Abstract
A novel species of metal amidoborane ammoniate, [Al(NH2BH3)63−][Al(NH3)63+] has been successfully synthesized in up to 95% via the one-step reaction of AlH3·OEt2 with liquid NH3BH3·nNH3 (n = 1~6) at 0 °C. This solution based reaction method provides an alternative pathway to the traditional mechano-chemical ball milling methods, avoiding possible decomposition. MAS 27Al NMR spectroscopy confirms the formulation of the compound as an Al(NH2BH3)63− complex anion and an Al(NH3)63+ cation. Initial dehydrogenation studies of this aluminum based M-N-B-H compound demonstrate that hydrogen is released at temperatures as low as 65 °C, totaling ~8.6 equivalents of H2 (10.3 wt %) upon heating to 105 °C. This method of synthesis offers a promising route towards the large scale production of metal amidoborane ammoniate moieties.
1. Introduction
A critical challenge facing the advancement of hydrogen fuel cells for automotive applications is the development of safe and energy efficient hydrogen storage materials. Metal amidoboranes (MNH2BH3, MAB) and metal borohydride ammoniates (MBH4·nNH3, MBA) are currently among the most promising candidate materials [1,2,3,4,5,6]. Recent demonstration of the regeneration of ammonia borane derivatives using hydrazine in liquid ammonia point to the feasibility of off-board reversibility [7,8,9]. Substitution of one protic H atom in the [NH3] of NH3BH3 by a metal atom leads to the formation of MAB complexes.
Aluminum amidoborane (Al(NH2BH3)3, AlAB), first synthesized by Hawthorne et al. [10], possesses one of the highest theoretical hydrogen capacities among MABs (12.9 wt % H), capable of releasing 6 wt % H2 at 190 °C and approximately 8 wt % H2 in the presence of an ionic liquid at lower temperatures [10]. As such, this material has already experienced intensive explorations, although up to now only a few reports have identified its existence owing to its poor stability and spontaneous H2 loss caused by the chemically vulnerable Lewis-acidic Al3+ center [10,11,12]. The improved dehydrogenation properties of AlAB (Al χp = 1.5), relative to ammonia borane [10], makes the Al-N-B-H systems attractive, albeit difficult to synthesize. Recently, Guo et al. reported on the stability of [Al(NH3)6](BH4)3 in air, which differs quite significantly from the analogous volatile liquid Al(BH4)3 [13,14]. Strong N-Hδ+∙∙∙−δH-B dihydrogen bonds contribute to the stability of this compound resulting in its long term stability in air. Another recently reported Al based amidoborane complex includes Li3AlH6·n(NH2BH3) which releases 9 wt % H2 at a temperature of 130 °C [15].
A variety of B-N amidoborane ammoniates, have previously been synthesized by reacting MAB and NH3, including LiNH2BH3·NH3 [16], Mg(NH2BH3)2·3NH3 [17], and Ca(NH2BH3)2·NH3 [18]. However, to the best of our knowledge, there has been no prior report of the synthesis of an aluminum analog. Herein we report the first synthesis of aluminum amidoborane ammoniate, [Al(NH2BH3)63−][Al(NH3)63+].
2. Results and Discussion
The synthesis of [Al(NH2BH3)63−][Al(NH3)63+] (according to Equations (1) and (2)) was achieved using a specially-designed polytetrafluoroethylene reactor, which allowed the reactants to be stirred at low temperature under ammonia pressure. Under these conditions, ammonia borane reversibly absorbs up to 6 equivalents of NH3, forming liquid NH3BH3·nNH3 (n = 1–6) complexes [19]. AlH3·OEt2, which is insoluble in NH3BH3·nNH3, was utilized as a highly reactive Al source [20,21]. Immersing the Al source in ammonia borane ammoniate complex permits the selective uptake of ammonia in a one-step synthesis of Al(NH2BH3)3·3NH3 as a solid precipitate that can be isolated in up to 95% purity (based on AlH3·OEt2) by filtration (Equations (1) and (2), details in Section 3, Experimental Section). It should be emphasized that this method avoids the high-energy impact generally encountered in traditional ball milling methods and further prevents possible decomposition of components. This synthesis strategy may also be effective for other amidoborane ammoniates.
Figure 1a illustrates the XRD pattern obtained for a sample of Al(NH2BH3)3·3NH3 prepared via the method described above. The pattern does not index to any previously reported Al-N-B-H quaternary compound and contains at most, only very minor contributions from unreacted starting material. FTIR analysis of Al(NH2BH3)3·3NH3 featured a N-B stretch at 875 cm−1 and peaks at 426 and 461 cm−1 which were assigned to Al-N lattice vibrations, (Figure S1, Table S1). Attempts were also made to prepare Al(NH2BH3)3·3NH3 using ball milling techniques. As shown in Figure 1c, no new species evolved from a mixture of AlH3·OEt2 + 3NH3BH3, which was ball milled under ammonia atmosphere at 0 °C, at a speed of 150 rpm for at least 2 hours. Moreover, increasing the ball milling energy, such as higher rotational speed or temperature (>40 °C) during the synthesis causes dissociation of the ether adduct, which often leads to the production of γ-AlH3 (Figure 1b). This alane polymorph incidentally shows much lower reactivity in liquid NH3BH3·nNH3 than pure AlH3·OEt2, and inhibits the formation of an Al-N bond [22].
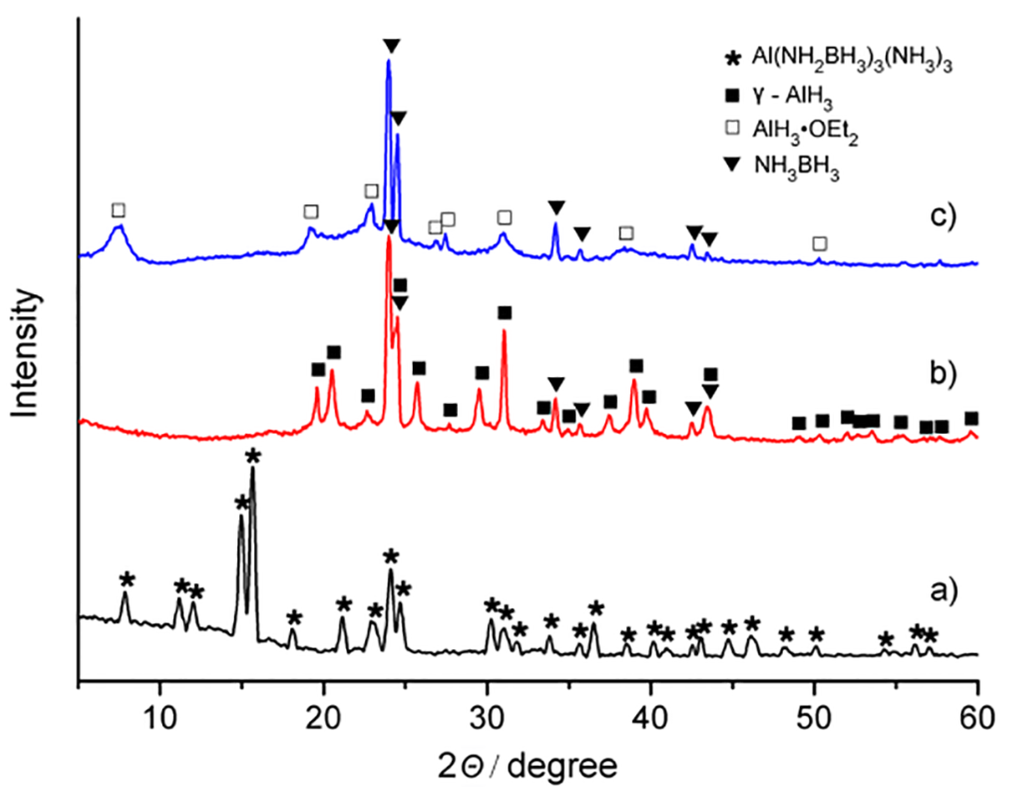
Figure 1.
XRD patterns of (a) as-prepared Al(NH2BH3)3·3NH3; (b) mixture of γ-AlH3 + 3NH3BH3; (c) ball milled AlH3·OEt2 + 3NH3BH3. λ = 1.5406 Å.
The 27Al MAS NMR spectrum (Figure 2b) verifies the formation of Al(NH2BH3)3·3NH3 and provides key information about its molecular structure. After reaction, only traces of the characteristic resonance for AlH3·OEt2 at 109.9 ppm are observed [23]. Two major resonances at 65.5 ppm and 33.6 ppm dominate the spectrum, clearly indicating that Al(NH2BH3)3·3NH3 contains equal amounts of aluminum in two very different coordination environments. The MAS 11B NMR spectrum of the product contains a major resonance for Al(NH2BH3)3·3NH3 at 19.6 ppm and a and a minor resonance at −38 ppm which is due the presence of [(NH3)2BH2+][BH4−] DADB or a related decomposition product that was also observed in the starting material [24]. As seen in Figure 2a, the 11B chemical shift of Al(NH2BH3)3·3NH3 is 2.7 ppm upfield from the 22.3 ppm shift observed for NH3BH3·nNH3. Similar upfield shifts have been observed for other metal amidoboranes and hence this observation confirms the substitution of an H atom by an Al atom in the ammonia borane molecule [25,26].
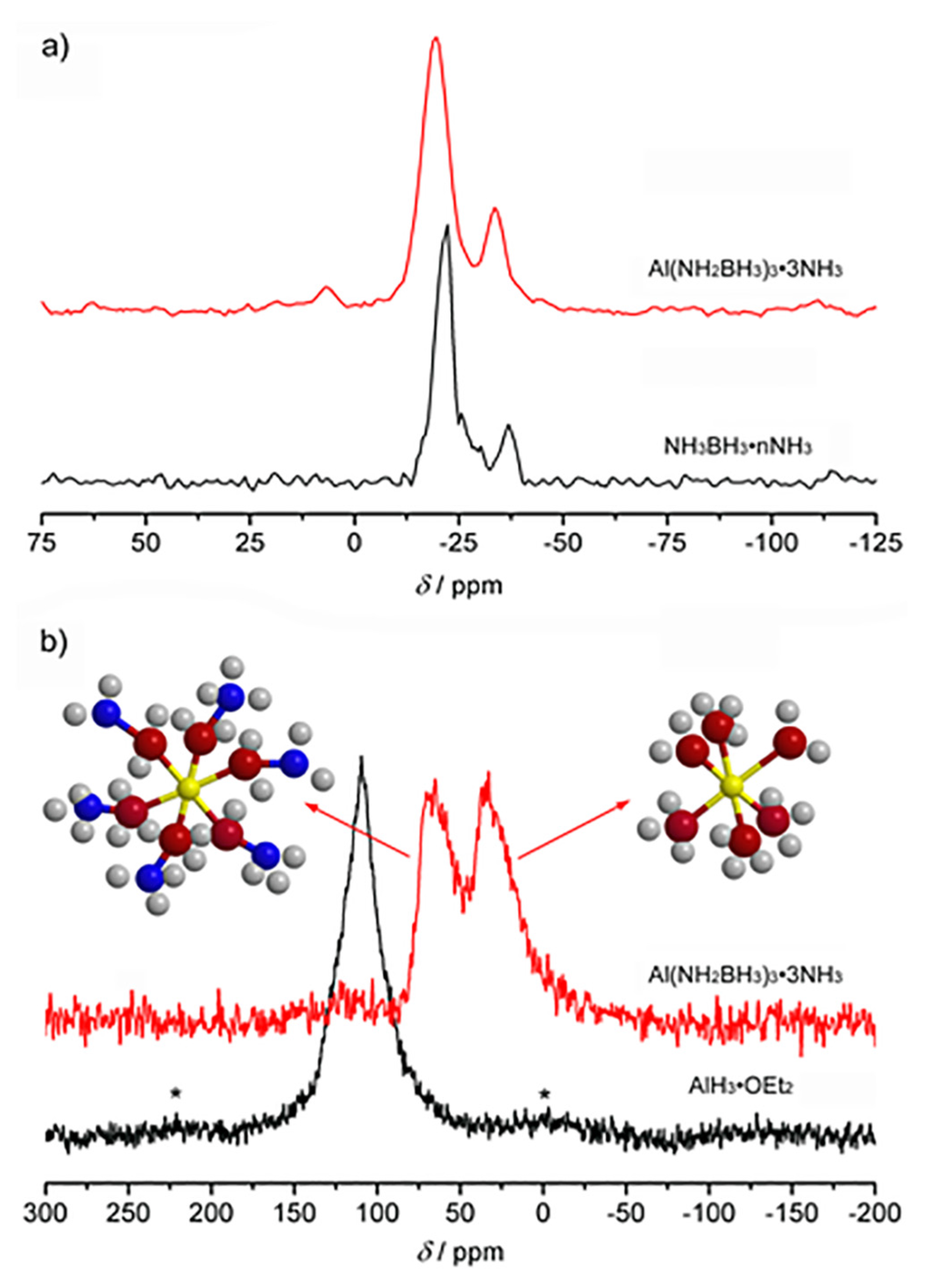
Figure 2.
(a) 11B MAS NMR spectra of [Al(NH2BH3)63−][Al(NH3)63+] and NH3BH3·nNH3; (b) 27Al MAS NMR spectra of [Al(NH2BH3)63−][Al(NH3)63+] and AlH3·OEt2. The molecular structure of the octahedral Al complexes are also depicted (yellow balls represent Al, red for N, blue for B, and grey for H).
Al3+ generally has either tetrahedral or octahedral coordination. Thus a priori there are four possible coordination geometries for Al(NH2BH3)3·3NH3: 1) Al coordinates three (NH2BH3)− anions and three ammonia molecules to give a neutral Al(NH3)3(NH2BH3)3 complex; 2) Al coordinates octahedrally with only ammonia giving a hexamminealuminum cation [27], Al(NH3)63+ and leaving three free (NH2BH3)− anions; 3) Al coordinates tetrahedrally with (NH2BH3)− anions, forming an Al(NH2BH3)4− anion and three of these anions pair with one Al(NH3)63+; and 4) Al octahedrally coordinates with (NH2BH3)− anions giving a Al(NH2BH3)63− complex anion and ion pairs with the Al(NH3)63+ cation. The observation of two peaks with equal intensity in the 27Al MAS NMR spectrum is consistent with only the [Al(NH2BH3)63−][Al(NH3)63+] formulation and as such the 27Al NMR resonances are assigned as follows: Al(NH2BH3)63− at 65.5 ppm and Al(NH3)63+ at 33.6 ppm (Figure 2b). This is quite similar to the reported structure of Mg(NH2BH3)2·3NH3 where Mg2+ exhibits both tetrahedral and octahedral coordination [17]. Elemental analysis (Table S2) also shows the ratio of Al:N:B is 1:6:3, and supports the [Al(NH2BH3)63−][Al(NH3)63+] formulation.
No apparent reaction was observed after exposure of a sample of [Al(NH2BH3)63−][Al(NH3)63+] to dry air for 3 days (Figure S2). The time-programmed-desorption/mass spectroscopy (TPD/MS) results reveal that the thermal decomposition of [Al(NH2BH3)63−][Al(NH3)63+] occurs in the temperature range of 65–180 °C, with the release corresponding to 7.5 wt % (Figure 3a,c). The desorbed gaseous product comprises of both H2 and NH3.
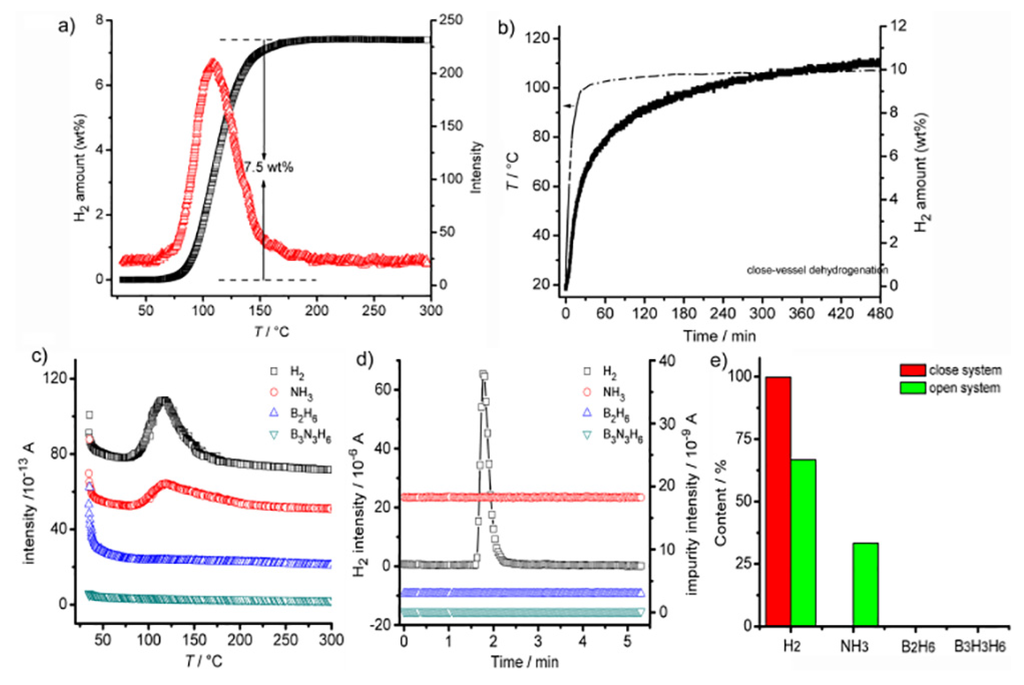
Figure 3.
(a) TPD (∆) and gas release (□) profiles of [Al(NH2BH3)63−][Al(NH3)63+] at a heating rate of 5 °C min−1 under argon flow; (b) Isothermal desorption of [Al(NH2BH3)63−][Al(NH3)63+] in a closed vessel; the temperature ramping shown by dash dot line; (c) MS signals in (a): □ H2, ○ NH3, ∆ B2H6, ▽ B3N3H6; (d) MS signals in (b) measured using Calibration Injection Mode: □ H2, ○ NH3, ∆ B2H6, ▽ B3N3H6; (e) H2 purity comparison between different systems.
Figure 4 shows the N 1s XPS results of AlAB·3NH3 before and after thermal decomposition (experimental details described in Supplemental Information). The peaks at ~396.6, ~398.0 and ~399.6 eV are attributed to N-Al, N-B and NH3, respectively. After decomposition, the evolution of NH3 and the corresponding peak at ~189.8 eV in B 1s XPS (Figure S3) suggests the formation of an Al-N-B matrix. Combined with the remaining B-H vibrations in micro-FTIR (Figure S4, Table S3), the reaction under dynamic inert gas flow can be described by Equation (3). The anticipated borazine-derivated structure is illustrated in Figure S5 representing AlN3B3H6.
[Al(NH2BH3)63−][Al(NH3)63+] → 2AlN3B3H3 + 6NH3 + 12H2
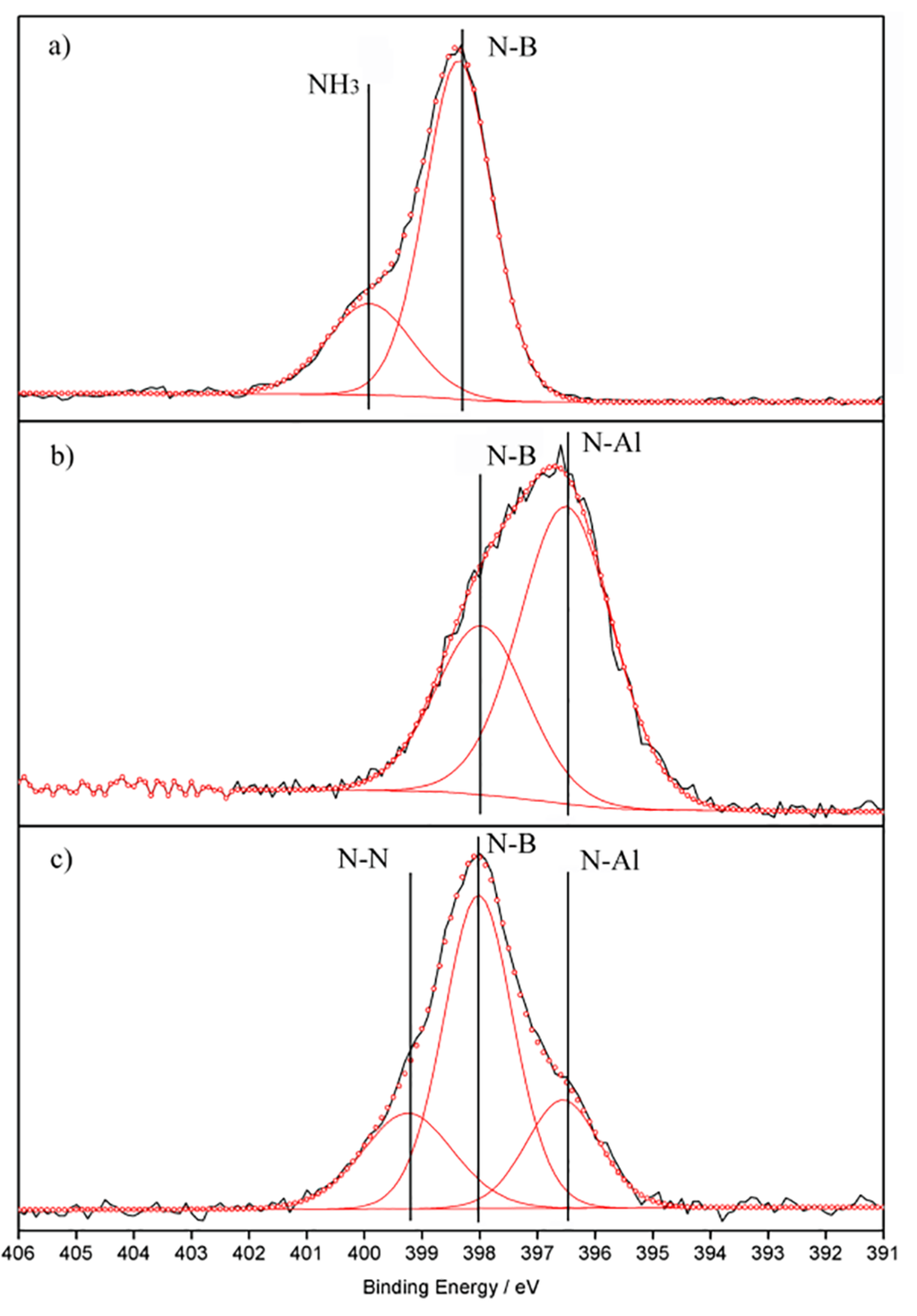
Figure 4.
The N 1s XPS results of Al(NH2BH3)3·3NH3 (1) before (a) and after thermal decomposition in an open system (b) and in a closed system (c). The experiment data are in black, while the fitted ones are in red.
The isothermal desorption in a closed vessel was examined using a Sieverts method at 105 °C (experimental details described in Supplemental Information). The gas evolved is calculated to be 10.3 wt % (Figure 3b), while only 0.05% NH3 is detectable (Figure 3d). Obviously, the mass difference (Figure 3e) indicates that a significant partial pressure of NH3 in a closed system suppresses further NH3 desorption. This phenomenon is in accordance with the decomposition pathways of other metal amidoborane ammoniates [17,28,29]. Element analysis shows that the composition of Al, N, B and H are 15.83%, 48.18%, 18.36% and 3.83%, respectively, indicating an empirical formula of AlN6B3H6.5*. Similarly, N-H or B-H vibrations are not observed in the micro-FTIR spectrum, while Al-N stretching vibrations and weak H wagging vibrations are apparent (Figure S4 and Table S3). Meanwhile, the peaks at 1367 and 1627 cm−1 are typical of N-B stretching in h-BN [30]. The N 1s XPS peak ~396.4 eV (Figure 4c) and is attributed to the formation of an Al-N bond, while the two overlapped B 1s XPS peaks in Figure S3 suggests that the decomposed product comprises of not only [AlNBH] but also another B moiety. On the other hand, the 11B MAS NMR spectrum presents at least two overlapping resonances at 6.3 ppm and 18.3 ppm (Figure S6), which is due to the second-order quadrupolar interaction. Thus, the B atoms are likely in a BN3 or BN2H environment [31,32]. The N 1s peak at ~399.1 eV is possibly a N-N bond. Clearly, the decomposition mechanism of [Al(NH2BH3)63−][Al(NH3)63+] in a closed system is much more complicated than that of an open system. On the basis of 8.6 equivalents of H2, the dehydrogenation process can be briefly described by Equation (4).
[Al(NH2BH3)63−][Al(NH3)63+] → AlN6B3H6.5* + (8.5~9.0)H2
3. Experimental Section
All starting materials, LiAlH4 99% (Sigma-Aldrich, Shanghai, China), AlCl3 99.99%, NH3BH3 99% (Sigma-Aldrich), and NH3 (Alfa Aesar, Shanghai, China), were obtained commercially and used without further purification. All manipulations were carried out under inert atmosphere conditions, either in an argon-filled glovebox or using standard Schlenk line techniques under a nitrogen atmosphere. The organometallic synthesis of AlH3·Et2O is a chemically simple process, but a brief summary is presented. Generally, AlCl3 was reacted with LiAlH4 in diethyl ether with the LiCl precipitate being removed by filtration [21,33]. The excess diethyl ether was then removed under dynamic vacuum. AlH3·Et2O was ground in a mortar with excess NH3BH3 and then sealed in a self-designed polytetrafluoroethylene (PTFE) reactor. The reactor was attached to the gas/vacuum manifold and rapidly evacuated/backfilled with 0.3–0.5 MPa NH3. The system was cooled to −70 °C using acetone and dry ice, and gradually warmed to 0 °C in an ice bath. At this temperature and under the NH3 atmosphere, ammonia borane reversibly absorbed up to at least 6 equivalents of NH3, forming liquid NH3BH3·nNH3 (n = 1–6) complexes. AlH3·OEt2 was dissolved in liquid NH3BH3·nNH3, and the solution stirred for 2 h until the reaction was complete. The internal temperature and pressure of the reactor and manifold were recorded for the duration of the experiment. The ammonia and reaction produced hydrogen were then removed in vacuo at room temperature. Anhydrous diethyl ether was then added to the remaining products, thereby dissolving the excess NH3BH3 of which was removed by filtration. The residual solid [Al(NH2BH3)63−][Al(NH3)63+] was then heated to 45 °C for 12h to remove residual solvent to yield a solid white powder.
4. Conclusions
In summary, a novel aluminum amidoborane ammoniate, [Al(NH2BH3)63−][Al(NH3)63+], has been successfully synthesized. A reaction vessel has been designed that allows a one-step synthesis from the reaction of AlH3·OEt2 with liquid NH3BH3·nNH3 (n = 1~6) at 0 °C. MAS 27Al NMR spectroscopy confirms the formulation of the compound as an Al(NH2BH3)63− complex anion and a Al(NH3)63+ cation. This aluminum based M-N-B-H compound begins to release hydrogen at 65 °C, amounting to ~8.6 equivalents of H2 (10.3 wt %) upon heating to 105 °C. This method of synthesis offers a promising route towards the large scale production of metal amidoborane ammoniate moieties.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1996-1073/8/9/9107/s1.
Acknowledgments
The authors would like to acknowledge the financial support given by the MOST of China (No. 2010CB631301, 2009CB939902 and 2012CBA01207) and the NSFC (No. U1201241 and 51071003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.S.; Chen, P.; Wu, G.; Xiong, Z. Development of amidoboranes for hydrogen storage. Chem. Commun. 2011, 47, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Wolverton, C. Crystal structures, phase stabilities, and hydrogen storage properties of metal amidoboranes. J. Phys. Chem. C 2012, 116, 14224–14231. [Google Scholar] [CrossRef]
- Zheng, X.L.; Wu, G.T.; Li, W.; Xiong, Z.T.; He, T.; Guo, J.P.; Chen, H.; Chen, P. Releasing 17.8 wt% H2 from lithium borohydride ammoniate. Energy Environ. Sci. 2011, 4, 3593–3600. [Google Scholar] [CrossRef]
- Guo, Y.H.; Wu, H.; Zhou, W.; Yu, X.B. Dehydrogenation tuning of ammine borohydrides using double-metal cations. J. Am. Chem. Soc. 2011, 133, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ley, M.B.; Lee, Y.-S.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jørgensen, J.E.; Besenbacher, F.; Jensen, T.R.; et al. Boron-nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef]
- Sutton, A.D.; Burrell, A.K.; Dixon, D.A.; Garner, E.B.; Gordon, J.C.; Nakagawa, T.; Ott, K.C.; Robinson, P.; Vasiliu, M. Regeneration of ammonia borane spent fuel by direct reaction with hydrazine and liquid ammonia. Science 2011, 331, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.W.; Tan, Y.B.; Chen, X.W.; Yu, X.B. Regenerable hydrogen storage in lithium amidoborane. Chem. Commun. 2012, 48, 9296–9298. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, Z.; Gong, Q.; Yu, X.; Beaumont, P.R.; Jensen, C.M. Phenyl introduced ammonium borohydride: Synthesis and reversible dehydrogenation properties. J. Mater. Chem. 2012, 22, 21017–21023. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Jalisatgi, S.S.; Safronov, A.V.; Lee, H.B.; Wu, J. Chemical Hydrogen Storage Using Polyhedral Borane Anions and Aluminum-Ammonia-Borane Complexes. Available online: http://www.osti.gov/scitech//servlets/purl/990217-xUxbgx/ (accessed on 28 June 2015).
- Harder, S.; Spielmann, J. Unprecedented reactivity of an aluminium hydride complex with ArNH2BH3: Nucleophilic substitution versus deprotonation. Chem. Commun. 2011, 47, 11945–11947. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Ketchum, D.R.; Hamilton, E.J.M.; Florian, P.A.; Vermillion, K.E.; Grandinetti, P.J.; Shore, S.G. Reactions of aluminum hydride derivatives with ammonia-borane: A new approach toward AlN/BN materials. Chem. Mater. 1996, 8, 2839–2842. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, X.; Sun, W.; Sun, D.; Yang, W. The hydrogen-enriched Al-B-N system as an advanced solid hydrogen-storage candidate. Angew. Chem. Int. Ed. 2011, 50, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Jiang, Y.X.; Xia, G.L.; Yu, X.B. Ammine aluminium borohydrides: An appealing system releasing over 12 wt% pure H2 under moderate temperature. Chem. Commun. 2012, 48, 4408–4410. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Tan, Y.; Chen, X.; Guo, Z.; Liu, H.; Yu, X. Mixed-metal (Li, Al) amidoborane: Synthesis and enhanced hydrogen storage properties. J. Mater. Chem. A 2013, 1, 1810–1820. [Google Scholar] [CrossRef]
- Xia, G.L.; Yu, X.B.; Guo, Y.H.; Wu, Z.; Yang, C.Z.; Liu, H.K.; Dou, S.X. Ammine lithium amidoborane Li(NH3)NH2BH3: A new coordination compound with favorable dehydrogenation characteristics. Chem. Eur. J. 2010, 16, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.D.; Wu, H.; Luo, J.H.; Zhou, W.; Wang, P. A simple and efficient approach to synthesize amidoborane ammoniates: Case study for Mg(NH2BH3)2(NH3)3 with unusual coordination structure. J. Mater. Chem. 2012, 22, 13174–13179. [Google Scholar] [CrossRef]
- Chua, Y.S.; Wu, H.; Zhou, W.; Udovic, T.J.; Wu, G.T.; Xiong, Z.T.; Wong, M.W.; Chen, P. Monoammoniate of calcium amidoborane: Synthesis, structure, and hydrogen-storage properties. Inorg. Chem. 2012, 51, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fang, H.C.; Li, Z.H.; Yu, X.B.; Fan, K.N. Liquefaction of solid-state BH3NH3 by gaseous NH3. Inorg. Chem. 2011, 50, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Graetz, J.; Chaudhuri, S.; Wegrzyn, J.; Celebi, Y.; Johnson, J.R.; Zhou, W.; Reilly, J.J. Direct and reversible synthesis of AlH3-triethylenediamine from Al and H2. J. Phys. Chem. C 2007, 111, 19148–19152. [Google Scholar] [CrossRef]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and properties of aluminum-hydride. J. Am. Chem. Soc. 1976, 98, 2450–2453. [Google Scholar] [CrossRef]
- Giannasi, A.; Colognesi, D.; Fichtner, M.; Rohm, E.; Ulivi, L.; Ziparo, C.; Zoppi, M. Temperature behavior of the AlH3 polymorph by in situ investigation using high resolution raman scattering. J. Phys. Chem. A 2011, 115, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Humphries, T.D.; Munroe, K.T.; Decken, A.; McGrady, G.S. Lewis base complexes of AlH3: Prediction of preferred structure and stoichiometry. Dalton Trans. 2013, 42, 6965–6978. [Google Scholar] [CrossRef] [PubMed]
- Stowe, A.C.; Shaw, W.J.; Linehan, J.C.; Schmid, B.; Autrey, T. In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: Mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material. Phys. Chem. Chem. Phys. 2007, 9, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Zhang, Y.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Solid state NMR study on the thermal decomposition pathway of sodium amidoborane NaNH2BH3. J. Mater. Chem. 2011, 21, 2609–2615. [Google Scholar] [CrossRef]
- Shimoda, K.; Doi, K.; Nakagawa, T.; Zhan, Y.; Miyaoka, H.; Ichikawa, T.; Tansho, M.; Shimizu, T.; Burrell, A.K.; Kojima, Y. Comparative study of structural changes in NH3BH3, LiNH2BH3, and KNH2BH3 during dehydrogenation process. J. Phys. Chem. C 2012, 116, 5957–5964. [Google Scholar] [CrossRef]
- Semenenko, K.N.; Shilkin, S.P.; Polyakova, V.B. Vibrational spectra and structure of the di- and tetraammoniate of aluminum borohydride. Bull. Acad. Sci. USSR Div. Chem. Sc. 1978, 27, 859–864. [Google Scholar] [CrossRef]
- Chua, Y.S.; Wu, G.T.; Xiong, Z.T.; Karkamkar, A.; Guo, J.P.; Jian, M.X.; Wong, M.W.; Autrey, T.; Chen, P. Synthesis, structure and dehydrogenation of magnesium amidoborane monoammoniate. Chem. Commun. 2010, 46, 5752–5754. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.S.; Wu, G.T.; Xiong, Z.T.; He, T.; Chen, P. Calcium amidoborane ammoniate-synthesis, structure, and hydrogen storage properties. Chem. Mater. 2009, 21, 4899–4904. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.; Rupprecht, G. Normal modes in hexagonal boron nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Marchetti, P.S.; Kwon, D.K.; Schmidt, W.R.; Interrante, L.V.; Maciel, G.E. High-field B11 magic angle spinning NMR characterization of boron nitrides. Chem. Mater. 1991, 3, 482–486. [Google Scholar] [CrossRef]
- Jeschke, G.; Hoffbauer, W.; Jansen, M. A comprehensive NMR study of cubic and hexagonal boron nitride. Solid State Nucl. Magn. Reson. 1998, 12, 1–7. [Google Scholar] [CrossRef]
- Humphries, T.D.; Munroe, K.T.; DeWinter, T.M.; Jensen, C.M.; McGrady, G.S. NMR spectroscopic and thermodynamic studies of the etherate and the α, α’, and γ phases of AlH3. Int. J. Hydrog. Energy 2013, 38, 4577–4586. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).