Abstract
Microbial fuel cell (MFC) is a novel and environmentally friendly technology for wastewater treatment and pollutant resource utilization. Although advances have been made in various aspects including electrode materials and synthetic biology approaches, the overall performance of MFC still requires improvement, with mass transfer efficiency and structural stability of biofilms emerging as key bottlenecks constraining their practical applications. This study investigated the regulation of substrate type and electrode potential during bioanode culture to optimize biofilm structure and enhance MFC performance. Results demonstrated that bioanodes cultured with glucose at −0.3 V formed vertically ordered biofilms that exhibited significant advantages in mass transfer characteristics, electrocatalytic activity, and structural stability. Under these culture conditions, enriched fermentative microorganisms facilitated the construction of porous biofilm scaffolds, while the electric field generated by the −0.3 V potential further induced vertical orientation and ordered arrangement of the biofilm. The superior mass transfer characteristics enabled the inner, middle, and outer layers of the biofilm to maintain high microbial activity (>50%), thereby maximizing the catalytic activity of electroactive microorganisms in each layer and enhancing biofilm structural stability. This study proposes a bioanode culture strategy centered on biofilm structural optimization, providing new theoretical foundations and technical pathways for achieving long-term stable and efficient MFC operation.
1. Introduction
With the escalating global energy crisis and growing environmental governance demands, organic matter in municipal wastewater not only constitutes a significant pollution source but also harbors tremendous energy potential. Research indicates that when wastewater contains organic matter exceeding 300 mg COD L−1, its theoretical energy recovery can reach 1.65 kWh m−3, which is 5.7 times higher than the current average energy consumption for municipal wastewater treatment (0.29 kWh m−3) [1,2]. Microbial fuel cells (MFCs), as a renewable energy technology that simultaneously addresses pollutant removal and energy recovery, demonstrate broad application prospects in wastewater treatment, environmental remediation, and biosensing. In recent years, researchers have enhanced both pollutant degradation efficiency and power generation in MFCs through various approaches, including electrode modification [3,4], functional microbial screening and community construction [5,6], and electron transfer pathway optimization [7,8]. However, during long-term operation, anodic biofilms in MFCs still commonly encounter challenges, including mass transfer limitations, declining electroactivity, and structural instability [9].
During the operation of MFCs, electrogenic microorganisms typically exhibit preferential attachment to electrode surfaces to access electron acceptors, thereby forming a dense inner biofilm layer. Outer-layer microorganisms subsequently adhere to this active layer to achieve more efficient electron transfer through microbial nanowires or electron mediators [10]. As outer-layer microorganisms continue to accumulate and extracellular polymeric substances (EPS) secretion increases, the biofilm gradually thickens and evolves into a multi-layered, densely packed structure [11,12]. However, this stratified architecture further prolongs the diffusion pathways for substrates and protons within the biofilm [13], leading to progressive deactivation of inner-layer microorganisms due to substrate depletion and localized acidification [14]. The progressively thickening layers of dead cells and EPS deposition not only clog pore channels but also weaken the structural connectivity between the biofilm and electrode interface, disrupting electron transfer pathways from outer-layer exoelectrogens to the electrode [15]. This ultimately results in biofilm structural instability and declining electroactivity during long-term operation. Therefore, regulating biofilm structure and mass transfer characteristics to maintain inner-layer exoelectrogenic microbial activity has emerged as a critical direction for enhancing both MFC performance and long-term stability.
Substrates serve as the primary carbon source and electron donor for electrogenic microorganisms, representing a critical factor in determining microbial metabolic pathways and biofilm community structure. Consequently, substrate regulation is recognized as an effective strategy for optimizing bioanode electricity-generation performance. Previous studies have employed various organic compounds (such as acetate, glucose, amino acids, lactate, etc.) as culture substrates to compare the electricity-generation performance of different bioanodes [16,17,18]. For instance, Yogesh Sharma et al. [19] reported that bioanodes cultured with acetate exhibited approximately 38% higher coulombic efficiency compared to those cultured with glucose, while their output power density was approximately 9% lower. Yu et al. [20] further confirmed that glucose-cultured MFCs generated approximately 21% higher output current density than those cultured with acetate. These performance variations were commonly attributed to substrate-induced changes in microbial community composition, which subsequently affected the abundance and functional performance of dominant electrogenic microorganisms, leading to differences in extracellular electron transfer (EET) efficiency or fermentation-related metabolic pathways [21,22]. Recently, Lu et al. proposed that adding 10% L-aspartic acid to glucose substrates could promote the formation of dense electroactive inner biofilms while effectively inhibiting excessive accumulation of oxygen-consuming outer biofilms, thereby enhancing overall MFC performance [11]. This finding indicated the potential feasibility of substrate regulation in optimizing biofilm structure. However, whether substrate regulation can achieve directional control over biofilm microstructure, as well as the underlying mechanisms affecting mass transfer characteristics and bioanode structural stability, remains to be further investigated.
Electric fields represent a critical physical factor in bioelectrochemical systems, directly providing the driving force for interfacial electron transfer between microorganisms and electrodes. Early studies demonstrated that ammonia treatment to enhance positive charge density on electrode surfaces resulted in approximately 50% improvement in bioanode electricity generation, revealing for the first time the pivotal role of electric field forces in the enrichment process of electrogenic microorganisms [23]. Recent investigations have further elucidated the significant role of electric fields in the formation and evolution of electroactive biofilms. For instance, Yalcin et al. [24] reported that electric fields could stimulate Geobacter to secrete cytochrome OmcZ nanowires, thereby enhancing EET efficiency. Simultaneously, electric fields facilitated the selective enrichment of dominant electrogenic microorganisms. For example, Geobacter anodireducens and G. sulfurreducens exhibited distinct adaptive strategies under electric field conditions, resulting in differential relative abundances during long-term culture [25]. Notably, electric field effects extended beyond electrogenic microorganisms. In anaerobic membrane bioreactors, moderate applied electric fields (such as 0.5 V) significantly promoted the activity and metabolic upregulation of non-electroactive bacteria, consequently accelerating biofilm formation [26]. Therefore, electrode potential regulation may provide a viable pathway for optimizing electrode biofilm physical structure.
In this study, substrate and electrode potential regulations were used to change the electrode biofilm structure to improve the mass transfer characteristics of the bioanode and then obtain a bioanode with high electricity-generation performance and structural stability. Cyclic voltammetry, chronoamperometry, and degradation kinetics tests were used to analyze the differences in electricity-generation performances of bioanodes; linear sweep voltammetry in different substrate solutions was used to analyze catalytic characteristics of different bioanodes; electrochemical impedance spectroscopy and the Tafel test were used to evaluate mass transfer characteristics of different bioanodes; open-circle operation was used to test the structural stability of electrode biofilms. 16s rRNA high-throughput sequencing, SEM, and FISH were used to characterize the microbial community and biofilm structure of bioanodes, respectively, to explain the differences in electricity-generation performance, catalytic activity, mass transfer characteristics and structural stability of different bioanodes. This study established a bioanode culture strategy oriented to biofilm structure optimization.
2. Materials and Methods
2.1. Inoculation, and Cultivation of the Bioanode
The bioanode was cultured in a bioelectrochemical system (BES) in order to facilitate the control of operating parameters, including substrate and electrode potential. A dual-chamber bioelectrochemical reactor with three-electrode configuration was assembled and interfaced with an electrochemical analyzer (Bio-Logic, Claix, France), following the methodology described in the previous study [27]. Microbial enrichment was conducted on a circular graphite sheet (20 mm diameter, 2 mm thickness) serving as the bioanode. The reactor configuration included an air cathode as the counter electrode [28] and a cation exchange membrane separating the two compartments (Figure S1). The anodic and cathodic chambers had working volumes of 30 mL and 10 mL, respectively. The reference electrode consisted of Ag/AgCl (+197 mV vs. SHE) positioned in the anodic compartment. All electrode potentials reported herein were referenced to the Ag/AgCl electrode.
Bioanode inoculation was performed using a 1:1 (v/v) mixture of anoxic sludge obtained from municipal wastewater treatment facilities and anodic medium. The anodic medium consisted of 50 mM phosphate buffer solution (PBS; 11.466 g L−1 Na2HPO4∙12H2O, 2.75 g L−1 NaH2PO4∙2H2O) supplemented with trace minerals (12.5 mL L−1) [29], NH4Cl (0.62 g L−1), vitamins (5 mL L−1), KCl (0.26 g L−1) and carbon substrate (1.50 g COD L−1 glucose or acetate). Daily replacement of the inoculum mixture was performed during the initial 5-day inoculation period, after which electrodes were cultured using anodic medium alone in fed-batch mode with daily medium renewal. Prior to addition to the anode chamber, all solutions were deoxygenated by nitrogen sparging for 20 min. The cathode chamber was supplied with substrate-free PBS medium (50 mM) containing equivalent concentrations of trace minerals, NH4Cl, vitamins and KCl, with medium replacement performed every 7 days. During the bioanode culture, the working electrode potentials were set to −0.2 V, −0.3 V and −0.4 V, respectively. The cultured bioanodes were referred to as A-02 (acetate; −0.2 V), A-03 (acetate; −0.3 V), A-04 (acetate; −0.4 V), G-02 (glucose; −0.2 V), G-03 (glucose; −0.3 V) and G-04 (glucose; −0.4 V) according to the substrate and electrode potential used. All experiments were carried out in a constant temperature environment of 30 °C.
2.2. Electrochemical Characterization
Bioelectrochemical characterization employed multiple electrochemical techniques. Cyclic voltammetry (CV) assessed bioanode electroactivity at 2 mV s−1 scan rate across −0.6 to −0.1 V potential window in 50 mM PBS supplemented with 500 mg COD L−1 substrate (acetate or glucose corresponding to culture conditions). Substrate degradation kinetics were evaluated through chronoamperometry (CA) at culture-specific potentials (−0.2 V, −0.3 V, or −0.4 V) in PBS containing 1500 mg COD L−1 acetate or glucose. Catalytic performance was analyzed using linear sweep voltammetry (LSV) following chamber solution replacement and 2 h CA operation. LSV measurements spanned −0.55 to −0.1 V at 1 mV s−1 in PBS containing substrate concentrations ranging from 0 to 1500 mg COD L−1. Mass transfer characteristics were determined through polarization analysis of assembled MFC configurations, where bioanode and air cathode were connected via variable external resistances (150–1000 Ω) with 20 min equilibration periods. Power density calculations were derived from voltage–current relationships across the resistance range. Electrochemical kinetics were evaluated through Tafel analysis conducted at 2 mV s−1 within ±0.3 V versus open-circuit potential in PBS containing 1000 mg COD L−1 substrate. Charge transfer resistance was quantified using electrochemical impedance spectroscopy (EIS) over 0.01 Hz to 100 kHz frequency range at −0.2 V applied potential in acetate-supplemented PBS (500 mg COD L−1), comparing bioanodes before and after 12 h of open circuit operation (OCO). Chemical oxygen demand analysis employed spectrophotometric detection at 620 nm wavelength using a DR2800 spectrophotometer (HACH CO., Chicago, IL, USA). All experiments were performed in triplicate to ensure reproducibility. Data analysis was conducted using MATLAB software (R2024a, MathWorks, Natick, MA, USA).
2.3. Characterization of Electrode Biofilm
Biofilm structural characterization employed fluorescence in situ hybridization (FISH) and scanning electron microscopy (SEM) techniques. Cell viability assessment was conducted using LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen, Carlsbad, CA, USA) containing SYTO9 and propidium iodide fluorescent dyes, which differentiate viable cells (green fluorescence) from membrane-compromised cells (red fluorescence) based on membrane integrity [14]. Confocal laser scanning microscopy was performed using a TCS SPE system with DMI 4000B microscope (Leica, Solms, Germany) at excitation/emission wavelengths of 485/535 nm and 498/617 nm [30].
For SEM analysis, biofilm samples underwent fixation with 2.5% glutaraldehyde followed by phosphate buffer rinsing (100 mM, pH 7.0). Secondary fixation was achieved using 1% osmium tetroxide for 1–2 h, followed by triple phosphate buffer washing. Dehydration was performed through graded ethanol series (30–100%). Critical-point drying with liquid CO2 and gold sputter coating preceded examination using a ZEISS GEMINI (New York, NY, USA) 300 scanning electron microscope at 3.0 kV acceleration voltage, with digital image capture at 1000–10,000× magnification.
Microbial community analysis utilized 16S rRNA high-throughput sequencing of biofilm samples scraped from graphite electrodes and collected in sterile centrifuge tubes. Genomic DNA extraction employed isolation kit (PowerSoil, Superior, WI, USA), with biomass quantification based on total DNA concentration [31]. Community profiling involved PCR amplification targeting V3-V4 hypervariable regions using primer pairs F: 5’-ACTCCTACGGGAGGCAGCAG-3’ and R: 5’-GGACTACHVGGGT-WTCTAAT-3’, followed by amplicon purification, library preparation, and Illumina MiSeq sequencing [14].
3. Results
3.1. Effects of Substrate and Electrode Potential on Bioanode Performances
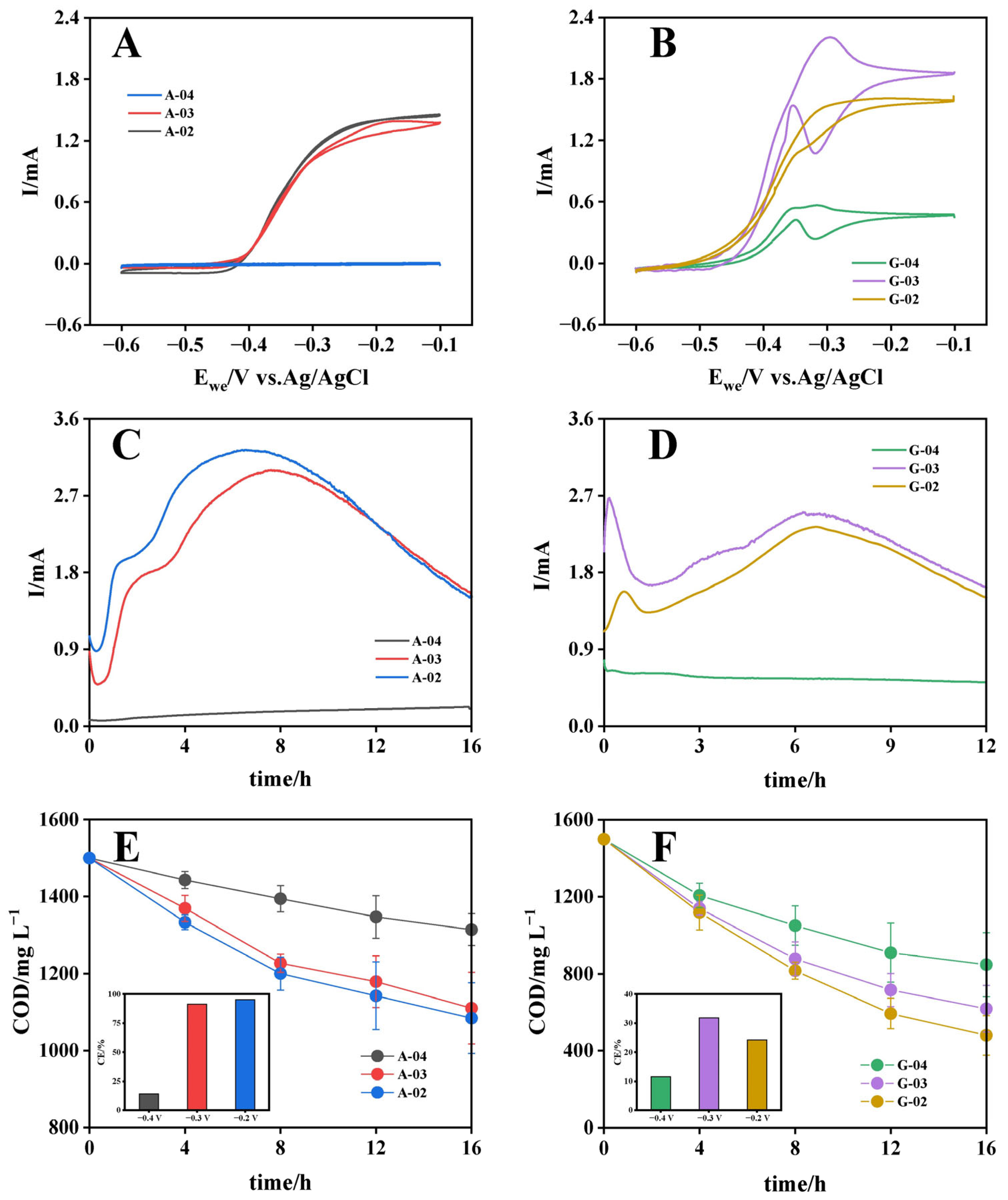
To evaluate the effects of substrate type and electrode potential on bioanode performance, bioanodes cultured under different conditions were subjected to CV in acetate solution, with biooxidation electroactivity quantified by maximum oxidation current. As shown in Figure 1A, acetate-cultured bioanodes exhibited potential-dependent bioelectroactivity, with maximum oxidation currents of 1.45 mA (−0.2 V) > 1.38 mA (−0.3 V) > 0.006 mA (−0.4 V). In contrast, glucose-cultured bioanodes demonstrated distinct performance characteristics, achieving peak oxidation current of 2.2 mA at −0.3 V, followed by 1.66 mA at −0.2 V and 0.56 mA at −0.4 V (Figure 1B). The reduced electroactivity at −0.4 V could be attributed to two aspects: first, as the applied potential became more negative, the increased overpotential reduced the kinetic efficiency of extracellular electron transfer (EET) processes in electrogenic bacteria [32]; second, from a microbial energetics perspective, the Gibbs free energy (ΔG) available to microorganisms depends on the potential difference between electron donor oxidation and electron acceptor reduction, and the excessively negative anode potential might have created thermodynamically unfavorable conditions for electrogenic bacteria, thereby affecting their metabolic activity [33]. The highest bioelectroactivity achieved with glucose cultivation exceeded that of acetate by 52%, consistent with previous reports [19,20], demonstrating the superior capacity of glucose for developing highly electroactive bioanodes. Notably, acetate-cultured bioanodes performed similarly at −0.2 V and −0.3 V with only 5% variation, whereas glucose-cultured bioanodes exhibited 33% higher bioelectroactivity at −0.3 V compared to −0.2 V. Differences in the optimal potential between acetate- and glucose-cultured bioanodes suggested that substrate type and electrode potential interacted to determine bioanode performance. This finding highlights the need to further investigate their interaction to develop high-performance bioanode culture strategies.
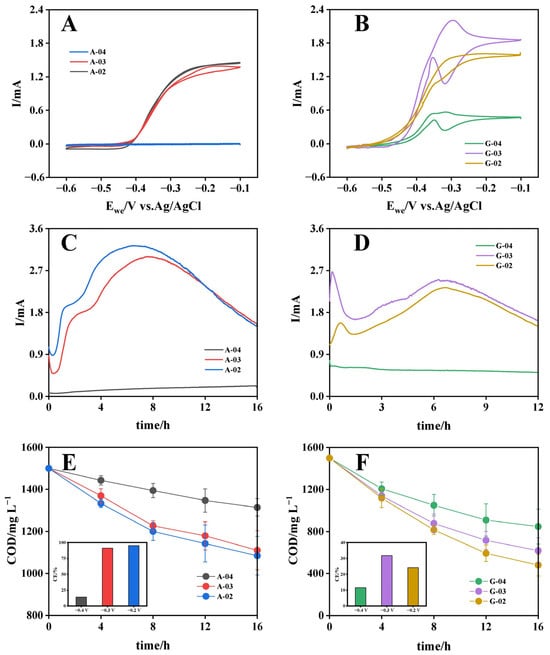
Figure 1.
Electrochemical performance of bioanodes cultured with acetate (A,C,E) and glucose (B,D,F) at different potentials. (A,B) Cyclic voltammograms; (C,D) chronoamperometric current profiles; (E,F) COD degradation kinetics with coulombic efficiency (insets).
CA tests were conducted on different bioanodes under their respective culture conditions (Figure 1C,D). The acetate-cultured bioanodes (A-02 and A-03) exhibited current outputs that initially increased to peak currents (3.2 mA and 2.8 mA, respectively) at 6–8 h, then decreased progressively as substrates were consumed. A-04 maintained consistently low current output below 0.4 mA throughout the test period. Compared to acetate-cultured bioanodes, the glucose-cultured bioanodes demonstrated more sustained and stable current output at relatively high levels, which might be attributed to the multi-step metabolic pathways of glucose degradation, with intermediate metabolites continuously supplying electron donors for electricity generation. Degradation kinetics were further analyzed. For acetate-cultured bioanodes, the average organic matter removal rates (calculated as COD) were 26.0 mg COD L−1 h−1 (−0.2 V), 24.4 mg COD L−1 h−1 (−0.3 V) and 11.6 mg COD L−1 h−1 (−0.4 V), respectively, accounting for only 41%, 44%, and 28.4% of the rates observed for glucose-cultured bioanodes at the corresponding potentials (63.8 mg COD L−1 h−1 (−0.2 V), 55.1 mg COD L−1 h−1 (−0.3 V) and 40.8 mg COD L−1 h−1 (−0.4 V)) (Figure 1E,F). Despite demonstrating higher organic matter removal rates across all potentials, glucose-cultured bioanodes exhibited significantly lower coulombic efficiency (CE) compared to acetate-cultured bioanodes. This discrepancy might be attributed to the extensive participation of non-electrogenic microorganisms during glucose metabolism and incomplete utilization of intermediate metabolic products by electrogenic microorganisms. Based on the comprehensive assessment of biooxidation electroactivity and substrate utilization efficiency (CE), A-02 and G-03 represented the bioanodes with optimal electricity-generation performance in the acetate and glucose groups [34], respectively, suggesting that the optimal culture potential for bioanodes was substrate-dependent. However, despite the enrichment of more non-electrogenic microorganisms and the presence of organic components that were difficult for electrogenic microorganisms to utilize electrochemically, glucose-cultivated bioanodes still demonstrated higher biooxidation electroactivity, the underlying mechanisms of which warranted further investigation.
3.2. Effects of Substrate and Electrode Potential on Bioanode Catalytic Properties and Microbial Community
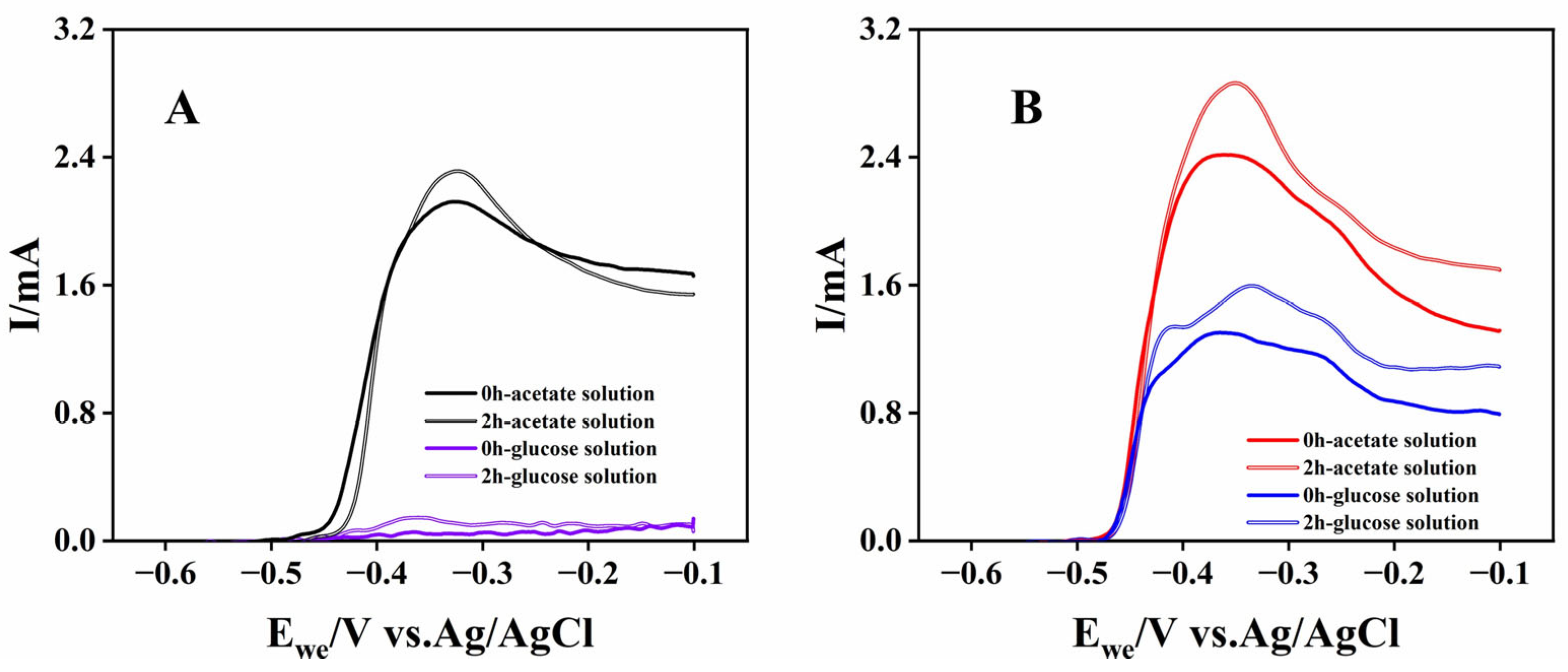
The bioanodes with high electricity-generation performance (A-02 and G-03) were evaluated for their catalytic properties using LSV in acetate and glucose solutions. Immediately after replacing the working electrode chamber solution, the glucose-cultured bioanode exhibited substantial oxidation currents in both acetate and glucose media, with maximum values of 2.42 mA and 1.30 mA, respectively (Figure 2B), indicating its ability to utilize both substrates for electricity generation. In contrast, the acetate-cultured bioanode generated detectable oxidation current only in acetate solution (Figure 2A). In the absence of reports on electrogenic microorganisms catalyzing direct bioelectrochemical glucose oxidation, this finding suggested that the glucose-cultured bioanode might enrich not only electrogenic microorganisms but also microorganisms capable of degrading macromolecular organics, such as fermentation bacteria. Furthermore, several weak oxidation current peaks were observed in the potential range of −0.46 V to −0.1 V in the LSV of the glucose-cultured bioanode, likely originating from the bioelectrochemical oxidation of intermediate metabolites produced during glucose metabolism. These results indicated that glucose-cultured bioanodes possessed the capacity to degrade a wider range of organic compounds, making them more suitable for application in actual wastewater with complex organic compositions. After 2 h of CA operation, the oxidation currents of both acetate- and glucose-cultured bioanodes were higher than those measured immediately after solution replacement, suggesting that their bioelectrochemical oxidation might involve indirect electron transfer processes, which were enhanced by increased secretion of electron mediators.
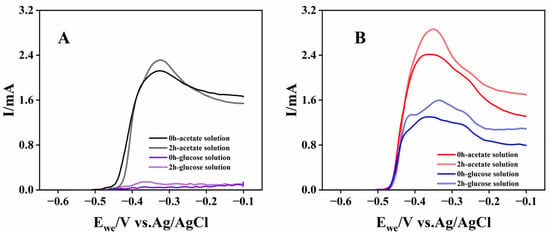
Figure 2.
LSV curves of acetate-cultured bioanodes (A) and glucose-cultured bioanodes (B) at different operating times and in different solutions.
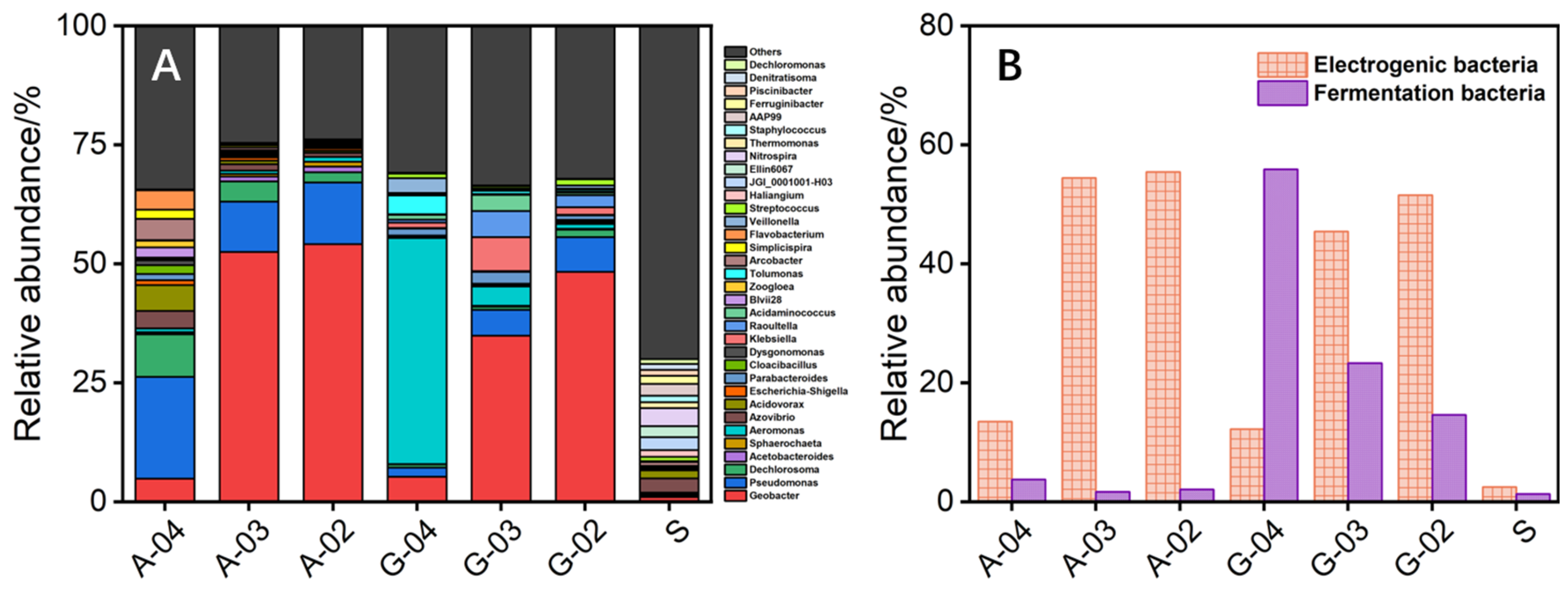
The microbial community showed that bioanodes cultured with acetate and glucose were gradually enriched with electrogenic microorganisms, including Geobacter [35], Escherichia-Shigella [36], Parabacteroides [37], Klebsiella [38], Zoogloea [39], Tolumonas [40], and Arcobacter [41], among which Geobacter was the main electrogenic microorganism on bioanodes with the relative abundance exceeding 36% (Figure 3A). The relative abundances of electrogenic microorganisms on bioanodes gradually increased with the positive shift of the culture-used electrode potential. Specifically, the relative abundance of electrogenic microorganisms on acetate-cultured bioanodes was 55.4% (−0.2 V) > 54.4% (−0.3 V) > 13.5% (−0.4 V), and that on the glucose-cultured bioanodes was 51.5% (−0.2 V) > 45.4% (−0.3 V) > 12.2% (−0.4 V). It can be found that the relative abundance of electrogenic microorganisms and the biooxidation electroactivity of the bioanode did not show a complete positive correlation. Similarly, G-03 had the highest biooxidation electroactivity among tested bioanodes, but the relative abundance of electrogenic microorganisms on the bioanode was lower than that of G-02 (35.2% vs. 48.2%). In addition, the relative abundance of fermentation microorganisms (including Aeromonas [40], Parabacteroides [42], Dysgonomonas [43], Klebsiella [44], Raoultella [43], Acidaminococcus [43], Veillonella [45], and Streptococcus [46]) in the glucose-cultured bioanode was significantly higher than the acetate-cultured bioanode, with the highest relative abundance reaching 55.8% (Figure 3B). Different relative abundance of fermentation microorganisms explained the difference in the ability of acetate-cultured and glucose-cultured bioanodes to use glucose to produce electricity (0.03 mA vs. 1.30 mA) (Figure 2). Enrichment of fermentation microorganisms could partly explain the low relative abundance of electrogenic microorganisms on the glucose-cultured bioanode with high biooxidation electroactivity. However, most of the fermentation microorganisms belonged to heterotrophic microorganisms that could compete with electrogenic microorganisms for substrates. From the perspective of substrate competition, the G-02 possessed a lower relative abundance of fermentation microorganisms and a higher relative abundance of electrogenic microorganisms should have higher biooxidation electroactivity than that of G-03, but the results were quite the opposite. Therefore, the microbial community may also affect the electricity-generation performance of bioanode in other aspects and needs to be further explored.
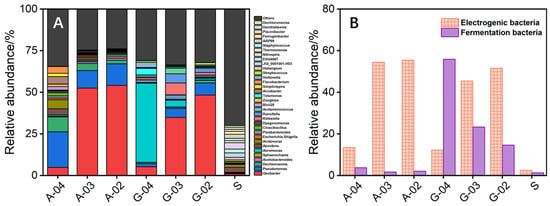
Figure 3.
The genus level of microbial community in sludge inoculum (S) and bioanodes (A); and the relative abundance of electrogenic and fermentation microorganisms in S and bioanodes (B).
3.3. Effect of Substrate and Electrode Potential on Biofilm Structure
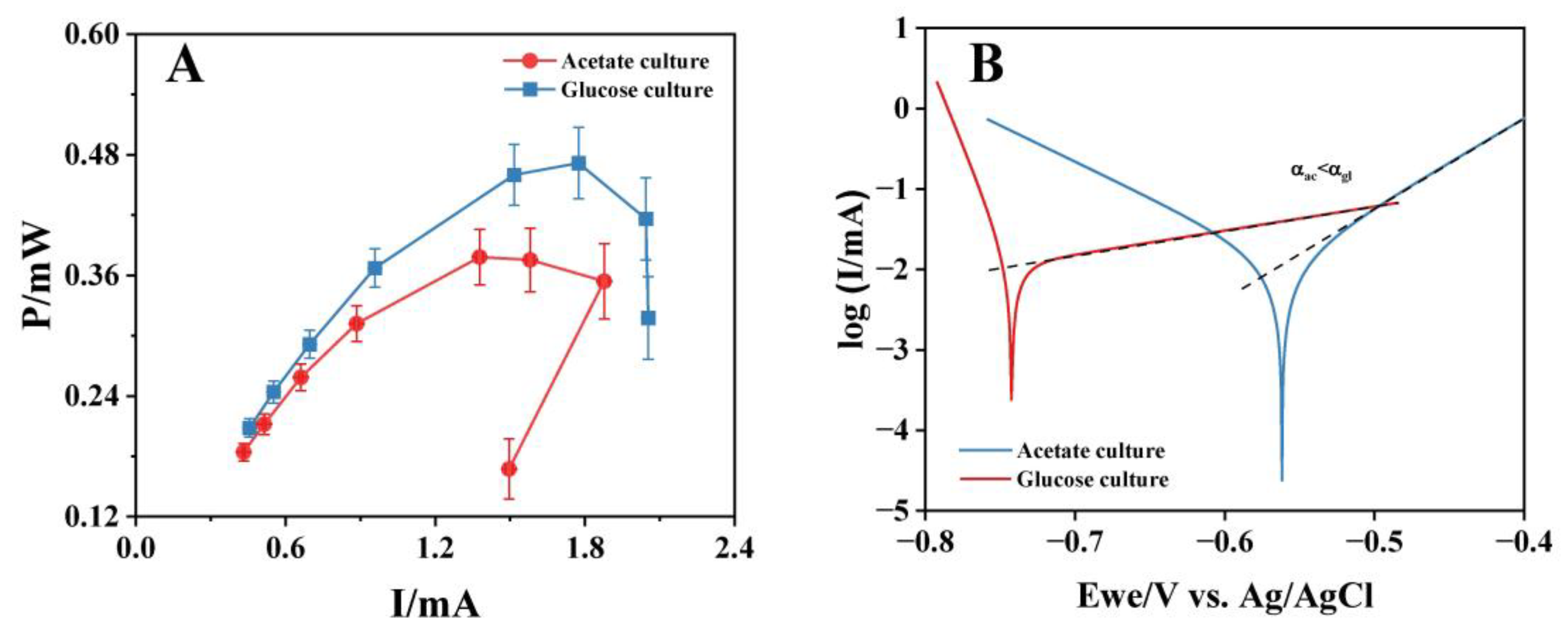
The bioanodes with high electricity-generation performance (A-02 and G-03) were used to construct MFCs for polarization tests. The polarization curves (Figure 4A) showed that the maximum output power of the MFC with the glucose-cultured bioanode was 0.472 mW, 1.21 times that of the MFC with the acetate-cultured bioanode (0.389 mW). The tested MFCs used the same cathode, so it could be considered that the difference in the maximum output power came from different bioanode performances. Notably, the output power of MFCs dropped suddenly after reaching the maximum output current (1.88 mA/2.05 mA; A-02/G-03). The rapid decrease of MFC output power at a high current value was due to the fact that the mass transfer on the electrode surface cannot meet the requirements of a high bioelectrochemical reaction rate [47]. Therefore, the above phenomenon suggested that the bioanode cultured with glucose had better mass transfer characteristics than acetate. Tafel analysis was then performed on bioanodes cultured with acetate and glucose in acetate solutions. The results showed that the glucose-cultured bioanode exhibited a higher charge-transfer coefficient (αgl = 11.789) compared to the acetate-cultured bioanode (αac = 2.944), indicating more favorable electrochemical kinetics at the electrode–biofilm interface.
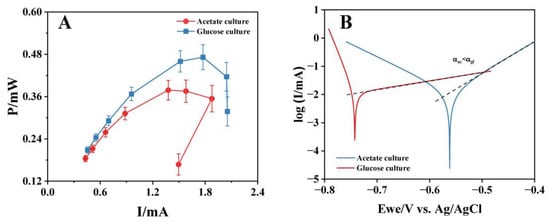
Figure 4.
The polarization curves (A) and Tafel curves (B) of bioanodes cultured with acetate and glucose. Dashed lines represent the linear fitting region of the Tafel slope.
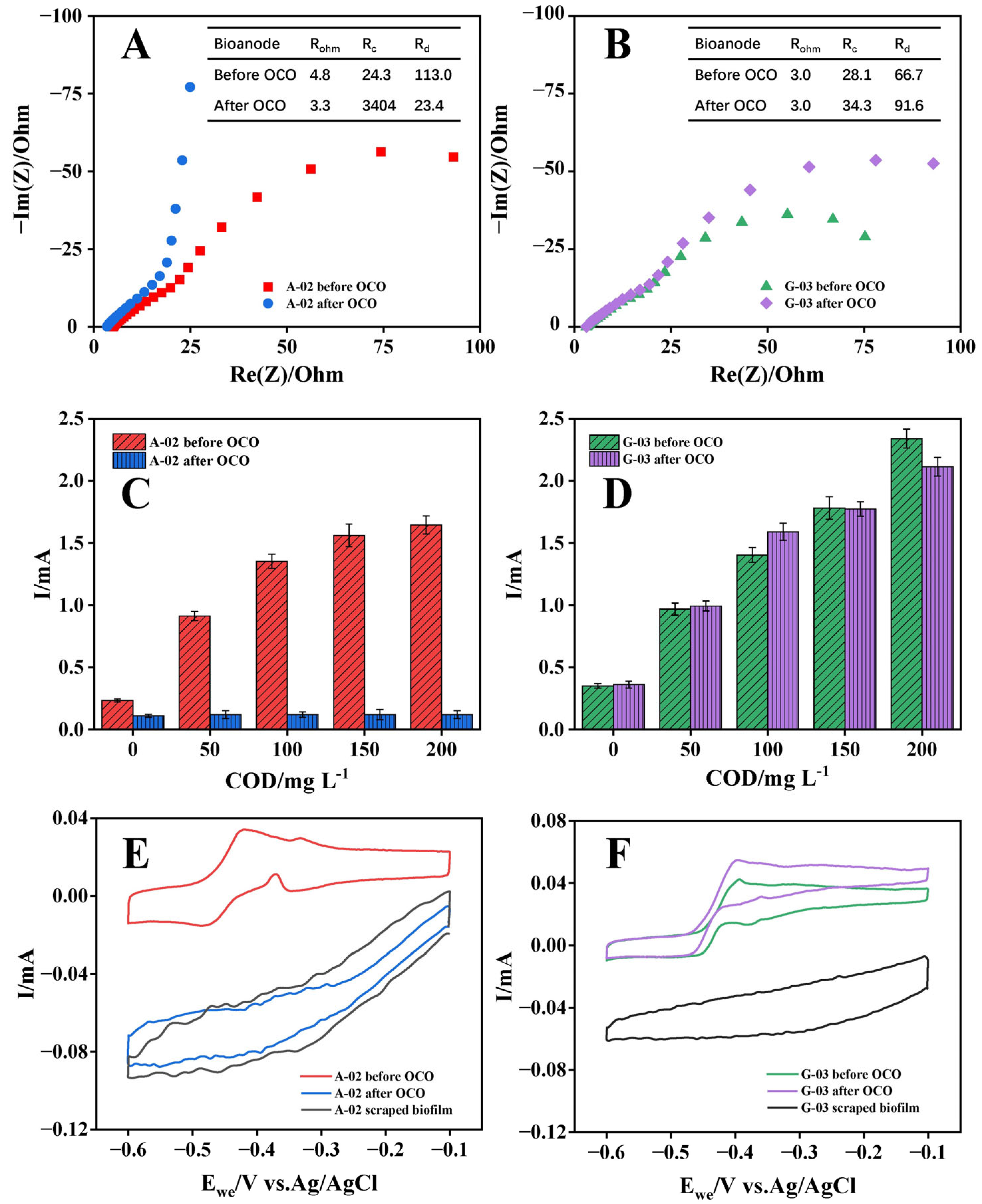
Bioanodes were operated under open-circuit conditions (OCO) for 12 h to evaluate changes in their performance and assess the structural stability of the electrode biofilm after removal of the electric field force. The electricity-generation performance of the acetate-cultured bioanode declined sharply after 12 h OCO, whereas the performance decrease in the glucose-cultured bioanode was minimal (Figure 5). EIS revealed that the electron transfer resistance (Rc) of the acetate-cultured bioanode increased dramatically from 24.3 Ω to 3404 Ω after OCO. Consistent with these findings, the LSV oxidation currents of the acetate-cultured bioanode at various substrate concentrations were markedly lower than those before OCO, with the maximum reduction exceeding 90%. These results indicated that the biooxidation electroactivity of the acetate-cultured bioanode was severely impaired following 12 h OCO. In contrast, both Rc and LSV oxidation currents of the glucose-cultured bioanode remained relatively stable after OCO, with negligible change in oxidation current at low substrate concentrations. Given that bioelectrochemical reaction rates at low substrate concentrations were diffusion-controlled [48], these observations suggested that the mass transfer characteristics of the glucose-cultured bioanode were essentially unaffected by 12 h OCO.
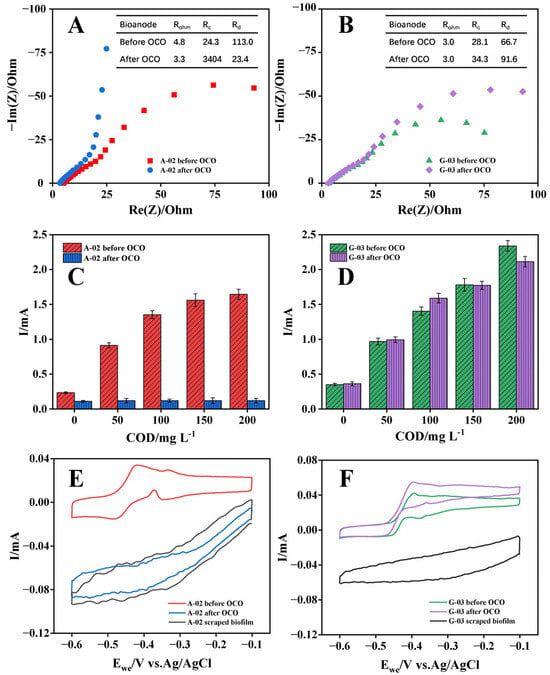
Figure 5.
EIS curves in 50 mM PBS adding 1000 mg COD L−1 acetate, LSV curves under different substrate concentrations of 0~200 mg COD L−1 and CV scan curves in 50 mM PBS of the A-02 (A,C,E) and G-03 (B,D,F) before and after OCO. In (A,B), the symbols represent the test values, the lines represent the fitted values, and the inset table shows the fitting results of the impedance in the bioelectrochemical oxidation of bioanodes for the equivalent circuit.
To explore the reasons for the change of bioanode performance, the redox-active substances and biofilm structures of the bioanodes were characterized before and after OCO. For the acetate-cultured bioanode, the CV curve obtained in 50 mM PBS after 12 h OCO was similar to that of the electrode with its biofilm removed, indicating that the surface redox functionality was almost completely lost. In contrast, the glucose-cultured bioanode exhibited only a 28% decrease in CV oxidation current after 12 h OCO, which could be attributed to the microbial respiration during OCO consuming bioelectrochemically degradable compounds such as extracellular polymeric substances (EPS). CLSM and SEM imaging further revealed that, after 12 h OCO, the biofilm of the acetate-cultured bioanode experienced severe detachment and structural collapse, whereas the glucose-cultured bioanode retained a relatively intact biofilm architecture, with only a reduction in polysaccharide content (Figure S1). These findings indicated that glucose-cultured biofilms exhibited stronger attachment to the electrode surface and greater structural stability than acetate-cultured biofilms.
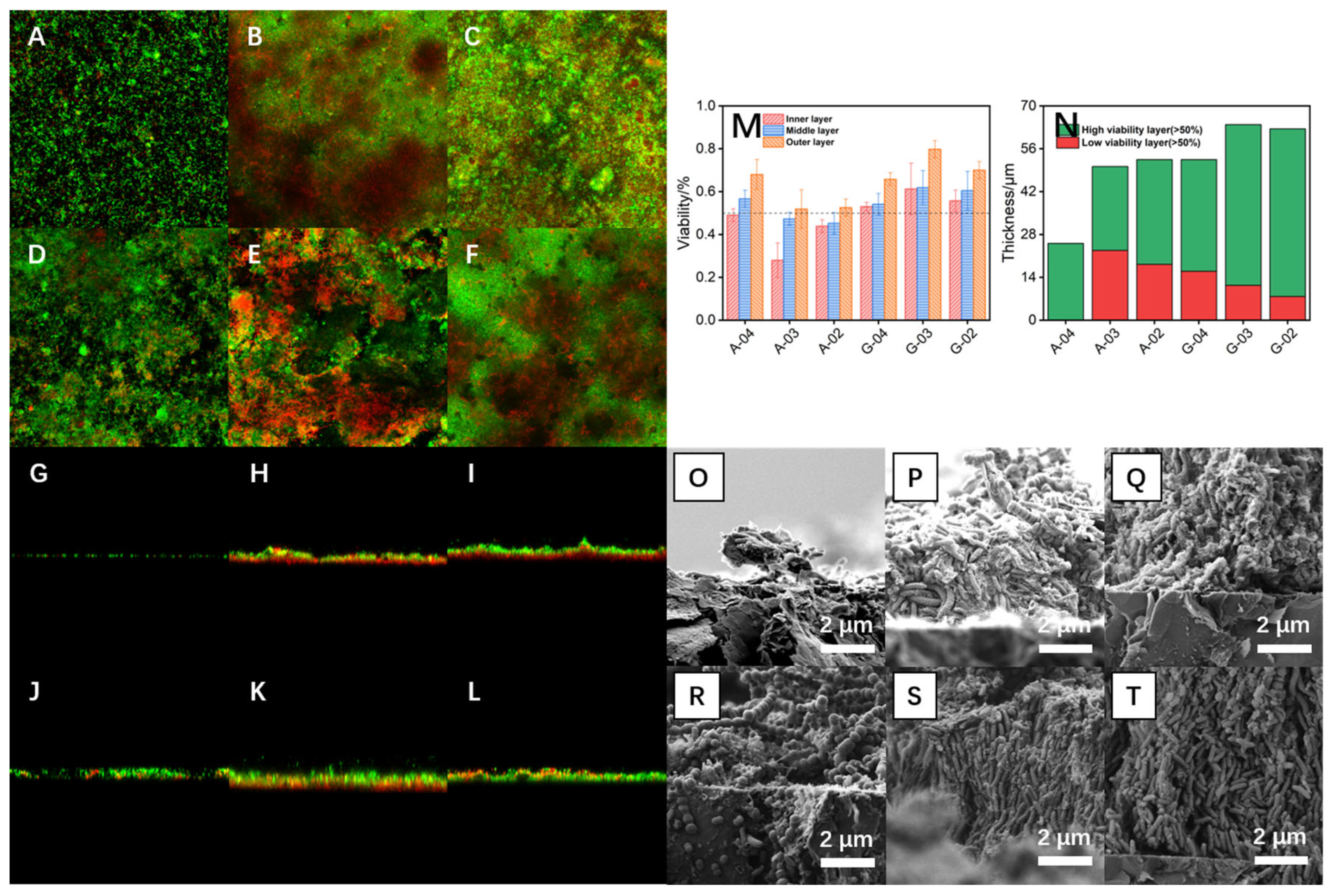
To investigate the reasons underlying the differences in mass transfer characteristics and structural stability of bioanodes, the distribution of live and dead microorganisms as well as the biofilm morphology under different substrate and electrode potential culture conditions were examined using CLSM and SEM, respectively. As shown in Figure 6A–F, acetate-cultured bioanodes (except A-04) formed a compact and dense biofilm structure on the electrode surface, whereas glucose-cultured bioanodes also enriched a large number of microorganisms but exhibited significantly higher biofilm porosity compared to the acetate-cultured group. In acetate-cultured bioanodes, a large number of dead microorganisms were uniformly distributed in the inner layer of the biofilm, predominantly rod-shaped and consistent with Geobacter, the main electrogenic bacterium. In contrast, glucose-cultured bioanodes contained fewer and more scattered dead microorganisms in the inner layer, mainly filamentous in shape and consistent with Aeromonas, the dominant fermentative bacterium. CLSM cross-sectional images (Figure 6G–L) revealed that the biofilms of acetate-cultured bioanodes (A-02 and A-03) contained a distinct dead microorganism-dominated layer adjacent to the electrode surface, with a thickness exceeding one-third of the total biofilm thickness. Due to the weak adhesion between this layer and the electrode surface, the biofilm was prone to detachment after losing the effect of the electric field force, ultimately leading to the marked decline in electricity-generation performance observed after 12 h OCO. In contrast, glucose-cultured biofilms exhibited vertically oriented pore structures and a lower proportion of dead microorganisms, which facilitated the formation of efficient mass transfer channels, maintained the activity of microorganisms in the inner biofilm layer, and enhanced structural stability. Among them, G-03 showed higher biofilm thickness and porosity compared with G-04 and G-02.
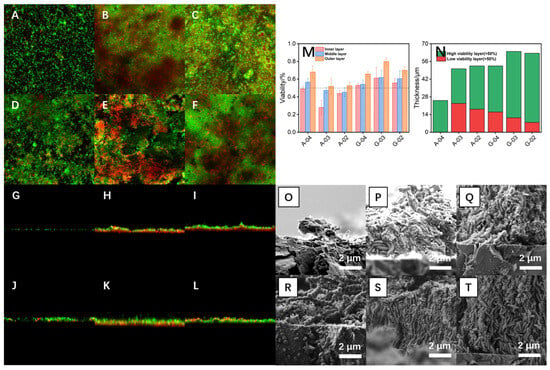
Figure 6.
CLSM plan views (A–F), CLSM section views (G–L)) and SEM section views (O–T) of bioanodes A-04, A-03, A-02, G-04, G-03 and G-02. The microbial activity in inner, middle and outer layers of different bioanode biofilms (M) and the distribution of highly and weakly active layers of biofilms (N).
As shown in Figure 6M, each biofilm was divided into inner, middle, and outer layers based on its total thickness, and the microbial activity of each layer was quantified using Fiji (ImageJ 1.53t, National Institutes of Health, Bethesda, MD, USA). The activity percentage was calculated by analyzing the ratio of green fluorescence (live cells) to total fluorescence (green + red) intensity from CLSM live/dead staining images. Layers with microbial activity below 50% were defined as low-activity layers and those above 50% as high-activity layers, to enable quantitative comparison of biofilm functionality under different culture conditions. At −0.4 V, no significant differences in activity were observed between the layers, likely due to the relatively thin biofilm and favorable mass transfer characteristics. At −0.2 V and −0.3 V, the activity of all layers in acetate-cultured bioanodes was lower than that in glucose-cultured bioanodes, particularly in the inner and middle layers where activity was below 50%, corresponding to the dead microorganism-dominated layer near the electrode side observed in CLSM cross-sections. Figure 6N shows the total biofilm thickness and the relative proportion of high-activity versus low-activity layers under different culture conditions. The results showed that glucose-cultured bioanodes possessed thinner low-activity layers and thicker high-activity layers, consistent with their superior electricity-generation performance and stable biofilm structure. Furthermore, the thickness of the low-activity layer decreased with a positive shift in electrode potential, possibly because higher overpotentials promoted bioelectrochemical oxidation reactions by electrogenic microorganisms for energy harvesting, while the stronger electric field enhanced mass transfer and provided sufficient substrates for microbial growth.
High-magnification SEM images (Figure 6O–T) demonstrated that microorganisms in acetate-cultured biofilms were stacked and irregularly arranged, whereas those in glucose-cultured biofilms were vertically and orderly arranged, forming efficient mass transfer channels within the bioanode and ensuring the activity of inner-layer microorganisms. Abundant filamentous microorganisms were observed on the surface of glucose-cultured bioanodes, interweaving with other microorganisms to form highly porous biofilm structures. Based on microbial relative abundance and attachment relationship analysis, these filamentous microorganisms were inferred to be fermentative bacteria. In this synergistic system, fermentative bacteria first decomposed glucose into intermediate metabolites such as acetate, lactate, or ethanol, which were subsequently oxidized by non-fermentative electrogenic bacteria (such as Geobacter spp.) that transferred electrons to the electrode. Therefore, the growth and metabolism of electrogenic bacteria depended on electron donors provided by fermentative bacteria, forming a biofilm structure based on fermentative bacterial scaffolds. This metabolic interaction mode offered dual advantages: it provided electrogenic bacteria with stable electron donor sources, promoting their enrichment and activity maintenance at the electrode surface; meanwhile, the three-dimensional scaffold structure constructed by filamentous fermentative bacteria significantly enhanced biofilm porosity and mass transfer channel connectivity, improved the diffusion efficiency of substrates and metabolic products, and thereby enhanced overall mass transfer characteristics. Among glucose-cultured bioanodes, biofilms formed at −0.3 V exhibited the highest degree of vertical and ordered arrangement, likely because this potential ensured efficient bioelectrochemical oxidation while avoiding the adverse effects of excessive electric field force on structural order. The optimal biofilm architecture of G-03 provided the best mass transfer characteristics, explaining its highest electricity-generation performance. A schematic comparison of the biofilm architectures of bioanodes cultured with glucose and acetate is provided in Figure S3, offering a visual summary of these structural differences. And a performance comparison with related MFC studies is provided in Table S1 [49,50,51,52,53,54,55], demonstrating the competitive electrochemical performance of the optimized bioanode.
However, it should be noted that although structural optimization markedly enhanced the electricity-generation performance and stability of bioanodes, the presence of abundant non-electrogenic bacteria in glucose-cultured systems led to lower coulombic efficiency, thereby constraining overall energy conversion. In practical applications, coulombic efficiency could be further improved by optimizing substrate concentration to balance microbial metabolism, regulating the ratio of fermentative to electrogenic bacteria to enhance electron transfer efficiency, and adopting staged feeding strategies to prevent substrate accumulation and intermediate losses. These strategies would help maintain the structural advantages of glucose-cultured bioanodes while achieving higher net energy recovery.
4. Conclusions
This study successfully constructed high-performance bioanodes with vertically ordered structures through the synergistic regulation of substrate type and electrode potential. Compared with acetate-fed systems, glucose-cultured bioanodes exhibited approximately 52% higher bioelectroactivity and demonstrated superior mass transfer efficiency. Structural and microbial analyses revealed that glucose-cultured bioanodes developed composite biofilms in which filamentous fermentative bacteria acted as structural scaffolds for the attachment and sustained activity of electrogenic bacteria such as Geobacter spp., thereby enhancing biofilm porosity and structural stability. Under appropriate electrode potentials, the combined effects of electric field forces and microbial interactions promoted the formation of vertically ordered biofilm structures with efficient mass transfer channels and improved inner-layer activity. These findings indicate that a structure-oriented culture strategy based on coordinated control of substrate and potential can effectively enhance electricity-generation performance and biofilm stability, providing technical guidance for the development of efficient and long-term stable bioelectrochemical systems applicable to complex organic wastewater treatment and real-time biosensing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en18215796/s1, Figure S1. Schematic photograph of the dual-chamber three-electrode bioelectrochemical system (BES) reactor used for bioanode culture. Figure S2. CLSM images (A-02 before OCO: A; G-03 before OCO: B; A-02 after OCO: C; G-03 after OCO: D) and SEM images (A-02 before OCO: E; G-03 before OCO: F; A-02 after OCO: G; G-03 after OCO: H) of bioanodes. Figure S3. Schematic illustration comparing the biofilm structures of acetate-cultured (left) and glucose-cultured (right) bioanodes. Table S1. Performance comparison of MFCs with literature analogues.
Author Contributions
Conceptualization, X.H., S.C. and Z.L.; Methodology, X.H., S.C., Z.L., Y.L. and Y.Z.; Validation, Y.Z.; Formal analysis, X.H., Y.L. and Y.Z.; Investigation, X.H.; Resources, Z.L., Y.L. and Y.Z.; Data curation, X.H., Z.L. and Y.L.; Writing – original draft, X.H.; Writing – review & editing, S.C.; Supervision, S.C.; Project administration, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 52070162) and the National Key Research and Development Program of China (2018YFA0901300).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garrido, J.M.; Fdz-Polanco, M.; Fdz-Polanco, F. Working with energy and mass balances: A conceptual framework to understand the limits of municipal wastewater treatment. Water Sci. Technol. 2013, 67, 2294–2301. [Google Scholar] [CrossRef]
- Hernández-Sancho, F.; Molinos-Senante, M.; Sala-Garrido, R. Energy efficiency in Spanish wastewater treatment plants: A non-radial DEA approach. Sci. Total Environ. 2011, 409, 2693–2699. [Google Scholar] [CrossRef]
- Thapa, K.; Liu, W.Y.; Zhang, Y.W.; Westenberg, D.; Zhou, Y.S.; Wang, R.S. Boosting the Power Performance of Microbial Fuel Cells by Using Dual Nanomaterial-Modified Carbon Felt Electrodes. Energy Fuels 2024, 38, 21412–21422. [Google Scholar] [CrossRef]
- Li, L.X.; He, X.Y.; Li, H.H.; Lu, Y.; Song, H.; Cheng, S.A. Enhancing Performance of Microbial Fuel Cell by Binder-Free Modification of Anode with Reduced Graphene Oxide through One-Step Electrochemical Exfoliation and In Situ Electrodeposition. ACS Appl. Bio Mater. 2024, 8, 642–651. [Google Scholar] [CrossRef]
- Nong, Y.Z.; Xu, M.; Liu, B.C.; Li, J.F.; He, D.Y.; Li, C.F.; Lin, P.Y.; Luo, Y.; Dang, C.Y.; Fu, J. Low temperature acclimation of electroactive microorganisms may be an effective strategy to enhance the toxicity sensing performance of microbial fuel cell sensors. Water Res. 2024, 256, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Liao, C.M.; Meng, X.Y.; Zhao, Q.; Yan, X.J.; Tian, L.L.; Liu, Y.; Li, N.; Wang, X. Switchover of electrotrophic and heterotrophic respirations enables the biomonitoring of low concentration of BOD in oxygen-rich environment. Water Res. 2023, 235, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Ding, W.Q.; Sun, L.M.; Wang, L.; Liu, C.G.; Song, H. Engineered Shewanella oneidensis-reduced graphene oxide biohybrid with enhanced biosynthesis and transport of flavins enabled a highest bioelectricity output in microbial fuel cells. Nano Energy 2018, 50, 639–648. [Google Scholar] [CrossRef]
- Min, D.; Cheng, L.; Zhang, F.; Huang, X.N.; Li, D.B.; Liu, D.F.; Lau, T.C.; Mu, Y.; Yu, H.Q. Enhancing Extracellular Electron Transfer of Shewanella oneidensis MR-1 through Coupling Improved Flavin Synthesis and Metal-Reducing Conduit for Pollutant Degradation. Environ. Sci. Technol. 2017, 51, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Netsch, A.; Latussek, I.; Horn, H.; Wagner, M. Detecting Excess Biofilm Thickness in Microbial Electrolysis Cells by Real-Time In-Situ Biofilm Monitoring. Biotechnol. Bioeng. 2025, 122, 2049–2062. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef]
- Lu, Y.; He, X.Y.; Li, H.H.; Chen, H.; Li, L.X.; Zhu, J.L.; Chen, K.; Ding, Z.Y.; Sun, S.Y.; Cheng, S.A. Highly boosting performance of glucose-fed microbial fuel cells by adding L-aspartic acid combined with constructing and in-situ observing electroactive biofilms. Chem. Eng. J. 2025, 518, 10. [Google Scholar] [CrossRef]
- Angelaalincy, M.J.; Krishnaraj, R.N.; Shakambari, G.; Ashokkumar, B.; Kathiresan, S.; Varalakshmi, P. Biofilm Engineering Approaches for Improving the Performance of Microbial Fuel Cells and Bioelectrochemical Systems. Front. Energy Res. 2018, 6, 12. [Google Scholar] [CrossRef]
- Babauta, J.T.; Nguyen, H.D.; Harrington, T.D.; Renslow, R.; Beyenal, H. pH, redox potential and local biofilm potential microenvironments within Geobacter sulfurreducens biofilms and their roles in electron transfer. Biotechnol. Bioeng. 2012, 109, 2651–2662. [Google Scholar] [CrossRef]
- Sun, D.; Cheng, S.A.; Wang, A.J.; Li, F.J.; Logan, B.E.; Cen, K.F. Temporal-Spatial Changes in Viabilities and Electrochemical Properties of Anode Biofilms. Environ. Sci. Technol. 2015, 49, 5227–5235. [Google Scholar] [CrossRef]
- Sun, D.; Chen, J.; Huang, H.B.; Liu, W.F.; Ye, Y.L.; Cheng, S.A. The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int. J. Hydrogen Energy 2016, 41, 16523–16528. [Google Scholar] [CrossRef]
- You, J.; Walter, X.A.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Stability and reliability of anodic biofilms under different feedstock conditions: Towards microbial fuel cell sensors. Sens. Bio-Sens. Res. 2015, 6, 43–50. [Google Scholar] [CrossRef]
- Bratkova, S.; Alexieva, Z.; Angelov, A.; Nikolova, K.; Genova, P.; Ivanov, R.; Gerginova, M.; Peneva, N.; Beschkov, V. Efficiency of microbial fuel cells based on the sulfate reduction by lactate and glucose. Int. J. Environ. Sci. Technol. 2019, 16, 6145–6156. [Google Scholar] [CrossRef]
- Jung, S.; Regan, J.M. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 2007, 77, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Li, B.K. The variation of power generation with organic substrates in single-chamber microbial fuel cells (SCMFCs). Bioresour. Technol. 2010, 101, 1844–1850. [Google Scholar] [CrossRef]
- Yu, J.; Park, Y.; Kim, B.; Lee, T. Power densities and microbial communities of brewery wastewater-fed microbial fuel cells according to the initial substrates. Bioprocess Biosyst. Eng. 2015, 38, 85–92. [Google Scholar] [CrossRef]
- Salvian, A.; Farkas, D.; Ramirez-Moreno, M.; Torruella-Salas, D.; Berná, A.; Avignone-Rossa, C.; Varcoe, J.R.; Esteve-Núñez, A.; Gadkari, S. Resilience of anodic biofilm in microbial fuel cell biosensor for BOD monitoring of urban wastewater. Npj Clean Water 2024, 7, 12. [Google Scholar] [CrossRef]
- Srinak, N.; Chiewchankaset, P.; Kalapanulak, S.; Panichnumsin, P.; Saithong, T. Metabolic cross-feeding interactions modulate the dynamic community structure in microbial fuel cell under variable organic loading wastewaters. PLoS Comput. Biol. 2024, 20, 30. [Google Scholar] [CrossRef]
- Cheng, S.A.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Yalcin, S.E.; O’Brien, J.P.; Gu, Y.Q.; Reiss, K.; Yi, S.M.; Jain, R.; Srikanth, V.; Dahl, P.J.; Huynh, W.; Vu, D.; et al. Electric field stimulates production of highly conductive microbial OmcZ nanowires. Nat. Chem. Biol. 2020, 16, 1136. [Google Scholar] [CrossRef]
- Tian, L.L.; Yan, X.J.; Wang, D.B.; Du, Q.; Wan, Y.X.; Zhou, L.A.; Li, T.; Liao, C.M.; Li, N.; Wang, X. Two key Geobacter species of wastewater-enriched electroactive biofilm respond differently to electric field. Water Res. 2022, 213, 10. [Google Scholar] [CrossRef]
- Zhou, L.J.; Wu, F.; Ou, P.X.; Li, H.X.; Zhuang, W.Q. Non-electroactive bacteria behave variously in AnMBR biofilm control using electric field. Water Res. 2025, 268, 9. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, S.; Yu, Z.; Yang, J.; Huang, H.; Sun, Y. Enhancing bio-cathodic nitrate removal through anode-cathode polarity inversion together with regulating the anode electroactivity. Sci. Total Environ. 2020, 764, 142809. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Wu, J.C. Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells. Bioelectrochemistry 2013, 92, 22–26. [Google Scholar] [CrossRef]
- Sun, D.; Cheng, S.A.; Zhang, F.; Logan, B.E. Current density reversibly alters metabolic spatial structure of exoelectrogenic anode biofilms. J. Power Sources 2017, 356, 566–571. [Google Scholar] [CrossRef]
- Clementi, E.A.; Marks, L.R.; Roche-Hakansson, H.; Hakansson, A.P. Monitoring Changes in Membrane Polarity, Membrane Integrity, and Intracellular Ion Concentrations in Streptococcus pneumoniae Using Fluorescent Dyes. Jove-J. Vis. Exp. 2014, 17, 51008. [Google Scholar]
- Liebeskind, M.; Dohmann, M. Improved Method of Activated-Sludge Biomass Determination. Water Sci. Technol. 1994, 29, 7–13. [Google Scholar] [CrossRef]
- Liu, F.B.; Ma, B.Y.; He, Z.; Bai, P. Electron transfer kinetics at anode interface in microbial electrochemical systems. Electrochim. Acta 2022, 432, 7. [Google Scholar] [CrossRef]
- Picioreanu, C.; Head, I.M.; Katuri, K.P.; van Loosdrecht, M.C.M.; Scott, K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007, 41, 2921–2940. [Google Scholar] [CrossRef]
- Zhang, S.H.; Qiu, C.H.; Fang, C.F.; Ge, Q.L.; Hui, Y.X.; Han, B.; Pang, S. Characterization of Bacterial Communities in Anode Microbial Fuel Cells Fed with Glucose, Propyl Alcohol and Methanol. Appl. Biochem. Microbiol. 2017, 53, 250–257. [Google Scholar] [CrossRef]
- Esteve-Nunez, A.; Busalmen, J.P.; Berna, A.; Gutierrez-Garran, C.; Feliu, J.M. Opportunities behind the unusual ability of geobacter sulfurreducens for exocellular respiration and electricity production. Energy Environ. Sci. 2011, 4, 2066–2069. [Google Scholar] [CrossRef]
- Li, X.J.; Wang, X.; Wan, L.L.; Zhang, Y.Y.; Li, N.; Li, D.S.; Zhou, Q.X. Enhanced biodegradation of aged petroleum hydrocarbons in soils by glucose addition in microbial fuel cells. J. Chem. Technol. Biotechnol. 2016, 91, 267–275. [Google Scholar] [CrossRef]
- Vijay, A.; Chhabra, M.; Vincent, T. Microbial community modulates electrochemical performance and denitrification rate in a biocathodic autotrophic and heterotrophic denitrifying microbial fuel cell. Bioresour. Technol. 2019, 272, 217–225. [Google Scholar] [CrossRef]
- Kong, D.Y.; Yun, H.; Cui, D.; Qi, M.Y.; Shao, C.Y.; Cui, D.C.; Ren, N.Q.; Liang, B.; Wang, A.J. Response of antimicrobial nitrofurazone-degrading biocathode communities to different cathode potentials. Bioresour. Technol. 2017, 241, 951–958. [Google Scholar] [CrossRef]
- Yang, L.H.; Zhu, T.T.; Cai, W.W.; Haider, M.R.; Wang, H.C.; Cheng, H.Y.; Wang, A.J. Micro-oxygen bioanode: An efficient strategy for enhancement of phenol degradation and current generation in mix-cultured MFCs. Bioresour. Technol. 2018, 268, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Suzuki, S.; Yamanaka, Y.; Wu, A.; Nealson, K.H.; Bretschger, O. Population dynamics of electrogenic microbial communities in microbial fuel cells started with three different inoculum sources. Bioelectrochemistry 2017, 117, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhan, G.Q.; Wu, T.T.; Zhang, Y.Y.; Jiang, Q.R.; Li, D.P.; Xiang, Y.Y. Effect of air-exposed biocathode on the performance of a Thauera-dominated membraneless single-chamber microbial fuel cell (SCMFC). J. Environ. Sci. 2018, 66, 216–224. [Google Scholar] [CrossRef]
- An, C.; Yazaki, T.; Takahashi, H.; Kuda, T.; Kimura, B. Diet-induced changes in alginate- and laminaran-fermenting bacterial levels in the caecal contents of rats. J. Funct. Foods 2013, 5, 389–394. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.F.; Ren, N.Q.; Logan, B.E. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells. Bioresour. Technol. 2012, 124, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Cao, X.X.; Liang, P.; Huang, X.; Yang, S.P.; Zhao, G.G. Electricity generation from glucose by a Klebsiella sp in microbial fuel cells. Appl. Microbiol. Biotechnol. 2010, 87, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Lee, E.H.; Yoon, H.S.; Lee, J.H.; Kim, J.Y.; Kang, K.; Park, J.S.; Jin, J.B.; Ko, G.; Pan, C.H. Effects of fermented milk treatment on microbial population and metabolomic outcomes in a three-stage semi-continuous culture system. Food Chem. 2018, 263, 216–224. [Google Scholar] [CrossRef]
- Garault, P.; Faurie, J.; Quere, G. Sucrose Negative Streptococcus Thermophilus for Use in Preparation of Fermented Products; Compagnie Gervais Danone SA: Paris, France, 2021. [Google Scholar]
- Moon, H.; Chang, I.S.; Kim, B.H. Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Bioresour. Technol. 2006, 97, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cheng, S.; Li, H.; Li, L. A novel, rapidly preparable and easily maintainable biocathode electrochemical biosensor for the continuous and stable detection of nitrite in water. Sci. Total Environ. 2022, 806 Pt 4, 150945. [Google Scholar] [CrossRef]
- Jiang, J.W.; Wang, H.N.; Zhang, S.X.; Li, S.N.; Zeng, W.L.; Li, F.X. The influence of external resistance on the performance of microbial fuel cell and the removal of sulfamethoxazole wastewater. Bioresour. Technol. 2021, 336, 8. [Google Scholar] [CrossRef]
- Liu, J.; Guo, T.; Wang, D.; Ying, H.J. Clostridium beijerinckii mutant obtained atmospheric pressure glow discharge generates enhanced electricity in a microbial fuel cell. Biotechnol. Lett. 2015, 37, 95–100. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, L.Y.; Zhang, Y.M.; Yu, Y.J. Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution. Int. J. Environ. Res. Public Health 2022, 19, 12. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.J.; Ren, N.Q.; Wang, H.M.; Lee, H.; Li, N.; Zhao, Q.L. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Yu, J.; Park, H.; Park, Y.; Lee, T. Power Generation and Microbial Community Shift According to Applied Anodic Potential in Electroactive Biofilm Reactors Treating Synthetic and Domestic Wastewater. Energies 2022, 15, 12. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Min, B.; Huang, L.P.; Angelidaki, I. Electricity generation and microbial community response to substrate changes in microbial fuel cell. Bioresour. Technol. 2011, 102, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.P.; Tokash, J.C.; Hong, Y.Y.; Logan, B.E. Controlling the occurrence of power overshoot by adapting microbial fuel cells to high anode potentials. Bioelectrochemistry 2013, 90, 30–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).