Abstract
The transition to sustainable energy sources has intensified interest in lignocellulosic biomass (LCB) as a feedstock for second-generation biofuels. However, the inherent structural recalcitrance of LCB requires the utilization of an effective pretreatment to enhance enzymatic hydrolysis and subsequent fermentation yields. This manuscript presents a novel, single-step, and optimized nitrogen explosive decompression system (NED 3.0) designed to address the critical limitations of earlier NED versions by enabling the in situ removal of inhibitory compounds from biomass slurry and fermentation inefficiency at elevated temperatures, thereby reducing or eliminating the need for post-treatment detoxification. Aspen wood (Populus tremula) was pretreated by NED 3.0 at 200 °C, followed by enzymatic hydrolysis and fermentation. The analytical results confirmed substantial reductions in common fermentation inhibitors, such as acetic acid (up to 2.18 g/100 g dry biomass) and furfural (0.18 g/100 g dry biomass), during early filtrate recovery. Hydrolysate analysis revealed a glucose yield of 26.41 g/100 g dry biomass, corresponding to a hydrolysis efficiency of 41.3%. Fermentation yielded up to 8.05 g ethanol/100 g dry biomass and achieved a fermentation efficiency of 59.8%. Inhibitor concentrations in both hydrolysate and fermentation broth remained within tolerable limits, allowing for effective glucose release and sustained fermentation performance. Compared with earlier NED configurations, the optimized system improved sugar recovery and ethanol production. These findings confirm the operational advantages of NED 3.0, including reduced inhibitory stress, simplified process integration, and chemical-free operation, underscoring its potential for scalability in line with the EU Green Deal for bioethanol production from woody biomass.
1. Introduction
The global reliance on fossil fuels has created critical environmental and economic challenges. As a result, there is a swift change and rapid growth toward renewable and sustainable energy solutions. Bioethanol production has proven to be a suitable renewable alternative to fossil fuels. Currently, lignocellulosic biomass (LCB) offers a compelling alternative for bioethanol production. Its abundance, sustainability, low cost, and non-competitive nature with food production make it a prime alternative to fossil-based resources. Lignocellulosic biomass, such as wood waste and agricultural residues, which are of low quality and underused, can be considered suitable candidates for biochemicals production. The conversion of LCB can be broken down into the following three steps: pretreatment, hydrolysis, and fermentation. Finally, a distillation of the bioethanol is performed. The LCB complex matrix comprises the following three main parts: cellulose, hemicellulose, and lignin. Indeed, these components are tightly bound in the native state of LCB. Its interwoven structure (a lignin-encased polysaccharide matrix) makes it tough for hydrolytic enzymes or acids to reach the carbohydrate (cellulose and hemicellulose) polymers in order to break them down to fermentable sugars [1,2,3,4,5,6,7]. Hence, pretreatment is necessary to crack this structure and facilitate acidic or enzymatic hydrolysis to alleviate cellulose and hemi-cellulose from the barrier.
LCB pretreatment is conventionally based on physical [8], biological [9,10,11,12], chemical [12], and physico-chemical methods [13,14,15], along with their combinations [11]. These methods use organic solvents, alkaline agents, acids, water, enzymes, microorganisms, or a combination of them in breaking lignin–carbohydrate bonds by removing or redistributing the lignin, reducing cellulose crystallinity, and increasing porosity and surface area [16,17,18,19].
However, conventional pretreatment methods, such as steam explosion, acid hydrolysis, and organosolv, are often limited by high operational costs, severe environmental burdens due to chemical usage or effluent generation, and the frequent need for detoxification steps, which further increase the complexity and energy demand. These challenges underscore the need for a more efficient, cost-effective, and environmentally friendly alternative [14,17,18]. Unlike these conventional techniques, NED 3.0 offers an improved system. The new system encompasses a condenser and expansion vessel for instantaneous removal and recovery of volatile inhibitors in the biomass pretreatment step for increased total sugar recovery. Unlike other versions, including NED 1.0 and 2.0, which had inhibitor formation at high temperatures, NED 3.0 overcomes such limitations by minimizing the formation of injurious byproducts, concurrently maximizing fermentable sugar yields.
Conventional nitrogen explosive decompression (NED) is one of the methods that has shown promise by being chemical-free and effective at disrupting biomass structures. The sudden pressure release at elevated temperature ensures opening of the biomass structure. Raud et al. [17] were among the first to propose this NED strategy for lignocellulosic ethanol production. Their results on barley straw demonstrated that while glucose and ethanol yields peaked at 150 °C, performance declined significantly at 175 °C and 200 °C due to inhibitor formation. Building on this, Rooni et al. [2] applied NED to common aspen. Similarly, they observed that while glucose release increased with temperature, ethanol yields dropped to zero at 200 °C, likely due to elevated acetic acid and furan concentrations. For example, Rooni et al. [2] reported a maximum glucose yield of 81.06 g/kg at 175 °C, but ethanol production was completely inhibited at 200 °C, despite high sugar availability.
Sjulander et al. [7] aimed to address the limitations of conventional one-step nitrogen explosive decomposition (NED) pretreatment. They developed a two-step, industry-oriented pretreatment process using NED, designed to enhance sugar yields and ensure the fermentability of the resulting hydrolysate, regardless of the pretreatment temperature, while minimizing the formation of inhibitors. Although this approach reduced inhibitor formation and eliminated waste-intensive steps, such as washing and drying, it relied on filtration for detoxification. Filtration is an expensive downstream operation, leading to sugar losses during the two-step biomass pretreatment. Beyond sugar losses, Sjulander et al. [7] found that this two-step pretreatment still did not facilitate ethanol production from either softwood or hardwood at temperatures above 175 °C. Therefore, the ongoing need for detoxification and prevention of sugar loss highlights the limitations of the process. Consequently, there is a critical need for an improved NED pretreatment method.
In this context, the present study introduces a novel single-step NED 3.0 pretreatment process explicitly optimized for in situ inhibitor reduction and enhanced sugar yields. This manuscript presents a novel, single-step, and engineering-optimized nitrogen explosive decompression system (NED 3.0), whose hardware and operational parameters, including vessel design, real-time decompression, and in situ inhibitor recovery, were systematically redesigned based on limitations observed in the NED 1.0 and 2.0 systems. Please note that the term ‘optimized’ here refers to the technical and mechanical improvements made to the system rather than a statistical design of experiments. This optimized process leverages precise control of thermomechanical parameters and integrated volatiles recovery systems, fundamentally overcoming previous NED limitations. Table 1 shows the comparative parameters of the modified NED (3.0) and previous NED systems (1.0 and 2.0). Earlier NED configurations (1.0 and 2.0) presented their own limitations in terms of process control, efficiency, and scalability. The comparative parameters of all three NED configurations are presented in Table 1, which includes vessel size, heating method, control, decompression, mixing, and other relevant details. In contrast to 1.0 and 2.0 systems, the newly developed NED 3.0 system introduces several critical improvements and modifications, which distinguish the new optimized system in this study from the previous NED 1.0 [19] and NED 2.0 [2,7,17,20]. For instance, it uses a larger vessel; fully automated sensor-based temperature control with dual thermocouples; precise slurry-phase decompression at the incubation temperature, with speed regulation via nozzle geometry; integrated mixing; and a built-in condenser system for real-time recovery of volatile inhibitors. These innovations enable in situ detoxification, better reproducibility, and smoother integration with downstream hydrolysis and fermentation.

Table 1.
Configuration and comparative parameters of the newly modified NED (3.0) and previous NED systems (1.0 and 2.0).
This paper aims to investigate the effect of NED 3.0 pretreatment on sugar recovery from aspen biomass, including filtrate sugar profiles, hydrolysis sugar yields, and fermentation sugar outputs. These outcomes are then further compared with existing NED pretreatment strategies to assess the efficiency of the system in minimizing inhibitor interference while maximizing fermentable sugar yields.
2. Materials and Methods
2.1. Biomass
Aspen wood (Populus tremula) was used as a feedstock for the NED pretreatment. The biomass was collected in the spring of 2023 from a forest located near Tartu, Estonia. The raw biomass was dried at 45 °C in a drying oven until achieving a moisture content of less than 5%. The dried biomass was further milled with a cutting mill (Cutting Mill SM 100 comfort, Retsch GmbH, Haan, Germany) to a particle size of less than 1 mm. Scanning electron microscopy (SEM, Waltham, MA, USA) imaging was conducted on untreated, pretreated, hydrolyzed, and fermented biomass following the method described by Rooni et al. [2,7].
The hemicellulose, cellulose, lignin, and extractives contents of the raw biomass were determined using the ANKOM 2000 Automated Fiber Analyzer (Macedon, NY, USA) in accordance with its analytical protocols, the acid detergent fiber (ADF) method (Method 14) and neutral detergent fiber (NDF) method (Method 15). These ADF and NDF protocols have been published in peer-reviewed journals by previous authors [7,17,20]. The ash content was determined following the NREL/TP-510-42622 protocol. The moisture content of the samples was assessed using the automated infrared moisture analyzer (Kern MLS 50-3D, Balingen, Germany). All measurements were carried out at least in triplicate.
2.2. Pretreatment with the NED 3.0 System
The proposed modified nitrogen explosive decompression pretreatment system was developed to disrupt the biomass cell structure, expose cellulose fibrils, and remove inhibitory compounds, thereby enhancing subsequent enzymatic treatment and fermentation. A simplified schematic of the NED 3.0 setup is shown in Figure 1, and the experimental design is shown in Figure 2. The system comprises a 5 L pressure vessel, coupled with a heater, motor, and stirrer, and a 270 L expansion vessel coupled with a plate condenser unit. The exact slurry mixing speeds during the heating up, incubation time, and explosion were 173, 140, and 123 rpm, respectively. Temperature and pressure control mechanisms, including real-time monitoring, regulation protocols, and decompression trigger settings, took place in the control panel.
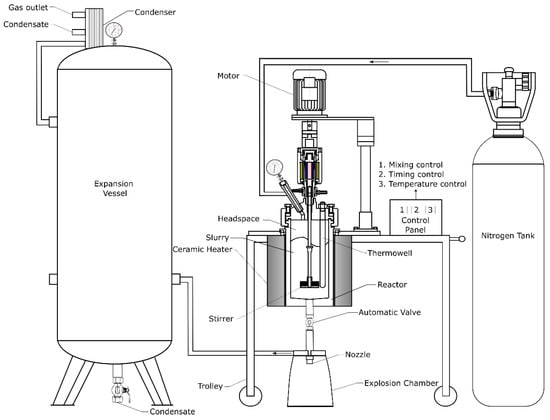
Figure 1.
The newly modified nitrogen explosive decompression (NED 3.0) setup.
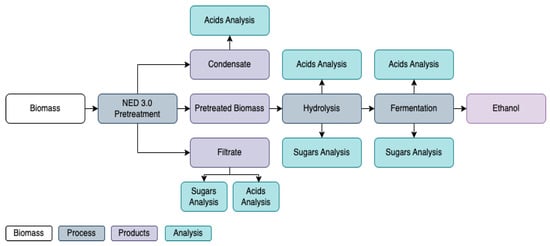
Figure 2.
Overview of the experimental workflow for the NED 3.0 pretreatment and downstream processing of aspen biomass.
The reactor was loaded with 100 g of dry biomass, which was then mixed with 800 mL of deionized water (DW). After the biomass–water mixture was introduced into the reactor chamber, the system was sealed to ensure pressure integrity during the NED 3.0 pretreatment. The reactor head space was then pressurized to 10 bars with nitrogen gas, which serves as the operating gas in the NED 3.0 system. Upon reaching the pre-set temperature, an incubation period of 1 min was automatically initiated. Post-pretreatment, the condensate was collected from the condenser and stored at −20 °C in a freezer for subsequent HPLC analysis.
The pretreated biomass slurry and filtrate were collected from the catcher bucket. The pretreated biomass was dried at ≤60 °C for three days in a ventilated drying oven. Although the standard NREL protocol recommends drying at 45 °C, we initially adhered to this; however, mold formation was observed under prolonged low-temperature drying conditions. Therefore, to prevent microbial contamination and ensure biomass preservation, the drying temperature was raised to ≤60 °C. This adjustment was applied uniformly across all samples to maintain internal consistency. The dried biomass was used directly for enzymatic hydrolysis and subsequent fermentation. All experiments were conducted in triplicate. The collected condensate and filtrate samples were stored at −20 °C in a freezer until the HPLC analysis was performed. All experiments were conducted in triplicate.
2.3. Enzymatic Hydrolysis
After the pretreatment, 100 g of the dried pretreated aspen samples was subjected to enzymatic hydrolysis using the enzyme complex ACCELLERASE 1500 (Genencor, Wilmington, DE, USA). For this, 30 mL of enzyme was used at a ratio of 0.3 mL per 1 g of dry biomass. The biomass was placed in a sterilized conical incubation flask and, subsequently, reconstituted with 800 mL of distilled water before the addition of the enzyme. The overall volume was then adjusted with distilled water to 1 L. The incubation flask containing the enzyme–water–pretreated biomass mixture was incubated at 50 °C and 250 rpm for 72 h on an IKA KS 4000i shaker–incubator. Samples were harvested every 24 h. The theoretical glucose yield and hydrolysis efficiency (Ehyd.) were calculated according to Tutt et al. [19] and Rooni et al. [2]. The enzyme loading of 0.3 mL/g dry biomass was selected to maintain consistency with earlier NED 1.0 and NED 2.0 studies [2,7,17,19], thereby allowing for a direct performance comparison across the three system configurations.
2.4. Fermentation
The hydrolyzed biomass was transferred into sterilized 1 L round flasks. A total of 2.5 g/L of dry yeast, Saccharomyces cerevisiae (Superjæst T3, Tartu, Estonia), was added as the fermenting organism to inoculate the hydrolysate [2,7]. The reaction vessel was sealed with an S-lock to maintain anaerobic conditions. Fermentation was initiated at a pH of approximately 5.5, which is considered optimal for S. cerevisiae. While the pH was monitored throughout the fermentation period, it was not actively controlled but measured. The fermentation process lasted for 28 days at room temperature. Samples were collected on the 7th, 14th, 21st, and 28th days. The bioethanol yield and fermentation efficiency (Efer) were calculated as the mass percentage, according to Tutt et al. [19].
2.5. HPLC Analysis
The HPLC analysis was carried out according to the method described by Rooni et al. [2]. Liquid samples were collected from the following three different stages of the process: (i) the condensate and filtrate obtained immediately after NED 3.0 pretreatment; (ii) the enzymatic hydrolysates sampled on 24, 48, and 72 h; and (iii) the fermentation broth sampled on days 7, 14, 21, and 28. These media samples were stored in a −20 °C freezer. Before the HPLC analysis, the samples were thawed at room temperature and centrifuged twice at 10,000 rpm for 10 min, and the supernatant was filtered through a 0.2 μm PTFE centrifuge filter (Thermo Scientific, Waltham, MA, USA, PTFE, 0.2 μm) before being transferred to HPLC vials. The HPLC system (Shimadzu Prominence-i LC-2030 3D Plus, Kyoto, Japan), equipped with two detectors, a refractive index detector (RID) and a photodiode array detector (PDA), was used for the analysis. For sugar analysis, an Aminex HPX-87P column (300 × 7.8 mm, Bio-Rad, Hercules, CA, USA) with an RID detector was used. A Micro-Guard De-Ashing pre-column (BIO-RAD, Hercules, CA, USA) was used to protect the main column. Autoclaved, degassed, and deionized water was used as the mobile phase. The flow rate was set at 0.6 mL/min, and the oven temperature was maintained at 80 °C. The sample injection volume was 5 μL, and the duration for analyzing each sample was 50 min. A Rezex™ ROA-Organic Acid H+ column (300 × 7.8 mm, Torrance, CA, USA) was used for inhibitors and biomolecule analyses. The mobile phase was an autoclaved, degassed, and deionized 5 mM H2SO4 solution. The column oven and detectors were heated to 50 °C, while the mobile phase was pumped at a 0.5 mL/min flow rate, and the duration for analyzing each sample was 75 min.
2.6. Statistical Analysis
All statistical analyses were performed using Design-Expert® software version 13 (Stat-Ease Inc., Minneapolis, MN, USA), Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA), and GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA) to analyze the variance of the results (ANOVA) and to evaluate the significance of the experimental factors at a 95% confidence level (p < 0.05). Initially, the descriptive statistics were calculated to determine the mean, standard deviation, and variability across experimental replicates. To assess the distribution characteristics of the data sets, normality and log-normality tests were applied. These tests informed the choice of appropriate inferential statistical procedures. For data sets that followed a normal distribution, a one-way analysis of variance (ANOVA) was conducted to determine the significance of differences among treatment groups at a 95% confidence level (p < 0.05). In cases where normality assumptions were not met, non-parametric tests (e.g., Kruskal–Wallis test) were used as alternatives. Post hoc multiple comparison tests were applied to identify specific group differences. To explore potential interdependencies among chemical and process variables, a correlation matrix analysis was performed. This matrix quantified the degree of linear association between inhibitors, sugars, and fermentation products. The results of the correlation matrix were visualized using a heat map, which facilitated pattern recognition and provided insights into covariation trends and inverse relationships among variables across different process streams. Appendix C (Table A1, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7 and Table A8 and Figure A1) compiles detailed statistical analyses, including Spearman correlation coefficients, significance testing, and ANOVA outputs for inhibitors and sugar profiles. These supplementary results provide further validation of the observed trends in sugar release and inhibitor suppression across the NED 3.0 process.
3. Results and Discussion
3.1. Fiber Analysis
The fiber analysis results for untreated and pretreated aspen wood at 200 °C using NED 3.0 are presented in Table 2. The cellulose content of the pretreated material was approximately 8.2% higher (65.3 ± 1.2%) than the untreated (60.3 ± 1.9%). This mass percentage increase in the cellulose content implies that NED 3.0 is efficient at deconstructing the hemicellulose–lignin complex, which improves cellulose accessibility while retaining biomass structural integrity for fermentation. Compared with NED 2.0, in which the highest cellulose content (48.8 ± 1.2%) was obtained at a reaction temperature of 175 °C [2], NED 3.0 offers a significant improvement. This improvement in cellulose availability, reflected by the elevated glucose yields after hydrolysis at 200 °C, suggests that NED 3.0 induces a more effective deconstruction of the lignocellulosic matrix. This structural breakdown, driven by intensified thermomechanical disruption and nitrogen permeation, will enhance enzymatic access to cellulose microfibrils. As a result, NED 3.0 not only surpasses the optimal cellulose exposure reported for NED 2.0 at 175 °C but also establishes a new performance benchmark for single-step pretreatment of hardwoods.

Table 2.
Fiber analysis results for untreated and pretreated aspen wood at 200 °C using NED 3.0.
Inversely to the increase in cellulose content, the hemicellulose content decreased from 20.9 ± 1.3% for untreated to 6.1 ± 0.3% for pretreated aspen. The reduced hemicellulose content implies an enhanced solubilization and removal of hemicellulosic fractions, presumably due to its thermal vulnerability and solubilization during the process.
The NED 3.0 pretreatment at 200 °C resulted in a lignin content of 12.5 ± 0.3%, which is comparable to that of untreated samples (11.7 ± 0.8%). These results suggest limited lignin removal under these reaction conditions, which may also be attributed to a moderate pretreatment severity and the relative enrichment of lignin (not absolute increase), which is especially noticeable following extensive hemicellulose solubilization.
The concentration of extractives increased substantially from 7.06 ± 0.10% (untreated biomass) to 16.2 ± 1.3% for samples pretreated at 200 °C. This increase is likely due to the thermal breakdown and solubilization of hemicellulose and low-molecular-weight lignin fragments, which contribute to the accumulation of soluble organic compounds in the extractives fraction. This increase reflects the elevated severity of the pretreatment at this temperature, which facilitates cell wall deconstruction.
The pretreatment resulted in an almost 100% decrease in the mineral content of the biomass, with values varying between 1.46 ± 0.02% for untreated biomass and 0.83 ± 0.02% for biomass pretreated at 200 °C. This suggests a partial leaching or volatilization of the inorganic components during the explosive decompression process, as previously observed in high-temperature and steam-based pretreatment systems [21,22,23]. This decrease in the mineral content is beneficial, as it can help minimize the catalyst inhibition during hydrolysis, reduce equipment scaling and corrosion, and alleviate fermentation disturbances caused by mineral toxicity, particularly from ions such as potassium, sodium, and calcium [21,22,23].
3.2. SEM Characterization
SEM analysis provided compelling visual evidence of the morphological transformations induced by NED 3.0 pretreatment at 200 °C. As it can be seen from Figure 3, the untreated aspen wood samples (A, B, and C) had compact and intact fiber structures, with smooth surfaces and minimal porosity across all magnifications (860×, 360×, and 220×). In contrast, the pretreated samples (D, E, and F) revealed severe structural ruptures, enhanced fibrillation, and expanded pore structures. These are clear indicators of a disrupted lignocellulosic structure, which can be attributed to the synergistic action of the temperature, nitrogen saturation, and explosive decompression, which amplified cell wall disintegration and increased surface accessibility.
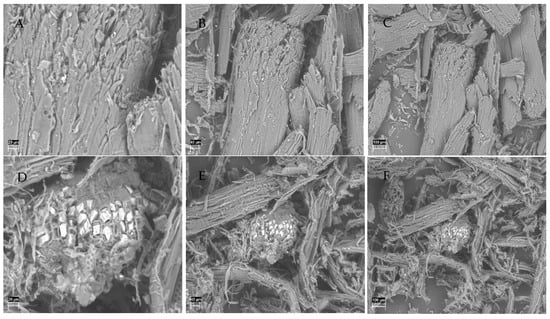
Figure 3.
Untreated aspen samples presented at magnifications of 860× (A), 360× (B), and 220× (C) and pretreated aspen samples at 200 °C presented at magnifications of 860× (D), 360× (E), and 220× (F).
Rooni et al. [2] previously observed similar structural changes in aspen pretreated with NED 2.0 at lower temperatures. The findings obtained in this study extend this observation. Utilizing a reaction temperature of 200 °C in NED 3.0 significantly amplified these effects, resulting in deeper fissuring and fiber liberation, as evident in the high-resolution SEM images.
These morphological changes correlate well with the improved enzymatic hydrolysis and fermentation efficiencies reported for NED 3.0. The disrupted and more accessible matrix allowed for deeper enzyme penetration, thus better saccharification.
3.3. Condensate Inhibitors
Appendix A summarizes all of the inhibitors and byproducts from NED 3.0, outlining their yield, relative composition, classification, and inhibitory potentials. Overall, high amounts of short-chain organic acids, primarily formic and acetic acids, are present in the samples, which aligns well with earlier observations by [2], who reported similar acid-dominated compositions arising from hemicellulose degradation during nitrogen explosive decompression (NED) at elevated temperatures [2]. Figure 4 shows the composition of the condensate separated during the aspen pretreatment at 200 °C using NED 3.0. As can be seen from Figure 4, formic acid emerged as the most abundant compound, representing 34.5% of the total condensate profile and yielding approximately 0.60 g/100 g dry biomass. This yield was statistically significantly different (p < 0.05) from the other condensate inhibitors. Its formation likely resulted from the thermal degradation of xylose and glucose under high-pressure conditions. Despite its moderate inhibitory potential, formic acid at this stage signals effective hemicellulose breakdown, a desired pretreatment outcome. This suggests partial oxidative cleavage of C–C bonds in sugar-derived intermediates. While traditional formic acid synthesis typically requires oxidative conditions with strong oxidants or catalysts in the presence of O2, Deng et al. [24] noted that thermal cleavage of the aldehyde-terminal carbon bond in carbohydrates can result in formic acid generation, even under aqueous conditions. The likely mechanism involves the breakdown of glucose or fructose into triose intermediates, which undergo further cleavage and oxidation [24]. The production of formic acid in the NED 3.0 system in the absence of added catalysts implies that the thermal gradient and pressure differential induced by the NED pretreatment caused equivalent bond cleavages.
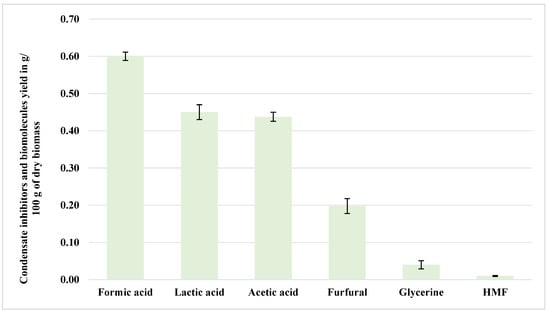
Figure 4.
Condensate inhibitors and biomolecules from aspen samples pretreated at 200 °C (NED 3.0).
Lactic acid (Figure 4) contributed to 25.9% of the total profile (0.45 g/100 g dry biomass), and its presence was also statistically significantly higher (p < 0.05) among the quantified inhibitory compounds in the condensates, indicating potential biochemical conversion of sugars. Importantly, lactic acid is non-inhibitory and has market value as a platform chemical in bioplastics and food additives, introducing possibilities for co-product valorization. In the United States, the price for nutraceutical-grade lactic acid reached approximately USD 1440 per metric ton (USD/MT) in June 2024 [25]. Lactic acid formation from lignocellulosic material typically involves a complex sequence of reactions, as follows: hydrolysis of cellulose to glucose, isomerization to fructose, retro-aldol cleavage to trioses, and, finally, conversion to lactic acid [24]. Deng et al. [24] described that metal salts (such as Pb2+, Al3+, and VO2+) can catalyze this transformation efficiently, with the retro-aldol step acting as the rate-determining barrier. While such metal catalysts were not present in the NED 3.0 process, the occurrence of lactic acid in the condensate may reflect the partial breakdown of trioses (glyceraldehyde and dihydroxyacetone) under thermal- and pressure-driven stress. This suggests that the pretreatment system can emulate the early steps of these tandem reactions, likely promoting isomerization and fragmentation through heat-activated pathways to produce high-value biomolecules.
Acetic acid (Figure 4) constituted about 25.3% of the condensate (0.44 g/100 g dry biomass) and was also statistically significantly different (p < 0.05) from the untreated samples. It primarily originates from the deacetylation of hemicellulose, especially xylan. Although acetic acid poses moderate fermentation inhibition risks, microorganisms tolerate it at low levels. Concentrations above 5 g/L can significantly inhibit microbial adaptation, growth, and metabolism [26]. Together, these three acids account for over 85% of the condensate, reinforcing the acid-centric degradation behavior of the NED 3.0 system at 200 °C. Deng et al. [24] noted that low acetic acid yields (~16%) are typical in non-catalytic systems, but improved yields (~26%) have been obtained using a two-step process, as follows: hydrolysis of cellulose to intermediates followed by catalytic oxidation. In the NED 3.0 system, where no catalysts were used, the observed acetic acid content (25.3%) suggests a significant extent of acetyl group hydrolysis from hemicellulose or the lignin–carbohydrate complex and, possibly, the further degradation of C3–C6 intermediates. This supports the view that the NED 3.0 enables partial oxidative fragmentation or hydrolytic cleavage at elevated temperatures and rapid decompression, mimicking aspects of tandem reaction steps previously only achieved under controlled catalytic conditions.
A key advantage of the NED 3.0 process is its significantly reduced production of potent inhibitors, such as furan-based compounds (furfural and HMF). Furfural, formed from pentose dehydration, was present at a moderate concentration (0.20 g/100 g dry biomass, p < 0.05), significantly lower than those reported for previous pretreatment methods, which accounts for 11.5% of the condensate chemical yield. However, HMF (5-hydroxymethylfurfural)—an inhibitor of particular concern in microbial fermentation—was detected at only 0.01 g/100 g dry biomass, approximately 0.6% of the total condensate, a statistically significantly low level (p < 0.05). Low HMF content in the condensate could result from the suppression of HMF formation by the NED 3.0 system. This low yield contrasts favorably with previous studies, such as those by [2], who reported increased furan derivative formation with increasing pretreatment severity, significantly inhibiting subsequent fermentation efficiency [2]. Sjulander and Kikas [7] reported that the formation of furfural and HMF from lignocellulose harms both hydrolysis and fermentation ability. The NED 3.0 process directly addresses these problems by lowering the number of different inhibitors. The lower HMF production indicates that the NED 3.0 method reduces the degradation of sugars.
Glycerin, though a minor component (2.3%, 0.04 g/100 g dry biomass), contributes to the chemical complexity of the condensate. It could originate from the partial hydrolysis of lipids or glycerol-like intermediates during the NED pretreatment process and could be exploited as a low-value co-product. Supporting our observation of minor glycerin formation and potential co-product valorization, Sjulander and Kikas [7] also emphasized that valorizing byproducts can significantly improve the economic feasibility of biomass processing. Detailed statistical results for inhibitors, biomolecules, and sugars, including p-value significance tests, Spearman correlations, and variance diagnostics, are compiled in Appendix C (Table A1, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7 and Table A8 and Figure A1). These results complement the interpretations presented in this section and the following sections on filtrate, hydrolysate, fermentation inhibitors, biomolecules, and sugar profiles. Descriptive statistics (Table A1) summarize the variability in condensate inhibitor yields, highlighting the predominance of formic acid and acetic acid. Normality testing, shown in Table A2, confirms that most inhibitor concentrations deviate from a Gaussian distribution, justifying the use of non-parametric methods in this study. Multiple comparisons (Table A3) show statistically significant differences between inhibitor concentrations and process variables.
3.4. Filtrate Inhibitors and Sugars Profile
The biomass pretreatment at 200 °C generated a slurry-like mixture. The slurry was vacuum-filtered to separate the biomass and liquid fraction (filtrate). The filtrate contains significantly higher (p < 0.05) quantities of organic acids and minor inhibitory compounds that impact downstream processes. Analysis of the filtrate revealed that acetic acid was the most abundant organic acid, comprising 2.18 g/100 g dry biomass (37%), followed by lactic acid at 1.35 g/100 g (23%) and levulinic acid at 1.09 g/100 g (19%). Figure 5 shows the inhibitors, acids, and biomolecules of the aspen filtrate pretreated with NED 3.0 at 200 °C. Acetic acid primarily results from acetyl group cleavage in hemicellulose during high-temperature pretreatments, and it exhibits moderate inhibitory effects on microbial fermentation [18]. As reviewed by Deng et al. [24], levulinic acid typically forms through a multi-step process involving glucose isomerization to fructose, dehydration to 5-hydroxymethylfurfural (HMF), and subsequent rehydration and rearrangement. Although most studies employ Brønsted acids or sulfonated solid catalysts to drive this sequence, the presence of levulinic acid in the NED 3.0 filtrate implies that the water, elevated temperature, and high-pressure used in the pretreatment are sufficient to promote this reaction cascade non-catalytically. This observation highlights the potential of NED 3.0 to replicate critical biomass degradation reactions without requiring harsh acid catalysts or extensive downstream purification.
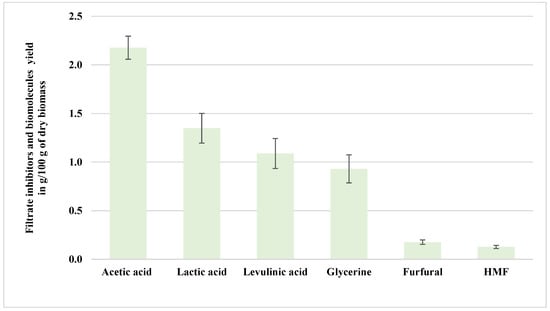
Figure 5.
Filtrate inhibitors and biomolecules from aspen samples pretreated at 200 °C (NED 3.0).
Additionally, glycerin, a biomolecule derived from the thermal degradation of cellulose and hemicellulose, constituted 0.93 g/100 g (16%) of the filtrate composition. Its presence is indicative of LCB polymer breakdown. However, glycerin itself presents relatively low inhibitory potential and typically does not significantly hinder fermentation performance. The filtrate also contained minor yet impactful inhibitory furan derivatives, specifically HMF at 0.13 g/100 g (2%) and furfural at 0.18 g/100 g (3%), which are statistically significantly lower (p < 0.05) than other filtrate inhibitors. Despite their lower quantities, these furans are notable inhibitors of microbial metabolism, negatively affecting fermentation efficiency by reducing cell growth and ethanol yields [18,27]. Overall, the filtrate showed reduced levels of inhibitors, affirming the effectiveness of the reactor in minimizing inhibitor carryover into the hydrolysate. Lower inhibitor concentrations in the filtrate indicate that the resulting media after pretreatment are more suitable for subsequent enzymatic hydrolysis and fermentation. Statistical details for the filtrate stream (Table A1, Table A2 and Table A3) further validate the observed dominance of acetic and levulinic acids. Spearman correlation analysis (Table A4) shows that filtrate acetic acid is positively correlated with pretreatment temperature (ρ = 0.89, p < 0.001), while furfural and HMF showed weak or inverse correlations.
Figure 6 shows the filtrate sugar profile obtained from the aspen samples, pretreated at 200 °C with NED 3.0. The filtrate exhibited a modest release of soluble sugars, with xylose (2.40 g/100 g dry biomass) and glucose (1.45 g/100 g dry biomass) as the predominant monosaccharides. Lower amounts of arabinose (1.30 g/100 g dry biomass), mannose (0.97 g/100 g dry biomass), galactose (0.98 g/100 g dry biomass), and cellobiose (0.39 g/100 g dry biomass) were also detected. These results suggest a partial solubilization of hemicellulosic sugars and depolymerized oligosaccharides during autohydrolysis under high-pressure nitrogen saturation before the explosive decompression step. A statistical comparison of the quantified sugars revealed that the differences were statistically significant (p < 0.05), with xylose concentrations being over six times higher than cellobiose. This pattern aligns with the findings of Sjulander and Kikas [7], who observed that the two-step NED approach solubilized hemicellulose more effectively in barley straw than in softwoods, where xylose and arabinose dominated the filtrate composition. This profile also aligns with solvolytic cleavage of xylan and other hemicellulosic fractions during high-pressure thermal pretreatment, as previously observed in hydrothermal and dilute acid processes [28,29,30,31]. However, the filtrate sugar concentrations in the NED 3.0 system were notably higher than those reported by Rooni et al. [2], where nitrogen explosion at 175 °C resulted in only trace filtrate sugar release due to shorter heating profiles and reduced residence times. The enhanced yield could be attributed to optimized temperature retention and nitrogen saturation, which likely intensified the solvolytic cleavage of hemicellulosic linkages.
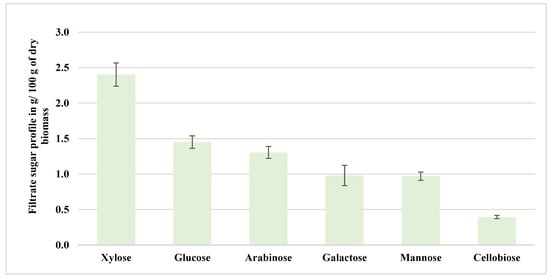
Figure 6.
Filtrate sugar profile obtained from the aspen samples pretreated at 200 °C (NED 3.0).
Compared with NED 2.0, where only trace amounts of filtrate sugar yields were recorded under similar biomass conditions, the present results represent a considerable advancement. Sluiter et al. [32] highlighted that even mild hot-water pretreatments typically yield low glucose in filtrates unless the structural barrier is significantly compromised [32]. The elevated xylose and glucose concentrations observed here suggest that NED 3.0 achieves a more profound disruption of hemicellulose without requiring acid catalysts. This feature distinguishes it from classical dilute acid or oxalic acid pretreatments, which often require detoxification of acid-rich filtrates [33].
Moreover, the presence of glucose in the filtrate at this stage may indicate partial cellulose disruption, a phenomenon also noted by Raud et al. [17], who reported that high-pressure NED 2.0 could initiate cellulose depolymerization when operated above 180 °C. Raud noted that while beneficial for immediate use, these soluble sugars could also contribute to fermentation inhibition if retained with acid byproducts. Hence, the recovery and detoxification of the filtrate are critical for downstream processes. The prevalence of xylose highlights the selective hydrolysis of hemicellulose under these pretreatment conditions. These findings align well with those reported by Sjulander and Kikas [7], who demonstrated that a two-step NED pretreatment of aspen and barley straw resulted in significant xylose extraction in the NED 2.0 pretreatment system due to effective hemicellulose depolymerization. Table A5 confirms that xylose and glucose were the most abundant sugars in the filtrate.
Additionally, compared to steam-explosion-based hemicellulose solubilization methods [34,35], the NED 3.0 system’s ability to recover filtrate sugars under near-neutral conditions, coupled with low levels of accompanying inhibitors, represents a strategic improvement for downstream enzymatic hydrolysis and fermentation.
3.5. Hydrolysate Inhibitors and Sugars Profile
Aspen wood hydrolysates pretreated at 200 °C (NED 3.0) revealed significant variation in the profile of inhibitors and biomolecules compared with the untreated samples (Figure 7). The primary inhibitor across all samples was acetic acid, which had a relatively high yield (1.25–1.46 g/100 g dry biomass), accounting for up to 78.5% of the total detected compounds (pretreated 48 h). The acetic acid concentrations at 24 h in the treated samples (1.40 g/100 g dry biomass) were markedly higher compared with the untreated samples (0.41 g/100 g dry biomass). Similarly, at 48 h, the acetic acid concentration remained statistically significantly elevated in the pretreated samples (1.46 g/100 g dry biomass) relative to the untreated samples (0.39 g/100 g dry biomass). At 72 h, acetic acid concentrations in the pretreated samples (1.25 g/100 g dry biomass) continued to show statistically significant differences from the untreated samples (0.39 g/100 g dry biomass). Acetic acid released from hemicellulose during hydrolysis is a significant inhibitory compound derived from hemicellulose during hydrolysis [36]. Taherzadeh and Karimi [37] stated that the acetic acid yield in the hydrolysis does not significantly depend on the severity of the hydrolysis process and that it can be formed at concentrations even higher than 10 g/L, which supports the relatively high acetic acid observed in the hydrolysates.
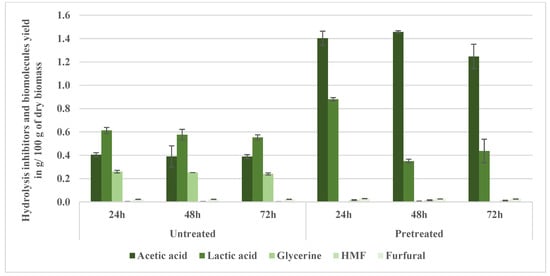
Figure 7.
Hydrolysis inhibitor and biomolecule yields of aspen wood pretreated at 200 °C (NED 3.0).
Lactic acid, a less inhibitory biomolecule, showed notably different trends (p < 0.05) among hydrolysis intervals. Initially, at 24 h, the lactic acid concentration in the pretreated samples (0.88 g/100 g dry biomass) was markedly higher than in the untreated samples (0.61 g/100 g dry biomass). However, at 48 h, lactic acid concentrations statistically significantly dropped in the pretreated samples (0.35 g/100 g dry biomass), compared with both the 24 h pretreated samples and the 48 h untreated samples (0.58 g/100 g dry biomass). At 72 h, the lactic acid in the pretreated samples partially recovered (0.44 g/100 g dry biomass) yet remained notably different compared with the untreated samples (0.55 g/100 g dry biomass). Lactic acid’s fluctuating yields, peaking at 0.88 g/100 g dry biomass in the pretreated 24 h sample, suggest a shift in sugar degradation pathways under severe pretreatment conditions [37].
Glycerin, a minor fermentation byproduct, was notably absent in the NED-treated samples but consistently present in the untreated samples. The glycerin concentrations in the pretreated samples were notably different from the untreated samples for all measured intervals. It was undetectable (0.00 g/100 g dry biomass) in the pretreated samples, in stark contrast to the untreated samples, which consistently maintained glycerin levels of around 0.24–0.26 g/100 g dry biomass across the hydrolysis periods (24, 48, and 72 h). Glycerol (glycerin) appears in hydrolysates usually due to thermal decomposition or side reactions. Its presence or absence indicates distinct reaction pathways influenced by pretreatment parameters [38]. Glycerin’s notable absence in the NED-treated samples suggested altered pathways at higher pretreatment severities or selective degradation under higher temperatures and pretreatment pressures. This contrasts with traditional acid-catalyzed systems, which often trigger sugar degradation and generate non-fermentable byproducts [35,39,40]. Furan-based inhibitors, namely, hydroxymethylfurfural (HMF) and furfural, showed a minimal yet consistent presence at notably different concentrations between the untreated and pretreated samples. Despite their relatively low concentrations (0.01–0.03 g/100 g dry biomass), they are known to maintain high inhibitory potential [18,27]. Table A6 and Table A7 confirm that the sugar data from hydrolysates are not normally distributed (p < 0.01), supporting the application of non-parametric statistics. Figure 8 shows the sugar profile of the hydrolyzed aspen pretreated at 200 °C. The biomass hydrolysis showed a substantial enhancement in sugar yields compared with the untreated samples. Specifically, glucose concentrations were notably different (p < 0.05) between the untreated samples and pretreated samples for all measured hydrolysis durations. After 24 h, glucose significantly increased from 12.28 g/100 g dry biomass in the untreated samples to 21.70 g/100 g dry biomass in the pretreated samples; at 48 h, from 12.73 g/100 g dry biomass in the untreated samples to 26.51 g/100 g dry biomass in the pretreated samples; and at 72 h, from 12.79 g/100 g dry biomass in the untreated samples to 26.40 g/100 g dry biomass in the pretreated samples (p < 0.05). Similarly, xylose also demonstrated statistically significant increases for all hydrolysis durations. After 24 h, xylose concentrations rose significantly from 2.05 g/100 g dry biomass in the untreated samples to 10.64 g/100 g in the pretreated samples; at 48 h, from 2.25 g/100 g in the untreated samples to 11.94 g/100 g in the pretreated samples; and at 72 h, from 2.32 g/100 g dry biomass in the untreated samples to 10.63 g/100 g in the pretreated samples (p < 0.05). Cellobiose showed statistically significant differences after 24 h (0.26 g/100 g in the untreated samples vs. 0.88 g/100 g in the pretreated samples), particularly at 48 h (0.19 g/100 g in the untreated samples vs. 2.17 g/100 g dry biomass in the pretreated samples) and at 72 h (0.00 g/100 g in the untreated samples vs. 1.39 g/100 g dry biomass in the pretreated samples) (p < 0.05).
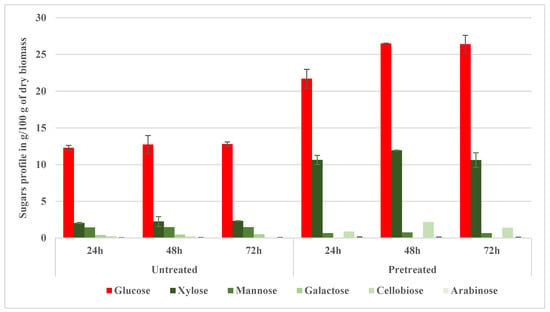
Figure 8.
Sugar profile of hydrolyzed aspen pretreated at 200 °C (NED3.0).
At 72 h, glucose (26.40 g/100 g dry biomass) and xylose (10.63 g/100 g dry biomass) were liberated, confirming substantial disruption of the lignocellulosic structure and supporting Raud et al.’s [17] observations regarding the effectiveness of NED 2.0. This enzymatic enhancement is consistent with the literature, which reports that biomass pretreated using mechanical or physicochemical means (such as AFEX or steam) shows improved digestibility when cellulose is exposed and lignin is restructured [12,34,41,42]. In sulphite pretreatment to overcome the recalcitrance of lignocellulose-(SPORL) pretreated hardwoods, similar glucose yields (25–30 g/100 g) were reported under optimized enzymatic conditions [43,44]. Additionally, mannose, arabinose, and cellobiose were present in smaller quantities. These results exhibit substantial improvement over earlier reports by Rooni et al. [2], where glucose yields from NED 2.0 pretreated aspen at 175 °C were 8.11 g/100 g dry biomass. Dunn’s post hoc test (Table A7) reveals no statistically significant difference in glucose between 48 h and 72 h hydrolysis, aligning with the observed plateau in glucose yield in Figure 7. These findings support the process optimization at 48 h of hydrolysis.
Aspen pretreated by NED 3.0 at 200 °C yielded higher glucose (26.4 g/100 g) and ethanol (8.1 g/100 g) than by steam explosion, which produced 14.96 g/100 g glucose at the same temperature [2]. These results demonstrate the superior efficiency of NED 3.0 under comparable conditions. The glucose and xylose results in this study are also comparable with those obtained by one-step pretreatment over Sjulander and Kikas [7], where pretreating aspen twice at 175 °C and 200 °C with NED 2.0 followed by filtration after each pretreatment, led to overall glucose and xylose yields of 29.19 g/100 g and 12.99 g/100 g dry biomass, respectively. Despite using sequential NED pretreatments and filtration steps, Sjulander and Kikas’ two-step system compares to the yield of the single-step NED 3.0 system used in this study. Firstly, the explosive decompression at high temperature with NED 3.0 facilitates a more complete disruption of the lignin–carbohydrate matrix in a single, optimized exposure, reducing the need for multiple pretreatments. Secondly, while the two-step pretreatment strategy in the NED 2.0 system effectively reduced inhibitors through filtration, it comes with an additional cost, is energy intensive, and leads to sugar loss. This can lead to a net reduction in the fermentable sugar pool if the recovery steps are not fully optimized. Therefore, the new approach simplifies the pretreatment protocol and enhances sugar recovery, primarily due to the effective separation of volatiles during the decompression stage. This distinct advantage is achieved by evaporating and subsequently condensing the volatile components in a separate vessel, making it a more viable and efficient platform for industrial-scale bioconversion.
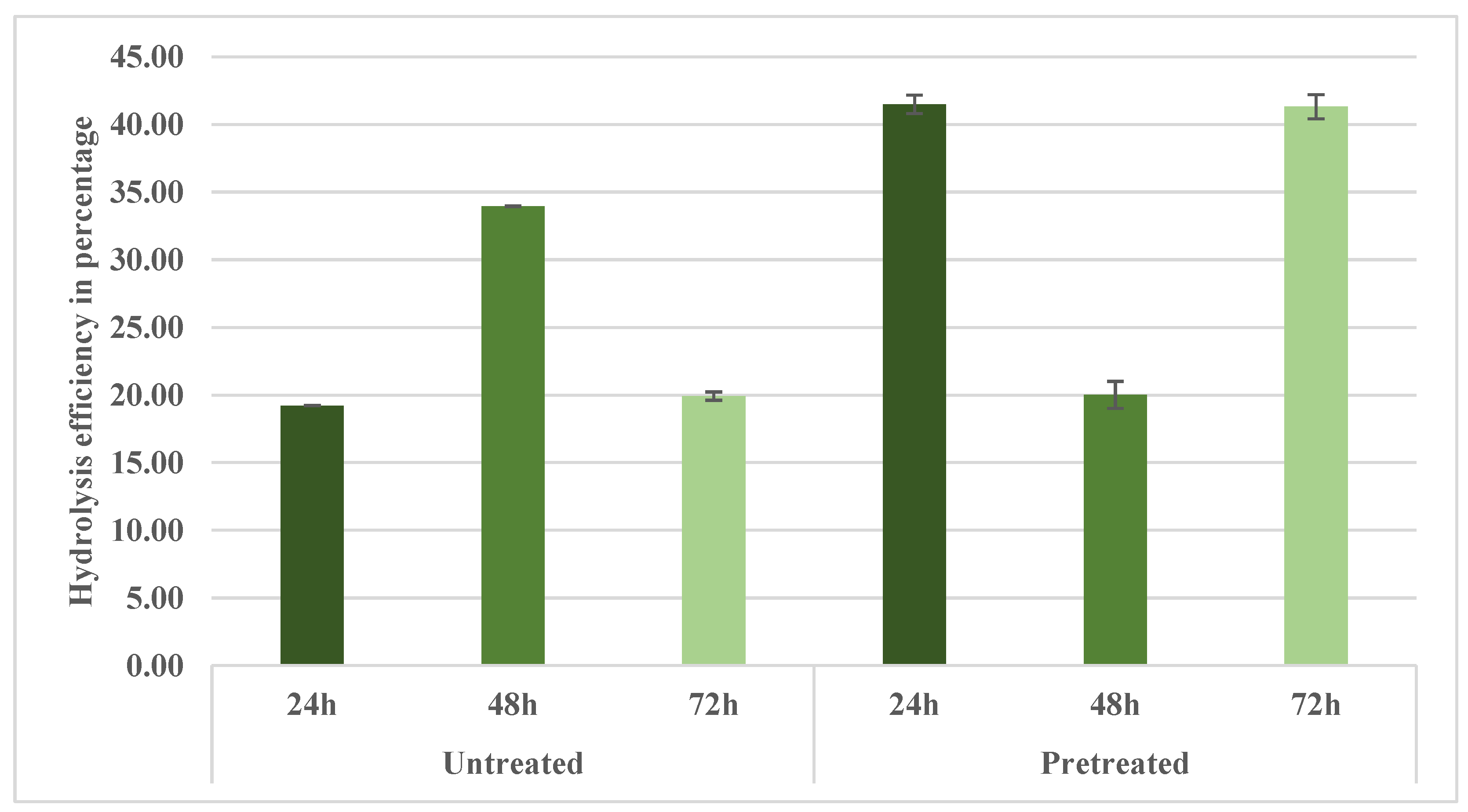
Appendix B shows the hydrolysis efficiency of aspen biomass. The general trend observed indicates that hydrolysis efficiency progressively improves with increasing hydrolysis duration. After 24 h, the hydrolysis efficiency in the pretreated samples (33.95 mass%) was notably different (p < 0.05) from the untreated (19.22 mass%). At 48 h, hydrolysis efficiency notably increased in the pretreated samples (41.48 mass%), showing a notably different result (p < 0.05) compared with the untreated samples (19.92 mass%). However, after 48h of hydrolysis, the efficiency of the process tends to slightly decrease, although no statistically significant difference exists between 48 h (41.48%) and 72 h (41.38%). Yet, after 72 h, the hydrolysis efficiency of the pretreated samples (41.31 mass%) was notably different (p < 0.05) compared with the untreated samples (20.01 mass%).
Compared with previously published data, these hydrolysis efficiencies align closely with the findings reported by Rooni et al. [2]. Rooni et al. [2] evaluated the hydrolysis efficiency of aspen biomass pretreated by NED 2.0 and found values of approximately 29.5% at 200 °C. Further, compared to the data presented by Raud et al. [17], who reported hydrolysis efficiencies of 74.1% for barley straw pretreated under optimal conditions (150 °C and pressures between 10 and 30 bar) using NED 2.0, the efficiencies achieved in the current study for aspen biomass are predictably lower. The difference arises due to inherent disparities in biomass recalcitrance, with hardwood biomass such as aspen being structurally more complex and resistant to enzymatic attack than herbaceous biomass like barley straw. Given these structural differences, the efficiencies obtained with aspen are considered acceptable and expected, underscoring the efficacy of NED 3.0 pretreatment in hardwood processing.
3.6. Fermentation Inhibitors, Residual Sugars, and Ethanol Profiles
Figure 9 illustrates the profiles of the fermentation broth inhibitors. The production of acetic acid, a known moderate inhibitor due to its impact on intracellular pH [36], significantly increased (p < 0.05) in the pretreated samples (fermentation broth), reaching 0.99 g/100 g dry biomass in 7 days, markedly higher (p < 0.05) than in the untreated samples, which had 0.33 g/100 g dry biomass. Acetic acid accounted for approximately 43% of the total quantified inhibitors in the fermentation broth, compared with 25% in the untreated samples. This increase is likely due to enhanced deacetylation of hemicelluloses during NED. Despite this increase, ethanol production was not adversely affected, indicating either the robust inhibitor tolerance of the fermentation system or that inhibitory thresholds were not breached. Similarly, lactic acid, another moderate inhibitor primarily affecting osmotic pressure, was present at 1.00 g/100 g dry biomass in the fermentation broth samples compared with 0.92 g/100 g dry biomass in the untreated samples after 7 days. This difference was not statistically significant (p > 0.05). However, by day 28, the lactic acid concentration in the fermentation broth (0.98 g/100 g) was significantly reduced (p < 0.05) compared to the untreated samples (1.70 g/100 g), suggesting reduced accumulation of this metabolite in the pretreated samples, potentially due to improved redox balance or sugar utilization efficiency.
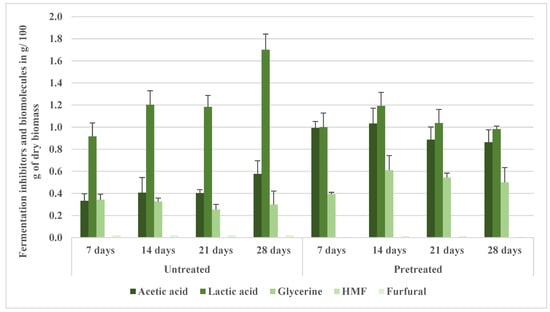
Figure 9.
Fermentation broth inhibitors and biomolecules from aspen samples pretreated at 200 °C (NED 3.0).
The sugar alcohol glycerin, often regarded as a low-toxicity overflow metabolite involved in redox balancing [45], increased only marginally, from 0.34 g/100 g dry biomass in the untreated samples to 0.39 g/100 g in the fermentation broth at 7 days, and was notably different (p > 0.05). However, at 14, 21, and 28 days, glycerin concentrations were markedly higher in the fermentation broth (0.61, 0.54, and 0.50 g/100 g, respectively) than in the untreated samples (0.33, 0.25, and 0.30 g/100 g, respectively), at p < 0.05, suggesting enhanced NADH reoxidation and metabolic adaptation in response to the NED treatment. Interestingly, the relative contribution of glycerin to total inhibitors declined slightly in the fermentation broth, from 7.6% in the untreated samples to 5.4%, which may reflect lower metabolic stress in the pretreated samples.
Notably, HMF and furfural, both high-toxicity furanic inhibitors, were either absent or present in negligible amounts (<0.01 g/100 g dry biomass) across all conditions. These results strongly support the proposition that NED 3.0’s rapid decompression at 200 °C reduces sugar degradation and suppresses furanic inhibitor generation, which aligns with previous reports on thermal dynamics and inhibitor suppression during optimized pretreatment [46].
Although pH control was not a variable in this study, pH was monitored during the fermentation process to track potential shifts related to microbial activity and acid production. The pH of the slurry after pretreatment at 200 °C was measured at approximately 5.5. During the 28-day fermentation period, a consistent decline was observed, with the final pH reaching 3.4. This reduction is likely attributed to the accumulation of organic acids (e.g., lactic and acetic acids) produced or released during fermentation. While the pH was not actively adjusted or maintained at a set point, this observation suggests an acidic environment that may influence long-term microbial stability in extended fermentation processes.
Figure 10 illustrates the amount of residual sugars available in the fermented samples. When compared to the untreated samples, there was increase in the fermentation efficiency after seven days of fermentation. Specifically, on day 7, the fermented samples retained substantial quantities of xylose (6.68 g/100 g dry biomass) and mannose (3.93 g/100 g dry biomass), reflecting slower fermentation kinetics for pentoses and some hexoses. At 7 days, glucose (1.66 g/100 g dry biomass), xylose, mannose, and cellobiose (0.44 g/100 g dry biomass) concentrations in the fermentation broth were markedly higher (p < 0.05) compared with the untreated samples (0.00, 1.26, 2.28, and 0.15 g/100 g dry biomass, respectively). By day 14, the xylose and mannose concentrations slightly increased, reaching 6.71 g/100 g and 7.20 g/100 g dry biomass, respectively. These xylose, mannose, galactose (0.51 g/100 g dry biomass), and arabinose (0.08 g/100 g dry biomass) concentrations in the fermentation broth were markedly higher (p < 0.05) compared with the untreated samples. These findings are corroborated by a report by Burton et al. (2010), which states that the most common polysaccharides found in hardwood hemicellulose are glucuronoxylan and glucomannan [47]. Furthermore, a report by the U.S. Forest Service’s “Cell Wall Chemistry” details that hardwoods predominantly contain glucuronoxylan (15–30%) and glucomannan (2–5%) as their main hemicelluloses [48]. The presence of glucuronoxylan and glucomannan in hardwood could explain the high concentrations of xylose and mannose sugars in the fermentation’s residual sugar. This suggests that as continuous saccharification and fermentation occur, the polysaccharides glucuronoxylan and glucomannan in hardwood are broken down, leading to an abundance of xylose and mannose sugars, which Saccharomyces cerevisiae does not metabolize. Unlike xylose, glucose was nearly completely fermented by day 14, decreasing significantly to 0.24 g/100 g dry biomass. After 21 and 28 days, xylose, mannose, galactose, arabinose, and glucose concentrations remained markedly higher (p < 0.05) in the fermentation broth compared with the untreated samples.
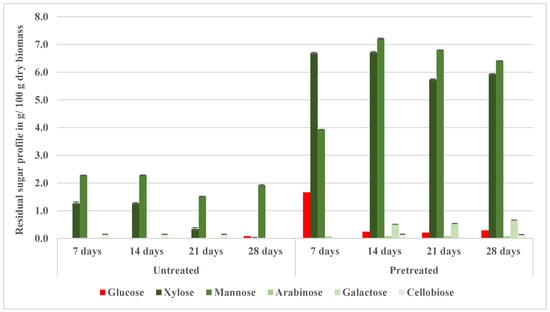
Figure 10.
Residual sugars profiles of the untreated and pretreated (NED3.0 at 200 °C) fermented aspen samples.
This fermentation pattern closely mirrors the findings of Sjulander and Kikas [7], where efficient glucose utilization was accompanied by slower pentose consumption under similar conditions. The formation and persistence of xylose after fermentation underscores the need for improved microbial strains or process conditions capable of utilizing pentoses more efficiently, an aspect also emphasized by Sjulander and Kikas [7].
The fermentation efficiency (Efer) of the aspen biomass pretreated at 200 °C using the newly configured NED 3.0 system was assessed over various fermentation durations and benchmarked against untreated samples (Figure 11). NED 3.0 consistently outperformed the untreated samples in converting fermentable sugars to ethanol. The highest fermentation efficiency was observed on the 14th day, reaching 59.79%, corresponding to an empirical ethanol yield of 8.00 g per 100 g dry biomass. At 200 °C, the NED 3.0 pretreatment process achieved significant in situ detoxification of fermentation inhibitors. Furfural concentrations in the fermentation broth remained consistently low at 0.01 g/100 g dry biomass, which is well below the inhibitory threshold of 0.5–1.0 g/L for Saccharomyces cerevisiae [36]. This indicates that furanic aldehydes were effectively removed during the condensation process. However, acetic acid concentrations peaked at 1.03 g/100 g dry biomass (~1.03 g/L) by day 14 of fermentation. While this level is below the critical inhibitory thresholds reported by [49,50], which range from 2 to 6 g/L, depending on the yeast tolerance, it may still present challenges for less robust or genetically engineered strains that lack resistance to weak acids. This represents a notable limitation of the NED 3.0 system: although it drastically reduces the presence of furan compounds and partially mitigates acid accumulation, residual acetic acid persists and could impair microbial fermentation performance under specific conditions. To address this, future improvements may include automatic in situ pH control of the hydrolysis and fermentation systems or the use of acetic-acid-tolerant strains to further enhance the system’s biocompatibility without compromising sugar recovery or process simplicity. In contrast, untreated samples under the same duration peaked at only 36.03%. Even by day 28, the NED 3.0 system maintained a high efficiency of 57.49%, whereas for untreated samples it declined further to 31.57%. The fermentation efficiencies obtained from the fermentation broth for 14, 21, and 28 days were statistically significantly different (p < 0.05) and notably higher than their respective untreated samples (36.03%, 22.69%, and 30.36%). The improved yield relationship in the present study reflects the advantages of the NED 3.0 system’s more regulated severity profile and efficient separation strategy. Solid–liquid separation following pretreatment and enzymatic hydrolysis helped to reduce the accumulation of fermentation inhibitors. Based on these findings, acetic acid—the dominant inhibitor—peaked at 2.18 g/100 g dry biomass in the filtrate and remained under 1.03 g/100 g dry biomass in the fermentation broths, well below the inhibitory thresholds reported in previous studies. Similarly, concentrations of furfural and HMF, two potent furan-based inhibitors, remained low, with maximum observed values of 0.20 g/100 g and 0.13 g/100 g dry biomass, respectively. These values are significantly lower than the 0.4 g/100 g limit referenced by Sjulander and Kikas [7], who emphasized that S. cerevisiae can tolerate and metabolize small amounts of these inhibitors under controlled pH and nutrient conditions. Comparable ethanol yields under detoxified steam-exploded aspen systems required extensive filtration and pH conditioning [51,52,53]. Furthermore, the fermentation results align with recent work on engineered S. cerevisiae strains exhibiting elevated tolerance to acetic acid and furans [54,55]. However, such genetic modifications are not required in the NED 3.0 system, as its design inherently suppresses inhibitor accumulation during the pretreatment phase. The moderate accumulation of formic acid (0.60 g/100 g) and lactic acid (1.7 g/100 g dry biomass) further supports the system’s ability to limit fermentation stress, especially given the low inhibitory potential of these compounds.
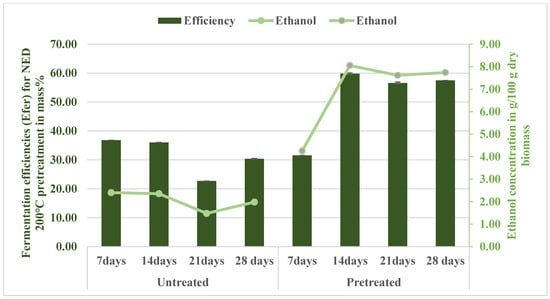
Figure 11.
Fermentation efficiency (Efer) and ethanol yields from aspen samples pretreated at 200 °C (NED 3.0).
These results highlight that NED 3.0 pretreatment significantly improves fermentation outcomes, likely by enhancing sugar availability and mitigating inhibitor impact. The performance aligns with theoretical expectations based on stoichiometric yields (0.51 g ethanol/g sugar), where the empirical fermentation efficiency reached 59.4% based solely on glucose and 42.4% when including xylose in the calculations. Compared to the work of Rooni et al. [2], where the fermentation efficiency for the NED 2.0-treated aspen did not exceed ~47%, the NED 3.0 configuration offers a marked improvement. Similarly, while Sjulander and Kikas [7] emphasized detoxification steps to overcome fermentation inhibition, our findings indicate that NED 3.0 at 200 °C can achieve a high fermentation efficiency without requiring post-pretreatment filtration, provided the pH control and fermentation time are optimized.
Figure 11 shows the ethanol yields for aspen samples pretreated with NED 3.0 at 200 °C. The ethanol yield in the fermentation broth was statistically significantly higher (p < 0.05) compared with the untreated for all fermentation durations measured, starting from 7 days (4.25 g/100g dry biomass vs. 2.40 g/100g dry biomass), peaking notably at 14 days (8.05 g/100g dry biomass vs. 2.35 g/100g dry biomass), and remaining significantly higher at both 21 days (7.62 g/100g dry biomass vs. 1.48 g/100g dry biomass) and 28 days (7.74 g/100g dry biomass vs. 1.98 g/100g dry biomass). The time-course pattern observed here suggests that a 14–21-day fermentation window is optimal for maximizing yeast performance before ethanol inhibition or acid build-up curtails conversion. Table A8 consolidates the ethanol production and fermentation efficiency data, showing that NED 3.0 achieves a maximum ethanol yield of 8.05 g/100 g dry biomass with a fermentation efficiency of 59.8%. The low residual glucose and weak correlation with inhibitory furans (p < −0.3) in the heatmap further confirm that the detoxification strategy minimized inhibitor interference. In addition, the statistical analyses in Appendix C provide robust support for the observed improvements in sugar yield, inhibitor suppression, and fermentation efficiency. The combined use of non-parametric tests and correlation mapping strengthens the reliability of NED 3.0 as an effective in situ detoxification pretreatment. This insight offers a valuable operational lever for process optimization in NED-based biorefinery applications, especially when balancing yield, inhibitor avoidance, and energy input for pretreatment severity.
4. Current Challenges and Future Trends in Lignocellulosic Biomass Pretreatment
Current challenges in lignocellulosic biomass pretreatment primarily revolve around developing feedstock-agnostic processes that can efficiently extract cellulose, hemicellulose, and lignin [56]. The inherent complexity and recalcitrance of lignocellulosic biomass demand extensive and efficient pretreatment to enhance enzymatic hydrolysis and fermentation yields. Conventional pretreatment methods, such as steam explosion, acid hydrolysis, and organosolv, face limitations due to high operational costs, substantial environmental burdens, and generation of fermentation inhibitors, notably acetic acid, furfural, and HMF [56,57]. These inhibitors severely impede microbial activity during fermentation, thus requiring effective detoxification strategies. Furthermore, traditional pretreatment methods often demand substantial water and energy inputs, leading to increased overall capital costs, which significantly limit scalability and environmental sustainability [56]. These concerns are echoed in bacterial cellulose production studies, where the pretreatment of lignocellulosic residues was reported to be energy-intensive, inhibitor-generating, and cost-prohibitive without optimization [58]. These challenges underscore the urgent need for more efficient, cost-effective, and environmentally benign pretreatment technologies.
Future development trends in pretreatment technology are steering toward the design of universal or feedstock-agnostic solvents and methods, emphasizing recyclability and minimal environmental impact [57]. Advances in pretreatment technologies underscore the necessity to address microbial inhibitors effectively while significantly reducing energy and water consumption [56]. Emerging strategies involve employing membrane-based or enzymatic detoxification processes prior to fermentation, aiming to enhance downstream process efficiency [57]. Furthermore, hybrid and integrated biorefinery systems combining physical, chemical, and biological pretreatments are increasingly gaining attention for their potential to fully valorize biomass components, thereby significantly improving overall economic viability and sustainability [58].
Specifically, novel technologies such as the nitrogen explosive decompression (NED 3.0) pretreatment system have demonstrated substantial potential. Unlike previous NED configurations, NED 3.0 incorporates an in situ inhibitor removal mechanism, which significantly reduces the concentrations of inhibitory compounds, such as acetic acid and furfural, thereby enhancing sugar recovery and the subsequent fermentation efficiency. This advanced configuration ensures precise thermal and pressure control, improved structural breakdown, and minimized sugar loss, collectively improving bioconversion efficiency compared to conventional methods. The integration of in situ detoxification processes in pretreatment steps, such as NED 3.0, represents a critical advancement toward practical scalability and alignment with broader sustainability objectives, including those outlined in the EU Green Deal.
Overall, these developments underscore a transition toward more integrated, environmentally friendly, and economically viable pretreatment technologies. Future research should emphasize optimization through statistical methods, such as response surface methodology (RSM), extensive techno-economic analysis (TEA), and comprehensive life cycle assessment (LCA), to enhance further the commercial and environmental prospects of biomass pretreatment methods.
5. Conclusions
This study demonstrates the novel efficacy and significant industrial promise of the newly developed single-step nitrogen explosive decompression (NED 3.0) pretreatment system for lignocellulosic biomass valorization. The unique integration of optimized thermal exposure, controlled decompression kinetics, and simultaneous inhibitor removal in NED 3.0 distinctly advances biomass processing beyond previous NED methodologies.
The key novelty lies in the system’s ability to simultaneously separate and recover volatile and soluble inhibitors directly from biomass during the pretreatment stage, substantially lowering the levels of potent inhibitors. This is enabled by the system’s design, which allows volatile compounds to vaporize and be captured in the integrated condenser and expansion vessel as condensate. Meanwhile, non-volatile but water-soluble inhibitors are recovered in the filtrate fraction through vacuum separation of the biomass slurry.
Our findings confirm that this dual phase separation enables early-phase removal of furanic and organic acid inhibitors, reducing their downstream accumulation in hydrolysate and fermentation broths. By achieving effective in situ removal of inhibitors such as HMF and furfural into condensate and filtrate fractions, NED 3.0 reduces downstream toxicity, enhancing both hydrolysis and fermentation processes. The process significantly improves fermentable sugar recovery, particularly glucose and xylose, while maintaining low acetic acid levels and producing microbial-compatible byproducts like lactic acid and glycerin.
Despite these promising results, certain limitations remain. The current study focused solely on aspen biomass, and additional evaluations are required to validate the method’s broader applicability across diverse lignocellulosic feedstocks. Moreover, while the environmental and economic advantages of NED 3.0 appear evident, comprehensive life-cycle and techno-economic analyses remain necessary for full industrial validation.
Further work should explore the applicability of NED 3.0 across various biomass types, including softwoods, and developing advanced kinetic models to predict inhibitor release and sugar yields. Crucially, the establishment of closed-loop nitrogen recycling systems could further enhance the environmental performance and economic viability of the process. In alignment with the goals of the European Union’s Green Deal, the adoption of NED 3.0 could significantly contribute to sustainable bioethanol production and integrated biorefinery systems, fostering the transition toward renewable energy and bio-based economies.
Author Contributions
D.O.: Designing the experiments, investigation, writing—original draft, conceptualization, visualization, software, formal analysis, methodology, and data curation; L.R.-M.: co-supervision, writing—review and editing, and visualization; V.R.: co-supervision, writing—review and editing, and visualization; T.K.: conceptualization, supervision, writing—review and editing, project administration, resources, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by PRG project number PRG2730, “Production of lignin-based pharmaceutical intermediates and FDCA from lignocellulosic biomass”.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Comprehensive Summary of All Inhibitors and Byproducts from NED 3.0
| Compound | Yield (g/100 g Dry Biomass) | Relative Composition (%) | Classification | Inhibitory Potential | Source | Sample |
| Formic acid | 0.60 | 34.5 | Organic acid | Moderate | Condensate | – |
| Lactic acid | 0.45 | 25.9 | Biomolecule | Low | Condensate | – |
| Acetic acid | 0.44 | 25.3 | Organic acid | Moderate | Condensate | – |
| Furfural | 0.20 | 11.5 | Furan-based inhibitor | High | Condensate | – |
| Glycerin | 0.04 | 2.3 | Biomolecule (byproduct) | Low | Condensate | – |
| HMF | 0.01 | 0.6 | Furan-based inhibitor | High | Condensate | – |
| Levulinic acid | 1.09 | 19 | Organic acid | Moderate | Filtrate | – |
| Acetic acid | 2.18 | 37 | Organic acid | Moderate | Filtrate | – |
| Lactic acid | 1.35 | 23 | Organic acid | Moderate | Filtrate | – |
| Glycerin | 0.93 | 16 | Biomolecule (byproduct) | Low | Filtrate | – |
| HMF | 0.13 | 2 | Furan-based inhibitor | High | Filtrate | – |
| Furfural | 0.18 | 3 | Furan-based inhibitor | High | Filtrate | – |
| Acetic acid | 0.41 | 31.5 | Organic acid | Moderate | Hydrolysate | Untreated samples 24 h |
| Acetic acid | 0.39 | 31.5 | Organic acid | Moderate | Hydrolysate | Untreated samples 48 h |
| Acetic acid | 0.39 | 32.5 | Organic acid | Moderate | Hydrolysate | Untreated samples 72 h |
| Acetic acid | 1.40 | 60.1 | Organic acid | Moderate | Hydrolysate | Pretreated 24 h |
| Acetic acid | 1.46 | 78.5 | Organic acid | Moderate | Hydrolysate | Pretreated 48 h |
| Acetic acid | 1.25 | 72.3 | Organic acid | Moderate | Hydrolysate | Pretreated 72 h |
| Lactic acid | 0.61 | 46.9 | Biomolecule | Low | Hydrolysate | Untreated samples 24 h |
| Lactic acid | 0.58 | 46.8 | Biomolecule | Low | Hydrolysate | Untreated samples 48 h |
| Lactic acid | 0.55 | 45.8 | Biomolecule | Low | Hydrolysate | Untreated samples 72 h |
| Lactic acid | 0.88 | 37.8 | Biomolecule | Low | Hydrolysate | Pretreated 24 h |
| Lactic acid | 0.35 | 18.8 | Biomolecule | Low | Hydrolysate | Pretreated 48 h |
| Lactic acid | 0.44 | 25.4 | Biomolecule | Low | Hydrolysate | Pretreated 72 h |
| Glycerin | 0.26 | 20.0 | Biomolecule (byproduct) | Low | Hydrolysate | Untreated samples 24 h |
| Glycerin | 0.25 | 20.2 | Biomolecule (byproduct) | Low | Hydrolysate | Untreated samples 48 h |
| Glycerin | 0.24 | 20.0 | Biomolecule (byproduct) | Low | Hydrolysate | Untreated samples 72 h |
| Glycerin | 0.00 | 0.0 | Biomolecule (byproduct) | Low | Hydrolysate | Pretreated 24 h |
| Glycerin | 0.00 | 0.0 | Biomolecule (byproduct) | Low | Hydrolysate | Pretreated 48 h |
| Glycerin | 0.00 | 0.0 | Biomolecule (byproduct) | Low | Hydrolysate | Pretreated 72 h |
| HMF | 0.00 | 0.0 | Furan-based inhibitor | High | Hydrolysate | Untreated samples 24 h |
| HMF | 0.00 | 0.0 | Furan-based inhibitor | High | Hydrolysate | Untreated samples 48 h |
| HMF | 0.00 | 0.0 | Furan-based inhibitor | High | Hydrolysate | Untreated samples 72 h |
| HMF | 0.02 | 0.9 | Furan-based inhibitor | High | Hydrolysate | Pretreated 24 h |
| HMF | 0.02 | 1.1 | Furan-based inhibitor | High | Hydrolysate | Pretreated 48 h |
| HMF | 0.01 | 0.6 | Furan-based inhibitor | High | Hydrolysate | Pretreated 72 h |
| Furfural | 0.02 | 1.5 | Furan-based inhibitor | High | Hydrolysate | Untreated samples 24 h |
| Furfural | 0.02 | 1.6 | Furan-based inhibitor | High | Hydrolysate | Untreated samples 48 h |
| Furfural | 0.02 | 1.7 | Furan-based inhibitor | High | Hydrolysate | Untreated samples 72 h |
| Furfural | 0.03 | 1.3 | Furan-based inhibitor | High | Hydrolysate | Pretreated 24 h |
| Furfural | 0.03 | 1.6 | Furan-based inhibitor | High | Hydrolysate | Pretreated 48 h |
| Furfural | 0.03 | 1.7 | Furan-based inhibitor | High | Hydrolysate | Pretreated 72 h |
| Acetic acid | 0.33 | 20.5 | Organic acid | Moderate | Fermentation broth | Untreated samples 7 days |
| Acetic acid | 0.41 | 20.9 | Organic acid | Moderate | Fermentation broth | Untreated samples 14 days |
| Acetic acid | 0.4 | 21.6 | Organic acid | Moderate | Fermentation broth | Untreated samples 21 days |
| Acetic acid | 0.58 | 22.3 | Organic acid | Moderate | Fermentation broth | Untreated samples 28 days |
| Acetic acid | 0.99 | 41.4 | Organic acid | Moderate | Fermentation broth | Pretreated 7 days |
| Acetic acid | 1.03 | 36.3 | Organic acid | Moderate | Fermentation broth | Pretreated 14 days |
| Acetic acid | 0.89 | 35.9 | Organic acid | Moderate | Fermentation broth | Pretreated 21 days |
| Acetic acid | 0.86 | 36.6 | Organic acid | Moderate | Fermentation broth | Pretreated 28 days |
| Lactic acid | 0.92 | 57.1 | Biomolecule | Low | Fermentation broth | Untreated samples 7 days |
| Lactic acid | 1.2 | 61.2 | Biomolecule | Low | Fermentation broth | Untreated samples 14 days |
| Lactic acid | 1.18 | 63.8 | Biomolecule | Low | Fermentation broth | Untreated samples 21 days |
| Lactic acid | 1.7 | 65.4 | Biomolecule | Low | Fermentation broth | Untreated samples 28 days |
| Lactic acid | 1.0 | 41.8 | Biomolecule | Low | Fermentation broth | Pretreated 7 days |
| Lactic acid | 1.19 | 41.9 | Biomolecule | Low | Fermentation broth | Pretreated 14 days |
| Lactic acid | 1.04 | 41.9 | Biomolecule | Low | Fermentation broth | Pretreated 21 days |
| Lactic acid | 0.98 | 41.7 | Biomolecule | Low | Fermentation broth | Pretreated 28 days |
| Glycerin | 0.34 | 21.1 | Biomolecule (byproduct) | Low | Fermentation broth | Untreated samples 7 days |
| Glycerin | 0.33 | 16.8 | Biomolecule (byproduct) | Low | Fermentation broth | Untreated samples 14 days |
| Glycerin | 0.25 | 13.5 | Biomolecule (byproduct) | Low | Fermentation broth | Untreated samples 21 days |
| Glycerin | 0.3 | 11.5 | Biomolecule (byproduct) | Low | Fermentation broth | Untreated samples 28 days |
| Glycerin | 0.39 | 16.3 | Biomolecule (byproduct) | Low | Fermentation broth | Pretreated 7 days |
| Glycerin | 0.61 | 21.5 | Biomolecule (byproduct) | Low | Fermentation broth | Pretreated 14 days |
| Glycerin | 0.54 | 21.8 | Biomolecule (byproduct) | Low | Fermentation broth | Pretreated 21 days |
| Glycerin | 0.5 | 21.3 | Biomolecule (byproduct) | Low | Fermentation broth | Pretreated 28 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 7 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 14 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 21 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 28 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Pretreated 7 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Pretreated 14 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Pretreated 21 days |
| HMF | 0.0 | 0.0 | Furan-based inhibitor | High | Fermentation broth | Pretreated 28 days |
| Furfural | 0.02 | 1.2 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 7 days |
| Furfural | 0.02 | 1.0 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 14 days |
| Furfural | 0.02 | 1.1 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 21 days |
| Furfural | 0.02 | 0.8 | Furan-based inhibitor | High | Fermentation broth | Untreated samples 28 days |
| Furfural | 0.01 | 0.4 | Furan-based inhibitor | High | Fermentation broth | Pretreated 7 days |
| Furfural | 0.01 | 0.4 | Furan-based inhibitor | High | Fermentation broth | Pretreated 14 days |
| Furfural | 0.01 | 0.4 | Furan-based inhibitor | High | Fermentation broth | Pretreated 21 days |
| Furfural | 0.01 | 0.4 | Furan-based inhibitor | High | Fermentation broth | Pretreated 28 days |
| The % composition is relative to the total concentration of all six compounds in each treatment group. | ||||||
Appendix B. Hydrolysis Efficiency of Aspen Samples Pretreated with NED 3.0 at 200 °C
Appendix C. Results of the Statistical Analysis

Table A1.
Descriptive statistics for inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
Table A1.
Descriptive statistics for inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
| Temperature (°C) | Hydrolysis Time (Hour) | Fermentation Time (Days) | Formic Acid | Lactic Acid | Acetic Acid | Furfural | Glycerin | HMF | Levulinic Acid | Ethanol | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | 0.000 | 0.000 | 0.000 | 0.000 | 0.3500 | 0.3300 | 0.01000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Maximum | 200.0 | 72.00 | 28.00 | 0.6000 | 1.700 | 2.180 | 0.2000 | 0.9300 | 0.1300 | 1.090 | 8.050 |
| Range | 200.0 | 72.00 | 28.00 | 0.6000 | 1.350 | 1.850 | 0.1900 | 0.9300 | 0.1300 | 1.090 | 8.050 |
| Mean | 112.5 | 18.00 | 8.750 | 0.03750 | 0.9013 | 0.8381 | 0.04063 | 0.3113 | 0.01188 | 0.06813 | 2.242 |
| Std. Deviation | 102.5 | 27.01 | 10.69 | 0.1500 | 0.3792 | 0.5278 | 0.05882 | 0.2513 | 0.03229 | 0.2725 | 3.031 |
| Std. Error of Mean | 25.62 | 6.753 | 2.673 | 0.03750 | 0.09481 | 0.1320 | 0.01470 | 0.06282 | 0.008074 | 0.06813 | 0.7578 |
The concentration of the inhibitors and biomolecules is in g/100 g dry biomass.

Table A2.
Results of the normality test (Shapiro–Wilk test) for inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
Table A2.
Results of the normality test (Shapiro–Wilk test) for inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
| Test for Normal Distribution | Temperature (°C) | Hydrolysis Time (Hours) | Fermentation Time (Days) | Formic Acid | Lactic Acid | Acetic Acid | Furfural | Glycerin | HMF | Levulinic Acid | Ethanol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W | |||||||||||
| p-Value | 0.6383 | 0.6978 | 0.7864 | 0.2727 | 0.9525 | 0.8520 | 0.5084 | 0.9181 | 0.4166 | 0.2727 | 0.7389 |
| Passed normality test (alpha = 0.05)? | No | No | No | No | Yes | No | No | Yes | No | No | No |
| p-Value summary | p ≤ 0.0001 | p ≤ 0.001 | p ≤ 0.01 | p ≤ 0.0001 | p > 0.05 (non-significant) | p ≤ 0.05 | p ≤ 0.0001 | p > 0.05 (non-significant) | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.001 |
The concentrations of the inhibitors and biomolecules are presented as g/100 g dry biomass.

Table A3.
Results of the Dunn’s multiple comparisons test for inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
Table A3.
Results of the Dunn’s multiple comparisons test for inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
| Mean Rank Difference | Significant? | Summary | |
|---|---|---|---|
| Temperature (°C) vs. Hydrolysis time (hour) | 31.50 | No | ns |
| Temperature (°C) vs. Fermentation time (days) | 13.00 | No | ns |
| Temperature (°C) vs. Formic acid | 67.50 | Yes | p ≤ 0.05 |
| Temperature (°C) vs. Lactic acid | −18.50 | No | ns |
| Temperature (°C) vs. Acetic acid | −9.500 | No | ns |
| Temperature (°C) vs. Furfural | 22.50 | No | ns |
| Temperature (°C) vs. Glycerin | 22.50 | No | ns |
| Temperature (°C) vs. HMF | 61.00 | No | ns |
| Temperature (°C) vs. Levulinic acid | 69.50 | Yes | p ≤ 0.05 |
| Temperature (°C) vs. Ethanol | 21.00 | No | ns |
| Hydrolysis time (hours) vs. Fermentation time (days) | −18.50 | No | ns |
| Hydrolysis time (hour) vs. Formic acid | 36.00 | No | ns |
| Hydrolysis time (hour) vs. Lactic acid | −50.00 | No | ns |
| Hydrolysis time (hour) vs. Acetic acid | −41.00 | No | ns |
| Hydrolysis time (hour) vs. Furfural | −9.000 | No | ns |
| Hydrolysis time (hour) vs. Glycerin | −9.000 | No | ns |
| Hydrolysis time (hour) vs. HMF | 29.50 | No | ns |
| Hydrolysis time (hour) vs. Levulinic acid | 38.00 | No | ns |
| Hydrolysis time (hours) vs. Ethanol | −10.50 | No | ns |
| Fermentation time (days) vs. Formic acid | 54.50 | No | ns |
| Fermentation time (days) vs. Lactic acid | −31.50 | No | ns |
| Fermentation time (days) vs. Acetic acid | −22.50 | No | ns |
| Fermentation time (days) vs. Furfural | 9.500 | No | ns |
| Fermentation time (days) vs. Glycerin | 9.500 | No | ns |
| Fermentation time (days) vs. HMF | 48.00 | No | ns |
| Fermentation time (days) vs. Levulinic acid | 56.50 | No | ns |
| Fermentation time (days) vs. Ethanol | 8.000 | No | ns |
| Formic acid vs. Lactic acid | −86.00 | Yes | p ≤ 0.001 |
| Formic acid vs. Acetic acid | −77.00 | Yes | p ≤ 0.01 |
| Formic acid vs. Furfural | −45.00 | No | ns |
| Formic acid vs. Glycerin | −45.00 | No | ns |
| Formic acid vs. HMF | −6.500 | No | ns |
| Formic acid vs. Levulinic acid | 2.000 | No | ns |
| Formic acid vs. Ethanol | −46.50 | No | ns |
| Lactic acid vs. Acetic acid | 9.000 | No | ns |
| Lactic acid vs. Furfural | 41.00 | No | ns |
| Lactic acid vs. Glycerin | 41.00 | No | ns |
| Lactic acid vs. HMF | 79.50 | Yes | p ≤ 0.01 |
| Lactic acid vs. Levulinic acid | 88.00 | Yes | p ≤ 0.001 |
| Lactic acid vs. Ethanol | 39.50 | No | ns |
| Acetic acid vs. Furfural | 32.00 | No | ns |
| Acetic acid vs. Glycerin | 32.00 | No | ns |
| Acetic acid vs. HMF | 70.50 | Yes | p ≤ 0.01 |
| Acetic acid vs. Levulinic acid | 79.00 | Yes | p ≤ 0.01 |
| Acetic acid vs. Ethanol | 30.50 | No | ns |
| Furfural vs. Glycerin | 0.000 | No | ns |
| Furfural vs. HMF | 38.50 | No | ns |
| Furfural vs. Levulinic acid | 47.00 | No | ns |
| Furfural vs. Ethanol | −1.500 | No | ns |
| Glycerin vs. HMF | 38.50 | No | ns |
| Glycerin vs. Levulinic acid | 47.00 | No | ns |
| Glycerin vs. Ethanol | −1.500 | No | ns |
| HMF vs. Levulinic acid | 8.500 | No | ns |
| HMF vs. Ethanol | −40.00 | No | ns |
| Levulinic acid vs. Ethanol | −48.50 | No | ns |
ns means p > 0.05 (non-significant).

Table A4.
p-Values for Spearman correlations for the inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
Table A4.
p-Values for Spearman correlations for the inhibitors and biomolecules in the condensate, filtrate, hydrolysates, and fermentation broth.
| Temperature (°C) | Hydrolysis Time (Hours) | Fermentation Time (Days) | Formic Acid | Lactic Acid | Acetic Acid | Furfural | Glycerin | HMF | Levulinic Acid | Ethanol | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrolysis time (hours) | 8.7412 × 10−5 | 0.8678 | 0.7062 | 1 | 0.6064 | 3.4965 × 10−4 | 0.7147 | 0.7354 | 0.0336 | 1 | 0.6818 |
| Fermentation time (days) | 0.8678 | 1.3875 × 10−6 | 0.0065 | 1 | 0.0012 | 0.9647 | 0.1913 | 0.0027 | 0.3502 | 1 | 0.0064 |
| Formic acid | 0.7062 | 0.0065 | 3.0833 × 10−8 | 0.75 | 0.0048 | 0.56752 | 0.0041 | 0.0313 | 0.0214 | 0.75 | 2.1315 × 10−4 |
| Lactic acid | 1 | 1 | 0.75 | 0.0625 | 0.375 | 0.875 | 0.0625 | 0.5 | 0.3125 | 1 | 0.75 |
| Acetic acid | 0.6064 | 0.0012 | 0.0048 | 0.375 | 9.5589 × 10−14 | 0.8497 | 0.1360 | 0.0013 | 0.2251 | 0.25 | 0.0225 |
| Furfural | 3.4965 × 10−4 | 0.9647 | 0.5675 | 0.875 | 0.8497 | 1.9117 × 10−13 | 0.3561 | 0.8456 | 0.0027 | 0.125 | 0.8356 |
| Glycerin | 0.7147 | 0.1913 | 0.0041 | 0.0625 | 0.1360 | 0.3561 | 3.4687 × 10−8 | 0.0165 | 1.1446 × 10−4 | 0.125 | 8.0128 × 10−5 |
| HMF | 0.73548 | 0.0027 | 0.0313 | 0.5 | 0.0013 | 0.8456 | 0.0165 | 5.7353 × 10−5 | 0.1409 | 0.0625 | 0.0028 |
| Levulinic acid | 0.0336 | 0.3502 | 0.0214 | 0.3125 | 0.2251 | 0.0027 | 1.1446 × 10−4 | 0.1409 | 7.6312 × 10−6 | 0.0625 | 0.0213 |
| Ethanol | 1 | 1 | 0.75 | 1 | 0.25 | 0.125 | 0.125 | 0.0625 | 0.0625 | 0.0625 | 0.75 |
| Temperature (°C) | 0.6818 | 0.0064 | 2.1315 × 10−4 | 0.75 | 0.0225 | 0.8356 | 8.0128 × 10−5 | 0.0028 | 0.0213 | 0.75 | 1.9270 |
The concentrations of the inhibitors and biomolecules are presented as g/100 g dry biomass.

Table A5.
Descriptive statistics for sugars in the filtrate, hydrolysates, and fermentation broth.
Table A5.
Descriptive statistics for sugars in the filtrate, hydrolysates, and fermentation broth.
| Temperature (°C) | Hydrolysis Time (Hours) | Fermentation Time (Days) | Glucose | Arabinose | Xylose | Mannose | Galactose | Cellobiose | |
|---|---|---|---|---|---|---|---|---|---|
| Minimum | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.6600 | 0.000 | 0.000 |
| Maximum | 200.0 | 72.00 | 28.00 | 26.51 | 1.300 | 11.94 | 7.200 | 0.9800 | 2.170 |
| Range | 200.0 | 72.00 | 28.00 | 26.51 | 1.300 | 11.94 | 6.540 | 0.9800 | 2.170 |
| Mean | 106.7 | 19.20 | 9.333 | 7.756 | 0.1447 | 4.676 | 2.653 | 0.2713 | 0.4013 |
| Std. Deviation | 103.3 | 27.51 | 10.80 | 10.18 | 0.3229 | 3.983 | 2.303 | 0.3254 | 0.6190 |
| Std. Error of Mean | 26.67 | 7.104 | 2.789 | 2.627 | 0.08337 | 1.028 | 0.5947 | 0.08402 | 0.1598 |
The concentrations of sugars are presented in g/100 g dry biomass.

Table A6.
Results of the normality test (Shapiro–Wilk test) for sugars in the filtrate, hydrolysates, and fermentation broth.
Table A6.
Results of the normality test (Shapiro–Wilk test) for sugars in the filtrate, hydrolysates, and fermentation broth.
| Hydrolysis Time (Hours) | Fermentation Time (Days) | Glucose | Arabinose | Xylose | Mannose | Galactose | Cellobiose | |
|---|---|---|---|---|---|---|---|---|
| W | 0.6434 | 0.7181 | 0.8065 | 0.7510 | 0.4115 | 0.8841 | 0.7699 | 0.7873 |
| p-Value | <0.0001 | 0.0004 | 0.0045 | 0.0009 | <0.0001 | 0.0546 | 0.0015 | 0.0025 |
| Passed normality test (alpha = 0.05)? | No | No | No | No | No | Yes | No | No |
| p-Value summary | p ≤ 0.0001 | p ≤ 0.001 | p ≤ 0.01 | p ≤ 0.001 | p ≤ 0.0001 | p > 0.05 (not significant) | p ≤ 0.01 | p ≤ 0.01 |
The concentrations of sugars are presented as g/100 g dry biomass.

Table A7.
Results of the Dunn’s multiple comparisons test for sugars in the filtrate, hydrolysates, and fermentation broth.
Table A7.
Results of the Dunn’s multiple comparisons test for sugars in the filtrate, hydrolysates, and fermentation broth.
| Mean Rank Difference | Significant? | Summary | |
|---|---|---|---|
| Temperature (°C) vs. Hydrolysis time (hours) | 16.00 | No | ns |
| Temperature (°C) vs. Fermentation time (days) | 26.00 | No | ns |
| Temperature (°C) vs. Glucose | 4.000 | No | ns |
| Temperature (°C) vs. Arabinose | −3.000 | No | ns |
| Temperature (°C) vs. Xylose | −4.000 | No | ns |
| Temperature (°C) vs. Mannose | 18.00 | No | ns |
| Temperature (°C) vs. Galactose | 10.00 | No | ns |
| Temperature (°C) vs. Cellobiose | 14.00 | No | ns |
| Hydrolysis time (hours) vs. Fermentation time (days) | 10.00 | No | ns |
| Hydrolysis time (hours) vs. Glucose | −12.00 | No | ns |
| Hydrolysis time (hours) vs. Arabinose | −19.00 | No | ns |
| Hydrolysis time (hours) vs. Xylose | −20.00 | No | ns |
| Hydrolysis time (hours) vs. Mannose | 2.000 | No | ns |
| Hydrolysis time (hours) vs. Galactose | −6.000 | No | ns |
| Hydrolysis time (hours) vs. Cellobiose | −2.000 | No | ns |
| Fermentation time (days) vs. Glucose | −22.00 | No | ns |
| Fermentation time (days) vs. Arabinose | −29.00 | No | ns |
| Fermentation time (days) vs. Xylose | −30.00 | No | ns |
| Fermentation time (days) vs. Mannose | −8.000 | No | ns |
| Fermentation time (days) vs. Galactose | −16.00 | No | ns |
| Fermentation time (days) vs. Cellobiose | −12.00 | No | ns |
| Glucose vs. Arabinose | −7.000 | No | ns |
| Glucose vs. Xylose | −8.000 | No | ns |
| Glucose vs. Mannose | 14.00 | No | ns |
| Glucose vs. Galactose | 6.000 | No | ns |
| Glucose vs. Cellobiose | 10.00 | No | ns |
| Arabinose vs. Xylose | −1.000 | No | ns |
| Arabinose vs. Mannose | 21.00 | No | ns |
| Arabinose vs. Galactose | 13.00 | No | ns |
| Arabinose vs. Cellobiose | 17.00 | No | ns |
| Xylose vs. Mannose | 22.00 | No | ns |
| Xylose vs. Galactose | 14.00 | No | ns |
| Xylose vs. Cellobiose | 18.00 | No | ns |
| Mannose vs. Galactose | −8.000 | No | ns |
| Mannose vs. Cellobiose | −4.000 | No | ns |
| Galactose vs. Cellobiose | 4.000 | No | ns |
ns means p > 0.05 (non-significant).

Table A8.
p-Values for Spearman correlations for sugars in the filtrate, hydrolysates, and fermentation broth.
Table A8.
p-Values for Spearman correlations for sugars in the filtrate, hydrolysates, and fermentation broth.
| Temperature (°C) | Temperature (°C) | Hydrolysis Time (Hours) | Fermentation Time (Days) | Glucose | Arabinose | Xylose | Mannose | Galactose | Cellobiose |
|---|---|---|---|---|---|---|---|---|---|
| Hydrolysis time (hour) | 1.5540 × 10−4 | 0.9216 | 0.9020 | 0.1504 | 4.6620 × 10−4 | 3.1080 × 10−4 | 1 | 0.3342 | 0.4048 |
| Fermentation time (days) | 0.9216 | 2.2200 × 10−6 | 0.0024 | 1.2876 × 10−4 | 0.281 | 0.1705 | 0.0042 | 0.6404 | 0.0728 |
| Glucose | 0.9020 | 0.0024 | 6.1666 × 10−8 | 0.0014 | 0.0971 | 0.1419 | 0.0012 | 0.8750 | 0.0084 |
| Arabinose | 0.1504 | 1.2876 | 0.0014 | 4.5882 × 10−12 | 0.0083 | 0.0033 | 0.0065 | 0.9860 | 0.0432 |
| Xylose | 4.6620 × 10−4 | 0.2811 | 0.0971 | 0.0083 | 2.2023 × 10−10 | 8.3703 × 10−5 | 0.1595 | 0.1611 | 0.0724 |
| Mannose | 3.1080 × 10−5 | 0.1705 | 0.1419 | 0.0033 | 8.3703 × 10−5 | 1.5294 × 10−12 | 0.3852 | 0.7121 | 0.1122 |
| Galactose | 1 | 0.0042 | 0.0012 | 0.0065 | 0.1595 | 0.3853 | 3.0588 × 10−12 | 0.2724 | 0.0016 |
| Cellobiose | 0.3342 | 0.6404 | 0.8750 | 0.9860 | 0.1611 | 0.7121 | 0.2724 | 3.0833 × 10−8 | 0.4915 |
| Temperature (°C) | 0.4048 | 0.0728 | 0.0084 | 0.0432 | 0.0724 | 0.1121 | 0.0016 | 0.4915 | 4.4047 × 10−10 |
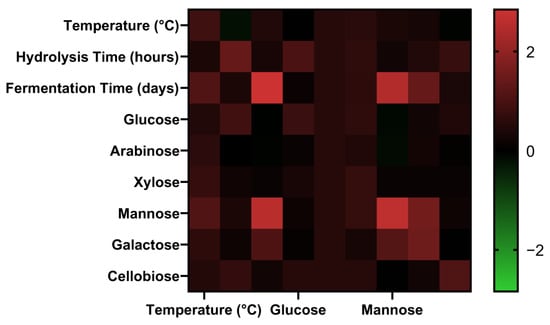
Figure A1.
Heat map: Spearman correlation results.
References
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Rooni, V.; Sjulander, N.; Cristobal-Sarramian, A.; Raud, M.; Rocha-Meneses, L.; Kikas, T. The efficiency of nitrogen explosion pretreatment on common aspen—Populus tremula: N2—VS steam explosion. Energy 2021, 220, 119741. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Arce, C.; Kratky, L. Mechanical pretreatment of lignocellulosic biomass toward enzymatic/fermentative valorization. iScience 2022, 25, 104610. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—A review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, K.; Zhang, Y.; Wang, M.; Yong, C.; Chen, L.; Qu, P.; Huang, H.; Sun, E.; Pan, M. Lignocellulose dissociation with biological pretreatment towards the biochemical platform: A review. Mater. Today Bio 2022, 16, 100445. [Google Scholar] [CrossRef]
- Woźniak, A.; Kuligowski, K.; Świerczek, L.; Cenian, A. Review of Lignocellulosic Biomass Pretreatment Using Physical, Thermal and Chemical Methods for Higher Yields in Bioethanol Production. Sustainability 2025, 17, 010287. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 112937. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Foston, M.; Leisen, J.; DeMartini, J.; Wyman, C.E.; Ragauskas, A.J. Determination of porosity of lignocellulosic biomass before and after pretreatment by using Simons’ stain and NMR techniques. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef]
- Raud, M.; Olt, J.; Kikas, T. N2 explosive decompression pretreatment of biomass for lignocellulosic ethanol production. Biomass Bioenergy 2016, 90, 1–6. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Tutt, M.; Raud, M.; Kahr, H.; Pointner, M.; Olt, J.; Kikas, T. Nitrogen explosion pretreatment of lignocellulosic material for bioethanol production. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1785–1789. [Google Scholar] [CrossRef]
- Raud, M.; Krennhuber, K.; Jäger, A.; Kikas, T. Nitrogen explosive decompression pre-treatment: An alternative to steam explosion. Energy 2019, 177, 175–182. [Google Scholar] [CrossRef]
- Meesters, K.; Elbersen, W.; Van Der Hoogt, P.; Hristov, H. Biomass Pre-Treatment for Bioenergy Case Study 5: Leaching as a Biomass Pre-Treatment Method for Herbaceous Biomass. Sugar Cane Trash and Palm Oil Mill Residues. Pressed, Dried and Milled EFB InterTask Project on Fuel Pretreatment of Biomass Residues in the Supply Chain for Thermal Conversion. 2018. Available online: https://www.ieabioenergy.com/wp-content/uploads/2018/11/CS5-Leaching-as-a-biomass-pre-treatment-method-for-herbaceous-biomass.pdf (accessed on 15 April 2024).
- Fitria; Liu, J.; Yang, B. Roles of mineral matter in biomass processing to biofuels. Biofuels Bioprod. Biorefining 2023, 17, 696–717. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical review of the role of ash content and composition in biomass pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Yan, N. Production of organic acids from biomass resources. Curr. Opin. Green Sustain. Chem. 2016, 2, 54–58. [Google Scholar] [CrossRef]
- IMARC Group. Lactic Acid Pricing Report: Price Trend, Demand & Forecast 2024–2029. 2024. Available online: https://www.imarcgroup.com/lactic-acid-pricing-report (accessed on 1 February 2025).
- Hua, S.; Wang, Y.; Wang, L.; Zhou, Q.; Li, Z.; Liu, P.; Wang, K.; Zhu, Y.; Han, D.; Yu, Y. Regulatory mechanisms of acetic acid, ethanol and high temperature tolerances of acetic acid bacteria during vinegar production. Microb. Cell Factories 2024, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance; conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Ko, J.K.; Enkh-Amgalan, T.; Gong, G.; Um, Y.; Lee, S.M. Improved bioconversion of lignocellulosic biomass by Saccha-romyces cerevisiae engineered for tolerance to acetic acid. GCB Bioenergy 2020, 12, 90–100. [Google Scholar] [CrossRef]
- Wen, L.; Tan, X.; Zhang, D.; Jia, Y.; Sheng, Y.; Lai, C.; Yong, Q. Effect of pretreatment severity on the inhibitory behaviors of larch lignins in enzymatic hydrolysis. Ind. Crops Prod. 2023, 197, 116660. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal pretreatment for the production of oligosaccharides: A review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Yoon, J.; Sim, S.; Myint, A.A.; Lee, Y.W. Kinetics of the hydrolysis of xylan based on ether bond cleavage in subcritical water. J. Supercrit. Fluids 2018, 135, 145–151. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Zhang, T.; Kumar, R.; Wyman, C.E. Sugar yields from dilute oxalic acid pretreatment of maple wood compared to those with other dilute acids and hot water. Carbohydr. Polym. 2013, 92, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, Y.; Lei, T.; Li, Y.; Shen, Y.; Wang, G.; Yang, M.; Wang, Y.; Zheng, H. Regulation of biomass physicochemical properties for fuel quality improvement by pretreatment technology: A review. Energy 360 2025, 3, 100015. [Google Scholar] [CrossRef]
- Gao, D.-M.; Zhang, X.; Liu, H.; Fujino, H.; Lei, T.; Sun, F.; Zhu, J.; Huhe, T. Critical approaches in the catalytic transformation of sugar isomerization and epimerization after Fischer—History, challenges, and prospects. Green Energy Environ. 2024, 9, 435–453. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-Based Hydrolysis Processes for Ethanol from Lignocellulosic Materials: Bioethanol Review. BioResources 2007, 2, 707–738. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Amit, K.; Nakachew, M.; Yilkal, B.; Mukesh, Y. A review of factors affecting enzymatic hydrolysis of pretreated ligno-cellulosic biomass. Res. J. Chem. Environ. 2018, 22, 62–67. [Google Scholar]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, W.; Gleisner, R.; Pan, X.J.; Zhu, J.Y. Comparisons of SPORL and dilute acid pretreatments for sugar and ethanol productions from aspen. Biotechnol. Prog. 2011, 27, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhu, J. An update on Sulfite Pretreatment (SPORL) of lignocellulosic biomass for effective production of cellulose ethanol. In Proceedings of the 16th International Symposium on Wood, Fiber and Pulping Chemistry, Tianjin, China, 8–10 June 2011; Available online: https://www.researchgate.net/publication/267958328 (accessed on 3 July 2025).
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+− dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bellissimi, E.; Van Dijken, J.P.; Pronk, J.T.; Van Maris, A.J.A. Effects of acetic acid on the kinetics of xylose fermentation by an engineered, xylose-isomerase-based saccharomyces cerevisiae strain. FEMS Yeast Res. 2009, 9, 358–364. [Google Scholar] [CrossRef]
- Huang, H.; Guo, X.; Li, D.; Liu, M.; Wu, J.; Ren, H. Identification of crucial yeast inhibitors in bio-ethanol and improvement of fermentation at high pH and high total solids. Bioresour. Technol. 2011, 102, 7486–7493. [Google Scholar] [CrossRef]
- Wyman, C.E. Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wang, B.; Feng, H. Detoxification of lignocellulosic hydrolysates. In Biofuels from Agricultural Wastes and Byproducts; Blaschek, H.P., Ezeji, T.C., Scheffran, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 233–250. [Google Scholar]
- Lynd, L.R.; Liang, X.; Biddy, M.J.; Allee, A.; Cai, H.; Foust, T.; Himmel, M.E.; Laser, M.S.; Wang, M.; Wyman, C.E. Cellulosic ethanol: Status and innovation. Curr. Opin. Biotechnol. 2017, 45, 202–211. [Google Scholar] [CrossRef]
- Agrawal, R. Emerging Technologies for Improvement of Microbial Strains Using Omic Sciences and Measuring Methods for Maximum Product Formation. In Textbook of Industrial Microbiology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 13–39. [Google Scholar]
- Aggarwal, N.; Pham, H.L.; Ranjan, B.; Saini, M.; Liang, Y.; Hossain, G.S.; Ling, H.; Foo, J.L.; Chang, M.W. Microbial engineering strategies to utilize waste feedstock for sustainable bioproduction. Nat. Rev. Bioeng. 2024, 2, 155–174. [Google Scholar] [CrossRef]
- Prasanthan, A.; Krishna Prasad, R.; Balu, M. Recent advancements and challenges in the lignocellulosic and algal biomass-based bioethanol production: A review. Biofuels 2025, 16, 499–518. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Fatima, A.; Manan, S.; Khattak, W.A.; Ullah, M.W.; Yang, G. Potential of Food and Agro-Industrial Wastes for Cost-Effective Bacterial Cellulose Production: An Updated Review of Literature. ES Food Agrofor. 2023, 13, 905. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).