Enhanced Photoluminescence and Photocatalytic Efficiency of La-Doped Bismuth Molybdate: Its Preparation and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Photocatalytic Activity Measurements
3. Results and Discussion
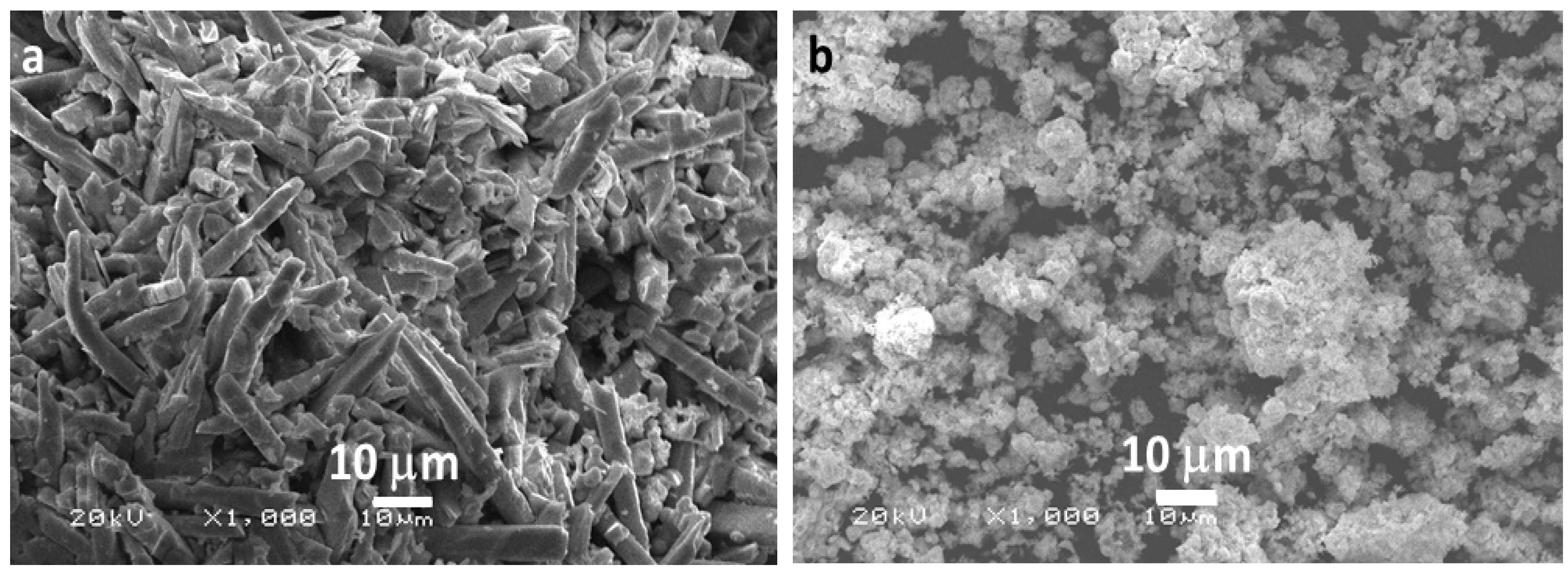
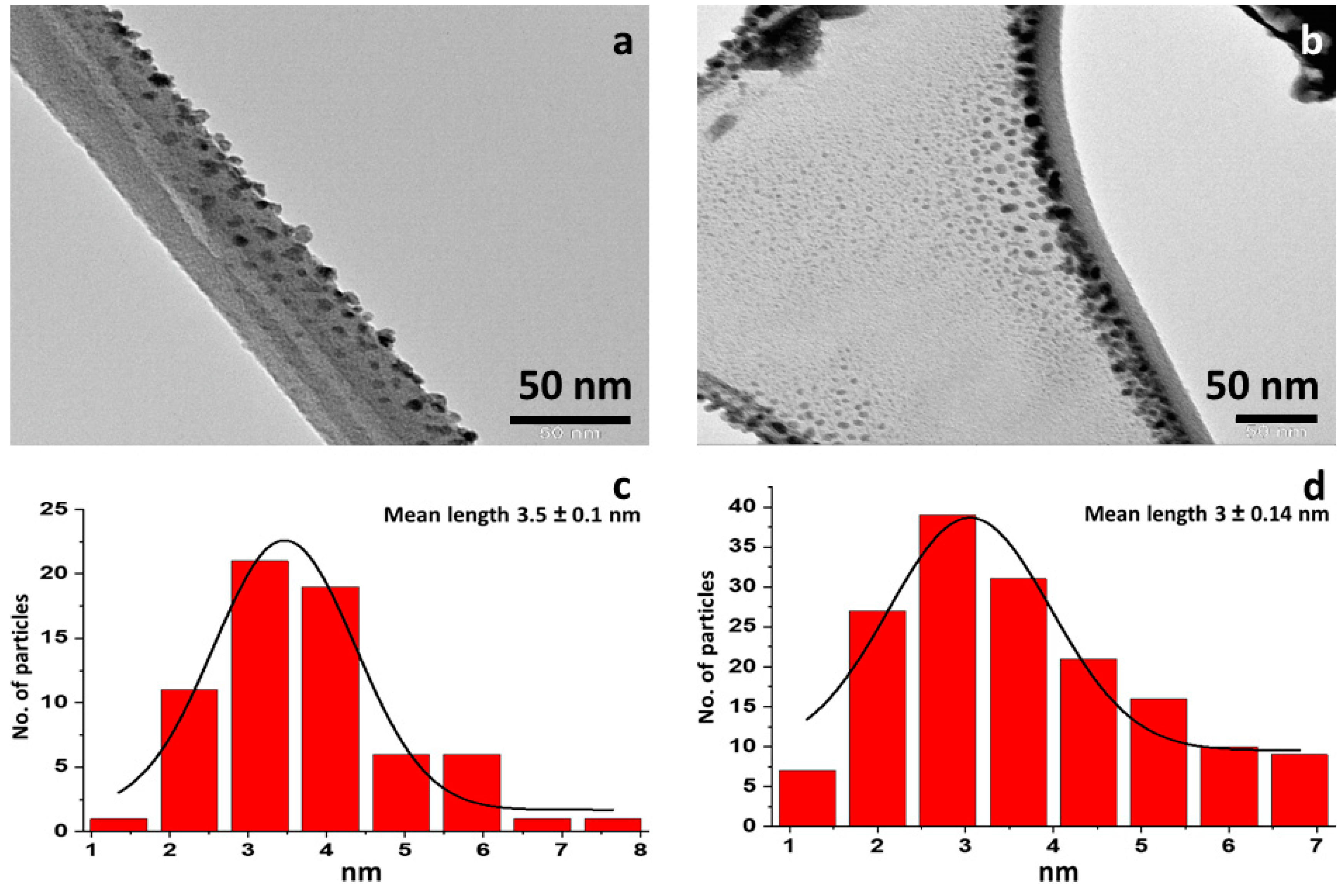
3.1. Microscopic Analysis
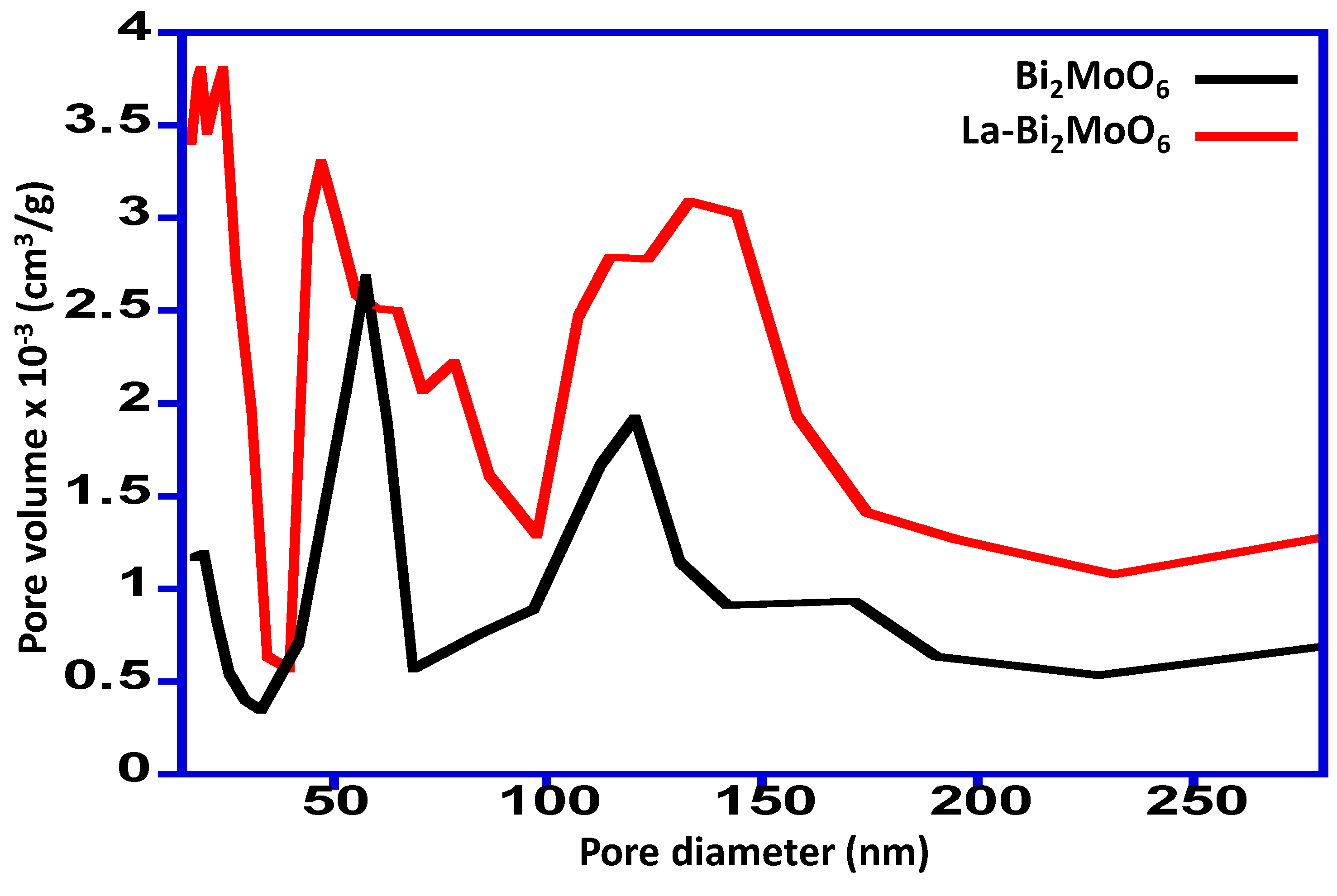
3.2. BET Analysis
3.3. FT-IR Spectral Analysis
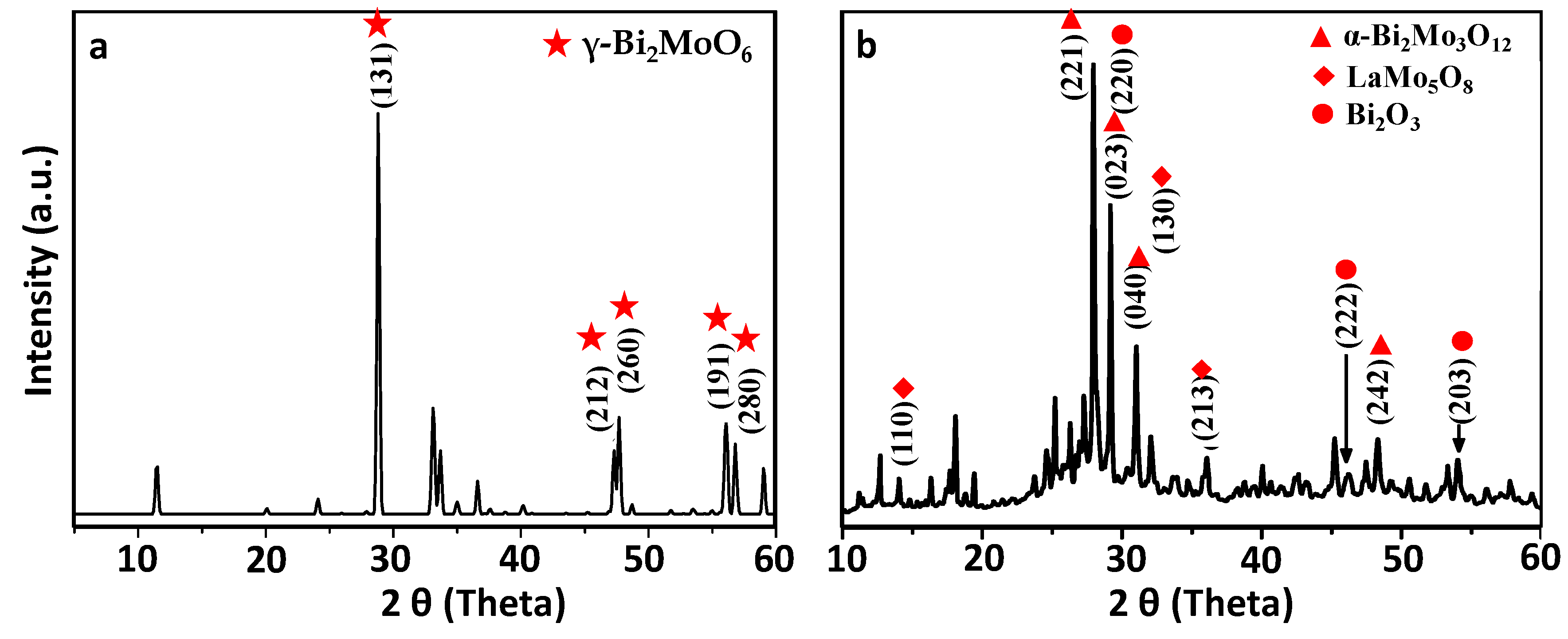
3.4. X-Ray Diffraction Spectroscopy
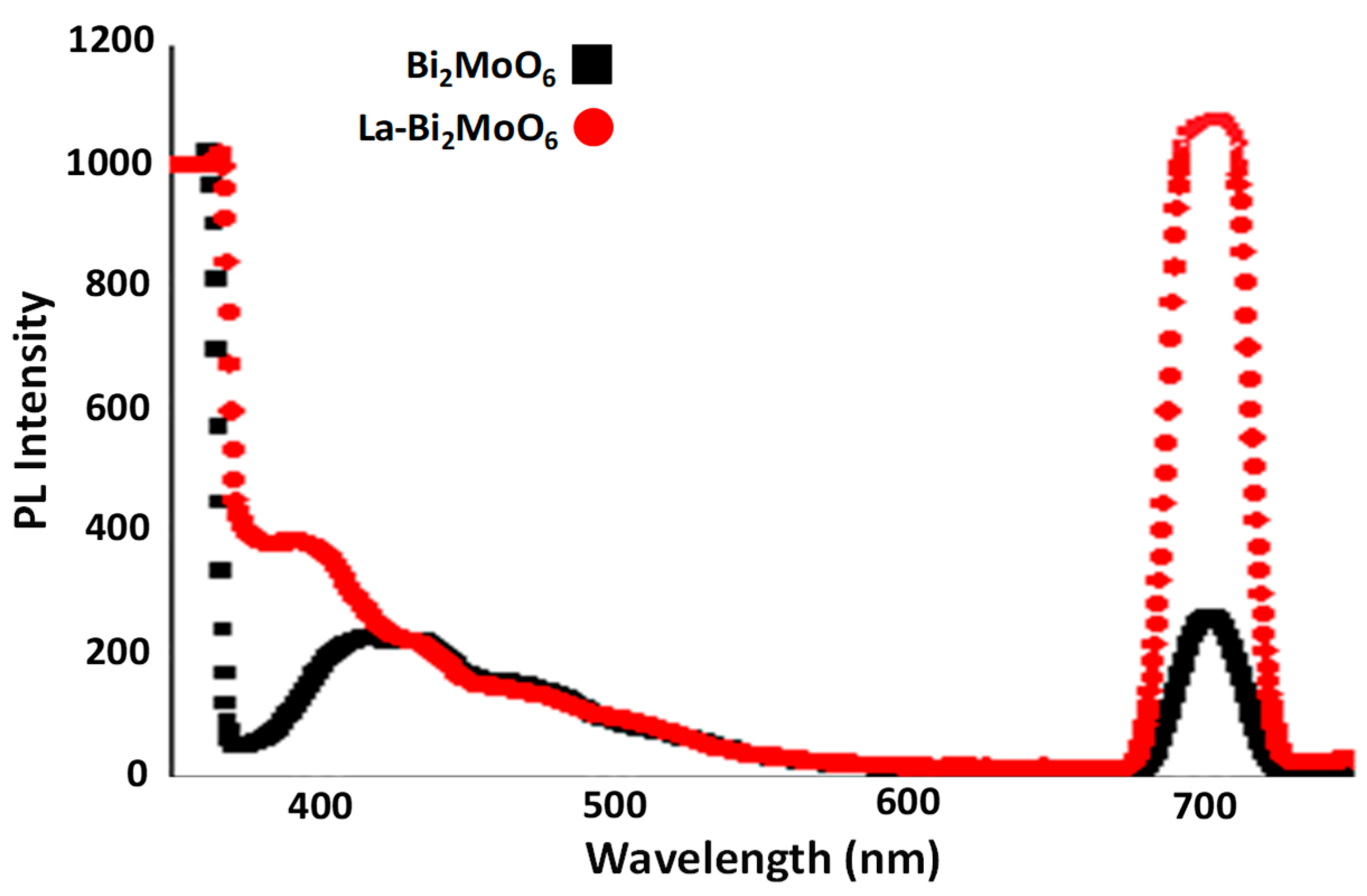
3.5. Luminescence
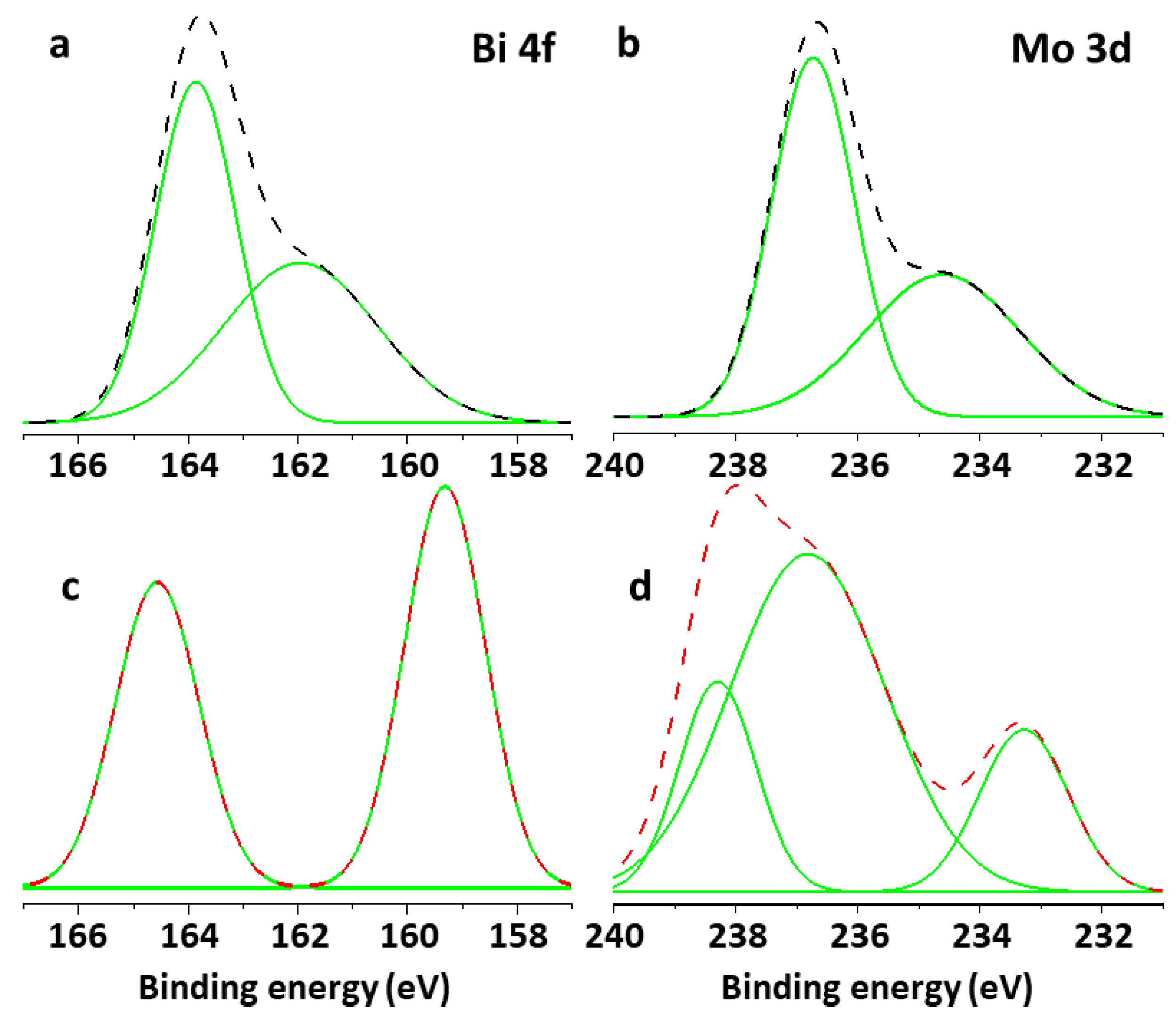
3.6. X-Ray Photoelectron Spectroscopy
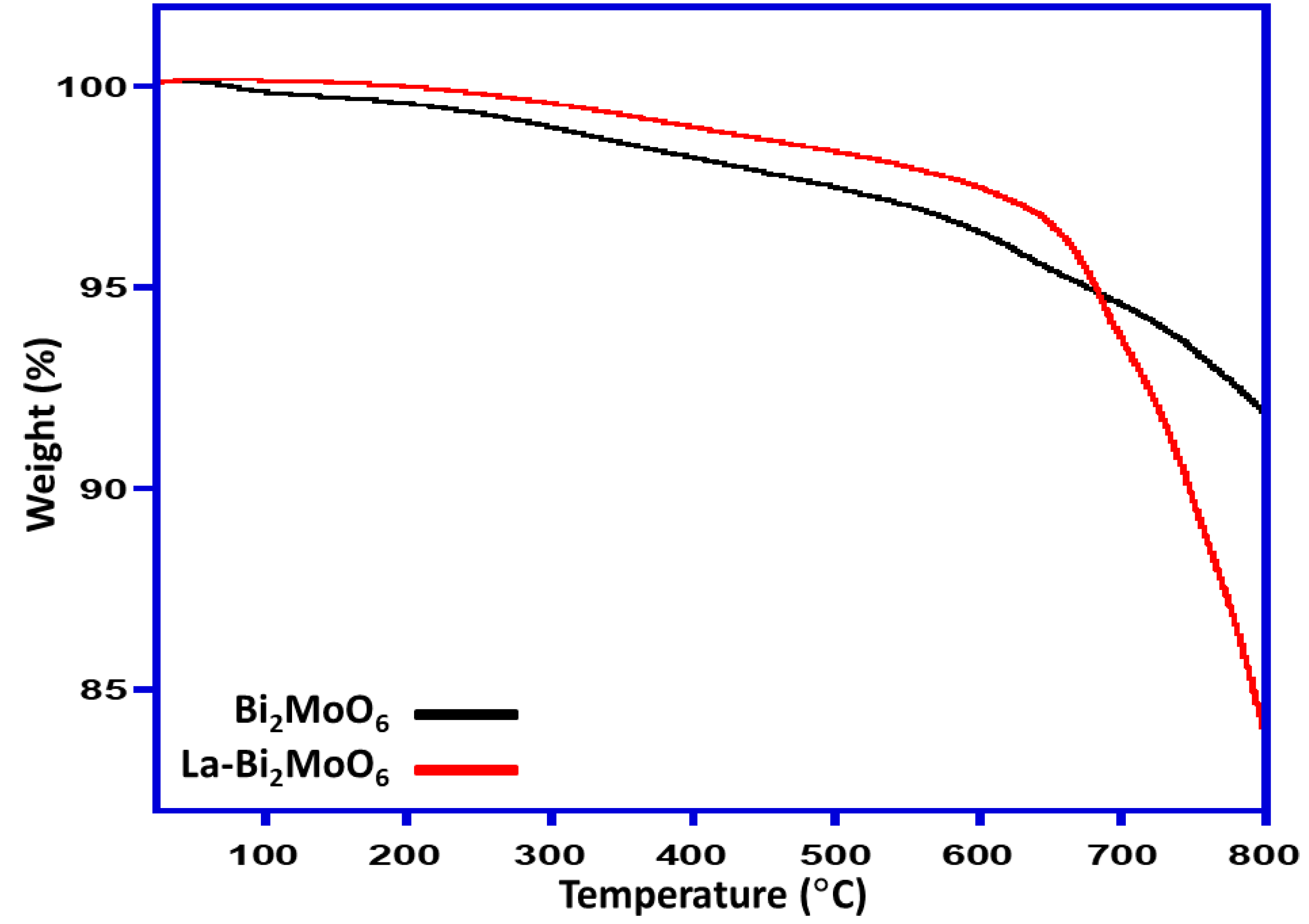
3.7. TGA Analysis
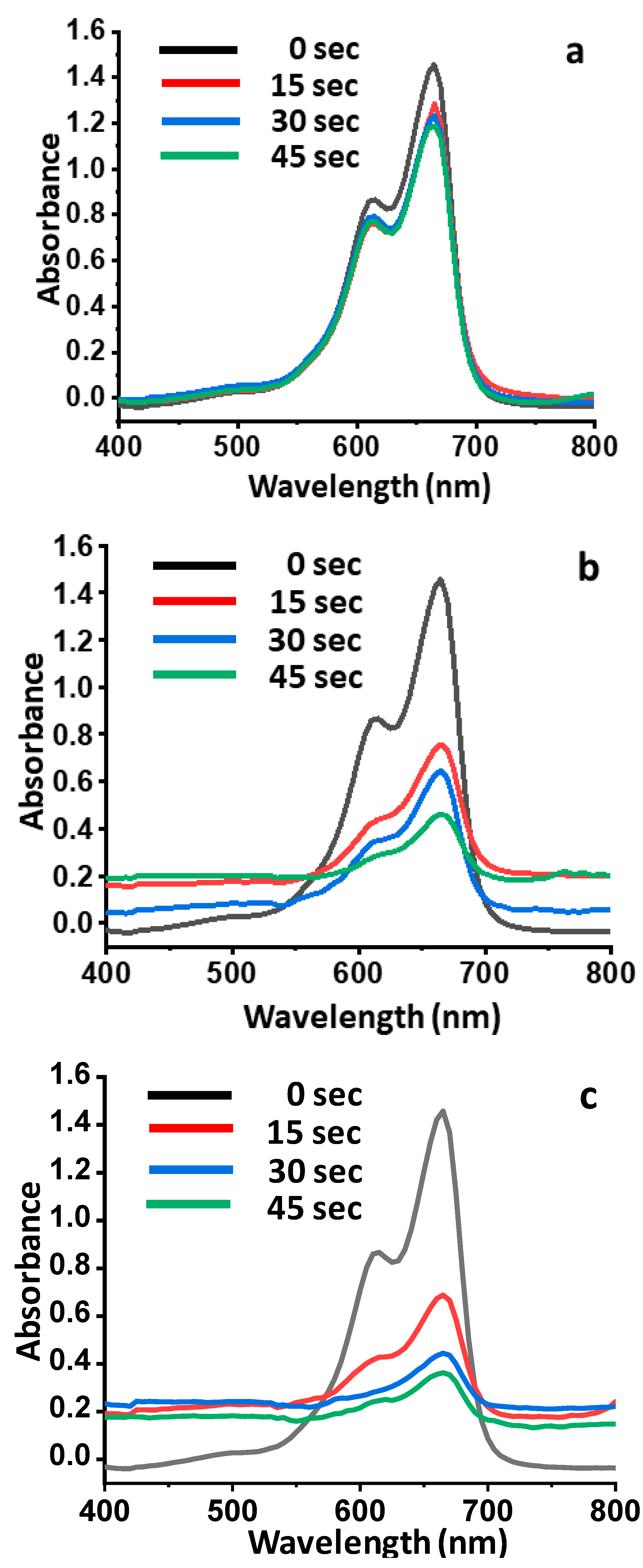
3.8. Photocatalytic Activity
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fouassier, C.E.; Fu, W.T.; Hagenmuller, P. Luminescent Mixed Borates Based on Rare Earths. U.S. Patent 4,946,621, 7 August 1990. [Google Scholar]
- Jayachandiran, M.; Annadurai, G.; Kennedy, S.M.M. Photoluminescence properties of red emitting Ba3Bi2 (PO4) 4: Eu3+ phosphor for WLEDs applications. J. Lumin. 2018, 201, 196–202. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Y.; Qiu, S.; Lercher, J.A.; Zhang, H. Coordination modulation induced synthesis of nanoscale Eu1-xTbx-metal-organic frameworks for luminescent thin films. Adv. Mater. 2010, 22, 4190–4192. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Ahmed, T.; Sarker, P.; Pakkanen, T.T.; Suvanto, M.; Horimoto, M.; Nakata, H. Structural, luminescence and photophysical properties of novel trimetallic nanocomposite CeO2· ZnO ZnAl2O4. J. Lumin. 2014, 148, 98–102. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Kuang, A.; Mao, Y. Persistent luminescent sub-10 nm Cr doped ZnGa 2 O 4 nanoparticles by a biphasic synthesis route. Chem. Commun. 2015, 51, 7372–7375. [Google Scholar] [CrossRef]
- Nayab Rasool, S.; Rama Moorthy, L.; Kulala Jayasankar, C. Optical and luminescence properties of Eu3+-doped phosphate based glasses. Mater. Express 2013, 3, 231–240. [Google Scholar] [CrossRef]
- Lin, H.; Liu, K.; Pun, E.; Ma, T.; Peng, X.; An, Q.; Yu, J.; Jiang, S. Infrared and visible fluorescence in Er3+-doped gallium tellurite glasses. Chem. Phys. Lett. 2004, 398, 146–150. [Google Scholar] [CrossRef]
- Niishiro, R.; Kato, H.; Kudo, A. Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions. Phys. Chem. Chem. Phys. 2005, 7, 2241–2245. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, S.; Xu, T.; Zhu, Y.; Chen, J. Photocatalytic degradation of RhB by fluorinated Bi2WO6 and distributions of the intermediate products. Environ. Sci. Technol. 2008, 42, 2085–2091. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Z.; Zhang, J.; Ma, J. Preparation of a novel Bi3. 64Mo0. 36O6. 55 nanophotocatalyst by molten salt method and evaluation for photocatalytic decomposition of rhodamine B. J. Alloys Compd. 2010, 503, 159–162. [Google Scholar] [CrossRef]
- Labib, S. Preparation, characterization and photocatalytic properties of doped and undoped Bi2O3. J. Saudi Chem. Soc. 2017, 21, 664–672. [Google Scholar] [CrossRef]
- Singh, P.; Raizada, P.; Sudhaik, A.; Shandilya, P.; Thakur, P.; Agarwal, S.; Gupta, V.K. Enhanced photocatalytic activity and stability of AgBr/BiOBr/graphene heterojunction for phenol degradation under visible light. J. Saudi Chem. Soc. 2019, 23, 586–599. [Google Scholar] [CrossRef]
- Lu, L.; Kobayashi, A.; Tawa, K.; Ozaki, Y. Silver nanoplates with special shapes: Controlled synthesis and their surface plasmon resonance and surface-enhanced Raman scattering properties. Chem. Mater. 2006, 18, 4894–4901. [Google Scholar] [CrossRef]
- Yu, J.; Kudo, A. Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Abe, R.; Arakawa, H. Photocatalytic decomposition of water with Bi2 InNbO7. Catal. Lett. 2000, 68, 235–239. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3 PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Chen, C.; Wang, X. Enhanced photocatalytic degradation of methylene blue under visible irradiation on graphene@ TiO2 dyade structure. Appl. Catal. B Environ. 2012, 111, 303–308. [Google Scholar] [CrossRef]
- Karaoglu, E.; Baykal, A. CoFe2O4–Pd (0) nanocomposite: Magnetically recyclable catalyst. J. Supercond. Nov. Magn. 2014, 27, 2041–2047. [Google Scholar] [CrossRef]
- Liyan, W.; Hongxia, W.; Aijie, W.; Min, L. Surface modification of a magnetic SiO2 support and immobilization of a nano-TiO2 photocatalyst on it. Chin. J. Catal. 2009, 30, 939–944. [Google Scholar]
- Baux, N.; Vannier, R.; Mairesse, G.; Nowogrocki, G. Oxide ion conductivity in Bi2W1− xMExO6− x/2 (ME = Nb, Ta). Solid State Ion. 1996, 91, 243–248. [Google Scholar] [CrossRef]
- Islam, M.S.; Lazure, S.; Vannier, R.-n.; Nowogrocki, G.; Mairesse, G. Structural and computational studies of Bi 2 WO 6 based oxygen ion conductors. J. Mater. Chem. 1998, 8, 655–660. [Google Scholar] [CrossRef]
- Karathanos, V.; Saravacos, G. Porosity and pore size distribution of starch materials. J. Food Eng. 1993, 18, 259–280. [Google Scholar] [CrossRef]
- Umapathy, V.; Manikandan, A.; Antony, S.A.; Ramu, P.; Neeraja, P. Structure, morphology and opto-magnetic properties of Bi2MoO6 nano-photocatalyst synthesized by sol–gel method. Trans. Nonferrous Met. Soc. China 2015, 25, 3271–3278. [Google Scholar] [CrossRef]
- Rambabu, U.; Han, S.D. Synthesis and luminescence properties of broad band greenish-yellow emitting LnVO4: Bi3+ and (Ln1, Ln2) VO4: Bi3+ (Ln= La, Gd and Y) as down conversion phosphors. Ceram. Int. 2013, 39, 701–708. [Google Scholar] [CrossRef]
- Adhikari, R.; Gyawali, G.; Cho, S.H.; Narro-García, R.; Sekino, T.; Lee, S.W. Er3+/Yb3+ co-doped bismuth molybdate nanosheets upconversion photocatalyst with enhanced photocatalytic activity. J. Solid State Chem. 2014, 209, 74–81. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, J.; Li, J. Er3+ doped bismuth molybdate nanosheets with exposed {0 1 0} facets and enhanced photocatalytic performance. Appl.Catal. B Environ. 2011, 110, 221–230. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, S.; Zhao, Y.; Xu, L.; He, D.; Jiao, H. Effects of organic additives on morphology and luminescent properties of Eu3+-doped calcium molybdate red phosphors. Powder Technol. 2015, 275, 1–11. [Google Scholar] [CrossRef]
- Haoran, W.; Keryn, L. The Development of Pseudocapacitive Molybdenum Oxynitride Electrodes for Super capacitors. ECS Trans. 2014, 58, 67–75. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqar, M.; Imran, M.; Adil, S.F.; Noreen, S.; Latif, S.; Khan, M.; Siddiqui, M.R.H. Enhanced Photoluminescence and Photocatalytic Efficiency of La-Doped Bismuth Molybdate: Its Preparation and Characterization. Materials 2020, 13, 35. https://doi.org/10.3390/ma13010035
Waqar M, Imran M, Adil SF, Noreen S, Latif S, Khan M, Siddiqui MRH. Enhanced Photoluminescence and Photocatalytic Efficiency of La-Doped Bismuth Molybdate: Its Preparation and Characterization. Materials. 2020; 13(1):35. https://doi.org/10.3390/ma13010035
Chicago/Turabian StyleWaqar, Muhammad, Muhammad Imran, Syed Farooq Adil, Sadia Noreen, Shoomaila Latif, Mujeeb Khan, and Mohammed Rafiq H. Siddiqui. 2020. "Enhanced Photoluminescence and Photocatalytic Efficiency of La-Doped Bismuth Molybdate: Its Preparation and Characterization" Materials 13, no. 1: 35. https://doi.org/10.3390/ma13010035
APA StyleWaqar, M., Imran, M., Adil, S. F., Noreen, S., Latif, S., Khan, M., & Siddiqui, M. R. H. (2020). Enhanced Photoluminescence and Photocatalytic Efficiency of La-Doped Bismuth Molybdate: Its Preparation and Characterization. Materials, 13(1), 35. https://doi.org/10.3390/ma13010035







