Abstract
The main results highlighted in this article underline the critical significance of hydrogen technologies in the move towards carbon neutrality. This research focuses on several key areas including the production, storage, safety, and usage of hydrogen, alongside innovative approaches for assessing hydrogen purity and production-related technologies. This study emphasizes the vital role of hydrogen storage technology for the future utilization of hydrogen as an energy carrier and the advancement of technologies that facilitate effective, safe, and cost-efficient hydrogen storage. Furthermore, bibliometric analysis has been instrumental in identifying primary research fields such as hydrogen storage, hydrogen production, efficient electrocatalysts, rotary engines utilizing hydrogen as fuel, and underground hydrogen storage. Each domain is essential for realizing a sustainable hydrogen economy, reflecting the significant research and development efforts in hydrogen technologies. Recent trends have shown an increased interest in underground hydrogen storage as a method to enhance energy security and assist in the transition towards sustainable energy systems. This research delves into the technical, economic, and environmental facets of employing geological formations for large-scale, seasonal, and long-term hydrogen storage. Ultimately, the development of hydrogen technologies is deemed crucial for meeting sustainable development goals, particularly in terms of addressing climate change and reducing greenhouse gas emissions. Hydrogen serves as an energy carrier that could substantially lessen reliance on fossil fuels while encouraging the adoption of renewable energy sources, aiding in the decarbonization of transport, industry, and energy production sectors. This, in turn, supports worldwide efforts to curb global warming and achieve carbon neutrality.
1. Introduction
Faced with the growing challenges of climate change and the need to decarbonize global energy systems, hydrogen is emerging as a key element of the future energy mix [1,2,3,4]. Its unique properties, including high energy density and the ability to burn cleanly, open up wide prospects for its use as an energy carrier in various sectors of the economy, such as transport, industry, home heating, and electricity production [5,6,7,8,9,10,11,12]. With hydrogen’s ability to be produced from renewable energy sources such as solar and wind, it can play a central role in the energy transition towards carbon neutrality [13,14,15,16,17,18,19,20,21,22].
Over the last decade, with global interest in the development of hydrogen technologies as one of the key elements of the future, the sustainable energy economy has increased significantly [23,24,25,26,27,28,29]. This is partly a response to the growing need to decarbonize the energy and industrial sectors, as well as to search for effective ways of storing renewable energy [30,31,32,33]. Figure 1 shows low-emission hydrogen production from 2010 to 2023, shedding light on the growing importance of and investment in hydrogen production technologies that minimize carbon emissions. The R-squared value of 88% indicates that the estimated exponential regression is statistically of a good quality and can satisfactorily represent low-carbon hydrogen production within this 14-year interval. These data highlight the dynamic development of hydrogen technologies and their potential to contribute to global climate goals, but at the same time, it should be noted that the full commercial use of hydrogen as an energy carrier still requires much investment and research due to its technological and economic challenges [34,35]. The most important include the need for the development of effective and cost-competitive methods of hydrogen production, especially those using green energy; the development of safe, efficient, and sustainable hydrogen storage and transport systems; and the adaptation of the existing energy infrastructure to use hydrogen as an energy carrier. Additionally, on the way to the wider implementation of hydrogen technologies is the need to overcome regulatory barriers, build social acceptance for new technologies, and ensure appropriate investments in research and development [36,37,38,39,40,41,42].
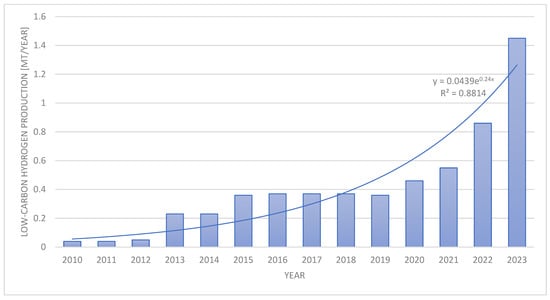
Figure 1.
Low-carbon hydrogen production [43].
The development of hydrogen technologies and their applications in energy and environmental engineering are the subject of intensive scientific research, which is reflected in the growing number of publications. In this context, bibliometric analysis is a valuable tool for understanding research trends, mapping the field of knowledge, and identifying key countries, collaborations, and potential development directions [44,45,46,47]. Using the CiteSpace program 6.2.R6 Advanced to conduct such an analysis allows the visualization of bibliometric data, which is invaluable in the process of synthesizing the current state of knowledge and in identifying research gaps and new research opportunities [48,49,50,51,52,53].
In order to understand the role of hydrogen in future energy systems, this article undertakes a bibliometric analysis of publications from the Web of Science database, focusing on the energy industry and environmental engineering [31,54,55,56]. This analysis covers publications from the last ten years, making it possible to identify the evolution of interest in hydrogen and the main research areas, as well as to predict future trends in this field [57,58,59,60,61,62].
2. Methodology
In the first step of the bibliometric analysis, a database of publications was downloaded from the Web of Science. For this purpose, the keyword “hydrogen” (hydrogen) was used, which was the basis for identifying publications related to research on hydrogen as an energy source, its applications in the energy industry, and its impact on the environment. Then, to ensure the specificity and relevance of the results, the search was limited to the “Energy and Fuels” and “Environmental Sciences” categories. This selection of categories allowed us to focus on publications that directly concern energy and environmental issues in the context of the use of hydrogen, taking into account both technological and ecological aspects. Additionally, a time period was defined from 2014 to 2024, which enabled the analysis of trends and research developments in the last decade. The choice of this time frame was motivated by the desire to understand how interest in hydrogen has evolved over recent years, especially in the context of global efforts for sustainable development and the fight against climate change [63,64].
In order to conduct a comprehensive bibliometric analysis of publications from the energy and environmental engineering industries included in the Web of Science database, the CiteSpace program was used [65,66,67,68]. Bibliometric analysis, as a method of quantitative research of scientific literature, allows the identification of the main trends; patterns of cooperation between authors, institutions, and countries; key topics; and dynamically developing fields. In this context, CiteSpace was used to visualize and analyze trends and patterns in bibliometric data [69,70,71,72,73,74]. This program enables the generation of collaborative networks, heat maps that indicate the intensity of research in given areas, and the identification of keywords indicating new and emerging research directions. CiteSpace is characterized by the ability to analyze data from various periods of time, which allows you to observe the evolution of a given scientific field and identify trends and changes in the flow of knowledge [75,76,77,78,79,80,81,82]. By using algorithms to detect the most frequently cited and co-occurring terms, this tool significantly supports the process of identifying areas with high development dynamics and potential directions for future research. This methodology, based on solid scientific foundations and highly developed analytical tools such as CiteSpace, provides scientists with valuable tips on the state of research in selected fields, enabling a deeper understanding of the structure and dynamics of science development in the context of global energy and environmental challenges [83,84,85,86,87,88,89,90].
Bibliometric analysis using CiteSpace includes a number of steps that allow for the effective identification, visualization, and analysis of research trends and key works in a given field of science. The detailed procedure for such an analysis is presented below:
- Determining the thematic and time scope of the analysis:
- Selection of keywords and phrases corresponding to an interesting research field;
- Determining the time frame of the publications to be analyzed.
- Collecting data from the Web of Science database:
- Conducting a search in the Web of Science database using previously defined keywords;
- Exporting search results to files that can be processed by CiteSpace.
- CiteSpace configuration and performing simulations:
- Importing exported data from Web of Science to CiteSpace;
- Setting analysis parameters such as time period, nodes (e.g., authors, institutions, keywords), and types of analysis (e.g., clustering, co-occurrence);
- Generating network maps that visualize relationships between authors, institutions, keywords, etc.;
- Analysis of the cluster structure, identification of main research areas and key works.
- Trend and keyword analysis:
- Leveraging CiteSpace tools to identify growing trends and hot topics in a given field;
- Analysis of keywords and their evolution over time, which allows you to understand changes in research areas.
- Identification of key authors, works, and institutions:
- Analyzing the centrality and power of nodes in the network to identify the most influential authors, most cited works, and leading research institutions.
- Interpretation of results:
- Interpreting visual network maps and statistical data to infer trends, research gaps, and potential future research directions.
- Preparation of a report or publication:
- Organizing the collected data and conclusions into a logical and coherent structure of a report or scientific article;
- Including network visualizations as elements illustrating key analysis conclusions.
- Discussion and conclusions:
- Discussion of the importance of discovered trends, key authors, and works for the further development of the researched field;
- Indication of potential research directions resulting from the bibliometric analysis.
The following research theses were formulated in this paper:
- “What are the main directions of research on the use of hydrogen in the energy industry and environmental engineering in the last decade?”
- “What are the key challenges and barriers in hydrogen research?”
- “How does the development of hydrogen technologies affect the achievement of sustainable development goals, especially in the context of climate change and the reduction of greenhouse gas emissions?”
3. Results
Figure 2 shows the number of publications used for analysis in individual years. The total number of analyzed publications is 26,169.
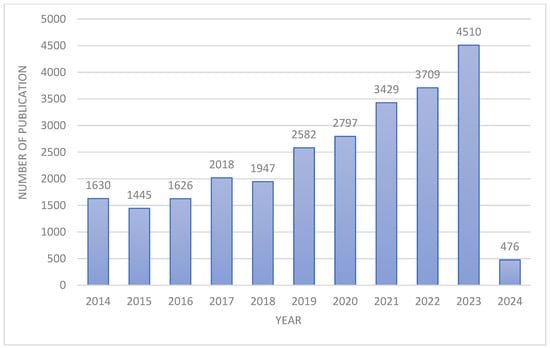
Figure 2.
Number of publications from 2014 to February 2024.
The first stage of the bibliometric analysis is the analysis of international cooperation in hydrogen research. The aim of this approach is to identify key countries and their role in the global scientific network, which will allow an understanding of how individual countries contribute to progress in hydrogen research. By analyzing the number of publications assigned to each country, it is possible to determine which countries are most active in generating new knowledge and innovation. The centrality index is also an important indicator in the analysis. The centrality indicator is a key element of network analysis, allowing the assessment of the role of individual nodes in the network of international cooperation in the context of renewable energy sources and environmental engineering. This metric mathematically expresses how often a given node (in this case, a country) appears on the shortest paths between pairs of other nodes in the network. The higher the value of the indirect centrality of a given country, the greater the role it plays in mediating and transferring knowledge between other countries, which may indicate its importance as a mediator or a key point of information flow in the global scientific network.
In the context of international cooperation, countries with high intermediate centrality often constitute key nodes in the network, integrating knowledge from different regions and disciplines. Such countries can have a significant influence on research directions and the development of new technologies because their position in the network allows them to act as main channels of communication and cooperation. Analyzing this indicator can also help in identifying potential barriers to science communication and suggesting areas where increased collaboration could contribute to greater research integration and efficiency.
The interpretation of the indirect centrality index in the bibliometric analysis of international cooperation allows for a deeper understanding of the network structure and dynamics of knowledge flow in the field of renewable energy sources. This enables researchers and policymakers to better understand how collaborative strategies and research investments can be directed to maximize scientific and innovation impact, and to identify leaders and key players in global scientific collaboration. The results of the analysis are presented in Figure 3. The size of a given node in the figure corresponds to the number of publications from a given country.
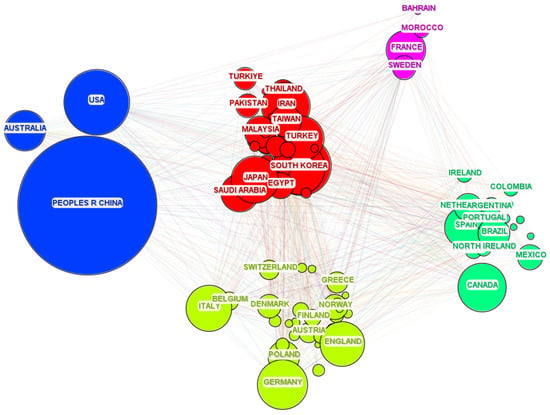
Figure 3.
Cooperation between countries.
Table 1 shows the top 10 countries with the highest centrality index and the number of publications.

Table 1.
Number of publications and centrality by country.
Analysis of the presented data indicates a clear dominance of China in the field of hydrogen research, both in terms of the number of publications and the centrality index. The significant number of publications (10,913) and the highest centrality index (0.24) demonstrate China’s strong position as a leader in this field, highlighting its key role in shaping global knowledge and innovation in the energy sector. The United States ranks second with significantly fewer publications (2312) but still a high centrality index (0.15), suggesting its important role as a center for knowledge exchange and international cooperation. European countries such as France, Germany, England, and Spain, although they have fewer publications compared to China and the USA, still maintain relatively high centrality indices, which indicates their significant participation in the international cooperation network and information flow. In turn, Brazil, Iran, India, and Australia, although they have lower centrality indices, their share in global scientific production proves the growing role of these countries in research on renewable energy sources. Overall, these data highlight that leaders in renewable energy research are also key nodes in global collaboration networks, acting as mediators and hubs in the flow of knowledge. The high number of publications and the centrality index of China suggest its dominant role in creating new knowledge and technologies in this field, while the strong presence of countries from both Europe and other regions of the world proves the global nature of research and cooperation in the hydrogen sector.
The next stage of the analysis will be a detailed examination of cooperation between universities in the area of hydrogen research, a key component in future renewable energy technologies. In this context, the analysis will focus on mapping academic collaboration networks, identifying leading research centers and assessing how these institutions contribute to innovation and knowledge development in the field of hydrogen technologies.
The analysis of the centrality index will allow us to determine which universities act as key nodes in the flow of information and knowledge between various research groups, which is important for promoting interdisciplinary and international research projects. Such analysis is expected to reveal a comprehensive network of collaborations among academic institutions that play a significant role in hydrogen research. Identifying universities that are leaders in generating knowledge and innovation in this field will help us to understand which institutions have the greatest impact on the development of hydrogen technologies and which academic partnerships may be most fruitful in accelerating progress in this critical field for the future of energy. This analysis will also be valuable for science policy and investment decisions as it can provide strategic directions for further supporting the research and development of hydrogen technologies. By identifying institutions that play a central role in the collaborative network and have a significant impact on innovation, it will be possible to more effectively direct funds and initiatives for hydrogen research, which is key to accelerating the transition to sustainable energy sources. The results are presented in Figure 4.
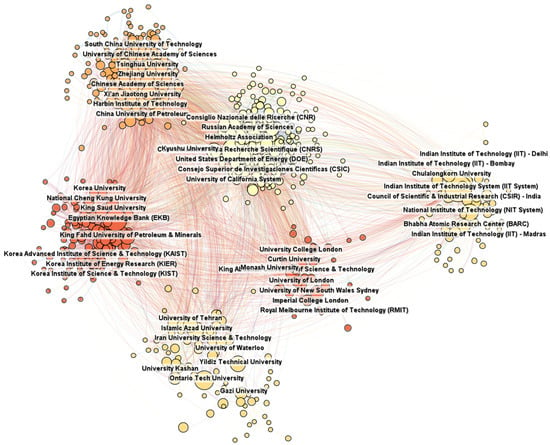
Figure 4.
Cooperation between universities.
Based on the analysis presented in Figure 4, Table 2 presents the top 10 institutes with the highest centrality index and the number of their publications.

Table 2.
Number of publications and centrality by institutes.
The dominant position of the Chinese Academy of Sciences, with the largest number of publications and the highest centrality index, highlights its central role in generating new knowledge and in the scientific cooperation network, demonstrating China’s strong commitment to research and development. At the same time, the high activity of European institutes, such as the Center National de la Recherche Scientifique and the Helmholtz Association, and American ones, such as the United States Department of Energy, indicates their important role in international cooperation and contribution to the global development of knowledge. The significant number of publications by institutes from developing countries, such as the Egyptian Knowledge Bank and the Indian Institute of Technology System, indicates the growing contribution of these regions to world science.
Figure 5 shows the analysis of institutes in terms of the “citation burst” indicator and the years in which this indicator was the highest. The “citation burst” indicator, which identifies periods of a sudden increase in the number of citations received by publications, researchers, or institutes, indicates moments when their works suddenly gain importance or are considered particularly influential in a given field of science. The interpretation of this indicator for institutes may reflect the moment when an institution’s research begins to be perceived as groundbreaking, which often involves the publication of high-quality research that answers important scientific questions or introduces innovative technologies. Institutes that achieve high citation burst values often gain recognition and prestige, which may attract the attention of other researchers and potential collaborators, as well as increase their chances of obtaining funding for future research projects. Additionally, this indicator may signal changes in research trends or growing interest in specific research topics, identifying institutes that may be leaders in shaping new research directions.
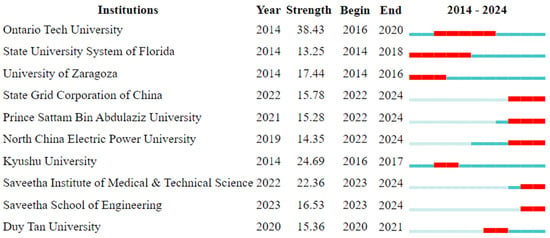
Figure 5.
Top 10 institutions with strongest citation burst.
Institutes with the highest “citation burst”, i.e., a sudden increase in the number of citations, may differ from those with the highest “centrality” indicator for several reasons that result from differences in the nature and interpretation of both indicators. Citation burst measures short-term interest in a particular institute’s work, which may indicate breakthrough discoveries or publications that are currently gaining popularity. In turn, the “centrality” indicator refers to the position of the institute in the scientific cooperation network, showing how important a role it plays in the flow of information and knowledge in a given field. An institute with a high centrality index may not experience equally high citation growth in the short term, but it plays an ongoing, key role in the scientific network. Institutes with the highest citation burst can be recognized for specific, significant scientific contributions in a short period of time that attract the attention of the scientific community. Institutes with a high “centrality” index, on the other hand, can be considered long-term influential, having stable and lasting connections in the scientific network that contribute to the development of the field over the years.
Differences may also result from the specificity of the research. Institutes that achieve high citation bursts often do so because of specific research that is attracting a lot of attention at the moment. On the other hand, a high “centrality” index may reflect the institute’s wide network of cooperation and influence in various research areas, which is not necessarily related to a specific, individual publication. Research trends can also change quickly, which affects the citation burst of individual institutes. Institutes that have focused their research on current hot topics may experience a sudden increase in citations. The “centrality” indicator, on the other hand, is more stable and reflects the long-term position of the institute in the scientific network, which does not always respond quickly to changes in trends. To sum up, the differences between institutes with the highest “citation burst” and those with the highest “centrality” indicator result from different aspects of scientific impact that these indicators measure. “Citation burst” refers to short-term interest in an institute’s work, while “centrality” indicates the long-term, central role of the institute in the network of scientific cooperation.
The next stage will be citation analysis, which is a key element in bibliometric research. By focusing on citations in the scientific literature, you can identify the most influential works, authors, and research directions that shape the development of a given field. This analysis allows for the selection of key works that constitute the foundation for current research. In the context of research on hydrogen technology, citation analysis will enable an understanding of which technologies or research methods dominate in the scientific literature. This method allows you to build solid foundations for future research, identifying those works that are most important for the development of a given field. The result of the reference analysis is presented in Figure 6.
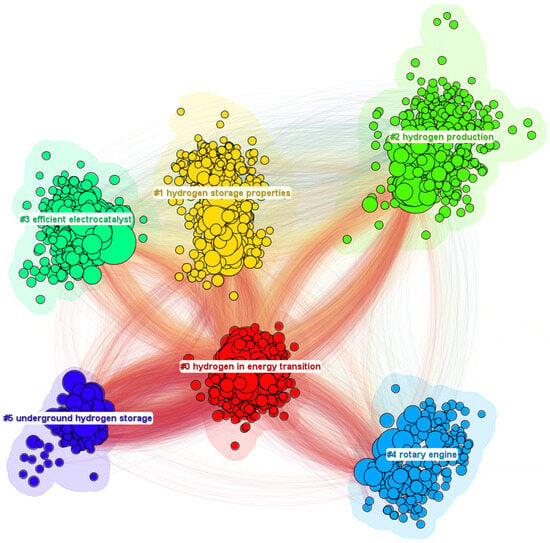
Figure 6.
Main areas of scientific interest.
Table 3 shows the silhouette values for the nodes shown in Figure 6. In CiteSpace, “silhouette value” is a measure used to assess the consistency and quality of clusters generated during bibliometric analysis. The silhouette value ranges from −1 to 1, where a value closer to 1 indicates high internal coherence of clusters and well-separated clusters from each other, meaning that elements within a cluster are more similar to each other than to elements from other clusters. In contrast, a value closer to −1 suggests that the clusters are poorly defined and may contain elements that would fit better into other clusters.

Table 3.
Silhouette values for the analyzed clusters.
Interpreting the silhouette value allows you to assess the quality of the clustering and identify those clusters that contain well-correlated works, authors, or topics. A high profile value for a given cluster indicates strong thematic coherence and may indicate areas that are currently well developed and have a clear research direction. In contrast, clusters with a low figure value may require further analysis to understand why they are less coherent, which may suggest interdisciplinary relationships or new research topics that have not yet crystallized into fully consolidated research areas.
In practice, the silhouette value is used as an indicator to optimize the grouping process in CiteSpace, allowing for a better understanding of the structure of bibliometric data and the identification of significant trends and research gaps in a given field of science. This is particularly useful in analyses that aim to map scientific research fronts, identify key actors, and assess progress in particular research areas. In the analyzed nodes, all silhouette values are above 0.7, which means there is high consistency in all clusters.
The next stage of the analysis will be to examine in what years particular research topics received the most attention. The result of the analysis is shown in Figure 7. The analysis was carried out from 2004 onwards.
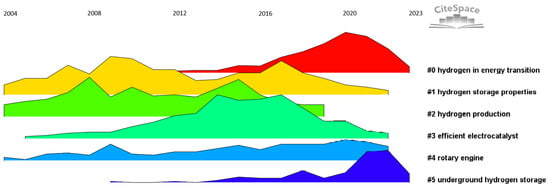
Figure 7.
Timeline of main areas of scientific interest.
Analyzing Figure 7, it can be seen that in the hydrogen in energy transition cluster (#0), the greatest interest occurred in the years 2019–2022, where this trend increased to the highest level compared to other clusters. The storage properties of hydrogen (#1) were most discussed around 2007, after which interest gradually decreased. Hydrogen production (#2) shows a stable trend with a slight increase in recent years. High-performance electrocatalysts (#3) enjoyed increased interest around 2013. Rotary engines (#4) were the most frequently discussed in 2004–2007. Underground hydrogen storage (#5) has gained popularity in recent years, with the most publications devoted to this topic in 2023.
The largest cluster resulting from the citation analysis is “hydrogen in energy transition”, which reflects the current interest and intensity of research in the field of hydrogen production, a key element in the transition to green energy. Hydrogen has the potential to play a key role in the energy transition, being a clean and versatile fuel that has the potential to decarbonize various sectors of the economy, including transport, industry, and energy production, enabling the transition to more sustainable and green energy sources. Table 4 shows three publications from the first cluster with the highest citation burst index. The value of this indicator and the corresponding time period help us to understand when and for how long scientific work had a particularly significant impact on a given area of research.

Table 4.
Top 3 publications from the #0 cluster with the highest citation burst index.
The first article presents a detailed analysis of hydrogen technologies and their applications in energy systems, focusing on aspects such as hydrogen production, storage, re-electrification, and conversion. Particular attention was paid to the potential of hydrogen as a key element in the energy transition towards a carbon-neutral society. The authors provide an overview of current and emerging technologies, with an emphasis on performance, durability, and cost. They note that current research is focused on optimizing water electrolysis processes and exploring new materials and catalysts that can reduce costs and increase the efficiency of hydrogen production. The discussion also covers the challenges related to hydrogen storage, including the development of technologies such as storage in liquid, solid, and high-pressure gas tanks. The article also highlights the importance of integrating hydrogen systems with existing energy networks and the potential of hydrogen in mobile and stationary applications, including transport and cogeneration. In light of these analyses, the authors point to a growing trend towards sustainable and economically feasible hydrogen solutions, emphasizing the need for further research and development to overcome existing technological and economic barriers [91]. The second article highlights key aspects of the development of hydrogen technologies as an essential element in the transition to sustainable energy sources. In the context of increasing energy demand caused by economic and demographic growth, the article highlights problems related to the excessive use of fossil fuels, such as increased greenhouse gas emissions and resource depletion. The authors argue for a global transition from conventional to renewable energy sources, pointing to hydrogen as a potential solution for using renewable energy thanks to its energy storage and transport capabilities. The work provides a critical analysis of hydrogen production methods, such as electrolysis powered by renewable energy (green hydrogen) and conventional methods with CO2 capture and storage (blue hydrogen), and discusses the use, storage, transport, and distribution of hydrogen. A comparative analysis of different hydrogen production systems based on renewable and non-renewable energy sources takes into account factors such as cost, global warming potential (GWP), infrastructure, and efficiency. The work also highlights key challenges and opportunities related to the commercial implementation of hydrogen-based systems, including the issue of storing hydrogen due to its low density and high production costs. In conclusion, the article points to hydrogen as a promising energy vector supporting decarbonization and sustainable development, highlighting the need for further research and technological innovation to enable the commercialization and full use of hydrogen’s potential in various sectors, including transport and energy production [92]. The third article discusses the role of hydrogen in variable renewable energy (RE) storage to achieve a fully renewable and sustainable hydrogen economy, presenting a hydrogen energy system covering hydrogen production, storage, safety, and utilization. This system was presented as four stages of an integrated whole, demonstrating the interconnections and dependencies between these stages. The hydrogen production route and the choice of specific technologies depend on the type of energy and raw material available and the required purity of the final product. Even though end-use hydrogen is a zero-carbon energy, its purity depends on the production path and the energy used to produce it, so guaranteeing the origin of hydrogen is essential for it to be considered clean energy. The article introduces an innovative model as a hydrogen purity index for further research and development. The article also explores the impact of continued global population and economic growth and rapid urbanization on increased energy demand, highlighting the need to decarbonize the energy supply through the use of clean, sustainable, and renewable energy sources. A key challenge in the transition to 100% RE is the variable and intermittent nature of these resources, requiring technical adaptations, especially in terms of balancing variable supply and varying energy demand. The increase in renewable energy penetration in current energy systems raises the need for large-scale energy storage systems to cope with the variable nature of RE sources. An elegant solution for storing RE is to use an energy carrier such as hydrogen, which is storable, transportable, and usable. Hydrogen can be produced from a wide range of resources using different pathways and technologies, including fossil fuels and RE resources. The paper explores the potential of hydrogen energy storage systems (HydESSs) as a cost-effective solution for large-scale RE energy storage, transportation, and export, pointing to HydESSs as a leader on the journey to a 100% renewable energy economy. The literature review shows that while a fully hydrogen economy is still under debate, it is beginning to gain potential. However, it was emphasized that most of the literature does not yet link storage technologies with the end use of hydrogen and specific safety guidelines with production routes. This review provides an overview of the role of hydrogen in the energy sector and the transition towards fully renewable, sustainable, green energy sources, integrating research findings on hydrogen production pathways and related technologies in the broader context of HydS, while limiting the discussion to hydrogen production for the energy sector only [93].
The second-largest cluster in the analyzed case is “hydrogen storage properties”, which emphasizes the importance of hydrogen storage technology for the future use of hydrogen as an energy carrier. The focus on hydrogen storage properties reflects the need to develop effective, safe, and economic methods for storing hydrogen to facilitate its widespread use in various sectors, including transport, industry, and energy. The development of these technologies is key to realizing the vision of a hydrogen-based economy. Table 5 shows the three publications from this cluster with the highest citation burst.

Table 5.
Top 3 publications from the #1 cluster with the highest citation burst index.
The first article presents hydrogen as an ideal energy carrier considered for future transport applications, with an emphasis on hydrogen storage as one of the key challenges in developing the hydrogen economy. According to the authors, storage methods such as high-pressure gas or liquid hydrogen do not meet future storage goals, which is why methods of storing hydrogen in materials through chemical or physical bonds are being investigated. Intensive research has been conducted on metal hydrides, which show potential in improving hydrogenation properties. The review presents the latest developments in the field of metal hydrides, including their hydrogen storage capacity, kinetics, cycling behavior, toxicity, and response to pressure and temperature. The magnesium-based hydride group is emerging as a promising candidate for a competitive hydrogen storage material with reversible hydrogen capacity up to 7.6 wt% for on-board applications. These activities are focused on lowering the desorption temperature of these materials, improving kinetics and cyclic life. The kinetics were improved by adding an appropriate catalyst to the system and by ball milling, which introduces defects that improve surface properties. The authors report promising results such as improved kinetics and lower decomposition temperatures; however, current materials are still far from achieving commercial goals for their transportation applications, so further research is needed to achieve goals through improvements in the hydrogenation, thermal, and hydride cycling behavior of the metal [94]. The second article focuses on research on complex hydrates as hydrogen storage materials, with particular emphasis on their application in transport. It includes a detailed analysis of alanates, amides, and borohydrides, their synthesis, atomic structure, dehydrogenation, and rehydrogenation reactions, and application prospects. In particular, alanates such as NaAlH4 are considered promising due to their relatively high hydrogen storage capacity and moderate rehydrogenation conditions, although they require improvement to meet all practical application requirements. Amides and borohydrides, although they have high hydrogen density, have their limitations related to the kinetics and thermodynamics of the reaction. The latest research results indicate that the development of effective materials for hydrogen storage requires further optimization of their properties, including improving the kinetics of dehydrogenation and rehydrogenation reactions and increasing the life cycle and safety. Despite intensive research, none of the materials currently under investigation, including Ti-doped NaAlH4, meet all the criteria necessary for practical applications yet, suggesting the need for further research to address the thermodynamic, kinetic, and cyclic capacities and safety issues. With respect to the latest research results, it is clear that despite progress, there are still significant challenges to overcome before the commercialization of complex hydrates as hydrogen storage materials. Further research is needed to understand and improve the interactions between hydrides and various admixtures and to increase their durability and performance under various operating conditions [95]. The third article provides an overview of potential magnesium-based hydrogen storage materials, highlighting their importance as promising hydrogen storage candidates, especially for transportation applications. It was emphasized that magnesium and its hydrates show promising properties due to their high hydrogen storage capacity and relatively low costs. However, the paper also highlights significant challenges associated with the use of these materials, such as the high temperature needed to release hydrogen and the slow reaction kinetics. The article highlights various strategies to improve the properties of magnesium as a hydrogen storage medium, including the addition of catalysts, nanocrystalline modifications by ball milling, and the formation of composites with other metals and oxides. Despite progress, these materials still do not meet all the required criteria for practical use, indicating the need for further research. To sum up, although magnesium and its hydrides are considered one of the most promising materials for hydrogen storage, further work is necessary to improve their thermal, kinetic, and cyclic properties. The paper suggests that future research should focus on the development of new catalysts, surface modification techniques, and composites to overcome these challenges and bring magnesium closer to practical applications in hydrogen storage [96].
The next cluster in the bibliometric analysis, “hydrogen production”, highlights the significant interest in and intensity of research on hydrogen production methods. This is a key area for achieving a sustainable hydrogen economy, as efficient and economical hydrogen production methods are the foundation for the widespread use of hydrogen as a clean energy source. Articles with the highest citation burst are presented in Table 6.

Table 6.
Top 3 publications from the #2 cluster with the highest citation burst index.
The first article offers a comprehensive look at current and emerging hydrogen production technologies, focusing on both fossil fuel and renewable biomass methods. The article begins by presenting the motivation for searching for alternative fuels and points to hydrogen as a potential solution to energy and environmental problems related to conventional energy sources. The authors discuss various methods of hydrogen production in detail, including hydrocarbon reforming (steam, partial oxidation, autothermal), pyrolysis, plasma reforming, water reforming (aqueous phase reforming), and methods that do not decompose hydrocarbons, such as biomass gasification, biological processes (including dark fermentation and photofermentation), and methods of producing hydrogen from water, such as electrolysis and photoelectrolysis. In the section on the production of hydrogen from hydrocarbons, they highlight challenges such as the need to desulfurize the feedstock and avoid the formation of coke, which is crucial to maintaining the efficiency of the catalysts and the durability of the process. They also point to the development of technologies such as plasma reforming and water reforming, which may offer lower operating temperatures and lower emissions. The part devoted to the production of hydrogen from biomass highlights the potential of biomass as a sustainable source of hydrogen, but also highlights the technological and economic difficulties associated with its processing. Various approaches to biological hydrogen production are discussed, from dark fermentation to photofermentation processes, with an emphasis on their current limitations and potential development paths. In the section on hydrogen production from water, the authors present electrolysis as a well-developed technology, but also highlight newer methods such as photoelectrolysis and thermochemical water splitting, which have the potential to change the energy landscape if their current technical and economic challenges are overcome. Overall, the paper presents a promising but complex hydrogen production technology landscape, highlighting the need for further research and development to achieve economically feasible, efficient, and environmentally friendly hydrogen production methods. The development of these technologies is crucial for the transition to a low-carbon economy and can help reduce dependence on fossil fuels and reduce greenhouse gas emissions [97]. The second article focuses on the potential of hydrogen separation technology using titanium dioxide (TiO2) nanograins for the production of hydrogen from solar sources. They point out that the current efficiency of converting solar energy into hydrogen is too low to make the technology economically viable. The main obstacles are the fast recombination of the electron–hole pair generated by light, feedback reactions, and the weak activation of TiO2 by visible light. In response to these problems, researchers are focusing on effective repair methods, including the addition of sacrificial reagents and carbonate salts to prevent rapid recombination and back reactions, and the modification of TiO2 through metal loading, ion doping, dye sensitization, formation of composite semiconductors, anion doping, and metal ion implantation. The authors review the latest developments in the above-mentioned technologies used for photocatalytic hydrogen production using TiO2. They state that doping with metal ions and sensitization with dyes are very effective methods for extending the activation spectrum to the visible range, which plays an important role in the development of effective photocatalytic hydrogen production. In conclusion, although hydrogen separation technology using TiO2 nanograins is promising due to its low cost and environmentally friendly aspects, there are many challenges that need to be overcome to make it economically feasible. Key areas include increasing the efficiency of converting solar energy into hydrogen, developing techniques to reduce the rate of electron and hole recombination, and making better use of visible light. Further research and development in these areas is necessary to accelerate the commercialization of this technology and contribute to the development of the hydrogen economy [98]. The third article provides an in-depth review of bioethanol reforming processes as a method for producing hydrogen from renewable sources. Bioethanol, produced mainly by biomass fermentation, is considered an attractive and promising renewable energy source due to its relatively high hydrogen content, availability, non-toxicity, and safe storage and handling. Reforming bioethanol, especially through steam and autothermal reforming processes, is a promising method for hydrogen production. These processes vary in terms of conversion efficiency and operating conditions. Steam reforming is more common due to its higher conversion efficiency, but it requires an external energy source to perform the endothermic reaction. On the other hand, autothermal reforming combines the features of steam reforming and partial oxidation, offering potential benefits such as lower external energy requirements and longer operational stability. The article also highlights the key role of catalysts in bioethanol reforming processes. Rhodymium (Rh) and nickel (Ni) have been distinguished as the most effective catalysts, but the selection of the appropriate support and catalyst preparation method significantly affects their activity. The development of bimetallic catalysts and two-bearing reactors is a promising research direction to increase hydrogen production and the long-term stability of the catalysts. The authors point out that although reforming bioethanol to produce hydrogen is in the early stages of research and development, the process shows great promise as a future application in fuel cells. However, to fully exploit the potential of bioethanol, further work is needed on the optimization of reforming processes and the development of catalysts and reactor technologies. To sum up, reforming bioethanol presents itself as a promising path to the production of clean and renewable hydrogen, crucial for the sustainable development of the hydrogen economy. Success in this field will depend on further advances in research on catalysts, processes, and reactor technologies to overcome current technological and economic challenges [99].
The next cluster is “efficient electrocatalyst”, which focuses on developing catalysts that enhance the efficiency of electrochemical reactions. This area is critical for various applications, including fuel cells, electrolysis for hydrogen production, and environmental remediation. Efficient electrocatalysts aim to lower energy consumption, increase reaction rates, and improve overall system sustainability. Research in this cluster likely explores materials science, nanoengineering, and surface chemistry to innovate catalysts with superior activity, durability, and selectivity. Table 7 shows the top three articles from this cluster.

Table 7.
Top 3 publications from the #3 cluster with the highest citation burst index.
The first article presents an overview of the technology for producing hydrogen from bioethanol, emphasizing that reforming bioethanol is a promising method of producing hydrogen from renewable sources. The authors point out that, apart from operational conditions, catalysts play a key role in the production of hydrogen by reforming ethanol, especially Rh and Ni, which are currently the best and most commonly used catalysts for reforming steam ethanol to produce hydrogen. They also emphasize that the appropriate selection of the catalyst support and the catalyst preparation methods significantly affect the activity of the catalysts. The authors suggest that the development of double-metal alloy catalysts and double-bed reactors may be promising to increase hydrogen production and long-term catalyst stability. Autothermal reforming of bioethanol has the advantages of lower external heat requirements and long-term stability, but its overall efficiency needs to be further improved because part of the ethanol feedstock is used to provide low-grade thermal energy. The development of a reactor with a contact time of milliseconds is presented as a cheap and effective method for reforming bioethanol and hydrocarbons to modernize fuels. Although this is an area of early research and development, reforming bioethanol into hydrogen shows promising prospects for future fuel cell applications [100]. The second article, examining various technological aspects related to water electrolysis as a hydrogen production method, delves into the challenges and advances in alkaline electrolysis and other electrolytic technologies. This paper examines the effects of operating conditions, selection of electrode materials, electrolyzer design, and electrolyte optimization on the performance of the electrolysis process in detail. It emphasizes that key factors such as temperature, pressure, electrolyte composition, and electrode surface characteristics have a significant impact on the rate and efficiency of hydrogen production. Particular attention is paid to the development of new electrode materials that are characterized by improved catalytic activity, durability, and corrosion resistance in alkaline environments. These advances include the use of advanced materials such as modern alloys and metallic composites, as well as nanostructured materials and noble metal catalysts. These changes are aimed at reducing the overpotential of the electrodes and increasing the speed of electrochemical reactions, which directly translates into increasing the energy efficiency of the electrolysis process. Moreover, the article discusses the optimization of the electrolyte composition in detail, emphasizing how the proper selection of ingredients, such as buffering additives or complexing compounds, can effectively reduce ionic resistance and improve diffusion, which is crucial for more effective and faster electrolysis. Techniques for managing the generation and release of gas bubbles are also being explored, which can help minimize disruptions in current flow between electrodes and increase the overall efficiency of electrolysis. In light of this research, the authors of the article point to significant progress in the field of water electrolysis, while emphasizing that there are many challenges to overcome, including the need to further reduce costs, improve durability, and increase the efficiency of hydrogen production. Therefore, continued research and the development of innovative solutions are necessary to fully exploit the potential of water electrolysis as a key technology for sustainable and ecological hydrogen production [101]. The third article presents an in-depth study on hydrogen generation using semiconductor photocatalytic materials. This work discusses the basic principles of photocatalytically generating hydrogen, including the fundamental mechanisms and main processes in photocatalytically generating hydrogen, such as light absorption by the semiconductor, the generation of electron–hole pairs, their recombination, separation, migration, and charged particle transfer and trapping. The authors analyze various approaches to modifying the band structure in order to collect visible light and effectively separate the generated charges, which is crucial for improving the efficiency of photocatalysis. The study focuses on various strategies to increase the efficiency of photogenerated charge generation and their separation, including the use of co-catalysts, semiconductor combinations, and modification of the crystal structure and morphology. Various photocatalytic hydrogen generation systems are also presented, including systems using sacrificial reagents and total water-splitting systems. Various methods for assessing the performance of photocatalysts are also presented, including photocatalytic activity and photocatalytic stability, and an overview of UV-active photocatalytic materials is presented, divided into four groups based on their electronic configuration. In summary, this review provides a comprehensive look at the current state of knowledge on hydrogen generation via semiconductor photocatalysis, with an emphasis on the performance achievements of visible light photocatalysts and their modification strategies. From the perspective of further research in the field of semiconductor photocatalysis for hydrogen generation, the key challenges are increasing the efficiency of visible light absorption and improving the durability and stability of photocatalysts. It is also important to discover new, cheaper photocatalytic materials that can be produced on a large scale. New methods for the synthesis of photocatalysts should be developed, which will enable precise control of their structure and properties. Furthermore, understanding the interfaces between the photocatalyst and the reaction medium can help in the design of better photocatalytic systems. Integrating photocatalytic systems with existing technologies for energy collection and use may also open new opportunities for the practical application of photocatalysis in hydrogen production. Finally, interdisciplinary collaboration between chemists, materials engineers, physicists, and energy engineers will be essential to overcome these challenges and accelerate the introduction of photocatalytic hydrogen production technologies into practical use [102].
The fourth cluster, “rotary engine”, concerns engines that use hydrogen as fuel. Hydrogen-powered engines could represent a breakthrough in ecological propulsion, combining the innovation of rotary engines with the clean combustion of hydrogen. These engines offer high efficiency and minimize emissions of harmful substances, which makes them an attractive alternative to conventional drive sources. The development of hydrogen rotary engine technology opens up new opportunities for the automotive and aviation industries striving to reduce their carbon footprint and improve sustainability. Table 8 shows the three articles with the highest citation burst from this cluster.

Table 8.
Top 3 publications from the #4 cluster with the highest citation burst index.
The first article discusses the use of hydrogen as a fuel in combustion engines, highlighting its advantages and disadvantages as an energy carrier. It raises the issue of the use of hydrogen in the context of reducing primary energy consumption and greenhouse gas emissions. The article describes in detail the basic properties of hydrogen and its combustion, mixture formation strategies and their impact on emissions, as well as the challenges related to non-standard combustion phenomena such as pre-ignition and knocking. Additionally, it presents the possibilities of designing engines or their conversion for the use of hydrogen, including safety aspects, and an overview of cars with hydrogen engines. The prospects for using hydrogen as a fuel in combustion engines are promising, mainly due to its high efficiency and low emissions of harmful substances. The challenges to be overcome include the need to adapt or completely redesign engine components to work with hydrogen, ensuring safe use due to the properties of hydrogen as a fuel, and the development of fuel infrastructure. The development of hydrogen storage and distribution technologies and further research on the optimization of hydrogen combustion processes in engines are crucial for the commercialization of this technology. Despite these challenges, hydrogen engines can be an important element in the pursuit of sustainable mobility and reducing the environmental impact of transport [103]. The second article presents a comprehensive review of hydrogen-powered internal combustion engine research, focusing mainly on light- and medium-duty engines. The first part of the article discusses the basic properties of hydrogen as a fuel and their impact on engine operation, pointing out that although hydrogen allows for clean and efficient combustion at low loads, at higher loads, problems appear such as premature ignition and an increase in the production of nitrogen oxides (NOx) due to high combustion temperatures. The authors point to progress in the development of advanced hydrogen engines that improve power density and reduce NOx emissions at high loads. Next, the reasons why the development of hydrogen engines seems promising and necessary are discussed, especially from the perspective of striving for sustainable development and pollution reduction. Various strategies for managing hydrogen engines are discussed, such as the use of advanced emission control systems and fuel blending strategies to maximize efficiency and minimize negative effects. The article summarizes significant achievements in the development of hydrogen-fueled combustion engines and H2ICE vehicles, emphasizing that simple H2ICE options are convenient and economically viable in the short term. The conclusion indicates that a hydrogen-powered internal combustion engine can serve as a transitional option for transport propulsion in a hydrogen economy, which is well illustrated in current applications. Looking ahead to future work, it will be important to continue the research and development of advanced hydrogen engines to understand their limitations, especially related to high loads and NOx control. This will require innovations in engine design, mixing strategies, and combustion management systems. Moreover, integration with exhaust gas treatment systems and the development of fuel infrastructure will be crucial for the broad commercialization of the H2ICE technology. This development paves the way for more sustainable and clean transport as the world strives to reduce dependence on fossil fuels and reduce greenhouse gas emissions [104]. The third article examines experimental research on the thermal efficiency and emission characteristics of an engine powered by a hydrogen-rich lean mixture and lean natural gas. This study focuses on the effect of hydrogen addition to natural gas (NG) on the performance of a spark ignition engine using variable hydrogen/CNG (HCNG) blend compositions. The results show that hydrogen enrichment significantly extends the lean limit, improves the engine’s ability to run on lean mixtures, and shortens combustion time. However, nitrogen oxides (NOx) have been found to increase with the addition of hydrogen if the ignition is not optimized according to the high combustion rate of hydrogen. Furthermore, if the ignition timing is constant, the addition of hydrogen actually increases heat transfer from the cylinder due to the shorter stall distance and higher combustion temperature, which is not beneficial for improving thermal efficiency if combined with the effect of a suboptimal ignition timing. But if the ignition is slowed to MBT (maximum braking torque), taking advantage of the high hydrogen combustion rate, NOx emissions show no obvious increase with the addition of hydrogen, and the engine thermal efficiency increases as the hydrogen fraction increases. A decrease in unburned hydrocarbons (HCs) is always observed as the hydrogen fraction increases. For further research, it is important to further understand the impact of hydrogen on combustion and emissions in natural gas engines, especially in the context of the development of low-emission and efficient propulsion systems. The development of optimal control strategies that maximize the benefits of hydrogen enrichment while limiting the negative impact of NOx emissions is crucial. Additionally, further innovations in fuel mixing and combustion management technologies may allow for better use of hydrogen’s properties. Equally important is understanding the long-term impact of hydrogen on engine durability and fuel system components, which is essential to ensuring the reliability and safety of HCNG vehicles. The development of effective NOx capture and removal systems and the integration of HCNG engines with vehicle propulsion systems to maximize overall energy efficiency and minimize environmental impact also remain challenges [105].
The last cluster in the bibliometric analysis is “underground hydrogen storage”. In this cluster, research focuses on storing hydrogen in underground formations for energy applications. This method is considered for its potential to enhance energy security and support the transition to sustainable energy systems. Studies in this cluster explore technical, economic, and environmental aspects of using geological formations, such as depleted oil and gas fields, aquifers, and salt caverns, for large-scale, seasonal, and long-term hydrogen storage. The research aims to address challenges related to hydrogen’s properties, storage efficiency, safety measures, and retrieval processes to make underground hydrogen storage a viable component of future hydrogen economies. Table 9 shows the results of the analysis of the top publications with the highest citation burst.

Table 9.
Top 3 publications from the #5 cluster with the highest citation burst index.
The first article presents a comprehensive overview of underground hydrogen storage (UHS) as an innovative energy storage method. The article focuses on potential storage locations and their characteristics, UHS mechanisms, and related challenges. Attention was drawn to the need to develop energy storage systems to balance the variable production of renewable energy such as wind and solar energy. The authors discuss various locations for storing hydrogen underground, including depleted hydrocarbon deposits, reservoirs, and hand-created underground caves (caverns). They analyze various UHS mechanisms, including hydrodynamic, geochemical, physicochemical, biochemical, and microbiological reactions occurring during hydrogen storage. The study also includes a review of model studies assessing the feasibility of the process. Global laboratory and field research and potential storage locations are discussed, and technical challenges are presented along with appropriate remediation techniques and economic evaluation. Finally, the article presents several possible strategies for the underground hydrogen storage process, which may be helpful in future research and development of UHS technologies. It was emphasized that storing hydrogen underground, due to its high capacity and lower costs, is gaining importance in the context of the transition from fossil fuels to renewable energy sources. However, to make UHS more feasible and economically viable, further research is needed in assessing site suitability, understanding physicochemical and microbiological processes, and developing effective monitoring and modeling methods. To sum up, storing hydrogen underground seems to be a promising direction of development aimed at supporting the integration of renewable energy sources with energy systems and contributing to the reduction of greenhouse gas emissions. However, challenges such as risk assessment, understanding the interactions between hydrogen and storage rocks, and operational costs require detailed research and development [106]. The second article presents a comprehensive look at storing hydrogen underground, taking into account various potential storage locations and their characteristics. It focuses on the mechanisms of underground hydrogen storage (UHS), considering numerous phenomena such as hydrodynamics, geochemical, physiological, biochemical, and microbiological reactions. The article also discusses modeling studies aimed at assessing the feasibility of the process. Global laboratory research, field studies, and potential storage sites are presented, and technical challenges are discussed along with appropriate remediation techniques and economic viability. It was emphasized that storing hydrogen underground in depleted hydrocarbon deposits, aquifers, and mine workings is a potential solution that may contribute to reducing dependence on fossil fuels and thus reducing greenhouse gas emissions. However, the success of these ventures depends on further research, development, and optimization of key parameters governing UHS storage. Key challenges were identified, including the need for thorough site assessment, understanding microbial reactions, and managing the risk of leakage and contamination. Model studies have been identified as necessary to understand the processes occurring during hydrogen storage and extraction, including the impact on the properties of storage rocks and the surrounding geosphere. Furthermore, the article highlights the economic viability of various storage methods, emphasizing that these approaches must be cost-effective to be widely used. The authors emphasize the need for a holistic approach to assessing potential hydrogen storage locations, which would include both technical and socio-economic aspects. In summary, storing hydrogen underground presents itself as a promising path to realizing a hydrogen economy, but it requires intensive interdisciplinary research and technological development to overcome existing barriers and challenges. In the conclusion, the authors call for further research and international cooperation to increase the understanding and efficiency of processes related to UHS storage, and draw attention to the need to develop security standards and legal regulations that will enable the effective and safe implementation of this technology [107]. The third article presents the scientific challenges of storing hydrogen on large scales in underground porous media (UHSP), thereby supporting the global hydrogen economy. This review discusses the need for safe and efficient energy storage solutions, particularly in the context of the variability and seasonal fluctuations of renewable energy. It focuses on storing hydrogen in porous geological formations such as saline reservoirs and depleted hydrocarbon reservoirs, which could provide the enormous storage capacity needed to manage long-term fluctuations between energy supply and demand. The authors emphasize that despite its potential, the technological maturity of UHSP is relatively low, leading to various uncertainties and challenges, especially in terms of security and economic viability. They indicate that hydrogen’s unique physical and chemical properties, combined with potential geochemical reactions in the subsurface, pose significant challenges. These include the formation of corrosive substances such as hydrogen sulfide, loss of hydrogen due to microbial activity, and changes in permeability due to geochemical interactions, which can affect the predictability of hydrogen flow. Additionally, the article discusses the need for multidisciplinary research spanning fields such as reservoir engineering, chemistry, geology, and microbiology to address these challenges. This is a more complex approach than required for methane or CO2 storage, but necessary for the safe and efficient mass implementation of UHSP. Overall, the paper provides an in-depth analysis of the scientific barriers to UHSP implementation and highlights the need for continued research and development to realize its potential as part of a sustainable energy future. The paper suggests that overcoming these challenges can make a significant contribution to decarbonizing the energy system, providing a viable solution for storing renewable energy on a large scale and on a seasonal basis [108].
In the context of current trends in hydrogen technologies, we are observing a clear increase in interest in both the scientific and industrial sectors. The development of hydrogen production methods, especially through water electrolysis powered by renewable energy, is becoming more and more profitable thanks to the decreasing costs of renewable sources and technological progress in the design and production of electrolyzers. At the same time, research on methods of producing hydrogen from biomass is intensifying, which could contribute to greater use of agricultural and municipal waste for energy purposes.
One of the key trends is also the development of infrastructure for storing and distributing hydrogen. Technologies such as storage in liquid forms, in chemical compounds, or in the form of metal–organic cage structures are being developed. In the context of transport, in addition to building a network of hydrogen refueling stations, research on technologies enabling the safe transport of hydrogen, including through pipelines, is important.
In the future, the development of hydrogen technologies is expected to be strongly focused on the integration of hydrogen systems with global energy systems. This includes not only production and storage but also the use of hydrogen as an energy carrier in various economic sectors. Particular attention is likely to be given to transport applications, including the development of fleets of fuel cell vehicles, as well as the use of hydrogen in heavy industry and energy as a means of reducing CO2 emissions. In addition, further safety research related to the use of hydrogen will be important in order to minimize potential risks and increase public acceptance. The development of standards and regulations governing the production, storage, transport, and use of hydrogen will be crucial for the rapid and safe implementation of hydrogen technologies.
One of the current research trends is also research on the use of natural hydrogen [4,109]. Research indicates the possibility of significant amounts of hydrogen produced by natural geological processes that could supply global hydrogen demand for thousands of years. However, access to these resources is limited by their location in deep layers of the earth or in hard-to-reach areas. Still, even a small fraction of this estimated volume could meet hydrogen demand for hundreds of years if it could be economically extracted. Exploring the possibilities of using natural hydrogen opens new perspectives for future energy systems. The use of natural hydrogen resources could be a breakthrough in the pursuit of carbon neutrality, given the potentially low emissions associated with its extraction. However, it should be noted that challenges such as technical aspects of extraction, economic issues, and environmental impact need to be addressed. Further research and technology development could enable these resources to be used in a sustainable and cost-effective manner, which would be a stepping stone towards a sustainable energy future.
4. Conclusions
In summary, hydrogen technologies and their application in energy systems highlight the critical importance of hydrogen as part of the transition to carbon neutrality. The main areas of research interest are highlighted, including the production, storage, safety, and use of hydrogen, as well as innovative models for assessing hydrogen purity and technologies related to its production. The article also draws attention to the importance of hydrogen storage technology for the future use of hydrogen as an energy carrier and the development of technologies enabling effective, safe, and economical hydrogen storage.
In addition, bibliometric analysis allows the identification of key research areas, such as hydrogen storage, hydrogen production, efficient electrocatalysts, rotary engines using hydrogen as a fuel, and underground hydrogen storage. Each of these areas plays a fundamental role in achieving a sustainable hydrogen economy, reflecting the intensity of research and development of hydrogen technologies.
Recent trends indicate a growing interest in storing hydrogen underground as a method of increasing energy security and supporting the transition to sustainable energy systems. Research in this area focuses on the technical, economic, and environmental aspects of using geological formations for large-scale, seasonal, and long-term hydrogen storage.
The main challenges and barriers in hydrogen research focus primarily on efficient, economic, and ecological hydrogen production. This challenge includes the development of advanced technologies that enable mass production of hydrogen with minimal impact on the environment, especially through the use of green energy from renewable sources such as solar, wind, and hydropower. An additional challenge is to develop effective methods of storing and distributing hydrogen, which are key to its economic and safe use as an energy carrier. Another problem is increasing the durability and efficiency of fuel cells and hydrogen-to-energy conversion systems, which is necessary for the wide commercialization of hydrogen technologies. Adapting the existing energy and industrial infrastructure to the use of hydrogen is also a significant barrier, requiring significant investments and regulatory changes. In addition to the technical and economic aspects, social challenges cannot be ignored, such as building awareness and trust among the public and solving safety issues related to the production, storage, and use of hydrogen. The development of hydrogen technologies is fundamental to achieving the sustainable development goals, especially in the context of fighting climate change and reducing greenhouse gas emissions. Hydrogen offers a unique opportunity to decarbonize various economic sectors, including transport, heating, energy production, and heavy industry, contributing to reducing global warming and achieving carbon neutrality. Thanks to its versatility, hydrogen can be a key element in the integration of energy systems, enabling the use of excess energy from renewable sources and increasing their share in the energy mix. However, to fully exploit the potential of hydrogen technologies in achieving sustainable development, further support for research and development, elimination of regulatory barriers, development of infrastructure, and increased public acceptance are necessary. It will also be crucial to support international cooperation and coordination on energy policy, which will contribute to the creation of a global hydrogen market and the achievement of global climate goals.
Author Contributions
Conceptualization, P.K. and K.P.-U.; methodology, P.K.; software, P.K.; validation, P.K. and K.P.-U.; formal analysis, K.P.-U.; investigation, P.K. and K.P.-U.; resources, P.K., K.P.-U. and M.Z.; writing—original draft preparation, P.K.; writing—review and editing, K.P.-U. and M.Z.; visualization, P.K.; supervision, K.P.-U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Su, W.; Li, Q.; Zheng, W.; Han, Y.; Yu, Z.; Bai, Z.; Han, Y. Enhancing Wind-Solar Hybrid Hydrogen Production through Multi-State Electrolyzer Management and Complementary Energy Optimization. Energy Rep. 2024, 11, 1774–1786. [Google Scholar] [CrossRef]
- Chelvam, K.; Hanafiah, M.M.; Woon, K.S.; Ali, K.A. A Review on the Environmental Performance of Various Hydrogen Production Technologies: An Approach towards Hydrogen Economy. Energy Rep. 2024, 11, 369–383. [Google Scholar] [CrossRef]
- Jesse, B.-J.; Kramer, G.J.; Koning, V.; Vögele, S.; Kuckshinrichs, W. Stakeholder Perspectives on the Scale-up of Green Hydrogen and Electrolyzers. Energy Rep. 2024, 11, 208–217. [Google Scholar] [CrossRef]
- Osselin, F.; Pichavant, M.; Champallier, R.; Ulrich, M.; Raimbourg, H. Reactive Transport Experiments of Coupled Carbonation and Serpentinization in a Natural Serpentinite. Implication for Hydrogen Production and Carbon Geological Storage. Geochim. Cosmochim. Acta 2022, 318, 165–189. [Google Scholar] [CrossRef]
- Singh, R.; Samuel, M.S.; Ethiraj, S.; Ashwini John, J.; Ravikumar, M.; Joseph Sekhar, S.; Le, T.T.H.; Mathimani, T. Advancement in Integrated Ammonia Synthesis, and Its Techno-Economic Analysis, Emission Index, and Contribution to the Hydrogen 2.0 Economy. Fuel 2024, 364, 131030. [Google Scholar] [CrossRef]
- Luo, X.; Tveit, S.; Gholami, R.; Andersen, P.Ø. Underground Hydrogen Storage (UHS) in Natural Storage Sites: A Perspective of Subsurface Characterization and Monitoring. Fuel 2024, 364, 131038. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, K.; Liu, N.; Xie, Y. Unlock the Aggregated Flexibility of Electricity-Hydrogen Integrated Virtual Power Plant for Peak-Regulation. Appl. Energy 2024, 360, 122747. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Xiao, M.; Yao, Y.; Lv, H. Performance Investigation of Cross-Regional Utilization and Production of Renewable Hydrogen. Appl. Therm. Eng. 2024, 243, 122567. [Google Scholar] [CrossRef]
- Zhu, M.; Ai, X.; Fang, J.; Cui, S.; Wu, K.; Zheng, L.; Wen, J. Optimal Scheduling of Hydrogen Energy Hub for Stable Demand with Uncertain Photovoltaic and Biomass. Appl. Energy 2024, 360, 122783. [Google Scholar] [CrossRef]
- The Future of Hydrogen—Analysis. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 28 March 2024).
- Hydrogen from Renewable Power: Technology Outlook for the Energy Transition. Available online: https://www.irena.org/publications/2018/sep/hydrogen-from-renewable-power (accessed on 28 March 2024).
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A Comprehensive Review of the Promising Clean Energy Carrier: Hydrogen Production, Transportation, Storage, and Utilization (HPTSU) Technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B.; Civelek Yörüklü, H.; Açıkalın, K.; Demirci, U.B.; Kantürk Figen, A. Boron-Based Hydrogen Storage Materials towards Power-to-X Technology on the Path to Carbon Neutrality. Int. J. Hydrogen Energy 2023, 48, 39389–39407. [Google Scholar] [CrossRef]
- Han, X.; Vercoulen, P.; Lee, S.; Lam, A.; Kato, S.; Morotomi, T. Policy Design for Diffusing Hydrogen Economy and Its Impact on the Japanese Economy for Carbon Neutrality by 2050: Analysis Using the E3ME-FTT Model. Energies 2023, 16, 7392. [Google Scholar] [CrossRef]
- Shim, J.-H.; Kim, S.-W.; Baik, J.M. Advanced Materials for Carbon Neutrality: Energy Conversion, Hydrogen Storage, and CO2 Capture and Conversion. Nano Energy 2023, 115, 108726. [Google Scholar] [CrossRef]
- Kong, H.; Sun, Y.; Li, Z.; Zheng, H.; Wang, J.; Wang, H. The Development Path of Direct Coal Liquefaction System under Carbon Neutrality Target: Coupling Green Hydrogen or CCUS Technology. Appl. Energy 2023, 347, 121451. [Google Scholar] [CrossRef]
- Su, B.-L. Photocatalytic Hydrogen Production toward Carbon Neutrality: Tracking Charge Separation. Natl. Sci. Rev. 2023, 10, nwad139. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wu, X.; Liu, L.; Ye, J.; Wang, T.; Fu, L.; Wu, Y. Prediction of Remaining Useful Life and State of Health of Lithium Batteries Based on Time Series Feature and Savitzky-Golay Filter Combined with Gated Recurrent Unit Neural Network. Energy 2023, 270, 126880. [Google Scholar] [CrossRef]
- Plazas-Niño, F.A.; Yeganyan, R.; Cannone, C.; Howells, M.; Borba, B.; Quirós-Tortós, J. Assessing the Role of Low-Emission Hydrogen: A Techno-Economic Database for Hydrogen Pathways Modelling. Data Brief 2024, 52, 109822. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, Production and Characterization of the Main Process Worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Liu, G.; Sheng, Y.; Ager, J.W.; Kraft, M.; Xu, R. Research Advances towards Large-Scale Solar Hydrogen Production from Water. EnergyChem 2019, 1, 100014. [Google Scholar] [CrossRef]
- Yilmaz, F.; Ozturk, M.; Selbas, R. Development and Assessment of a Newly Developed Renewable Energy-Based Hybrid System with Liquid Hydrogen Storage for Sustainable Development. Int. J. Hydrogen Energy 2024, 56, 406–417. [Google Scholar] [CrossRef]
- Qin, B.; Wang, H.; Liao, Y.; Liu, D.; Wang, Z.; Li, F. Liquid Hydrogen Superconducting Transmission Based Super Energy Pipeline for Pacific Rim in the Context of Global Energy Sustainable Development. Int. J. Hydrogen Energy 2024, 56, 1391–1396. [Google Scholar] [CrossRef]
- Cao, X. Strained Carbon Steel as a Highly Efficient Catalyst for Seawater Electrolysis. Energy Mater. 2022, 2, 200010. [Google Scholar] [CrossRef]
- Kim, J.; Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Yang, M. Energy, Material, and Resource Efficiency for Industrial Decarbonization: A Systematic Review of Sociotechnical Systems, Technological Innovations, and Policy Options. Energy Res. Soc. Sci. 2024, 112, 103521. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial Decarbonization via Hydrogen: A Critical and Systematic Review of Developments, Socio-Technical Systems and Policy Options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in Energy Transition: A Review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Zhang, Y.; Davis, D.; Brear, M.J. The Role of Hydrogen in Decarbonizing a Coupled Energy System. J. Clean. Prod. 2022, 346, 131082. [Google Scholar] [CrossRef]
- Amano, F.; Tsushiro, K. Proton Exchange Membrane Photoelectrochemical Cell for Water Splitting under Vapor Feeding. Energy Mater. 2024, 4, 400006. [Google Scholar] [CrossRef]
- Hussam, W.K.; Barhoumi, E.M.; Abdul-Niby, M.; Sheard, G.J. Techno-Economic Analysis and Optimization of Hydrogen Production from Renewable Hybrid Energy Systems: Shagaya Renewable Power Plant-Kuwait. Int. J. Hydrogen Energy 2024, 58, 56–68. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.; Gallachóir, B.Ó. The Role of Hydrogen in Low Carbon Energy Futures—A Review of Existing Perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Özcan, H. Comprehensive Review on the Techno-Economics of Sustainable Large-Scale Clean Hydrogen Production. J. Clean. Prod. 2019, 220, 593–609. [Google Scholar] [CrossRef]
- Shafiei, E.; Davidsdottir, B.; Leaver, J.; Stefansson, H.; Asgeirsson, E.I. Energy, Economic, and Mitigation Cost Implications of Transition toward a Carbon-Neutral Transport Sector: A Simulation-Based Comparison between Hydrogen and Electricity. J. Clean. Prod. 2017, 141, 237–247. [Google Scholar] [CrossRef]
- Nagar, R.; Srivastava, S.; Hudson, S.L.; Amaya, S.L.; Tanna, A.; Sharma, M.; Achayalingam, R.; Sonkaria, S.; Khare, V.; Srinivasan, S.S. Recent Developments in State-of-the-Art Hydrogen Energy Technologies—Review of Hydrogen Storage Materials. Sol. Compass 2023, 5, 100033. [Google Scholar] [CrossRef]
- Garzón Baquero, J.E.; Bellon Monsalve, D. From Fossil Fuel Energy to Hydrogen Energy: Transformation of Fossil Fuel Energy Economies into Hydrogen Economies through Social Entrepreneurship. Int. J. Hydrogen Energy 2024, 54, 574–585. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Mahmoud, M.; Mahmoud, M.S.; Elsaid, K.; Obaideen, K.; Rezk, H.; Eisa, T.; Chae, K.-J.; Sayed, E.T. Multiple-Criteria Decision-Making for Hydrogen Production Approaches Based on Economic, Social, and Environmental Impacts. Int. J. Hydrogen Energy 2024, 52, 854–868. [Google Scholar] [CrossRef]
- Häußermann, J.J.; Maier, M.J.; Kirsch, T.C.; Kaiser, S.; Schraudner, M. Social Acceptance of Green Hydrogen in Germany: Building Trust through Responsible Innovation. Energy Sustain. Soc. 2023, 13, 22. [Google Scholar] [CrossRef]
- Blohm, M.; Dettner, F. Green Hydrogen Production: Integrating Environmental and Social Criteria to Ensure Sustainability. Smart Energy 2023, 11, 100112. [Google Scholar] [CrossRef]
- Bade, S.O.; Tomomewo, O.S.; Meenakshisundaram, A.; Ferron, P.; Oni, B.A. Economic, Social, and Regulatory Challenges of Green Hydrogen Production and Utilization in the US: A Review. Int. J. Hydrogen Energy 2024, 49, 314–335. [Google Scholar] [CrossRef]
- Vallejos-Romero, A.; Cordoves-Sánchez, M.; Cisternas, C.; Sáez-Ardura, F.; Rodríguez, I.; Aledo, A.; Boso, Á.; Prades, J.; Álvarez, B. Green Hydrogen and Social Sciences: Issues, Problems, and Future Challenges. Sustainability 2022, 15, 303. [Google Scholar] [CrossRef]
- De-León Almaraz, S.; Rácz, V.; Azzaro-Pantel, C.; Szántó, Z.O. Multiobjective and Social Cost-Benefit Optimisation for a Sustainable Hydrogen Supply Chain: Application to Hungary. Appl. Energy 2022, 325, 119882. [Google Scholar] [CrossRef]
- Available online: https://www.iea.org/reports/global-hydrogen-review-2023/executive-summary (accessed on 14 February 2024).
- Kemeç, A.; Altınay, A.T. Sustainable Energy Research Trend: A Bibliometric Analysis Using VOSviewer, RStudio Bibliometrix, and CiteSpace Software Tools. Sustainability 2023, 15, 3618. [Google Scholar] [CrossRef]
- Peng, W.; Haron, N.A.; Alias, A.H.; Law, T.H. Knowledge Map of Climate Change and Transportation: A Bibliometric Analysis Based on CiteSpace. Atmosphere 2023, 14, 434. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Liu, J.; Wang, G. Science Mapping the Knowledge Domain of Electrochemical Energy Storage Technology: A Bibliometric Review. J. Energy Storage 2024, 77, 109819. [Google Scholar] [CrossRef]
- Oommen, J.G.; Roy, N.S.; Brittoraj, R.D. Exploring the Landscape of Energy Audits: A Bibliometric Analysis. Int. J. Energy Econ. Policy 2024, 14, 31–36. [Google Scholar] [CrossRef]
- Chen, C. Visualizing and Exploring Scientific Literature with CiteSpace: An Introduction. In Proceedings of the 2018 Conference on Human Information Interaction&Retrieval—CHIIR’18, New Brunswick, NJ, USA, 11–15 March 2018; ACM Press: New York, NY, USA, 2018; pp. 369–370. [Google Scholar]
- Ding, X.; Yang, Z. Knowledge Mapping of Platform Research: A Visual Analysis Using VOSviewer and CiteSpace. Electron. Commer. Res. 2022, 22, 787–809. [Google Scholar] [CrossRef]
- Zhao, X.; Nan, D.; Chen, C.; Zhang, S.; Che, S.; Kim, J.H. Bibliometric Study on Environmental, Social, and Governance Research Using CiteSpace. Front. Environ. Sci. 2023, 10, 1087493. [Google Scholar] [CrossRef]
- García-Lillo, F.; Sánchez-García, E.; Marco-Lajara, B.; Seva-Larrosa, P. Renewable Energies and Sustainable Development: A Bibliometric Overview. Energies 2023, 16, 1211. [Google Scholar] [CrossRef]
- Özköse, H. Bibliometric Analysis and Scientific Mapping of IoT. J. Comput. Inf. Syst. 2023, 63, 1438–1459. [Google Scholar] [CrossRef]
- Kut, P.; Pietrucha-Urbanik, K.; Tchórzewska-Cieślak, B. Reliability-Oriented Design of a Solar-PV Deployments. Energies 2021, 14, 6535. [Google Scholar] [CrossRef]
- Shklyarskiy, Y.; Skvortsov, I.; Sutikno, T.; Manap, M. The Optimization Technique for a Hybrid Renewable Energy System Based on Solar-Hydrogen Generation. Int. J. Power Electron. Drive Syst. 2024, 15, 639–650. [Google Scholar] [CrossRef]
- Elminshawy, N.A.S.; Diab, S.; Yassen, Y.E.S.; Elbaksawi, O. An Energy-Economic Analysis of a Hybrid PV/Wind/Battery Energy-Driven Hydrogen Generation System in Rural Regions of Egypt. J. Energy Storage 2024, 80, 110256. [Google Scholar] [CrossRef]
- Guerra, K.; Welfle, A.; Gutiérrez-Alvarez, R.; Freer, M.; Ma, L.; Haro, P. The Role of Energy Storage in Great Britain’s Future Power System: Focus on Hydrogen and Biomass. Appl. Energy 2024, 357, 122447. [Google Scholar] [CrossRef]
- Jamal, T.; Shafiullah, G.M.; Dawood, F.; Kaur, A.; Arif, M.T.; Pugazhendhi, R.; Elavarasan, R.M.; Ahmed, S.F. Fuelling the Future: An in-Depth Review of Recent Trends, Challenges and Opportunities of Hydrogen Fuel Cell for a Sustainable Hydrogen Economy. Energy Rep. 2023, 10, 2103–2127. [Google Scholar] [CrossRef]
- Rabiee, N.; Iravani, S. Nanosponges for Hydrogen Evolution Reaction: Current Trends and Future Perspectives. Sustain. Energy Fuels 2023, 7, 4825–4838. [Google Scholar] [CrossRef]
- Khan, T.; Yu, M.; Waseem, M. Review on Recent Optimization Strategies for Hybrid Renewable Energy System with Hydrogen Technologies: State of the Art, Trends and Future Directions. Int. J. Hydrogen Energy 2022, 47, 25155–25201. [Google Scholar] [CrossRef]
- Çınar, H.; Kandemir, I.; Donateo, T. Current Technologies and Future Trends of Hydrogen Propulsion Systems in Hybrid Small Unmanned Aerial Vehicles. In Hydrogen Electrical Vehicles; Wiley: Hoboken, NJ, USA, 2022; pp. 75–109. ISBN 978-1-394-16755-5. [Google Scholar]
- Iaquaniello, G.; Palo, E.; Salladini, A. An Overview of Today’s Industrial Processes to Make Hydrogen and Future Developments’ Trend. In Hydrogen Production and Energy Transition; De Gruyter: Berlin, Germany, 2021; Volume 1, pp. 137–170. ISBN 978-3-11-059625-0. [Google Scholar]
- Mishra, V.; Srivastava, I. Bio-Hydrogen Production: Current Trends and Future Prospects. In Bioenergy: Opportunities and Challenges; Academic Press: Cambridge, MA, USA, 2015; pp. 37–58. ISBN 978-1-4987-2205-6. [Google Scholar]
- Ogarek, P.; Wojtoń, M.; Słyś, D. Hydrogen as a Renewable Energy Carrier in a Hybrid Configuration of Distributed Energy Systems: Bibliometric Mapping of Current Knowledge and Strategies. Energies 2023, 16, 5495. [Google Scholar] [CrossRef]
- Rabczak, S.; Nowak, K. Evaluating the Efficiency of Surface-Based Air Heating Systems. Energies 2024, 17, 1252. [Google Scholar] [CrossRef]
- Raman, R.; Lathabhai, H.; Pattnaik, D.; Kumar, C.; Nedungadi, P. Research Contribution of Bibliometric Studies Related to Sustainable Development Goals and Sustainability. Discov. Sustain. 2024, 5, 7. [Google Scholar] [CrossRef]
- Junjia, Y.; Alias, A.H.; Haron, N.A.; Abu Bakar, N. A Bibliometric Review on Safety Risk Assessment of Construction Based on CiteSpace Software and WoS Database. Sustainability 2023, 15, 11803. [Google Scholar] [CrossRef]
- Jia, L.; Qiao, G. Quantification, Environmental Impact, and Behavior Management: A Bibliometric Analysis and Review of Global Food Waste Research Based on CiteSpace. Sustainability 2022, 14, 11293. [Google Scholar] [CrossRef]
- Zhang, P.; Du, Y.; Han, S.; Qiu, Q. Global Progress in Oil and Gas Well Research Using Bibliometric Analysis Based on VOSviewer and CiteSpace. Energies 2022, 15, 5447. [Google Scholar] [CrossRef]
- Zhang, Y.; You, X.; Huang, S.; Wang, M.; Dong, J. Knowledge Atlas on the Relationship between Water Management and Constructed Wetlands—A Bibliometric Analysis Based on CiteSpace. Sustainability 2022, 14, 8288. [Google Scholar] [CrossRef]
- Kut, P.; Pietrucha-Urbanik, K. Most Searched Topics in the Scientific Literature on Failures in Photovoltaic Installations. Energies 2022, 15, 8108. [Google Scholar] [CrossRef]
- Clerici Maestosi, P.; Salvia, M.; Pietrapertosa, F.; Romagnoli, F.; Pirro, M. Implementation of Positive Energy Districts in European Cities: A Systematic Literature Review to Identify the Effective Integration of the Concept into the Existing Energy Systems. Energies 2024, 17, 707. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Hu, Y.; Wang, Z.; Liu, C. Current Status and Future Directions of Building Information Modeling for Low-Carbon Buildings. Energies 2024, 17, 143. [Google Scholar] [CrossRef]
- Ferruzzi, G.; Delcea, C.; Barberi, A.; Di Dio, V.; Di Somma, M.; Catrini, P.; Guarino, S.; Rossi, F.; Parisi, M.L.; Sinicropi, A.; et al. Concentrating Solar Power: The State of the Art, Research Gaps and Future Perspectives. Energies 2023, 16, 8082. [Google Scholar] [CrossRef]
- Mavlutova, I.; Spilbergs, A.; Verdenhofs, A.; Kuzmina, J.; Arefjevs, I.; Natrins, A. The Role of Green Finance in Fostering the Sustainability of the Economy and Renewable Energy Supply: Recent Issues and Challenges. Energies 2023, 16, 7712. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Z.; Liu, S.; Tseng, H. Emerging Trends in Regenerative Medicine: A Scientometric Analysis in CiteSpace. Expert Opin. Biol. Ther. 2012, 12, 593–608. [Google Scholar] [CrossRef]
- Synnestvedt, M.B.; Chen, C.; Holmes, J.H. CiteSpace II: Visualization and Knowledge Discovery in Bibliographic Databases. AMIA Annu. Symp. Proc./AMIA Symp. AMIA Symp. 2005, 2005, 724–728. [Google Scholar]
- Wei, F.; Grubesic, T.H.; Bishop, B.W. Exploring the GIS Knowledge Domain Using CiteSpace. Prof. Geogr. 2015, 67, 374–384. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C. Visualization Analysis of Big Data Research Based on Citespace. Soft Comput. 2020, 24, 8173–8186. [Google Scholar] [CrossRef]
- Kut, P.; Pietrucha-Urbanik, K.; Zeleňáková, M. Bibliometric Analysis of Renewable Energy Research and Industrial Assets in Poland and Slovakia. In Proceedings of the CEE 2023, Rzeszów, Poland, 6–8 September 2023; Blikharskyy, Z., Koszelnik, P., Lichołai, L., Nazarko, P., Katunský, D., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 214–223. [Google Scholar] [CrossRef]
- Igliński, B.; Pietrzak, M.B. Renewable and Sustainable Energy: Current State and Prospects. Energies 2022, 15, 4735. [Google Scholar] [CrossRef]
- Cheba, K.; Bąk, I.; Pietrzak, M.B. Conditions of the Green Transformation. The Case of the European Union. Technol. Econ. Dev. Econ. 2022, 29, 438–467. [Google Scholar] [CrossRef]
- Wei, J.; Liang, G.; Alex, J.; Zhang, T.; Ma, C. Research Progress of Energy Utilization of Agricultural Waste in China: Bibliometric Analysis by Citespace. Sustainability 2020, 12, 812. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, R.; Maimaituerxun, M. Bibliometric Review of Carbon Neutrality with CiteSpace: Evolution, Trends, and Framework. Environ. Sci. Pollut. Res. 2022, 29, 76668–76686. [Google Scholar] [CrossRef]
- Wang, D.; Huangfu, Y.; Dong, Z.; Dong, Y. Research Hotspots and Evolution Trends of Carbon Neutrality—Visual Analysis of Bibliometrics Based on CiteSpace. Sustainability 2022, 14, 1078. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Wang, Z.; Maqbool, B.; Hussain, A.; Khan, W.A. Bibliometric Analysis of the Research on the Impact of Environmental Regulation on Green Technology Innovation Based on CiteSpace. Int. J. Environ. Res. Public Health 2022, 19, 13273. [Google Scholar] [CrossRef]
- Kut, P.; Pietrucha-Urbanik, K. Bibliometric Analysis of Renewable Energy Research on the Example of the Two European Countries: Insights, Challenges, and Future Prospects. Energies 2024, 17, 176. [Google Scholar] [CrossRef]
- Igliński, B.; Pietrzak, M.B.; Kiełkowska, U.; Skrzatek, M.; Gajdos, A.; Zyadin, A.; Natarajan, K. How to Meet the Green Deal Objectives—Is It Possible to Obtain 100% RES at the Regional Level in the EU? Energies 2022, 15, 2296. [Google Scholar] [CrossRef]
- Balcerzak, A.P.; Uddin, G.S.; Igliński, B.; Pietrzak, M.B. Global Energy Transition: From the Main Determinants to Economic Challenges. Equilib. Q. J. Econ. Econ. Policy 2023, 18, 597–608. [Google Scholar] [CrossRef]
- Pietrzak, M.B.; Igliński, B.; Kujawski, W.; Iwański, P. Energy Transition in Poland—Assessment of the Renewable Energy Sector. Energies 2021, 14, 2046. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A Review on Hydrogen Production and Utilization: Challenges and Opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal Hydride Materials for Solid Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen Storage in Mg: A Most Promising Material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A Review on Reforming Bio-Ethanol for Hydrogen Production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Zhang, D. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, S.; Wallner, T. Hydrogen-Fueled Internal Combustion Engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The Hydrogen-Fueled Internal Combustion Engine: A Technical Review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y.; Liu, H.; Li, Y.; Wang, J.; Zhao, S. Experimental Study on Thermal Efficiency and Emission Characteristics of a Lean Burn Hydrogen Enriched Natural Gas Engine. Int. J. Hydrogen Energy 2007, 32, 5067–5075. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground Hydrogen Storage: A Comprehensive Review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A Review on Underground Hydrogen Storage: Insight into Geological Sites, Influencing Factors and Future Outlook. Energy Rep. 2022, 8, 461–499. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Miocic, J.M.; Hangx, S.J.T.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.J.; Schmidt-Hattenberger, C.; et al. Enabling Large-Scale Hydrogen Storage in Porous Media—The Scientific Challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Osselin, F.; Soulaine, C.; Fauguerolles, C.; Gaucher, E.C.; Scaillet, B.; Pichavant, M. Orange Hydrogen Is the New Green. Nat. Geosci. 2022, 15, 765–769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).