Recent Progress in the Application of Hydroxyapatite for the Adsorption of Heavy Metals from Water Matrices
Abstract
:1. Introduction
2. Adsorption Process Modeling and Mechanism
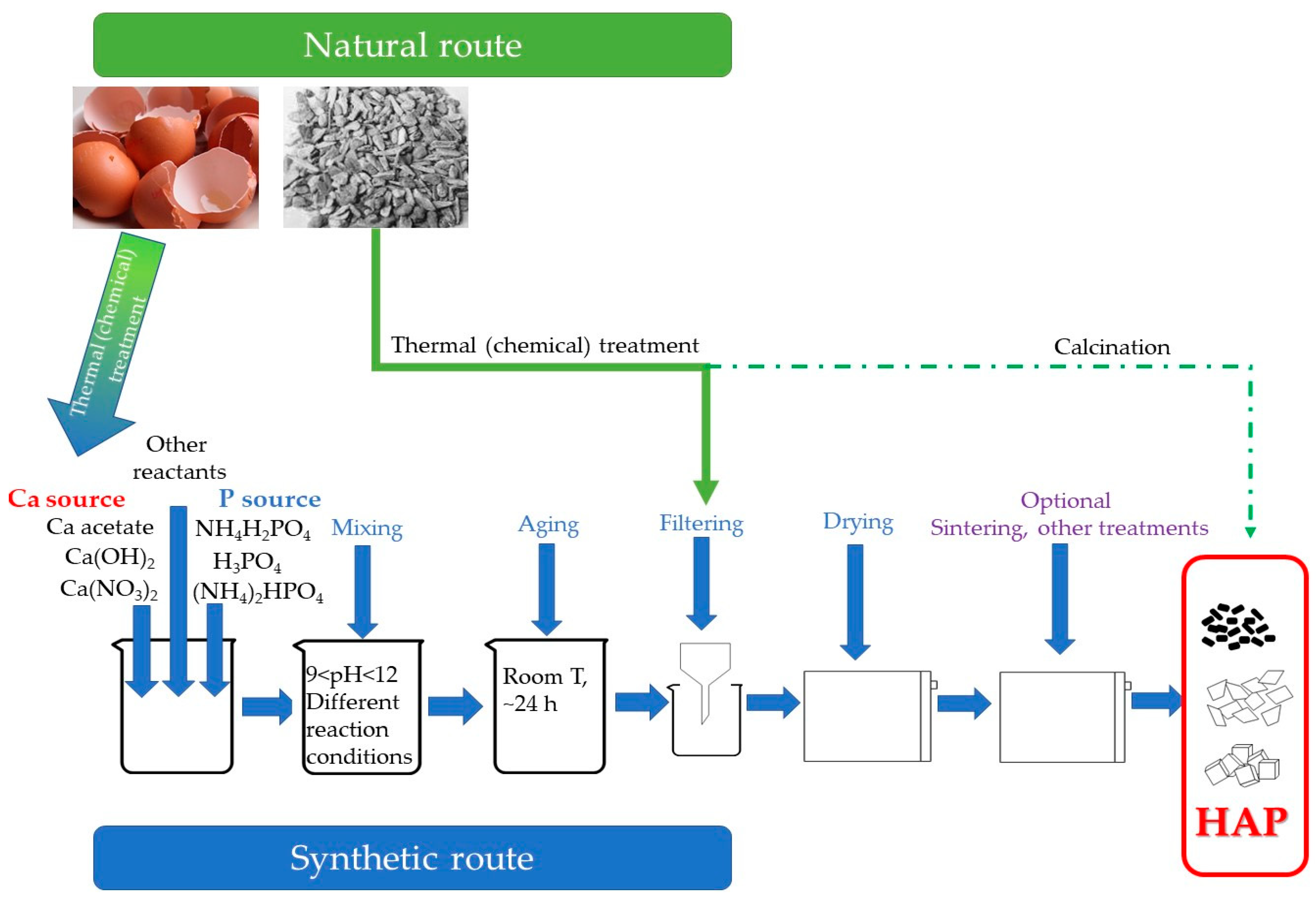
3. Application of Natural-Derived Hydroxyapatite for the Removal of Heavy Elements
4. Adsorption of Heavy Metals Using Synthesized Hydroxyapatite
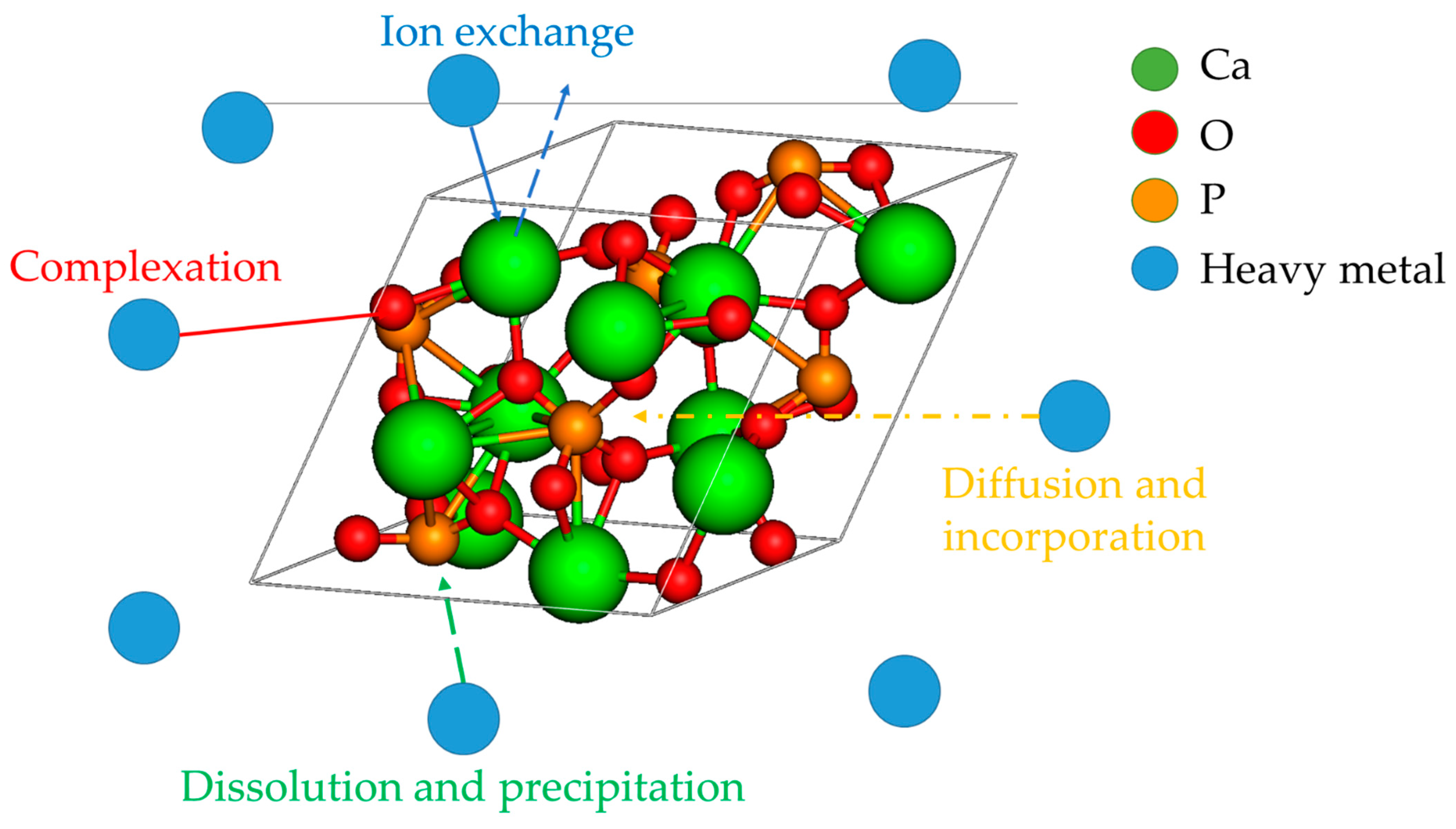
5. Mechanistic Aspects and Current Limitations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Ispas, G.C.; Manea, R.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C.; Raduly, M.F. Iron Oxide/Phosphatic Materials Composites with Potential Applications in Environmental Protection. Materials 2020, 13, 5034. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Garg, V.K.; Kadirvelu, K.; Sillanpää, M. Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons. Int. J. Environ. Sci. Technol. 2015, 13, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Osuna-Martínez, C.C.; Armienta, M.A.; Tiznado, M.E.B.; Páez-Osuna, F. Arsenic in waters, soils, sediments, and biota from Mexico: An environmental review. Sci. Total Environ. 2021, 752, 142062. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total. Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Bano, Z.; Mazari, S.; Saeed, R.Y.; Majeed, M.A.; Xia, M.; Memon, A.Q.; Abro, R.; Wang, F. Water decontamination by 3D graphene based materials: A review. J. Water Process. Eng. 2020, 36, 101404. [Google Scholar] [CrossRef]
- Sun, X.; Guo, P.; Sun, Y.; Cui, Y. Adsorption of Hexavalent Chromium by Sodium Alginate Fiber Biochar Loaded with Lanthanum. Materials 2021, 14, 2224. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Mongioví, C.; Morin-Crini, N.; Lacalamita, D.; Bradu, C.; Raschetti, M.; Placet, V.; Ribeiro, A.; Ivanovska, A.; Kostić, M.; Crini, G. Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties. Molecules 2021, 26, 4199. [Google Scholar] [CrossRef]
- Zhang, D.; Crini, G.; Lichtfouse, E.; Rhimi, B.; Wang, C. Removal of Mercury Ions from Aqueous Solutions by Crosslinked Chitosan-based Adsorbents: A Mini Review. Chem. Rec. 2020, 20, 1220–1234. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Rahman, S.; Sathasivam, K.V. Heavy Metal Adsorption ontoKappaphycussp. from Aqueous Solutions: The Use of Error Functions for Validation of Isotherm and Kinetics Models. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica AND Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Roginsky, S.Z.; Zeldovich, Y.B. Die Katalische Oxidation von Kohlenmonoxyd Auf Mangandioxyd. Acta Physiochim. URSS 1934, 1, 554–594. [Google Scholar]
- Dubinin, M.M. The Equation of the Characteristic Curve of Activated Charcoal. Proc. USSR Acad. Sci. 1947, 55, 327–329. [Google Scholar]
- Hobson, J.P. Physical adsorption isotherms extending from ultrahigh vacuum to vapor pressure. J. Phys. Chem. 1969, 73, 2720–2727. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar]
- Ho, Y.-S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 1995. [Google Scholar]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, J.W., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Willard Gibbs, J. A method of geometrical representation of the thermodynamic properties of substances by means of surfaces. In Transactions of the Connecticut Academy of Arts and Sciences; Connecticut Academy of Arts and Sciences: New Haven, CT, USA, 1873; Volume 2, pp. 382–404. [Google Scholar]
- Gueu, S.; Yao, B.; Adouby, K.; Ado, G. Kinetics and thermodynamics study of lead adsorption on to activated carbons from coconut and seed hull of the palm tree. Int. J. Environ. Sci. Technol. 2007, 4, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.-H.; Liu, S.-C.; Chen, C.-Y. Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lee, J.Y.; Park, Y.-T.; Baek, K.; Choi, J. Adsorption of As(III), As(V), Cd(II), Cu(II), and Pb(II) from Aqueous Solutions by Natural Muscovite. Sep. Sci. Technol. 2010, 45, 814–823. [Google Scholar] [CrossRef]
- Shahmohammadi-Kalalagh, S. Isotherm and Kinetic Studies on Adsorption of Pb, Zn and Cu by Kaolinite. Casp. J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Lasheen, M.R.; Ammar, N.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Putra, W.P.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies. J. Encapsul. Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Singh, A.; Sharma, A. Studies on the uptake of lead and zinc by lignin obtained from black liquor—A paper industry waste material. Environ. Technol. 1994, 15, 353–361. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Volesky, B.; Prasetyo, I. Cadmium removal in a biosorption column. Biotechnol. Bioeng. 1994, 43, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Tare, V.; Chaudhari, S.; Jawed, M. Comparative Evaluation of Soluble and Insoluble Xanthate Process for Heavy Metal Removal from Wastewaters. Water Sci. Technol. 1992, 26, 237–246. [Google Scholar] [CrossRef]
- Akpan, E.; Dauda, M.; Kuburi, L.; Obada, D.; Dodoo-Arhin, D. A comparative study of the mechanical integrity of natural hydroxyapatite scaffolds prepared from two biogenic sources using a low compaction pressure method. Results Phys. 2020, 17, 103051. [Google Scholar] [CrossRef]
- Hassan, H.S.; El-Kamash, A.M.; Ibrahim, H.A.-S. Evaluation of hydroxyapatite/poly(acrylamide-acrylic acid) for sorptive removal of strontium ions from aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 25641–25655. [Google Scholar] [CrossRef] [PubMed]
- Caballero, N.; Ozuna, P.C.; Monteiro, M. Kinetic Analysis of Lead Removal by Natural Hydroxyapatite from Aqueous Solution in High Concentration. Mater. Res. 2019, 22, 22. [Google Scholar] [CrossRef]
- Vahdat, A.; Ghasemi, B.; Yousefpour, M. Synthesis of hydroxyapatite and hydroxyapatite/Fe3O4 nanocomposite for removal of heavy metals. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100233. [Google Scholar] [CrossRef]
- Xia, X.; Shen, J.; Cao, F.; Wang, C.; Tang, M.; Zhang, Q.; Wei, S. A facile synthesis of hydroxyapatite for effective removal strontium ion. J. Hazard. Mater. 2019, 368, 326–335. [Google Scholar] [CrossRef]
- Núñez, D.; Serrano, J.A.; Mancisidor, A.; Elgueta, E.; Varaprasad, K.; Oyarzún, P.; Cáceres, R.; Ide, W.; Rivas, B.L. Heavy metal removal from aqueous systems using hydroxyapatite nanocrystals derived from clam shells. RSC Adv. 2019, 9, 22883–22890. [Google Scholar] [CrossRef] [Green Version]
- Meski, S.; Tazibt, N.; Khireddine, H.; Ziani, S.; Biba, W.; Yala, S.; Sidane, D.; Boudjouan, F.; Moussaoui, N. Synthesis of hydroxyapatite from mussel shells for effective adsorption of aqueous Cd(II). Water Sci. Technol. 2019, 80, 1226–1237. [Google Scholar] [CrossRef]
- Bernalte, E.; Kamieniak, J.; Randviir, E.P.; Bernalte-García, Á.; Banks, C.E. The preparation of hydroxyapatite from unrefined calcite residues and its application for lead removal from aqueous solutions. RSC Adv. 2019, 9, 4054–4062. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Tang, W.; Ding, C.; Yang, L.; Gong, D.; Kang, Z.; He, Z.; Wu, Y. Preparation of anionic-cationic co-substituted hydroxyapatite for heavy metal removal: Performance and mechanisms. J. Solid State Chem. 2019, 280, 120960. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siéwé, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of Cu(II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Kumar, P.S.; Woumfo, E.D.; Prasanth, S.M. Adsorption of Pb(II) and Cd(II) ions onto modified biogenic slaughterhouse waste: Equilibrium and kinetic analysis. Int. J. Environ. Anal. Chem. 2020, 1–20. [Google Scholar] [CrossRef]
- Omar, S.; Muhamad, M.S.; Chuan, L.T.E.; Rudi, N.N.; Hamidon, N.; Hamid, N.H.A.; Harun, H.; Sunar, N.M.; Ali, R. Effect of Hydroxyapatite (HAp) Adsorbent Dosage towards Lead Removal. Int. J. Emerg. Trends Eng. Res. 2020, 8, 201–205. [Google Scholar] [CrossRef]
- Ramdani, A.; Kadeche, A.; Adjdir, M.; Taleb, Z.; Ikhou, D.; Taleb, S.; Deratani, A. Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Pract. Technol. 2020, 15, 130–141. [Google Scholar] [CrossRef]
- Ramdani, A.; Taleb, Z.; Guendouzi, A.; Kadeche, A.; Herbache, H.; Mostefai, A.; Taleb, S.; Deratani, A. Mechanism study of metal ion adsorption on porous hydroxyapatite: Experiments and modeling. Can. J. Chem. 2020, 98, 79–89. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Bi, L.; Luan, X.; Geng, F.; Xu, X.; Chen, Y.; Zhang, F. Microwave-assisted synthesis of hollow microspheres with multicomponent nanocores for heavy-metal removal and magnetic sensing. ACS Appl. Mater. Interfaces 2020, 12, 46779–46787. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cocoletzi, H.; Salinas, R.A.; Águila-Almanza, E.; Rubio-Rosas, E.; Chai, W.S.; Chew, K.W.; Mariscal-Hernández, C.; Show, P.L. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ. Technol. Innov. 2020, 20, 101109. [Google Scholar] [CrossRef]
- Sricharoen, P.; Limchoowong, N.; Nuengmatcha, P.; Chanthai, S. Ultrasonic-assisted recycling of Nile tilapia fish scale biowaste into low-cost nano-hydroxyapatite: Ultrasonic-assisted adsorption for Hg2+ removal from aqueous solution followed by “turn-off” fluorescent sensor based on Hg2+-graphene quantum dots. Ultrason. Sonochem. 2020, 63, 104966. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, Y.M.; Andoshe, D.; Desissa, T.D. Composite of bentonite/CoFe2O4/hydroxyapatite for adsorption of Pb (II). Mater. Res. Express 2020, 7, 115501. [Google Scholar] [CrossRef]
- Hariani, P.L.; Riyanti, F.; Fatma, F.; Rachmat, A.; Herbanu, A. Removal of Pb(II) using Hydroxyapatite from Golden Snail Shell (Pomacea canaliculata L.) Modified with Silica. Molekul 2020, 15, 130–139. [Google Scholar] [CrossRef]
- Elsanafeny, H.A.; Aly, M.M.A.; Hasan, M.A.; Lasheen, Y.F.; Youssef, M.A. Synthesis and polymeric modification of hydroxyapatite from biogenic raw material for adsorptive removal of Co2+ and Sr2+. J. Radioanal. Nucl. Chem. 2020, 326, 1119–1133. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Ahmadi, A.; Akbari, A.; Farjadfard, S.; Ramavandi, B. Adsorption mercury, cobalt, and nickel with a reclaimable and magnetic composite of hydroxyapatite/Fe3O4/polydopamine. J. Environ. Chem. Eng. 2021, 9, 105709. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A.; Behjat, Y. Invent of a simultaneous adsorption and separation process based on dynamic membrane for treatment Zn(II), Ni(II) and, Co(II) industrial wastewater. Arab. J. Chem. 2021, 14, 103231. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adedapo, A.E.; Oladeji, O.B.; Sodiya, E.F.; Ibadin, F.H.; Zhang, D. Nano-rod hydroxyapatite for the uptake of nickel ions: Effect of sintering behaviour on adsorption parameters. J. Environ. Chem. Eng. 2021, 9, 105931. [Google Scholar] [CrossRef]
- Bambaeero, A.; Bazargan-Lari, R. Simultaneous removal of copper and zinc ions by low cost natural snail shell/hydroxyapatite/chitosan composite. Chin. J. Chem. Eng. 2021, 33, 221–230. [Google Scholar] [CrossRef]
- Ali, M.M.S.; Imam, D.M.; El-Nadi, Y.A. Vanadium(V) removal and recovery by adsorption onto modified activated carbon derived from natural hydroxyapatite. J. Iran. Chem. Soc. 2021, 18, 2771–2784. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hemmati, S.; Ahmadi, A.; Falletta, E.; Ramavandi, B.; Bianchi, C.L. Zn2+ removal from the aqueous environment using a polydopamine/hydroxyapatite/Fe3O4 magnetic composite under ultrasonic waves. RSC Adv. 2021, 11, 27309–27321. [Google Scholar] [CrossRef]
- Wei, W.; Han, X.; Shao, Y.; Xie, W.; Zhang, Y.; Yao, Y.; Zhao, W.; Han, R.; Li, S.; Zheng, C. Comparing the effects of humic acid and oxalic acid on Pb(II) immobilization by a green synthesized nanocrystalline hydroxyapatite. Chemosphere 2021, 285, 131411. [Google Scholar] [CrossRef]
- Ayodele, O.; Olusegun, S.J.; Oluwasina, O.O.; Okoronkwo, E.A.; Olanipekun, E.O.; Mohallem, N.D.; Guimarães, W.G.; de Gomes, B.L.M.; de Souza, G.O.; Duarte, H.A. Experimental and theoretical studies of the adsorption of Cu and Ni ions from wastewater by hydroxyapatite derived from eggshells. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100439. [Google Scholar] [CrossRef]
- Serhiienko, A.; Dontsova, T.; Yanushevska, O.; Lapinskyi, A.; Krymets, G. Synthesis and characterization of hydroxyapatite and composite based on it with collagen/alginate. Chem. Pap. 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Fierascu, I.; Fierascu, R.C.; Somoghi, R.; Ion, R.M.; Moanţă, A.; Avramescu, S.; Damian, C.M.; Ditu, L.M. Tuned apatitic materials: Synthesis, characterization and potential antimicrobial applications. Appl. Surf. Sci. 2018, 438, 127–135. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Guegan, R.; Beuran, M.; Costescu, A.; Predoi, D. Adsorption of Pb (II) Ions onto Hydroxyapatite Nanopowders in Aqueous Solutions. Materials 2018, 11, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanets, A.; Kitikova, N.; Shashkova, I.; Roshchina, M.; Srivastava, V.; Sillanpää, M. Adsorption performance of hydroxyapatite with different crystalline and porous structure towards metal ions in multicomponent solution. J. Water Process. Eng. 2019, 32, 100963. [Google Scholar] [CrossRef]
- Oulguidoum, A.; Bouyarmane, H.; Laghzizil, A.; Nunzi, J.-M.; Saoiabi, A. Development of sulfonate-functionalized hydroxyapatite nanoparticles for cadmium removal from aqueous solutions. Colloid Interface Sci. Commun. 2019, 30, 100178. [Google Scholar] [CrossRef]
- Doan, V.D.; Le, V.T.; Le, H.S.; Ta, D.H.; Nguyen, H.T. Effectiveness of Calcium Deficiency in Nanosized Hydroxyapatite for Removal of Fe(II), Cu(II), Ni(II) and Cr(VI) Ions from Aqueous Solutions. J. Nano Res. 2019, 56, 17–27. [Google Scholar] [CrossRef]
- De Resende, N.S.; Camargo, C.; Reis, P.C.; Perez, C.A.C.; Salim, V.M. Mechanisms of mercury removal from aqueous solution by high-fixation hydroxyapatite sorbents. Int. J. Environ. Sci. Technol. 2019, 16, 7221–7228. [Google Scholar] [CrossRef]
- Ferri, M.; Campisi, S.; Gervasini, A. Nickel and cobalt adsorption on hydroxyapatite: A study for the de-metalation of electronic industrial wastewaters. Adsorption 2019, 25, 649–660. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, Y.; Zhang, Z. Preparation of hydroxyapatite nanostructures with different morphologies and adsorption behavior on seven heavy metals ions. J. Contam. Hydrol. 2019, 226, 103538. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Le, T.P.T.; Do, H.T.; Vo, H.T.; Pham, N.T.; Nguyen, T.T.; Cao, H.T.; Nguyen, P.T.; Dinh, T.M.T.; Le, H.V.; et al. Fabrication of Porous Hydroxyapatite Granules as an Effective Adsorbent for the Removal of Aqueous Pb(II) Ions. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Yuan, L.; Yan, M.; Huang, Z.; He, K.; Zeng, G.; Chen, A.; Hu, L.; Li, H.; Peng, M.; Huang, T.; et al. Influences of pH and metal ions on the interactions of oxytetracycline onto nano-hydroxyapatite and their co-adsorption behavior in aqueous solution. J. Colloid Interface Sci. 2019, 541, 101–113. [Google Scholar] [CrossRef]
- Su, M.; Tsang, D.; Ren, X.; Shi, Q.; Tang, J.; Zhang, H.; Kong, L.; Hou, L.; Song, G.; Chen, D. Removal of U(VI) from nuclear mining effluent by porous hydroxyapatite: Evaluation on characteristics, mechanisms and performance. Environ. Pollut. 2019, 254, 112891. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Li, S.; Zhu, J.; Liu, W.; Zhang, Z. Self-templated microwave-assisted hydrothermal synthesis of two-dimensional holey hydroxyapatite nanosheets for efficient heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 30076–30086. [Google Scholar] [CrossRef] [PubMed]
- Shashkova, I.L.; Ivanets, A.I.; Kitikova, N.V. Sorption of Co2+, Pb2+, and Sr2+ Ions on Hydroxyapatite, Synthesized in the Presence of Oxyethylidenediphosphonic Acid. Russ. J. Appl. Chem. 2019, 92, 625–633. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, C.; Xu, Z.; Luo, P.; Fu, Z.; Zhang, J. Synthesis of bitter gourd-shaped nanoscaled hydroxyapatite and its adsorption property for heavy metal ions. Mater. Lett. 2019, 241, 176–179. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Wang, D.; Han, X. Electrospinning Synthesis of Hydroxyapatite Nanofibers Assembled from Nanorods and their Adsorption for Heavy Metal Ions. Pol. J. Environ. Stud. 2019, 28, 981–988. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Direct bromination of nano hydroxyapatite strategy towards particle brushes via surface-initiated ATRP for highly efficient heavy metal removal. Polymer 2019, 183, 121883. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Unexpectedly High Adsorption Capacity of Esterified Hydroxyapatite for Heavy Metal Removal. Langmuir 2019, 35, 16111–16119. [Google Scholar] [CrossRef]
- Peng, X.; Chen, W.; He, Z.; Li, D.; Liu, H.; Jin, H.; Zhou, G.; Xu, F. Removal of Cu(II) from wastewater using doped HAP-coated-limestone. J. Mol. Liq. 2019, 293, 293. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Li, L.; Han, R.; Wang, G.; Wei, W. Nanosized hydroxyapatite supported on natural sepiolite: A novel adsorbent for Cd(II) removal from simulated groundwater. Mater. Res. Express 2019, 6, 125518. [Google Scholar] [CrossRef]
- El-Maghrabi, H.; Younes, A.A.; Salem, A.; Rabie, K.; El-Shereafy, E.-S. Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): Preparation, characterization and adsorption optimization. J. Hazard. Mater. 2019, 378, 120703. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Kang, C.; Zhang, J.; Xu, Z.; Jiang, C.; Luo, P.; Fu, Z.; Ding, M.; Lv, Y. Synthesis of magnetic Fe-doped hydroxyapatite nanocages with highly efficient and selective adsorption for Cd2+. Mater. Lett. 2019, 253, 144–147. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Imran, M.; Muhammad, N.; Howari, F.M.; Alkhoori, S.A.; Khan, J.A.; Khan, Z.U.H.; Bhatnagar, A.; et al. Synergistic effects of activated carbon and nano-zerovalent copper on the performance of hydroxyapatite-alginate beads for the removal of As3+ from aqueous solution. J. Clean. Prod. 2019, 235, 875–886. [Google Scholar] [CrossRef]
- Ansari, A.; Vahedi, S.; Tavakoli, O.; Khoobi, M.; Faramarzi, M.A. Novel Fe3O4/hydroxyapatite/β-cyclodextrin nanocomposite adsorbent: Synthesis and application in heavy metal removal from aqueous solution. Appl. Organomet. Chem. 2019, 33, e4634. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Sun, K.; He, Y.; Song, P.; Zhang, D.; Wang, R. Preparation of hydroxyapatite-based porous materials for absorption of lead ions. Water Sci. Technol. 2019, 80, 1266–1275. [Google Scholar] [CrossRef]
- Long, Y.; Jiang, J.; Hu, J.; Hu, X.; Yang, Q.; Zhou, S. Removal of Pb(II) from aqueous solution by hydroxyapatite/carbon composite: Preparation and adsorption behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 471–479. [Google Scholar] [CrossRef]
- Jayaweera, H.D.A.C.; Siriwardane, I.; De Silva, K.M.N.; De Silva, R.M. Synthesis of multifunctional activated carbon nanocomposite comprising biocompatible flake nano hydroxyapatite and natural turmeric extract for the removal of bacteria and lead ions from aqueous solution. Chem. Cent. J. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Fox, J.T. Synthesis and appraisal of a hydroxyapatite/pectin hybrid material for zinc removal from water. RSC Adv. 2019, 9, 21095–21105. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.; de Almeida, O.; Brasil, H.; Moraes, D.; dos Reis, M. Adsorption of chromium (VI) on hydrotalcite-hydroxyapatite material doped with carbon nanotubes: Equilibrium, kinetic and thermodynamic study. Appl. Clay Sci. 2019, 172, 57–64. [Google Scholar] [CrossRef]
- Hokkanen, S.; Doshi, B.; Srivastava, V.; Puro, L.; Koivula, R. Arsenic (III) removal from water by hydroxyapatite-bentonite clay-nanocrystalline cellulose. Environ. Prog. Sustain. Energy 2019, 38, 13147. [Google Scholar] [CrossRef]
- Zhou, M.; Xie, F.; Li, G.; Wang, Q.; Tang, L.; Yan, M.; Bi, H.; Fei, X. Biocompatible HA@Fe3O4@N-CDs hybrids for detecting and absorbing lead ion. J. Biomed. Mater. Res. Part A 2019, 107, 1532–1540. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Majumdar, S.; Sarkar, S.; Kundu, B.; Sahoo, G.C. Performance investigation of Pb(II) removal by synthesized hydroxyapatite based ceramic ultrafiltration membrane: Bench scale study. Chem. Eng. J. 2019, 355, 510–519. [Google Scholar] [CrossRef]
- Thang, N.H.; Phong, D.T. Characterizations of Hydroxyapatite Synthesized from Calcium Hydroxide and Phosphoric Acid as Adsorbents of Lead in Wastewater. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2020; Volume 991, pp. 159–165. [Google Scholar]
- Jiang, J.; Long, Y.; Hu, X.; Hu, J.; Zhu, M.; Zhou, S. A facile microwave-assisted synthesis of mesoporous hydroxyapatite as an efficient adsorbent for Pb2+ adsorption. J. Solid State Chem. 2020, 289, 121491. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Song, X.; Wang, Y.; Fang, D.; Ge, S.; Zhang, R. Insights into dynamic adsorption of lead by nano-hydroxyapatite prepared with two-stage ultrasound. Chemosphere 2020, 253, 126661. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J. Experimental study on the adsorption of dissolved heavy metals by nano-hydroxyapatite. Water Sci. Technol. 2020, 82, 1825–1832. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, X.; Zhang, Y.; Wang, S.; Wang, M.; Wang, Y.; Weerakoon, W.M.S.B.; Sanginova, O. Study on immobilization of diatomite, Ca(H2PO4)2, CaCO3, HAP and nano-HAP for heavy metal contaminated sediment. Water Qual. Res. J. 2020, 55, 370–381. [Google Scholar] [CrossRef]
- Wu, M.; Mo, L.; Bi, E. Effects of fulvic acid and montmorillonite colloids at different concentrations on Cd(II) sorption onto nano-hydroxyapatite. Chemosphere 2020, 248, 125992. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Su, M.; Wei, L.; Wei, Y.; Liang, J.; Liu, Y.; Luo, Y. Comprehensive evaluation of the effectiveness on metals recovery and decontamination from MSWI fly ash by an integrating hydrometallurgical process in Guangzhou. Sci. Total Environ. 2020, 728, 138809. [Google Scholar] [CrossRef]
- Shi, Q.; Su, M.; Yuvaraja, G.; Tang, J.; Kong, L.; Chen, D. Development of highly efficient bundle-like hydroxyapatite towards abatement of aqueous U(VI) ions: Mechanism and economic assessment. J. Hazard. Mater. 2020, 394, 122550. [Google Scholar] [CrossRef]
- Aouay, R.; Jebri, S.; Rebelo, A.; Ferreira, J.M.F.; Khattech, I. Enhanced cadmium removal from water by hydroxyapatite subjected to different thermal treatments. J. Water Supply Res. Technol. 2020, 69, 678–693. [Google Scholar] [CrossRef]
- Zheng, N.; Yin, L.; Su, M.; Liu, Z.; Tsang, D.; Chen, D. Synthesis of shape and structure-dependent hydroxyapatite nanostructures as a superior adsorbent for removal of U(VI). Chem. Eng. J. 2020, 384, 123262. [Google Scholar] [CrossRef]
- Szenknect, S.; Mesbah, A.; Descostes, M.; Maihatchi-Ahamed, A.; Bonato, L.; Massonnet, M.; Ziouane, Y.; Vors, E.; Vercouter, T.; Clavier, N.; et al. Uranium removal from mining water using Cu substituted hydroxyapatite. J. Hazard. Mater. 2020, 392, 122501. [Google Scholar] [CrossRef]
- Ahmed, M.; Mansour, S.; Ramadan, R.; Afifi, M.; Mostafa, M.S.; El-Dek, S.; Uskoković, V. Tuning the composition of new brushite/vivianite mixed systems for superior heavy metal removal efficiency from contaminated waters. J. Water Process. Eng. 2020, 34, 101090. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, S.; Ma, J.; Wang, F.; Xu, H.; Xia, M. The facile synthesis of zoledronate functionalized hydroxyapatite amorphous hybrid nanobiomaterial and its excellent removal performance on Pb2+ and Cu2+. J. Hazard. Mater. 2020, 392, 122291. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liao, B.; Lu, T.; Wang, H.; Xu, L.; Li, Z.; Ye, C. Insight into immobilization of Pb2+ in aqueous solution and contaminated soil using hydroxyapatite/attapulgite composite. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125290. [Google Scholar] [CrossRef]
- Rout, S.; Muduli, B.; Kumar, A.; Pulhani, V. Removal of Uranium(VI) from Water Using Hydroxyapatite Coated Activated Carbon Powder Nanocomposite. J. Environ. Sci. Health Part A 2020, 55, 596–605. [Google Scholar] [CrossRef]
- El-Nagar, D.A.; Massoud, S.A.; Ismail, S.H. Removal of some heavy metals and fungicides from aqueous solutions using nano-hydroxyapatite, nano-bentonite and nanocomposite. Arab. J. Chem. 2020, 13, 7695–7706. [Google Scholar] [CrossRef]
- Das, K.C.; Dhar, S.S. Removal of cadmium(II) from aqueous solution by hydroxyapatite-encapsulated zinc ferrite (HAP/ZnFe2O4) nanocomposite: Kinetics and isotherm study. Environ. Sci. Pollut. Res. 2020, 27, 37977–37988. [Google Scholar] [CrossRef]
- Ma, J.; Xia, M.; Zhu, S.; Wang, F. A new alendronate doped HAP nanomaterial for Pb2+, Cu2+ and Cd2+ effect absorption. J. Hazard. Mater. 2020, 400, 123143. [Google Scholar] [CrossRef]
- Roque-Ruiz, J.H.; Garibay-Alvarado, J.A.; Medellín-Castillo, N.A.; Reyes-López, S.Y. Preparation of Electrospun Hydroxyapatite-Glass Fibers for Removal of Cadmium (Cd+2) and Lead (Pb+2) from Aqueous Media. Water Air Soil Pollut. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Zhang, A.; Zhang, H. Nano-hydroxyapatite incorporated gelatin/zein nanofibrous membranes: Fabrication, characterization and copper adsorption. Int. J. Biol. Macromol. 2020, 154, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Afifi, M.; Awwad, N.S.; Ibrahium, H.A. Pb(II) and Cd(II) removal, mechanical and morphological features of nanofibrous membranes of cellulose acetate containing fillers of hydroxyapatite, graphene oxide, and magnetite. Appl. Phys. A 2020, 126, 1–12. [Google Scholar] [CrossRef]
- UlAinab, Q.; Zhangab, H.; Yaseen, M.; Rasheed, U.; Liub, K.; Subhanac, S.; Tonga, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Al-Wafi, R.; Ahmed, M.; Mansour, S. Tuning the synthetic conditions of graphene oxide/magnetite/hydroxyapatite/cellulose acetate nanofibrous membranes for removing Cr(VI), Se(IV) and methylene blue from aqueous solutions. J. Water Process. Eng. 2020, 38, 101543. [Google Scholar] [CrossRef]
- Ma, K.; Cui, H.; Zhou, A.; Wu, H.; Dong, X.; Zu, F.; Yi, J.; Wang, R.; Xu, Q. Mesoporous hydroxyapatite: Synthesis in molecular self-assembly and adsorption properties. Microporous Mesoporous Mater. 2021, 323, 111164. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Design of hydroxyapatite aerogel with excellent adsorption performance to uranium. J. Environ. Chem. Eng. 2021, 9, 106364. [Google Scholar] [CrossRef]
- Nam, P.T.; Thanh, D.T.M.; Phuong, N.T.; Trang, N.T.T.; Hong, C.T.; Anh, V.T.K.; Lam, T.D.; Thom, N.T. Adsorption of Ag+ Ions Using Hydroxyapatite Powder and Recovery Silver by Electrodeposition. Vietnam J. Chem. 2021, 59, 179–186. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Jiang, X.; Sun, Y.; Yang, H.; Liu, Q.; Cao, Y.; Zhang, Y.; Cheng, H. Synthesis of strontium (Sr) doped hydroxyapatite (HAp) nanorods for enhanced adsorption of Cr (VI) ions from wastewater. Ceram. Int. 2021, 47, 16730–16736. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Li, Y.; Liu, J.; Yan, Z. Adsorption characteristics of Pb(II), Cd(II) and Cu(II) on carbon nanotube-hydroxyapatite. Environ. Technol. 2021, 42, 1560–1581. [Google Scholar] [CrossRef]
- Ferri, M.; Campisi, S.; Polito, L.; Shen, J.; Gervasini, A. Tuning the sorption ability of hydroxyapatite/carbon composites for the simultaneous remediation of wastewaters containing organic-inorganic pollutants. J. Hazard. Mater. 2021, 420, 126656. [Google Scholar] [CrossRef]
- Shen, X.; Gao, X.; Wei, W.; Zhang, Y.; Ma, L.; Liu, H.; Han, R.; Lin, J. Combined performance of hydroxyapatite adsorption and magnetic separation processes for Cd(II) removal from aqueous solution. J. Dispers. Sci. Technol. 2021, 42, 664–676. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Wang, F.; Ma, J. Experimental and theoretical study on the adsorption mechanism of Amino trimethylphosphate (ATMP) functionalized hydroxyapatite on Pb (II) and Cd (II). Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127029. [Google Scholar] [CrossRef]
- Ahmed, W.; Núñez-Delgado, A.; Mehmood, S.; Ali, S.; Qaswar, M.; Shakoor, A.; Chen, D.-Y. Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: Adsorption behavior and mechanism. Environ. Res. 2021, 201, 111518. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.S.; Wimalasiri, A.K.D.V.K.; Dziemidowicz, K.; Williams, G.R.; Koswattage, K.R.; Dissanayake, D.P.; de Silva, K.M.N.; de Silva, R.M. Biopolymer-Based Nanohydroxyapatite Composites for the Removal of Fluoride, Lead, Cadmium, and Arsenic from Water. ACS Omega 2021, 6, 8517–8530. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Zeng, W.-Y. Adsorption of Cu(II) by Poly-γ-glutamate/Apatite Nanoparticles. Polymers 2021, 13, 962. [Google Scholar] [CrossRef] [PubMed]
- Gibert, O.; Valderrama, C.; Martínez, M.; Darbra, R.; Moncunill, J.; Martí, V. Hydroxyapatite Coatings on Calcite Powder for the Removal of Heavy Metals from Contaminated Water. Water 2021, 13, 1493. [Google Scholar] [CrossRef]
- Yang, W.; Xi, D.; Li, C.; Yang, Z.; Lin, Z.; Si, M. “In-situ synthesized” iron-based bimetal promotes efficient removal of Cr(VI) in by zero-valent iron-loaded hydroxyapatite. J. Hazard. Mater. 2021, 420, 126540. [Google Scholar] [CrossRef]
- Billah, R.E.K.; Khan, M.A.; Park, Y.-K.; Am, A.; Majdoubi, H.; Haddaji, Y.; Jeon, B.-H. A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites. Polymers 2021, 13, 3427. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Liu, S.; Jiang, T.; Chen, W.; Li, D.; Yuan, J.; Xu, F. A Study of Adsorption Behaviour of Cu(II) on Hydroxyapatite-Coated-Limestone/Chitosan Composite. J. Polym. Environ. 2021, 29, 1727–1741. [Google Scholar] [CrossRef]
- Rajak, J.K.; Khandelwal, N.; Behera, M.P.; Tiwari, E.; Singh, N.; Ganie, Z.A.; Schäfer, T. Removal of Chromate Ions from Leachate-Contaminated Groundwater Samples of Khan Chandpur, India, Using Chitin Modified Iron-Enriched Hydroxyapatite Nanocomposite. Environ. Sci. Pollut. Res. Int. 2021, 28, 41760–41771. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Lan, G.; Qiu, H.; Xu, B.; Xu, Q.; Sun, N.; Zhang, L. Pb(II) adsorption characteristics of magnetic GO-hydroxyapatite and the contribution of GO to enhance its acid resistance. J. Environ. Chem. Eng. 2021, 9, 105310. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly enhanced adsorption performance to uranium(VI) by facile synthesized hydroxyapatite aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Zhou, C.; Song, X.; Wang, Y.; Wang, H.; Ge, S. The sorption and short-term immobilization of lead and cadmium by nano-hydroxyapatite/biochar in aqueous solution and soil. Chemosphere 2022, 286, 131810. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.R.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Chen, Z.; Zhou, X.; Lu, X.; Liu, J. Pollution characteristics and source analysis of heavy metals in surface sediments of Luoyuan Bay, Fujian. Environ. Res. 2022, 203, 111911. [Google Scholar] [CrossRef]
- Oulguidoum, A.; Bouiahya, K.; Bouyarmane, H.; Talbaoui, A.; Nunzi, J.-M.; Laghzizil, A. Mesoporous nanocrystalline sulfonated hydroxyapatites enhance heavy metal removal and antimicrobial activity. Sep. Purif. Technol. 2021, 255, 117777. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Yang, H.; Ye, B.-C. Hydrothermal synthesis of hierarchical hollow hydroxyapatite microspheres with excellent fluoride adsorption property. Microporous Mesoporous Mater. 2019, 289, 109620. [Google Scholar] [CrossRef]
- Bensalah, H.; Younssi, S.A.; Ouammou, M.; Gurlo, A.; Bekheet, M.F. Azo dye adsorption on an industrial waste-transformed hydroxyapatite adsorbent: Kinetics, isotherms, mechanism and regeneration studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- LoIacono, S.; Crini, G.; Martel, B.; Chanet, G.; Cosentino, C.; Raschetti, M.; Placet, V.; Torri, G.; Morin-Crini, N. Simultaneous removal of Cd, Co, Cu, Mn, Ni, and Zn from synthetic solutions on a hemp-based felt. II. Chemical modification. J. Appl. Polym. Sci. 2017, 134, 45138. [Google Scholar] [CrossRef]


| Model | Equation | Ref. |
|---|---|---|
| Langmuir | [13] | |
| [14] | ||
| Freundlich | [15] | |
| Temkin | [16] | |
| Dubinin–Radushkevich | [17] | |
| [18] | ||
| Pseudo-first-order model | [19] | |
| Pseudo-second-order model | [20] | |
| Elovich | [16] | |
| [21] | ||
| Intraparticle diffusion | [22] | |
| Thermodynamics | [12,23] |
| Adsorbent | Maximum Adsorption Capacity for Heavy Metals (mg Heavy Metal/g) | Ref. |
|---|---|---|
| Activated carbon (from coconut) | 4.56 mg Pb(II)/g | [24] |
| Activated carbon (seed hall of palm tree) | 3.58 mg Pb(II)/g | [24] |
| Epichlorohydrin-crosslinked chitosan | 34.13 mg Pb(II)/g, 35.46 mg Cu(II)/g, 10.21 mg Zn(II)/g | [25] |
| Hazelnut husk | 13.05 mg Pb(II)/g, 6.645 mg Cu(II)/g | [26] |
| Muscovite (natural) | 0.63 mg Pb(II)/g, 0.618 mg Cu(II)/g, 0.330 mg As(III)/g, 0.791 mg As(V)/g, 0.750 mg Cd(II)/g | [27] |
| Kaolinite | 7.75 mg Pb(II)/g, 4.42 mg Cu(II)/g, 4.95 mg Zn(II)/g | [28] |
| Modified orange peel | 73.53 mg Pb(II)/g, 15.27 mg Cu(II)/g | [29] |
| Coconut tree sawdust | 25 mg Pb(II)/g, 3.89 mg Cu(II)/g, 23.81 mg Zn(II)/g | [30] |
| Sugarcane bagasse | 21.28 mg Pb(II)/g, 3.65 mg Cu(II)/g, 40 mg Zn(II)/g | [30] |
| Lignin | 1865 mg Pb(II)/g, 95 mg Zn(II)/g | [31] |
| Chitosan | 518 mg Cd(II)/g | [32] |
| Seaweed brown algae | 67 mg Cd(II)/g | [33] |
| A. nodosum seaweed | 215 mg Cd(II)/g | [33] |
| Zeolites | 155.4 mg Pb(II)/g, 84.3 mg Cd(II)/g, 26 mg Cr(III)/g, 150.4 mg Hg(II)/g | [34] |
| Natural Source | Treatment | Application | Heavy Metal | Adsorption Parameters | Ref. |
|---|---|---|---|---|---|
| Bovine bones | Boiled, calcinated | Sorption studies using hydroxyapatite/poly (acrylamide-acrylic acid) composite | Sr(II) | Kinetics: pseudo-first-order model; ion exchange predominant model; Qmax = 53.59 mg Sr(II)/g | [36] |
| Bovine bones | Boiled, calcinated | Sorption studies; HAP characteristics: Ca/P ratio = 2, superficial area = 4.106 m2/g | Pb(II) | Kinetics: pseudo-second-order model; Qmax = 89 mg Pb(II)/g | [37] |
| Chicken bones | Carbonized, calcinated | Sorption studies using HAP and HAP/Fe3O4 composites | Pb(II) | Kinetics: pseudo-second-order model; Qmax = 105.26 mg Pb(II)/g HAP; Qmax = 109.89 Pb(II)/g HAP-Fe3O4 | [38] |
| Clam shells (Ca precursor) | Dissolved in water and nitric acid, addition of H3PO4 | Sorption studies; HAP: micrometric particle range, SSA 188.5–139.8 m2/g | Sr(II) | Kinetics: pseudo-second-order model; Qmax = 45.36 mg Sr(II)/g | [39] |
| Clam shells (Ca precursor) | Grinded, calcinated, addition of H3PO4 | Sorption studies | Pb(II), Cd(II), Cu(II) | Kinetics: pseudo-second-order model; Qmax = 265 mg Pb(II)/g; Qmax = 64 mg Cd(II)/g, Qmax = 55 mg Cu(II)/g | [40] |
| Mussel shells (Ca precursor) | Grinded, calcinated, addition of NH4H2PO4 | Sorption studies | Cd(II) | Kinetics: pseudo-second-order model, Langmuir isotherm; Qmax = 62.5 mg Cd(II)/g | [41] |
| Eggshells (Ca precursor) | Grinded, dissolved in HCl, addition of (NH4)2HPO4 | Sorption studies; HAP: hexagonal, 10 nm, SSA: 113 m2/g | Pb(II) | Kinetics: pseudo-second-order model; Qmax = 129.1 mg Pb(II)/g | [42] |
| Eggshells (Ca precursor) | Grinded, addition of H3PO4, Ca(OH)2, Na2CO3 and Na2SiO3 under ultrasounds to obtain Na-SiCHAP | Sorption studies; hexagonal, 10 nm, SSA: 79.09 m2/g, PD 21.32 nm, PV 0.40 cm3/g | Pb(II), Cd(II) | Kinetics: pseudo-second-order model, Langmuir isotherm model; Qmax = 698.68 mg Pb(II)/g, Qmax = 129.60 mg Cd(II)/g | [43] |
| Bovine horns core | Boiled, acetone soaking, drying, calcination | Sorption studies, using HAP with different characteristics, dependent on the calcination temperature | Cu(II) | Kinetics: pseudo-second-order model; Qmax = 99.98 mg Cu(II)/g | [44] |
| Bovine horns core | Boiled, acetone soaking, drying, calcination | Sorption studies, using HAP with different characteristics, dependent on the calcination temperature | Pb(II), Cd(II) | Kinetics: pseudo-second-order model; Qmax = 256.41 mg Pb(II)/g, Qmax = 105.26 mg Cd(II)/g | [45] |
| Fish scales | Soaked in HCl, treated with NaOH, heated | Sorption studies; HAP: Ca/P ratio = 1.96 | Pb(II) | 100% removal of 0.74 mg/L lead, after 10 min., using 4% HAP | [46] |
| Bovine femur bone | Washed with water, H2O2, HNO3, bleached, calcinated | Sorption studies, by comparison with commercial HAP; HAP: SSA: 46.8 m2/g, PD 25.5 nm, PV 0.18 cm3/g | Pb(II), Cd(II) | Kinetics: pseudo-second-order model; Qmax = 166.67 mg Pb(II)/g, Qmax = 138.89 mg Cd(II)/g | [47] |
| Bovine femur bone | Washed with water, H2O2, HNO3, bleached, boiled, calcinated | Sorption studies, by comparison with commercial HAP; HAP: SSA: 46.87 m2/g, PD 10 nm, PV 0.164 cm3/g | Cu(II), Fe(III) | Kinetics: pseudo-second-order model; Qmax = 102.35 mg Cu(II)/g, Qmax = 87.245 mg Fe(II)/g | [48] |
| Bovine cow bone | Dried, pyrolyzed, milled | Sorption studies; HAP: SSA: 313.09 m2/g, PD 6.46 nm, PV 0.4538 cm3/g | Cd(II), Cu(II), Pb(II) | Kinetics: pseudo-second-order model; Langmuir isotherm; Qmax = 165.77 mg Cd(II)/g, Qmax = 287.58 mg Cu(II)/g, Qmax = 558.88 mg Pb(II)/g | [49] |
| Chlorella powder | Added aq. NaOH and sodium dodecyl sulfate, microwave heated | Sorption studies in the form of hollow microspheres with multicomponent nanocores; HAP: Ca/P ratio = 1.72, PD 32.6 nm | Cd(II) | Kinetics: pseudo-second-order model; Langmuir isotherm; Qmax = 116.434 mg Cd(II)/g | [50] |
| Fish bones | Washed, dried, pulverized, sieved | Sorption studies; particle size 149–325 nm, PD 33–105 nm | Cu(II), Ni(II), Zn(II) | Langmuir isotherm (copper), Freundlich isotherm (nickel and zinc); >95% ion removal (30 mg/kg ion concentration) | [51] |
| Fish scales | Sonicated, dried, grinded | Sorption studies; HAP: SSA 102.2 m2/g, PD 9.14 nm, PV 0.28 cm3/g | Hg(II) | Kinetics: pseudo-second-order model; Langmuir isotherm; Qmax = 227.27 mg Hg(II)/g | [52] |
| Eggshells (Ca precursor) | Grinded, calcinated, addition of H3PO4 | Sorption studies using bentonite/CoFe2O4/HAP composite | Pb(II) | Kinetics: pseudo-second-order model; Langmuir isotherm; Qmax = 66 mg Pb(II)/g | [53] |
| Snail shells (Ca precursor) | Boiled, grinded, calcinated, addition of (NH4)2HPO4 | Sorption studies, using HAP and HAP-SiO2 composite; HAP: Ca/P ratio = 1.64 | Pb(II) | Kinetics: pseudo-second-order model; Langmuir isotherm; Qmax = 123 mg Pb(II)/g HAP; Qmax = 135.14 mg Pb(II)/g HAP-SiO2 | [54] |
| Eggshells (Ca precursor) | Dried, calcinated, addition of H3PO4 | Sorption studies, using HAP and polymeric modified HAP; HAP: Ca/P ratio = 1.63 | Co(II), Sr(II) | Kinetics: pseudo-second-order model; Freundlich isotherm; Qmax = 43.48 mg Co(II)/g, Qmax = 30.4 mg Sr(II)/g | [55] |
| Chicken thigh bones | Boiled, carbonized, calcinated | Sorption studies using HAP/Fe3O4/polydopamine composite; HAP: SSA: 16.722 m2/g, PV 0.008 cm3/g, PD 1.935 nm | Hg(II), Co(II), Ni(II) | Kinetics: Intraparticle diffusion model; Langmuir isotherm; Qmax = 51.73 mg Hg(II)/g, Qmax = 49.32 mg Co(II)/g, Qmax = 48.09 mg Ni(II)/g | [56] |
| Bovine bones | Boiled, crushed, calcinated | Sorption studies using a dynamic membrane of HAP, Sargassum glauscens nanoparticles, chitosan and polyvinyl alcohol | Zn(II), Co(II), Ni(II) | Over 90% removal efficiency | [57] |
| Fish scales | Boiled in NaOH, dried, calcinated (800 and 900 °C) | Sorption studies; HAP: SSA 88.73/103.46 m2/g, PV 0.38/0.36 cm3/g, PD 1.64/1.84 nm | Ni(II) | Kinetics: pseudo-first-order model; Langmuir isotherm; Qmax = 114.151 mg Ni(II)/g HAP (800 °C), Qmax = 181.321 mg Ni(II)/g HAP (800 °C) | [58] |
| Bovine cortical bones | Carbonized, calcinated | Sorption studies using HAP/chitosan/snail shell powder composite | Cu(II), Zn(II) | Kinetics: pseudo-second-order model; Langmuir/Temkin isotherms; Ion removal: 90%/60% (for 3 mg/L initial ions concentration) | [59] |
| Camel bones | Dried, grinded, soaked in H3PO4, treated with HNO3 and H2O2, dried | Sorption studies; HAP consisting material: SSA 19.29 m2/g, PV 0.054 cm3/g, PD 11.18 nm | V(V) | Kinetics: pseudo-second-order model; Langmuir isotherm; Qmax = 19.45 mg V(V)/g | [60] |
| Chicken bones | Dried, carbonized, calcinated | Sorption studies using HAP, HAP/Fe3O4 and polydopamine/HAP/Fe3O4, composites | Zn(II) | Kinetics: pseudo-second-order model; Freundlich isotherm; Qmax = 37.57 mg Zn(II)/g HAP, Qmax = 40.07 mg Zn(II)/g Hap-Fe3O4, Qmax = 46.37 mg Zn(II)/g poly-Hap-Fe3O4 | [61] |
| Eggshells (Ca precursor) | Washed, dried, calcinated, addition of H3PO4 | Sorption studies; HAP: Ca/P ratio = 1.65, SSA 63.7 m2/g, PV 0.1512 cm3/g | Pb(II) | Kinetics: pseudo-second-order model; Sips isotherm; Qmax = 518.46 mg Pb(II)/g | [62] |
| Eggshells (Ca precursor) | Washed, dried, calcinated, pulverized, addition of HNO3 and (NH4)2HPO4 | Sorption studies; HAP: Ca/P ratio = 1.74, SSA 32 m2/g | Cu(II), Ni(II) | Kinetics: pseudo-second-order model; Freundlich isotherm; Qmax = 10.58 mg Cu(II)/g, Qmax = 9.53 mg Ni(II)/g | [63] |
| Synthesis Method | Precursors, Post-Synthesis Steps | HAP Characteristics | Application | Adsorption Parameters | Ref. |
|---|---|---|---|---|---|
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, drying at 100 °C for 72 h | Rod shaped, SSA 71.97 m2/g | Adsorption of Pb(II), 0–100 mg/L | pH = 5.5, 0.2 g adsorbent, 20 mL solution volume; Langmuir isotherm model, removal efficiency 99.2% | [66] |
| Co-precipitation | Ca(NO3)2 × 4H2O, H3PO4, maturation for 24 h | SSA 72–127 m2/g, PV 0.233–0.516 cm3/g, increasing with the increase of inhibitors concentration. | Adsorption from multicomponent solution—Cd(II), Co(II), Cu(II), Fe(III), Ni(II), Pb(II), Zn(II), 0.13 to 1.28 mmol/g | Pseudo-second-order model, Qmax = 25.52 mg Pb(II)/g, Qmax = 7.5 mg Cu(II)/g, Qmax = 13.26 mg Cd(II)/g, Qmax = 0.98 mg Zn(II)/g, Qmax = 4.64 mg Ni(II)/g, Qmax = 0.59 mg Co(II)/g, | [67] |
| Dissolution/precipitation | Natural phosphate, HNO3, precipitated with NH4OH, aged for 24 h, functionalized with sodium benzene-1,3-disulphonate | SSA 100–196.5 m2/g, PV 35.3–46.8 cm3/g, PD 12–9 nm, in the order of functionalization degree increase | Adsorption of Cd(II), 0–1200 mg/L | pH = 5, pseudo-second-order model, Langmuir isotherm model, Qmax = 457.7 mg Cd(II)/g, | [68] |
| Co-precipitation | Ca(OH)2, H3PO4 | Rod-shaped, 20–30 nm diameter, 200–250 nm length | Adsorption of Fe(II), Cu(II), Ni(II), 20–240 mg/L, Cr(VI), 2–30 mg/L | pH = 5.5, Langmuir isotherm model, Qmax = 137.23 mg Fe(II)/g, Qmax = 128.02 mg Cu(II)/g, Qmax = 83.19 mg Ni(II)/g, Qmax = 2.92 mg Cr(VI)/g, | [69] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4 | Mesoporous, SSA 49 m2/g, PD 14.6 nm | Adsorption of Hg(II), 100–200 mg/L | pH = 3.5–5, Hg(II) up-take: approx. 75% | [70] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, thermal treatment | Mesoporous, SSA 100.5 m2/g, PD 5.21 nm, PV 0.272 cm3/g | Adsorption of Ni(II) and Co(II), 15–1200 mg/L | pH = 4–9, Langmuir isotherm model, Qmax = 18.61 mg Ni(II)/g, Qmax = 24.27 mg Co(II)/g | [71] |
| Hydrothermal | Ca(NO3)2 × 4H2O, P2O5, autoclaved | Various morphologies, dependent on the pH, reaction temperature, and reactant ratio | Adsorption of Pb(II), Cd(II), Cu(II), Co(II), Ni(II), Zn(II), Hg(II) | Efficiency (2 h) 99.61%, 30.95%, 71.08%, 70.36%, 79.56%, 98.60% and 99.90%. Pb(II): pseudo-second-order model, Langmuir isotherm model, Qmax = 252.53 mg Pb(II)/g, | [72] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, followed by sintering in the presence of polyvinyl alcohol | Granules, SSA 67.6–73 m2/g, proportional to the sintering temperature and PVA content | Adsorption of Pb(II), 30–60 mg/L | pH = 2.5–7.5, pseudo-second-order model, Langmuir isotherm model, Qmax = 7.99 mg Pb(II)/g | [73] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4 | Rod-shaped, 20–50 nm diameter, 100–200 nm length, SSA 55.24 m2/g, PV 0.19 cm3/g | Adsorption of Cu(II), Cd(II) and Pb(II), in the presence of oxytetracycline | Langmuir isotherm model, Qmax = 55.02 mg Cu(II)/g, Qmax = 101.17 mg Cd(II)/g, Qmax = 88 mg Pb(II)/g (in the presence of organic substance) | [74] |
| Sol-gel | Ca(NO3)2 × 4H2O, H3PO4 | SSA 140.41 m2/g, PD 10.2 nm | Adsorption of U(VI) | Pseudo-second-order model, Langmuir isotherm model, Qmax = 111.4 mg U(VI)/g, | [75] |
| Co-precipitation | CaCl2, Na2HPO4, in the presence of poly(allylamine hydrochloride) | Flower-like microsphere (diameter of 1–3 μm), nanosheets—8 to 12 nm, SSA 196.4 m2/g, PV 0.19 cm3/g | Adsorption of Pb(II), Cd(II), and Cu(II) | Pseudo-second-order model, Qmax = 210.5 mg Pb(II)/g, Qmax = 31.6 mg Cd(II)/g, Qmax = 24.9 mg Cu(II)/g | [76] |
| Co-precipitation | Ca(NO3)2 × 4H2O, H3PO4, with the post-synthesis addition of hydroxyethylidene diphosphonic acid | SSA 75–207 m2/g, PV 0.337–0.779 cm3/g, PD 10.9–27.6 nm | Adsorption of Co(II), Sr(II), and Pb(II) | Qmax = 38 mg Co(II)/g, Qmax = 24.54 mg Sr(II)/g, Qmax = 1740.48 mg Pb(II)/g | [77] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, with the addition of monododecyl phosphate potassium | Bitter gourd-shaped, 50–120 nm, SSA 77.25 m2/g, PV 0.1825 cm3/g | Adsorption of Pb(II), Cd(II), and Cr(II) | Qmax = 815 mg Pb(II)/g, Qmax = 291 mg Cd(II)/g, Qmax = 187 mg Cr(II)/g | [78] |
| Co-precipitation/electrospinning | Ca(NO3)2 × 4H2O, (NH4)2HPO4, capped with polyvinylpyrrolidone, followed by electrospinning and calcination | Nano-fibers, average diameters 500 nm | Adsorption of Cu(II), Cd(II), and Pb(II), 10–300 mg/L | pH = 6, pseudo-second-order model, Langmuir isotherm model, after 2 h, Qmax = 23 mg Cu(II)/g, Qmax = 36 mg Cd(II)/g, Qmax = 93 mg Pb(II)/g | [79] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, pH = 10, added 2-bromo- 2-methylpropionic acid, 24 h stirring, acrylonitrile | Not individually determined | Adsorption of Pb(II), 200 mg/L, as n-HAP-g-polyacrylonitrile | pH = 3–7, pseudo-second-order model, Langmuir isotherm model, Qmax = 950.5 mg Pb(II)/g | [80] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, added acetone and 2-bromo- 2-methylpropionic acid | Esterified HAP: rod-shaped, 10 nm, SSA 66.7 m2/g, PV 0.122 cm3/g, PD 5.3 nm | Adsorption of Pb(II) | pH = 3–7, pseudo-second-order model, Langmuir isotherm model, Qmax = 2398.33 mg Pb(II)/g | [81] |
| Co-precipitation | CaCO3 (limestone), (NH4)2HPO4, post-synthesis doping with Mg2+, respectively Sr2+ | SSA 17.7/71/57 m2/g, PV 0.09/0.35/0.28 cm3/g, PD 20.35/18.33/19.34 nm | Adsorption of Cu(II) | Pseudo-second-order model, Langmuir isotherm model, Qmax = 214.20 mg Cu(II)/g (Mg doped HAP) | [82] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of sepiolite | Spherical, rod-shaped, 50–100 nm, SSA 55.48–133.54 m2/g, PV 0.11–0.48 cm3/g, PD 7.94–17.63 nm, varying with the sepiolite amount | Adsorption of Cd(II), 5–50 mg/L, as n-HAP-supported on sepiolite | pH = 3–7, pseudo-second-order model, Sips isotherm model, Qmax = 46.81 mg Cd(II)/g | [83] |
| Co-precipitation | Ca(NO3)2 × 4H2O, H3PO4, pH = 10, followed by the incorporation of α-Fe2O3 phase | Rod-shaped, SSA 65 m2/g, PV 0.21 cm3/g, grain size 10–15 nm | Adsorption of U(VI), 150–300 mg/L | pH = 2–9, pseudo-second-order model, Langmuir isotherm model, Qmax = 310 mg U(VI)/g | [84] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2H PO4, in the presence of FeCl2 × 4H2O | Hollow core mesoporous, shell Fe-HAP, SSA 80.1973 m2/g, PV 0.43298 cm3/g | Adsorption of Cd(II) | Adsorption up-take 98% (at 296.7 mg/L initial concentration) | [85] |
| Commercial | Mixing activated carbon, copper nanoparticles, HAP, and sodium alginate | Composites: round structures, SSA 45.1 m2/g, PV 0.0443 cm3/g | Adsorption of As(III) | Pseudo-second-order model, Langmuir isotherm model, Qmax = 39.06 mg As(III)/g | [86] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4 in the presence of Fe3O4, followed by β-cyclodextrin binding | Not individually determined | Adsorption of Cd(II) and Cu(II), 20–200 mg/L, as HAP/Fe3O4 and HAP/Fe3O4/β-CD | pH = 2–6, pseudo-second-order model, Langmuir isotherm model, Qmax = 1000 mg Cd(II)/g composite, Qmax = 66.66 mg Cu(II)/g composite | [87] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4 in the presence of soy bean isolate, calcination | Calcinated at 600 °C: SSA 123.954 m2/g | Adsorption of Pb(II), 30–60 mg/L | Pseudo-second-order model, Freundlich isotherm model, Qmax = 25.84 mg Pb(II)/g | [88] |
| Co-precipitation | CaCl2, (NH4)2HPO4 in the presence of activated carbon, drying | C-HAP: SSA 60.42 m2/g, PV 0.18 cm3/g | Adsorption of Pb(II), 0.2–5 mM | pH = 3–7, pseudo-second-order model, Langmuir isotherm model, Qmax = 416.67 mg Pb(II)/g | [89] |
| Co-precipitation | Ca(NO3)2 × 4H2O, Na2HPO4 in the presence of activated carbon, further coated with turmeric | Nanoflakes | Adsorption of Pb(II), 400–700 mg/kg | pH = 4–7, Langmuir and Freundlich isotherm models gave similar correlations, Qmax = 29.4 mg Pb(II)/g | [90] |
| Commercial | Recrystallization in the presence of pectin | Flower-like particles consisting of sheet-like subunits | Adsorption of Zn(II), 180 mg/L | pH = 5, pseudo-second-order model, Langmuir isotherm model (under 50 mg/L), Freundlich isotherm model (above 60 mg/L), Qmax = 330.4 mg Zn(II)/g | [91] |
| Co-precipitation | Ca(OH)2, H3PO4, addition of hydrotalcite and carbon nanotubes | Not individually determined | Adsorption of Cr(VI), 1040 mg/L | pH = 6, 600 min., pseudo-second-order model, non-linear Freundlich isotherm model | [92] |
| Co-precipitation | CaCl2, NaH2PO4, in the presence of cellulose and bentonite | Not individually determined | Adsorption of As(III), 0.25–180 mg/L | pH = 2–9.3, pseudo-first-order model, Langmuir isotherm model, Qmax = 53.89 mg As(III)/g | [93] |
| Hydrothermal method | CaCl2, sodium dodecyl sulfate, Na2CO3, in the presence of Fe3O4 and N-carbon dots | Not individually determined | Adsorption of Pb(II), 10 mg/L | pH = 4–7, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 450.5 mg Pb(II)/g | [94] |
| Co-precipitation | Ca(OH)2, H3PO4, calcinated, coated over ceramic support | Capsule structure, 100 nm, porous | Adsorption and rejection of Pb(II), 1–10 mg/L | pH = 7.4, Q (g/m2) = 0.64 cation rejection 99.6% (at 5 mg/L) | [95] |
| Co-precipitation | Waste construction putty (as calcium source), (NH4)2HPO4, aging and drying | Leaf-like morphologies, SSA: up to 23.305 m2/g, depending on the aging temperature and time | Adsorption of Ni(II) | Qmax = 15 mg Ni(II)/g | [96] |
| Microwave-assisted | CaCl2, (NH4)2HPO4, grinded together and microwaved | Rod-shaped, SSA 8.08 m2/g, PV 0.05 cm3/g, PD 26.82 nm | Adsorption of Pb(II), 5–9 mM | pH = 2–6, pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 1438.85 mg Pb(II)/g | [97] |
| Sol-gel ultrasound assisted | Ca(NO3)2 × 4H2O, (NH4)2HPO4, ultrasonated, aged, freeze-dried | Irregular shapes, SSA 167.93 m2/g | Adsorption of Pb(II), 500–2200 mg/L | Pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 1303.48 mg Pb(II)/g | [98] |
| Commercial | - | Rod-shaped | Adsorption of Cu(II), Zn(II) | Freundlich adsorption isotherm model, Qmax = 59.03 mg Cu(II)/g, Qmax = 55.31 mg Zn(II)/g | [99] |
| Commercial | - | Micrometric and nanometric HAP | Adsorption of Zn(II), Mn(II), Pb(II), and Cd(II) | Superior effect of nanometric HAP | [100] |
| Commercial | - | - | Adsorption of Cd(II), in the presence of fulvic acid and montmorillonite colloids | pH = 7, pseudo-second-order model, Freundlich (for pure HAP and in the presence of montmorillonite), Langmuir (in the presence of fulvic acid) adsorption isotherm, Qmax = 45–18 mg Cd(II)/g, decreasing with the presence of colloids | [101] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of citric acid to control morphology, dried | Bundle shaped, length 1 μm, diameter 300 nm | Adsorption of Cd(II), Pb(II) | pH = 4–5, 99.9% adsorption rate | [102] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of citric acid to control morphology, aged, freeze-dried | Bound and fasciculated nanostructures, length 1 μm, diameter 300 nm, SSA 63.76 m2/g | Adsorption of U(VI), 0.02–0.2 g/L | pH = 2–6, pseudo-second-order model | [103] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, dried, calcinated (200/800 °C) | HAP (200/800 °C): SSA 58.1/10.8 m2/g, PV 0.237/0.014 cm3/g, PD 32.7/15.9 nm | Adsorption of Cd(II), 0.02–0.2 g/L | pH = 2–8, pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 217.4 mg Cd(II)/g | [104] |
| Hydrothermal | Ca(NO3)2 × 4H2O, (NH4)2HPO4, different reaction temperatures (120/150/180 °C), autoclaved, dried | Nanosheets, nanoribbons, blocky structures (depending on reaction temperature); SSA 5.31/9.42/10.79 m2/g, PV 0.022/0.031/0.033 cm3/g, PD 5.861/5.993/5.999 nm | Adsorption of U(VI), 37.36–148.44 mg/mL | pH = 1–7, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 336.58/378.26/403.91 mg U(VI)/g, depending on HAP synthesis temperature | [105] |
| Co-precipitation | Cu(OH)2, Ca(OH)2, H3PO4, dried | Cu-HAP–needle-shaped, SSA 147 m2/g | Adsorption of U(VI), 0.5–2.1 μM | 55–100% U(VI) up-take | [106] |
| Co-precipitation | CaCl2 × 2H2O, (NH4)2HPO4, in the presence of FeCl2 × 4H2O, aged, dried | Brushite/vivianite mixtures; 80% vivianite: 13.3 m2/g | Adsorption of Pb(II), Cr(II), 2 mg/L | pH = 2–8, 98/85% removal at 80% vivianite | [107] |
| Co-precipitation | CaCl2 × 2H2O, (NH4)2HPO4, with or without the presence of zoledronate (Z), aged, dried | HAP: rod-shaped, 100 nm, SSA 85.6 m2/g, PV 0.41 cm3/g, PD 13.79 nm; Z(10%)-HAP: microspherical, 50 nm, SSA 67.46 m2/g, PV 0.68 cm3/g, PD 33.99 nm | Adsorption of Pb(II), Cu(II), 100–1200/25–200 mg/L | pH = 3–5.5; pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 1009.9 mg Pb(II)/g HAP, Qmax = 106.96 mg Cu(II)/g HAP; Qmax = 1460.14 mg Pb(II)/g Z-HAP, Qmax = 226.33 mg Cu(II)/g Z-HAP | [108] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of attapulgite (A), aged, dried | - | Adsorption of Pb(II), 250–800 mg/L | pH = 2–6; pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 421.94 mg Pb(II)/g HAP | [109] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, with or without the presence of activated carbon (AC), aged, dried | HAP: needle shaped, SSA 48 m2/g; AC-HAP: needle-shapes accumulated over the AC surface, SSA 451 m2/g | Adsorption of U(VI), 250–800 mg/L | pH = 7, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 475 mg U(VI)/g HAP, Qmax = 464 mg U(VI)/g AC-HAP; Retention of 97.4% U(VI) from tap water at 150 μg/L | [110] |
| Ultrasonication | CaCO3, H3PO4, mixed post-synthesis with bentonite (B) and ultrasonicated | HAP: semi-spherical, 45 nm, SSA 24.5 m2/g, PD 1.1 nm; B-HAP: subspherical hydroxyapatite nanoparticles and bentonite nanosheet, 204.2 m2/g, PD 10.2 nm | Adsorption of Pb(II), Ni(II), 5–25 mg/L | pH = 7, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 5.83 mg Pb(II)/g HAP, Qmax = 5.99 mg Pb(II)/g B-HAP, Qmax = 3.72 mg Ni(II)/g HAP, Qmax = 1.01 mg Ni(II)/g B-HAP | [111] |
| Co-precipitation | ZnFe2O4 and cetrimonium bromide in ethanol, Ca(NO3)2 × 4H2O added, aged, dried to obtain HAP/ZnFe2O4 | HAP/ZnFe2O4: rod-shaped crystals of HAP-coated over ZnFe2O4 nanoparticles, SSA 60.189 m2/g | Adsorption of Cd(II), 100–300 mg/L | pH = 3–7, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 114 mg Cd(II)/g | [112] |
| Co-precipitation | CaCl2 × 2H2O, (NH4)2HPO4, with or without the presence of alendronate (A), aged, dried | HAP: rod-shaped, 100–200 nm, SSA 80.32 m2/g, PV 0.40 cm3/g, PD 13.54 nm; A-HAP: globular, 40 nm, SSA 77.87 m2/g, PV 0.63 cm3/g, PD 29.76 nm | Adsorption of Pb(II), Cu(II), Cd(II), 100–1200/25–200/50–400 mg/L | pH = 5, pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 1036.07 mg Pb(II)/g HAP, Qmax = 115.74 mg Cu(II)/g HAP, Qmax = 234.19 mg Cd(II)/g HAP, Qmax = 1529.85 mg Pb(II)/g A-HAP, Qmax = 243.31 mg Cu(II)/g A-HAP, Qmax = 465.12 mg Cd(II)/g A-HAP | [113] |
| Sol-gel, electrospinning | Ca(NO3)2 × 4H2O, triethyl phosphite hydrolyzed in ethanol, aged, polyvinylpyrrolidone, followed by electrospinning to obtain HAP/glass fibers (GF), thermal treatment | HAP–GF, segmented fiber structure formed by a chain of crystals, diameter 150 nm, SSA 6.57 m2/g, PV 0.025 cm3/g, PD 15.75 nm | Adsorption of Cd(II), Pb(II), 60–500 mg/L | pH = 5, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 93.3 mg Cd(II)/g, Qmax = 466.98 mg Pb(II)/g | [114] |
| Commercial | Incorporation of gelatin and zein in HAP by stirring | HAP agglomeration and nodules within the gelatin/zein fibers | Adsorption of Cu(II), 500 mg/L | Qmax = 67.8 mg Cu(II)/g, at 50% HAP (increasing with HAP addition) | [115] |
| Co-precipitation | Calcium and phosphate precursors, cellulose acetate (CA), graphene oxide (GO), magnetic nanoparticles (MNPs), electrospinning to obtain nanofibers | HAP: spherical, 0.9–2.5 μm; CA/HAP: non-oriented nanofibers; MNPs/HAP/GO/CA: random fibers with two diameter groups: 0.6–2.9 μm and 4.3–11.6 μm with spherical grains | Adsorption of Pb(II), Cd(II), 20 mg/L | pH = 8, removal efficiency (MNPs/HAP/GO/CA): 99.1/98.7% | [116] |
| Co-precipitation | CaCl2, Na3PO4, in the presence of magnetite/bentonite (M-B), N atmosphere, vacuum-dried to obtain M-B-HAP | HAP: flower-like structure; M-B-HAP: rough, compact and undulant surface, SSA 73.72 m2/g, PD 9.66 nm, PV 0.026 cm3/g | Adsorption of Cd(II), Pb(II), 20–2100 mg/L | pH = 2–10, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 309 mg Cd(II)/g, Qmax = 482 mg Pb(II)/g | [117] |
| Co-precipitation | CaCl2 × 2H2O, (NH4)2HPO4, cellulose acetate (CA), magnetite nanoparticles (M), graphene oxide (GO) electrospinning | CA-M-GO-HAP: non-oriented fibers, 0.11–0.29 μm, branched from intensive grains (4.57–5.71 μm) | Adsorption of Se(IV), Cr(VI), 25 mg/L | pH = 8, removal efficiency: 96/97.3% | [118] |
| Hydrothermal | CaCl2, (NH4)2HPO4, glucose alkaline solution, redispersed with surfactant, autoclaved, calcination | Mesoporous, 7–15 nm, pores 1.92 nm, SSA 48.81 m2/g, PD 1.93 nm, PV 0.38 cm3/g | Adsorption of Co(II), Ni(II), Cu(II), Zn(II), Cd(II), 50–1000 mg/L | pH = 5–7, pseudo-second-order model, Freundlich adsorption isotherm, Qmax = 299.46 mg Co(II)/g, Qmax = 309.35 mg Ni(II)/g, Qmax = 248.03 mg Cu(II)/g, Qmax = 276.11 mg Zn(II)/g, Qmax = 192.93 mg Cd(II)/g | [119] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, with Konjac gum (K), xanthan gum (X) or chitosan (C), aged, freeze-dried, calcinated, to obtain HAP aerogels | Uniform porous structure | Adsorption of U(VI), 50–1000 mg/L | Pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 2070.3 mg U(VI)/g K-HAP, Qmax = 1863.4 mg U(VI)/g X-HAP, Qmax = 1446.3 mg U(VI)/g C-HAP | [120] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4 | Cylinder-shaped, 18.29 nm, SSA 75 m2/g | Adsorption of Ag(II), 10–100 mg/L | pH = 2–8, pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 18.7 mg Ag(II)/g, | [121] |
| Hydrothermal | CaCl2, H3PO4, with or without the presence of SrCl2, autoclaved, to obtain HAP and SrHAP | Nanorods, approx. 4 nm SSA 321.5/378.9 m2/g, PD 1.93 nm, PV 0.38 cm3/g | Adsorption of Cr(VI), 1000 mg/L | pH = 3.5–7, Langmuir adsorption isotherm, Qmax = 322 mg Cr(VI)/g HAP, Qmax = 454 mg Cr(VI)/g SrHAP | [122] |
| Co-precipitation | CaCl2, Na2HPO4, in the presence of oxidized carbon nanotubes (CN) | - | Adsorption of Pb(II), Cd(II), Cu(II), 100 mg/L | pH = 5.5, pseudo-second-order model, Langmuir adsorption isotherm, Qmax = 1070–1522 mg Pb(II)/g, Qmax = 135–151 mg Cd(II)/g, Qmax = 167–180 mg Cu(II)/g (temperature- dependent) | [123] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of mesoporous carbon (MC), vacuum-aged, thermally treated | HAP: nano-platelets 5/40nm, SSA 101 m2/g, PV 0.306 cm3/g; MC-HAP: HAP crystallites over amorphous MC, SSA 130 m2/g, PV 0.221 cm3/g at 8% MC | Adsorption of Cu(II), Ni(II), 15–300 mg/L | pH = 5.5, Elovich adsorption model, Langmuir adsorption isotherm, Qmax = 79.5 mg Cu(II)/g, Qmax = 18.6 mg Ni(II)/g | [124] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, with or without the presence of γ-Fe2O3 (M), aged, dried | HAP: rod- or needlelike, diameter 5–10 nm, SSA 101 m2/g, PV 0.306 cm3/g; M-HAP: γ-Fe2O3 embedded in the needlelike matrix, SSA 130 m2/g, PV 0.221 cm3/g at 8% MC | Adsorption of Cd(II), 10–100 mg/L | pH = 3–10, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 277.78 mg Cd(II)/g, | [125] |
| Co-precipitation | CaCl2 × 2H2O, (NH4)2HPO4, with or without the presence of amino trimethylposphonate (AT), aged, dried | HAP: SSA 119.75 m2/g, PV 0.48 cm3/g, PD 15.85; AT-HAP: SSA 92.82 m2/g, PV 0.22 cm3/g, PD 10.31 nm | Adsorption of Pb(II), Cd(II), 10–1500/50–400 mg/L | pH = 2–6, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 884.96 mg Pb(II)/g HAP, Qmax = 1540.13 mg Pb(II)/g AT-HAP, Qmax = 181.82 mg Cd(II)/g HAP, Qmax = 367.65mg Cd(II)/g AT-HAP | [126] |
| Hydrothermal | CaCl2 × 2H2O, (NH4)2HPO4, in the presence of biochar (BC), autoclaved, dried | HAP on the surface of BC, compact, SSA 157.96 m2/g | Adsorption of U(VI), 50 mg/L | pH = 3.5–5.5, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 428.25 mg U(VI)/g, | [127] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of polymers: chitosan (CS), carboxymethyl cellulose (CC), alginate (Al), gelatin (Gl), aged, dried | HAP-CS: porous, with rod-shaped particles, 20–50 nm, SSA 191 m2/g, PV 0.402 cm3/g; HAP-CC: porous, agglomerated spherical particles, SSA 90.6 m2/g, PV 0.19 cm3/g; HAP-Al: less porous, nanoparticles embedded in polymer, SSA 83.8 m2/g, PV 0.12 cm3/g; HAP-Gl: less porous, larger particles, SSA 80 m2/g, PV 0.33 cm3/g; | Adsorption of Pb(II), Cd(II), As(V), 1500/400/1 mg/L | pH = 3–11, pseudo-second-order adsorption model, Freundlich adsorption isotherm, Qmax = 514.1 mg Pb(II)/g HAP-CS, Qmax = 478.8 mg Pb(II)/g HAP-CC, Qmax = 480.3 mg Pb(II)/g HAP-Al, Qmax = 579.8 mg Pb(II)/g HAP-Gl, Qmax = 114.1 mg Cd(II)/g HAP-CS, Qmax = 99 mg Cd(II)/g HAP-CC, Qmax = 102.5 mg Cd(II)/g HAP-Al, Qmax = 144.9 mg Cd(II)/g HAP-Gl, Qmax = 3.38 mg As(VI)/g HAP-CS, Qmax = 2.3 mg As(VI)/g JAP-CC, Qmax = 2.1 mg As(VI)/g HAP-Al, Qmax = 3.17 mg As(VI)/g HAP-Gl | [128] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of poly-γ-glutamate (PG), aged, dried | PG-HAP: particle dimensions: 79.7–92.8 nm (increased with PG content) | Adsorption of Cu(II), 100 mg/L | pH = 4–6, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 78.99 mg Cu(II)/g,.for PG/HAP ratio 1/20 | [129] |
| Co-precipitation | (NH4)2HPO4, over solid CaCO3, ethanol, aged, dried | Flower-like structures, deposited over calcite, SSA 58.25 m2/g, PV 0.0016 cm3/g | Adsorption of Zn(II), Cu(II), 5–120 mg/L | pH = 4.6, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 34.97 mg Zn(II)/g, Qmax = 60.24 mg Cu(II)/g, | [130] |
| Hydrothermal | Ca(OH)2, NH4H2PO4, autoclaved, dried, post-synthesis added Fe0 | Nanorods, diameter 2–10 nm, with Fe0 spherical nanoparticles on the surface, SSA 29.03 m2/g | Adsorption of Cr(VI), Co(II), Cu(II), Ni(II), 100 mg/L | pH = 5.25, pseudo-first-order adsorption model, Langmuir adsorption isotherm, Removal efficiency: 99.84/99.87/99.33/99.39% | [131] |
| Not declared | Mixing chitosan (CS), Si slurry and HAP, washed and dried to obtain CS-Si-HAP | Non-homogeneous surface, microstructures, embedded with Si particles, pores in the matrix | Adsorption of Cr(VI), 100 mg/L | pH = 1–7, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 212.76 mg Cr(VI)/g, | [132] |
| Co-precipitation | (NH4)2HPO4, over solid limestone, mixed with chitosan (CS), aged, dried | HAP-coated limestone, decreased roughness upon addition of CS; SSA 4.9–11.7 m2/g, PV 0.05–0.1 cm3/g, PD 25.8–38.2 nm (depending on CS content) | Adsorption of Cu(II), 10–50 mg/L | pH = 3–7, pseudo-second-order adsorption model, Freundlich adsorption isotherm, Qmax = 130.75 mg Cu(II)/g, (0.5% CS) | [133] |
| Co-precipitation | Ca(NO3)2 × 4H2O, KH2PO4, aged, dried, post-synthesis addition of Fe0 and chitin (CH) | Fe-CH-HAP: HAP nanorods, spherical iron particles | Adsorption of Cr(VI), 10–50 mg/L | pH = 3.5–6, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 39.7 mg Cr(VI)/g, | [134] |
| Not declared | Mixing Fe3O4 (M), graphene oxide (GO) and HAP, washed and dried to obtain CS-Si-HAP | Uniform morphology, SSA 158.72 m2/g, PV 0.25 cm3/g, PD 0.41 nm | Adsorption of Pb(II), 200–800 mg/L | pH = 1–8, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 249.64 mg Pb(II)/g, | [135] |
| Freeze-drying-calcination | Ca(NO3)2 × 4H2O, (NH4)2HPO4, in the presence of Konjac gum, lyophilized, calcinated | Porous structure, SSA 118.4 m2/g, PV 0.373 cm3/g | Adsorption of U(VI), 100 mg/L | pH = 4, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 2087.6 mg U(VI)/g, | [136] |
| Co-precipitation | Ca(NO3)2 × 4H2O, (NH4)2HPO4, under ultrasonication, in the presence of biochar (BC), aged, dried | HAP: SSA 157.914 m2/g, PV 0.762 cm3/g, PD 19.307 nm; BC-HAP: rod-shaped, 50/200 nm, attached on the surface of BC, SSA 144.762 m2/g, PV 0.353 cm3/g, PD 9.759 nm | Adsorption of Pb(II), Cd(II), 50–2000/50–600 mg/L | pH = 2.05–5.30/2–6.01, pseudo-second-order adsorption model, Langmuir adsorption isotherm, Qmax = 1257.03 mg Pb(II)/g HAP, Qmax = 770.15 mg Pb(II)/g BC-HAP, Qmax = 214.93 mg Cd(II)/g HAP, Qmax = 194.61 mg Cd(II)/g BC-HAP | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazdis, R.I.; Fierascu, I.; Avramescu, S.M.; Fierascu, R.C. Recent Progress in the Application of Hydroxyapatite for the Adsorption of Heavy Metals from Water Matrices. Materials 2021, 14, 6898. https://doi.org/10.3390/ma14226898
Brazdis RI, Fierascu I, Avramescu SM, Fierascu RC. Recent Progress in the Application of Hydroxyapatite for the Adsorption of Heavy Metals from Water Matrices. Materials. 2021; 14(22):6898. https://doi.org/10.3390/ma14226898
Chicago/Turabian StyleBrazdis, Roxana Ioana, Irina Fierascu, Sorin Marius Avramescu, and Radu Claudiu Fierascu. 2021. "Recent Progress in the Application of Hydroxyapatite for the Adsorption of Heavy Metals from Water Matrices" Materials 14, no. 22: 6898. https://doi.org/10.3390/ma14226898
APA StyleBrazdis, R. I., Fierascu, I., Avramescu, S. M., & Fierascu, R. C. (2021). Recent Progress in the Application of Hydroxyapatite for the Adsorption of Heavy Metals from Water Matrices. Materials, 14(22), 6898. https://doi.org/10.3390/ma14226898









