalt adsorption on hydroxyapatite: A study for the de-metalation of electronic industrial wastewaters. Adsorption 2019, 25, 649–660. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, Y.; Zhang, Z. Preparation of hydroxyapatite nanostructures with different morphologies and adsorption behavior on seven heavy metals ions. J. Contam. Hydrol. 2019, 226, 103538. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Le, T.P.T.; Do, H.T.; Vo, H.T.; Pham, N.T.; Nguyen, T.T.; Cao, H.T.; Nguyen, P.T.; Dinh, T.M.T.; Le, H.V.; et al. Fabrication of Porous Hydroxyapatite Granules as an Effective Adsorbent for the Removal of Aqueous Pb(II) Ions. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Yuan, L.; Yan, M.; Huang, Z.; He, K.; Zeng, G.; Chen, A.; Hu, L.; Li, H.; Peng, M.; Huang, T.; et al. Influences of pH and metal ions on the interactions of oxytetracycline onto nano-hydroxyapatite and their co-adsorption behavior in aqueous solution. J. Colloid Interface Sci. 2019, 541, 101–113. [Google Scholar] [CrossRef]
- Su, M.; Tsang, D.; Ren, X.; Shi, Q.; Tang, J.; Zhang, H.; Kong, L.; Hou, L.; Song, G.; Chen, D. Removal of U(VI) from nuclear mining effluent by porous hydroxyapatite: Evaluation on characteristics, mechanisms and performance. Environ. Pollut. 2019, 254, 112891. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Li, S.; Zhu, J.; Liu, W.; Zhang, Z. Self-templated microwave-assisted hydrothermal synthesis of two-dimensional holey hydroxyapatite nanosheets for efficient heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 30076–30086. [Google Scholar] [CrossRef] [PubMed]
- Shashkova, I.L.; Ivanets, A.I.; Kitikova, N.V. Sorption of Co2+, Pb2+, and Sr2+ Ions on Hydroxyapatite, Synthesized in the Presence of Oxyethylidenediphosphonic Acid. Russ. J. Appl. Chem. 2019, 92, 625–633. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, C.; Xu, Z.; Luo, P.; Fu, Z.; Zhang, J. Synthesis of bitter gourd-shaped nanoscaled hydroxyapatite and its adsorption property for heavy metal ions. Mater. Lett. 2019, 241, 176–179. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Wang, D.; Han, X. Electrospinning Synthesis of Hydroxyapatite Nanofibers Assembled from Nanorods and their Adsorption for Heavy Metal Ions. Pol. J. Environ. Stud. 2019, 28, 981–988. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Direct bromination of nano hydroxyapatite strategy towards particle brushes via surface-initiated ATRP for highly efficient heavy metal removal. Polymer 2019, 183, 121883. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Unexpectedly High Adsorption Capacity of Esterified Hydroxyapatite for Heavy Metal Removal. Langmuir 2019, 35, 16111–16119. [Google Scholar] [CrossRef]
- Peng, X.; Chen, W.; He, Z.; Li, D.; Liu, H.; Jin, H.; Zhou, G.; Xu, F. Removal of Cu(II) from wastewater using doped HAP-coated-limestone. J. Mol. Liq. 2019, 293, 293. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Li, L.; Han, R.; Wang, G.; Wei, W. Nanosized hydroxyapatite supported on natural sepiolite: A novel adsorbent for Cd(II) removal from simulated groundwater. Mater. Res. Express 2019, 6, 125518. [Google Scholar] [CrossRef]
- El-Maghrabi, H.; Younes, A.A.; Salem, A.; Rabie, K.; El-Shereafy, E.-S. Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): Preparation, characterization and adsorption optimization. J. Hazard. Mater. 2019, 378, 120703. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Kang, C.; Zhang, J.; Xu, Z.; Jiang, C.; Luo, P.; Fu, Z.; Ding, M.; Lv, Y. Synthesis of magnetic Fe-doped hydroxyapatite nanocages with highly efficient and selective adsorption for Cd2+. Mater. Lett. 2019, 253, 144–147. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Imran, M.; Muhammad, N.; Howari, F.M.; Alkhoori, S.A.; Khan, J.A.; Khan, Z.U.H.; Bhatnagar, A.; et al. Synergistic effects of activated carbon and nano-zerovalent copper on the performance of hydroxyapatite-alginate beads for the removal of As3+ from aqueous solution. J. Clean. Prod. 2019, 235, 875–886. [Google Scholar] [CrossRef]
- Ansari, A.; Vahedi, S.; Tavakoli, O.; Khoobi, M.; Faramarzi, M.A. Novel Fe3O4/hydroxyapatite/β-cyclodextrin nanocomposite adsorbent: Synthesis and application in heavy metal removal from aqueous solution. Appl. Organomet. Chem. 2019, 33, e4634. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Sun, K.; He, Y.; Song, P.; Zhang, D.; Wang, R. Preparation of hydroxyapatite-based porous materials for absorption of lead ions. Water Sci. Technol. 2019, 80, 1266–1275. [Google Scholar] [CrossRef]
- Long, Y.; Jiang, J.; Hu, J.; Hu, X.; Yang, Q.; Zhou, S. Removal of Pb(II) from aqueous solution by hydroxyapatite/carbon composite: Preparation and adsorption behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 471–479. [Google Scholar] [CrossRef]
- Jayaweera, H.D.A.C.; Siriwardane, I.; De Silva, K.M.N.; De Silva, R.M. Synthesis of multifunctional activated carbon nanocomposite comprising biocompatible flake nano hydroxyapatite and natural turmeric extract for the removal of bacteria and lead ions from aqueous solution. Chem. Cent. J. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Fox, J.T. Synthesis and appraisal of a hydroxyapatite/pectin hybrid material for zinc removal from water. RSC Adv. 2019, 9, 21095–21105. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.; de Almeida, O.; Brasil, H.; Moraes, D.; dos Reis, M. Adsorption of chromium (VI) on hydrotalcite-hydroxyapatite material doped with carbon nanotubes: Equilibrium, kinetic and thermodynamic study. Appl. Clay Sci. 2019, 172, 57–64. [Google Scholar] [CrossRef]
- Hokkanen, S.; Doshi, B.; Srivastava, V.; Puro, L.; Koivula, R. Arsenic (III) removal from water by hydroxyapatite-bentonite clay-nanocrystalline cellulose. Environ. Prog. Sustain. Energy 2019, 38, 13147. [Google Scholar] [CrossRef]
- Zhou, M.; Xie, F.; Li, G.; Wang, Q.; Tang, L.; Yan, M.; Bi, H.; Fei, X. Biocompatible HA@Fe3O4@N-CDs hybrids for detecting and absorbing lead ion. J. Biomed. Mater. Res. Part A 2019, 107, 1532–1540. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Majumdar, S.; Sarkar, S.; Kundu, B.; Sahoo, G.C. Performance investigation of Pb(II) removal by synthesized hydroxyapatite based ceramic ultrafiltration membrane: Bench scale study. Chem. Eng. J. 2019, 355, 510–519. [Google Scholar] [CrossRef]
- Thang, N.H.; Phong, D.T. Characterizations of Hydroxyapatite Synthesized from Calcium Hydroxide and Phosphoric Acid as Adsorbents of Lead in Wastewater. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2020; Volume 991, pp. 159–165. [Google Scholar]
- Jiang, J.; Long, Y.; Hu, X.; Hu, J.; Zhu, M.; Zhou, S. A facile microwave-assisted synthesis of mesoporous hydroxyapatite as an efficient adsorbent for Pb2+ adsorption. J. Solid State Chem. 2020, 289, 121491. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Song, X.; Wang, Y.; Fang, D.; Ge, S.; Zhang, R. Insights into dynamic adsorption of lead by nano-hydroxyapatite prepared with two-stage ultrasound. Chemosphere 2020, 253, 126661. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J. Experimental study on the adsorption of dissolved heavy metals by nano-hydroxyapatite. Water Sci. Technol. 2020, 82, 1825–1832. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, X.; Zhang, Y.; Wang, S.; Wang, M.; Wang, Y.; Weerakoon, W.M.S.B.; Sanginova, O. Study on immobilization of diatomite, Ca(H2PO4)2, CaCO3, HAP and nano-HAP for heavy metal contaminated sediment. Water Qual. Res. J. 2020, 55, 370–381. [Google Scholar] [CrossRef]
- Wu, M.; Mo, L.; Bi, E. Effects of fulvic acid and montmorillonite colloids at different concentrations on Cd(II) sorption onto nano-hydroxyapatite. Chemosphere 2020, 248, 125992. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Su, M.; Wei, L.; Wei, Y.; Liang, J.; Liu, Y.; Luo, Y. Comprehensive evaluation of the effectiveness on metals recovery and decontamination from MSWI fly ash by an integrating hydrometallurgical process in Guangzhou. Sci. Total Environ. 2020, 728, 138809. [Google Scholar] [CrossRef]
- Shi, Q.; Su, M.; Yuvaraja, G.; Tang, J.; Kong, L.; Chen, D. Development of highly efficient bundle-like hydroxyapatite towards abatement of aqueous U(VI) ions: Mechanism and economic assessment. J. Hazard. Mater. 2020, 394, 122550. [Google Scholar] [CrossRef]
- Aouay, R.; Jebri, S.; Rebelo, A.; Ferreira, J.M.F.; Khattech, I. Enhanced cadmium removal from water by hydroxyapatite subjected to different thermal treatments. J. Water Supply Res. Technol. 2020, 69, 678–693. [Google Scholar] [CrossRef]
- Zheng, N.; Yin, L.; Su, M.; Liu, Z.; Tsang, D.; Chen, D. Synthesis of shape and structure-dependent hydroxyapatite nanostructures as a superior adsorbent for removal of U(VI). Chem. Eng. J. 2020, 384, 123262. [Google Scholar] [CrossRef]
- Szenknect, S.; Mesbah, A.; Descostes, M.; Maihatchi-Ahamed, A.; Bonato, L.; Massonnet, M.; Ziouane, Y.; Vors, E.; Vercouter, T.; Clavier, N.; et al. Uranium removal from mining water using Cu substituted hydroxyapatite. J. Hazard. Mater. 2020, 392, 122501. [Google Scholar] [CrossRef]
- Ahmed, M.; Mansour, S.; Ramadan, R.; Afifi, M.; Mostafa, M.S.; El-Dek, S.; Uskoković, V. Tuning the composition of new brushite/vivianite mixed systems for superior heavy metal removal efficiency from contaminated waters. J. Water Process. Eng. 2020, 34, 101090. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, S.; Ma, J.; Wang, F.; Xu, H.; Xia, M. The facile synthesis of zoledronate functionalized hydroxyapatite amorphous hybrid nanobiomaterial and its excellent removal performance on Pb2+ and Cu2+. J. Hazard. Mater. 2020, 392, 122291. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liao, B.; Lu, T.; Wang, H.; Xu, L.; Li, Z.; Ye, C. Insight into immobilization of Pb2+ in aqueous solution and contaminated soil using hydroxyapatite/attapulgite composite. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125290. [Google Scholar] [CrossRef]
- Rout, S.; Muduli, B.; Kumar, A.; Pulhani, V. Removal of Uranium(VI) from Water Using Hydroxyapatite Coated Activated Carbon Powder Nanocomposite. J. Environ. Sci. Health Part A 2020, 55, 596–605. [Google Scholar] [CrossRef]
- El-Nagar, D.A.; Massoud, S.A.; Ismail, S.H. Removal of some heavy metals and fungicides from aqueous solutions using nano-hydroxyapatite, nano-bentonite and nanocomposite. Arab. J. Chem. 2020, 13, 7695–7706. [Google Scholar] [CrossRef]
- Das, K.C.; Dhar, S.S. Removal of cadmium(II) from aqueous solution by hydroxyapatite-encapsulated zinc ferrite (HAP/ZnFe2O4) nanocomposite: Kinetics and isotherm study. Environ. Sci. Pollut. Res. 2020, 27, 37977–37988. [Google Scholar] [CrossRef]
- Ma, J.; Xia, M.; Zhu, S.; Wang, F. A new alendronate doped HAP nanomaterial for Pb2+, Cu2+ and Cd2+ effect absorption. J. Hazard. Mater. 2020, 400, 123143. [Google Scholar] [CrossRef]
- Roque-Ruiz, J.H.; Garibay-Alvarado, J.A.; Medellín-Castillo, N.A.; Reyes-López, S.Y. Preparation of Electrospun Hydroxyapatite-Glass Fibers for Removal of Cadmium (Cd+2) and Lead (Pb+2) from Aqueous Media. Water Air Soil Pollut. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Zhang, A.; Zhang, H. Nano-hydroxyapatite incorporated gelatin/zein nanofibrous membranes: Fabrication, characterization and copper adsorption. Int. J. Biol. Macromol. 2020, 154, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Afifi, M.; Awwad, N.S.; Ibrahium, H.A. Pb(II) and Cd(II) removal, mechanical and morphological features of nanofibrous membranes of cellulose acetate containing fillers of hydroxyapatite, graphene oxide, and magnetite. Appl. Phys. A 2020, 126, 1–12. [Google Scholar] [CrossRef]
- UlAinab, Q.; Zhangab, H.; Yaseen, M.; Rasheed, U.; Liub, K.; Subhanac, S.; Tonga, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Al-Wafi, R.; Ahmed, M.; Mansour, S. Tuning the synthetic conditions of graphene oxide/magnetite/hydroxyapatite/cellulose acetate nanofibrous membranes for removing Cr(VI), Se(IV) and methylene blue from aqueous solutions. J. Water Process. Eng. 2020, 38, 101543. [Google Scholar] [CrossRef]
- Ma, K.; Cui, H.; Zhou, A.; Wu, H.; Dong, X.; Zu, F.; Yi, J.; Wang, R.; Xu, Q. Mesoporous hydroxyapatite: Synthesis in molecular self-assembly and adsorption properties. Microporous Mesoporous Mater. 2021, 323, 111164. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Design of hydroxyapatite aerogel with excellent adsorption performance to uranium. J. Environ. Chem. Eng. 2021, 9, 106364. [Google Scholar] [CrossRef]
- Nam, P.T.; Thanh, D.T.M.; Phuong, N.T.; Trang, N.T.T.; Hong, C.T.; Anh, V.T.K.; Lam, T.D.; Thom, N.T. Adsorption of Ag+ Ions Using Hydroxyapatite Powder and Recovery Silver by Electrodeposition. Vietnam J. Chem. 2021, 59, 179–186. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Jiang, X.; Sun, Y.; Yang, H.; Liu, Q.; Cao, Y.; Zhang, Y.; Cheng, H. Synthesis of strontium (Sr) doped hydroxyapatite (HAp) nanorods for enhanced adsorption of Cr (VI) ions from wastewater. Ceram. Int. 2021, 47, 16730–16736. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Li, Y.; Liu, J.; Yan, Z. Adsorption characteristics of Pb(II), Cd(II) and Cu(II) on carbon nanotube-hydroxyapatite. Environ. Technol. 2021, 42, 1560–1581. [Google Scholar] [CrossRef]
- Ferri, M.; Campisi, S.; Polito, L.; Shen, J.; Gervasini, A. Tuning the sorption ability of hydroxyapatite/carbon composites for the simultaneous remediation of wastewaters containing organic-inorganic pollutants. J. Hazard. Mater. 2021, 420, 126656. [Google Scholar] [CrossRef]
- Shen, X.; Gao, X.; Wei, W.; Zhang, Y.; Ma, L.; Liu, H.; Han, R.; Lin, J. Combined performance of hydroxyapatite adsorption and magnetic separation processes for Cd(II) removal from aqueous solution. J. Dispers. Sci. Technol. 2021, 42, 664–676. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Wang, F.; Ma, J. Experimental and theoretical study on the adsorption mechanism of Amino trimethylphosphate (ATMP) functionalized hydroxyapatite on Pb (II) and Cd (II). Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127029. [Google Scholar] [CrossRef]
- Ahmed, W.; Núñez-Delgado, A.; Mehmood, S.; Ali, S.; Qaswar, M.; Shakoor, A.; Chen, D.-Y. Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: Adsorption behavior and mechanism. Environ. Res. 2021, 201, 111518. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.S.; Wimalasiri, A.K.D.V.K.; Dziemidowicz, K.; Williams, G.R.; Koswattage, K.R.; Dissanayake, D.P.; de Silva, K.M.N.; de Silva, R.M. Biopolymer-Based Nanohydroxyapatite Composites for the Removal of Fluoride, Lead, Cadmium, and Arsenic from Water. ACS Omega 2021, 6, 8517–8530. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Zeng, W.-Y. Adsorption of Cu(II) by Poly-γ-glutamate/Apatite Nanoparticles. Polymers 2021, 13, 962. [Google Scholar] [CrossRef] [PubMed]
- Gibert, O.; Valderrama, C.; Martínez, M.; Darbra, R.; Moncunill, J.; Martí, V. Hydroxyapatite Coatings on Calcite Powder for the Removal of Heavy Metals from Contaminated Water. Water 2021, 13, 1493. [Google Scholar] [CrossRef]
- Yang, W.; Xi, D.; Li, C.; Yang, Z.; Lin, Z.; Si, M. “In-situ synthesized” iron-based bimetal promotes efficient removal of Cr(VI) in by zero-valent iron-loaded hydroxyapatite. J. Hazard. Mater. 2021, 420, 126540. [Google Scholar] [CrossRef]
- Billah, R.E.K.; Khan, M.A.; Park, Y.-K.; Am, A.; Majdoubi, H.; Haddaji, Y.; Jeon, B.-H. A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites. Polymers 2021, 13, 3427. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Liu, S.; Jiang, T.; Chen, W.; Li, D.; Yuan, J.; Xu, F. A Study of Adsorption Behaviour of Cu(II) on Hydroxyapatite-Coated-Limestone/Chitosan Composite. J. Polym. Environ. 2021, 29, 1727–1741. [Google Scholar] [CrossRef]
- Rajak, J.K.; Khandelwal, N.; Behera, M.P.; Tiwari, E.; Singh, N.; Ganie, Z.A.; Schäfer, T. Removal of Chromate Ions from Leachate-Contaminated Groundwater Samples of Khan Chandpur, India, Using Chitin Modified Iron-Enriched Hydroxyapatite Nanocomposite. Environ. Sci. Pollut. Res. Int. 2021, 28, 41760–41771. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Lan, G.; Qiu, H.; Xu, B.; Xu, Q.; Sun, N.; Zhang, L. Pb(II) adsorption characteristics of magnetic GO-hydroxyapatite and the contribution of GO to enhance its acid resistance. J. Environ. Chem. Eng. 2021, 9, 105310. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly enhanced adsorption performance to uranium(VI) by facile synthesized hydroxyapatite aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Zhou, C.; Song, X.; Wang, Y.; Wang, H.; Ge, S. The sorption and short-term immobilization of lead and cadmium by nano-hydroxyapatite/biochar in aqueous solution and soil. Chemosphere 2022, 286, 131810. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.R.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Chen, Z.; Zhou, X.; Lu, X.; Liu, J. Pollution characteristics and source analysis of heavy metals in surface sediments of Luoyuan Bay, Fujian. Environ. Res. 2022, 203, 111911. [Google Scholar] [CrossRef]
- Oulguidoum, A.; Bouiahya, K.; Bouyarmane, H.; Talbaoui, A.; Nunzi, J.-M.; Laghzizil, A. Mesoporous nanocrystalline sulfonated hydroxyapatites enhance heavy metal removal and antimicrobial activity. Sep. Purif. Technol. 2021, 255, 117777. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Yang, H.; Ye, B.-C. Hydrothermal synthesis of hierarchical hollow hydroxyapatite microspheres with excellent fluoride adsorption property. Microporous Mesoporous Mater. 2019, 289, 109620. [Google Scholar] [CrossRef]
- Bensalah, H.; Younssi, S.A.; Ouammou, M.; Gurlo, A.; Bekheet, M.F. Azo dye adsorption on an industrial waste-transformed hydroxyapatite adsorbent: Kinetics, isotherms, mechanism and regeneration studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- LoIacono, S.; Crini, G.; Martel, B.; Chanet, G.; Cosentino, C.; Raschetti, M.; Placet, V.; Torri, G.; Morin-Crini, N. Simultaneous removal of Cd, Co, Cu, Mn, Ni, and Zn from synthetic solutions on a hemp-based felt. II. Chemical modification. J. Appl. Polym. Sci. 2017, 134, 45138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).