Abstract
The thermal runaway (TR) behavior and combustion hazards of lithium-ion battery (LIB) packs directly determine the implementation of firefighting and flame-retardants in energy storage systems. This work studied the TR propagation process and dangers of large-scale LIB packs by experimental methods. The LIB pack consisted of twenty-four 60 Ah (192 Wh) LIBs with LiFePO4 (LFP) as the cathode material. Flame performance, temperature, smoke production, heat release rate (HRR), and mass loss were analyzed during the experiment. The results indicated that TR propagation of the LIB pack developed from the outside to the inside and from the middle to both sides. The development process could be divided into five stages corresponding to the combustion HRR peaks. In the initial stages, the main factor causing LFP battery TR under heating conditions was the external heat source. With the propagation of TR, heat conduction between batteries became the main factor. Hazard analysis found that the HRRmax of the LIB pack was 314 KW, more than eight times that of a single 60 Ah battery under heating conditions. The LIB pack had higher normalized mass loss and normalized THR (6.94 g/Ah and 187 KJ/Ah, respectively) than a single LFP battery. This study provides a reference for developing strategies to address TR propagation or firefighting in energy storage systems.
1. Introduction
Lithium-ion batteries, as a kind of electric energy storage carrier, have the advantages of high working voltage, high energy density, good life cycle, low self-discharge, etc. Nevertheless, LIBs cause thermal runaway (TR) under abusive conditions such as overheating and overcharging. The process of battery TR is accompanied by fire or explosion, threatening the safety of customers’ lives and property. Although some thermal management methods and intrinsically safe designs have been adopted to reduce the safety risks, the safety of LIBs remains a fundamental problem that restricts their large-scale application in energy storage systems.
Most of the safety accidents of LIBs in energy storage systems are caused by TR of the battery. During battery TR, severe electrochemical reactions are generated inside, accompanied by flame, flammable and toxic gases, and heat release. Feng et al. [1] studied the thermal runaway process of LIBs. They found that eight reactions occurred with increasing temperature, such as capacity decay under high-temperature, SEI decomposition, a negative electrode and electrolyte reaction, separator melting, a positive electrode and electrolyte reaction, electrolyte decomposition, a negative electrode and binder reaction, electrolyte combustion, and others. Wang [2,3,4] found that the decomposition temperature of the SEI was between 90 and 120 °C. SEI decomposition led to reactions between the negative electrode and electrolytes. When the temperature reached 130 to 150 °C, the separator began to melt and a micro short circuit occurred in the battery. With rising temperature, the cathode materials and electrolytes decomposed, producing flammable gases such as H2, CH4, CO, etc. Mao et al. [5] studied the gas and flame injection process of 18650-type LIBs with Li(Ni0.5Co0.2Mn0.3)O2 as the cathode material and found that when TR occurred, the gas generation rate was 2.724 g·s−1 and the peak speed of gas flow at the safety valve was 162.0 m·s−1. However, due to the shortage of internal space in the battery, the pressure in the battery at the time of TR only increased by 5600 Pa. Qin et al. [6] studied the gas production of 86 Ah prismatic LIBs with LFP cathodes. They found that the volume fraction of thermal runaway gases in the LIBs from high to low were H2, CO2, CO, ethylene, methane, and ammonia, in which the concentrations of H2 and CO2 were approximately 30.33% and 38.86%, respectively. Qi et al. [7] studied the TR hazards of 60 Ah prismatic LFP LIBs under different combustion conditions. The heat hazard of the TR process under combustion conditions was higher than that of the smoldering process. The comprehensive toxicity evaluation model was used to calculate the gas hazards, and the smoldering process showed a high gas hazard that reached 5.38 times the lethal concentration. Comparing the combustion heat of battery material measured by an oxygen bomb calorimeter with the HRR value measured by a cone calorimeter revealed that the combustion conditions significantly impact the battery’s HRR value and residual energy, and the proportion of residual energy under smoldering conditions was as high as 75.8%.
Much research has focused on the TR mechanism, heat generation, and gas generation characteristics of LIBs. In the application of LIBs, they are integrated into LIB packs to meet the power and capacity requirements of energy storage systems or electric vehicles. In the LIB pack, the energy released by battery TR accumulates, further leading to the chain process of TR propagation and resulting in TR of the entire LIB pack. Zhou et al. [8] studied the TR propagation of an LIB pack composed of four 50 Ah prismatic LFP batteries. They found that the heat released by battery TR was the primary heat source (52–67%) that triggered the TR of adjacent batteries. Wang et al. [9] studied the TR propagation of an LIB pack consisting of six cylindrical LIBs with Li(NiMnCo)1/3O2 cathodes and found that the TR of the LIB pack has a preheating stage; that is, TR of the first battery will preheat a battery that has not under the TR condition, which leads to the acceleration of TR propagation. They also found that the larger the battery size, the slower the TR propagation rate. Zhai et al. [10] studied the TR propagation of 18650-type batteries (Li(Ni0.5Co0.2Mn0.3)O2/Graphite) and found that the location of the first battery TR directly affected the TR propagation of the LIB pack. The process of TR propagation can be divided into four stages: trigger stage, heat accumulation stage, domino effect stage, and stop stage. In conclusion, previous works have researched the TR characteristics of LIBs and the TR propagation of small LIB packs, analyzed the heat and mass transfer characteristics of TR and TR propagation, discussed the hazards of the TR process, and expounded the general laws of TR of LIBs and small LIB packs. In LIB energy storage systems or energy storage facilities, LIBs form an LIB pack using the methods of two parallel 12 series or two parallel 16 series. When a battery causes TR, the whole pack will be burned down.
In this paper, for two parallel 12 series LIB packs in an LIB energy storage system, a cone calorimeter was used to study the heat release and smoke density of the TR process in order to clarify the generation and development process of TR of large-scale LIB packs. The fire behavior, temperature, heat release rate (HRR), smoke production rate (SPR), and mass loss were analyzed to help more deeply reveal the burning features and hazards of LIB packs. The results will help guide safety protection, firefighting, and smoke evacuation technologies in the large-scale integrated application of LIB systems.
2. Materials and Methods
2.1. Experimental Battery Sample and Pack
One kind of 60 Ah/192 Wh large-scale prismatic LIBs with LiFePO4 as the positive electrode material was selected as the sample in this study. Its charge/discharge cutoff voltages were 3.65 and 2.5 V, respectively. Each model was charged to 100% state of charge (SOC) at 0.5 °C to exhibit its maximum potential hazard. The size of the cell was 138 mm (L) × 209 mm (H) × 28 mm (W) and the total mass of the cell was 1850 ± 20 g.
Figure 1a illustrates that the equipped LIB pack had a sandwich structure consisting of steel plates, fixtures, and 24 LFP batteries. The size of the pack was 552 mm (L) × 209 mm (H) × 168 mm (W), the total mass of the pack was 55.45 kg, and the full capacity and energy of the pack were 1440 Ah and 4608 Wh, respectively. The outermost layers were composed of two steel plates, which were used to keep the LIB pack’s close arrangement.
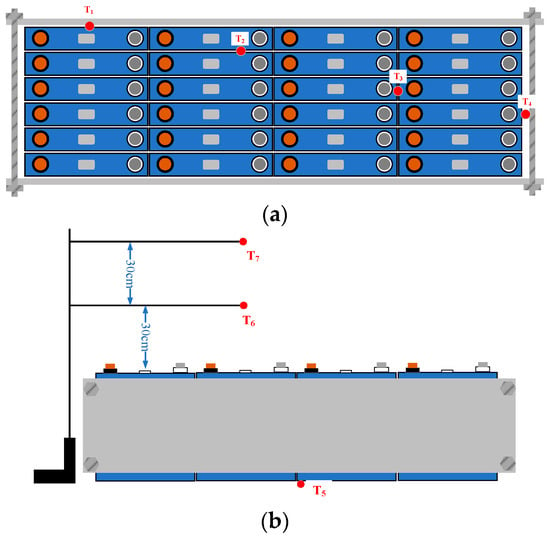
Figure 1.
Schematic diagram of the LFP batteries and thermocouple arrangements: (a) vertical view; (b) side view.
2.2. Experimental Battery Sample and Pack
An experimental system was built to investigate an LIB pack’s combustion and fume characteristics. The temperature, HRR, and gases were measured during the test using the apparatus shown schematically in Figure 2. The experimental system consisted of four parts, including a combustion chamber, exhaust gas analyzer, propane burner, and video cameras. During the test, the section was kept under well-ventilated conditions using a fan and gas duct with a mass flow rate of 0.512 m3/s. K-type thermocouples (measuring range: 0–1000 °C; error limit: ±0.75%; diameter: 3 mm) were fixed on the surface of the LIB pack with high-temperature resistant tape to measure the temperature variations, as shown in Figure 1a,b. The front surface temperature of the pack was measured by T1. T2 and T3 monitored the temperatures of the pack center. The temperatures of the side and bottom of the pack were measured by T4 and T5, respectively. Meanwhile, T6 and T7 were located 30 and 60 cm above the pack to measure the flame temperature. An 80 kW propane burner triggered the battery TR. A HIKVISION video camera was used to record the combustion processes of the LIB pack. An electronic balance was used to record the mass loss.

Figure 2.
Schematic illustration of experimental apparatus.
A paramagnetic analyzer was used to measure the concentration of O2 and the survey had a margin of error of less than 0.02%. The HRR was calculated based on the O2 consumption principle. The concentration of CO2 was determined via a non-dispersive infrared (NDIR) sensor with an accuracy of 0.1 ppm. A Thorlabs optical receiver and light bulb were used to reveal the distribution of obscuration after exhaust gas generation. The distribution of obscuration was calculated by the SPR.
3. Results and Discussion
3.1. Combustion Characteristic
The TR of a single LFP battery underwent the following processes: (1) heating, (2) safety valve rupture and ignition, (3) intense combustion, and (4) extinguishing [7,11,12]. The LFP battery temperature increased and several reactions occurred during the thermal runaway (TR) process [13]. Thus, various fumes were generated inside the battery, resulting in rupture of the safety valve. The fumes would ignite when they encountered open flame, and the ignited fume intensified the battery’s internal reactions, resulting in many particles being ejected. The flame was extinguished when the LFP battery’s internal reactions ended [8]. The TR process of the LIB pack was a collection of TRs of many single batteries. In the LIB pack, the batteries heated unevenly and the combustion position and intensity continuously changed. As shown in Figure 3, the heating was mainly concentrated in the pack’s center. Thus, various gases were generated inside the batteries in the pack center, resulting in increased internal pressure. At 513 s, the pressure reached a critical value and the safety valve ruptured, accompanied by ejected materials such as electrolytes and combustible gases. The ejected materials were ignited by the propane flame, forming aggressive fireballs immediately followed by a short extinguishment. Subsequently, reactions inside batteries in the pack center were intensified at the elevated heating level. The stable combustion turned into an intense jet fire with increasingly bright and lighter color at 845 s. At 1930 s, the combustible gases generated inside the batteries in the pack center gradually decreased, and the jet fire tapered off. Meanwhile, the batteries residing on both sides of the pack started to TR with high-speed jet fires. At 3330 s, the jet fire gradually weakened and extinguished naturally. The total combustion time of the LIB pack was 2787 s. Overall, the combustion characteristics of the LIB pack were similar to those of a single battery, with four elements: safety valve rupture, stable combustion, jet fire, and gradual extinction. TR propagation of the LIB pack had a trend from the middle to both sides of the pack. Compared with batteries in the pack center, the batteries on the outer sides of the pack were more prone to TR because of the more extensive heating range.

Figure 3.
Combustion behavior during the TR process of the LIB pack.
The exciting details of safety valve rupture were observed by video camera, as shown in Figure 4. The process of jet flame presented two stages. Stage one is demonstrated in Figure 4a–c; the electrolytes and combustible gases were ignited when ejected from the battery, the flame climbed rapidly with ejection, and the maximum height reached more than 1.2 m. The ejection speed at the safety valve was breakneck, and the top speed can reach 29.91 m·s−1 [14]. The hot gas ejected horizontally through the safety valve with high temperature/heat flux. It reacted with the ambient fresh air and moved upward due to the buoyancy provided by the flame itself. The turbulent air entrainment at the interface (envelope of the plume) between the outside hot plume and surrounding cold air resulted in mass exchange and mixing, which controlled the flame’s diffusive combustion and characteristic scale (shape and size) as well as the profile of the characteristic parameters (temperature, heat flux/radiation) [15]. Under the influence of high-speed airflow, the flame close to the safety valve was blown out. Stage two is shown in Figure 4d–g; the upper flame gradually weakened while the bottom ejection was ignited again, forming aggressive fireballs. The flame was demonstrated to have a two-section shape and the jet flame followed immediately. The shape of the jet flame was ellipsoid, accompanied by a large quantity of black smoke. After the jet flame, the ejections gradually burned out and combustion became stable.

Figure 4.
Combustion details of the safety valve rupturing process. (a) gas ejection; (b,c) gas ignited; (d–f) the first flame decreases and the second flame rises; (g) end of flame impact.
The safety valve rupturing times of different batteries are essential in understanding the TR process of the LIB pack. As shown in Figure 5, by linearly fitting the valve rupturing time and quantity, it was found that safety valve rupturing in the LIB pack had two stages. In the first stage, the change in time with the quantity of batteries undergoing safety valve rupture was given by Equation (1):
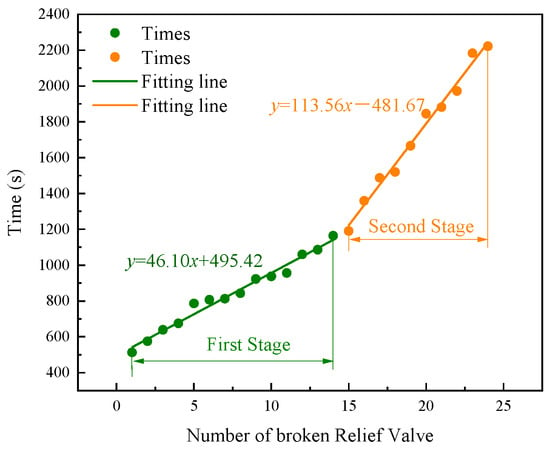
Figure 5.
The relationship between the number of broken safety valves and rupturing time.
In the second stage, the change in time with the quantity of batteries undergoing safety valve rupture was given by Equation (2):
where y is the rupture time of the battery safety valve, and x is the number of batteries with ruptured battery safety. Under heating conditions, the rupture time of battery safety valves with the same SOC and SOH is related to the heating power and heating area [16,17]. In this study, an 80 kW propane burner was used to heat the LIB pack. The heating power of every battery was similar. The difference in safety valve rupture times was mainly affected by the heating area of the battery. As shown in Figure 3, the flame formed by the burner covered the external surface of the LIB pack. The burner flame heated the bottom and side of the outer batteries, and the burner flame heated only the bottom of the internal batteries. The heating area of the external batteries was more significant than that of the internal batteries, so the battery safety valves ruptured before those of the internal batteries. The battery safety valves ruptured from the outside to the inside of the pack, showing a relationship between the safety valve rupturing time and number of batteries in stage 1. With the propagation of TR, affected by the coverage area of the burner flame, the flame was concentrated in the middle of the LIB pack. The safety valves of batteries in the pack’s center ruptured first, and the batteries on the two sides were less heated by the flame. The heat causing battery TR mainly came from heat transfer of the batteries. Due to the low thermal conductivity of the battery, the heat transfer speed was slow and the battery safety valve ruptured later. Thus, the safety valve rupturing speed became slower, indicated by the increased slope of the fitting curve in the graph. In a word, through the analysis of the combustion flame changes and the correlation between safety valve rupture sequence and time, it was found that the rupture sequence of the safety valves of the LIB pack occurred from the middle to the sides and from the outside to the inside.
3.2. The Temperature Responses of the Pack
The LIB pack’s temperature is the most intuitive and persuasive parameter indicating the TR characteristics. The temperature responses of the pack are shown in Figure 6. The pack’s temperature gradually increased during the heating stage, which promoted exothermic reactions in the batteries. In previous studies, the cell temperature dropped by approximately 20 °C when the safety valve ruptured, mainly because the high-temperature gas that accumulated inside the cell was released along with some heat [18,19]. During the heating process, there was no apparent temperature drop caused by the rupture of the safety valve, which was because of excessive heat accumulation inside the LIB pack. The surface temperature of the pack gradually increased with the effect of thermal conduction from the propane burner and thermal radiation from the flame above the safety valve. Qi et al. [7] pointed out that a temperature rise rate (dT/dt) of 1 °C/s was the trigger for TR of the LFP battery and an internal short circuit occurred simultaneously. T1 measured the side temperature of the pack, which was close to the propane flame, where the temperature rose earliest. When the temperature of T2 reached 102 °C, the temperature rise rate (dT/dt) was 1 °C/s at 892 s. The combustion turned into fierce combustion due to a great deal of heat generated, which was promoted by the severe internal reactions of the batteries. Thus, it was considered that TR occurred in the battery closest to T2. The surface temperature of the pack measured by T1 exhibited the same trend at 1299 s. T2 was close to the middle and outside pack batteries, and TR occurred earliest in the batteries next to T2 due to continuous heating by the propane flame. The temperature of T2 was an earlier signal reflecting the characteristics of TR than the temperature of T1. The pack’s inside and side temperatures were measured by T3 and T4, respectively, which lagged behind the temperatures of T1 and T2 due to the later TR times (1914 s and 1952 s, respectively). TR propagated from the batteries closest to the heat source to the layers inside and on both sides of the pack. At 2344 s, the dT/dt of T3 exceeded 1°C/s once again, and the temperature reached 376 °C due to continuous TR inside the LIB pack. As shown in Figure 6, the maximum temperatures inside the LIB pack (T2 and T3) were much higher than those outside the pack (T1 and T4). Zhang et al. [20] reported that the maximum surface temperature of the LFP battery under the TR process was lower than 400 °C. Because of the LIB pack’s close arrangement and poor heat dissipation capability, continuous heat accumulation occurred inside the LIB pack, leading to a higher TR temperature than that of a single LFP battery.

Figure 6.
(a) Temperature and (b) dT/dt responses of LIB pack.
As shown in Figure 7, the propane flame temperature at the bottom of the LIB pack was approximately 790 °C, which triggered the TR of the LIB pack. The maximum flame temperature of the LIB pack under the TR process was 939.7 °C, which was close to that reported during the TR of an LFP battery [20]. The temperatures of T6 and T7 were earlier signals reflecting the characteristics of TR or flame of the LIB pack than the temperatures of T1–T4. T6 and T7 were located above the middle of the LIB pack, and the safety valve rupture time and TR time of the batteries in the middle of the LIB pack were earlier than those of other batteries. Due to the LIB pack’s poor heat dissipation capability and uneven thermocouple distribution, it was not easy to transfer heat to the T1–T4 thermocouples. Therefore, T6 and T7 would be the first to receive temperature signals. After 1578 s, the flame temperature gradually decreased while TR of the LIB pack was still in progress. This was because the TR of the LIB pack propagated from the middle to both sides.

Figure 7.
Flame temperatures above the safety valve and propane burner.
3.3. HRR and Total Heat Release (THR) of the Pack
HRR, a vital parameter indicating the degree of combustion and for defining the fire hazard, was determined by the oxygen consumption principle. The heat release per mass oxygen was 13.1 MJ kg−1. By integrating the HRR curve, THR was calculated. As shown in Figure 8, five successive HRR peaks were observed, and the values of the HRR peaks were 166, 314, 288, 170, and 190 kW, respectively. The THR of LIB pack combustion was 269.5 MJ. In addition, there were three small pulses before the first peak, whose values were 36, 56, and 71 kW, respectively. The three small pulses were formed by flammable gas ignition after the safety valves opened. Previous studies indicated that these combustible gases mainly include compounds from SEI film decomposition and reaction of intercalated lithium with the organic solvent [21,22]. The HRR peak appeared when the TR of batteries was triggered and violent jet fires were emitted [23]. Compared with previous studies [7,24,25], the combustion of the LIB pack had a higher HRR peak; its values were 4–8 times that of a single battery and 6–10 times that of gasoline, thus indicating an extreme thermal hazard.

Figure 8.
HRR and THR variations for LIB pack at different combustion states.
The five peaks indicated five stages of LIB pack combustion and combustion processes between five different battery layers. The curve between peaks indicated stable combustion processes between separate battery layers. Figure 9 shows the LIB pack combustion evolution process.

Figure 9.
Five stages of LIB pack combustion.
Stage 1 occurred after the first safety valve rupture. As the propane flame continuously heated the bottom and front surfaces of batteries in layer 1, the first battery TR generated the first HRR peak. In stage 1, the energy that caused battery TR mainly came from propane flame and self-heating. As the intense jet fire of batteries in layer 1 turned to stable combustion, the value of HRR gradually decreased and stage 1 ended.
Stage 2 was the result of intense combustion of the batteries in layer 2. The batteries in layer 2 were composed of two parts. The first part was on both sides of the LIB pack, in which the heating area was much smaller than that of layer 1. Therefore, the TR times were slightly later than those in layer 1. The second part was protected by layer 1 and the propane flame heated only the bottom of the batteries. At this point, the second part was also subjected to continuous heat transfer from batteries in layer 1. Zhou et al. [12] investigated the impact of heating position on battery TR and found that compared with front surface heating, bottom surface heating caused more intense exothermic reactions and a later TR time. The bottom surface heating mode led to a higher and later HRR peak than in stage 1.
Stage 3 was mainly due to the intense combustion of batteries in layer 3 and controlled by the bottom surface heating mode and heat transfer of batteries in layer 2. The batteries in layer 2 turned to stable combustion at this stage. Since the time difference between the second and third peaks was less than 200 s, the heat generated by layer 2 still contributed significantly to stage 3 of combustion. In 1658 s, the propane burner was turned off.
Stages 4 and 5 featured the intense combustion of the batteries in layers 4 and 5, respectively, which was mainly caused by TR propagation. The bottom surface heating mode did not cause battery TR. In the process of TR propagation, heat transfer between batteries was mainly conducted through the front surface [26,27]. This heat transfer mode determined that the TR of batteries in layer 4 was earlier than that of batteries in layer 5. The combustion process of the LIB pack ended when the flame of layer 5 went out.
3.4. Smoke and Mass Loss Analyses of LIB Pack Combustion
Other essential features of LIB pack combustion were gas and smoke release from chemical reaction, ejection, and combustion of battery materials [28,29]. The presence of CO2 increases the absorption rate of asphyxiant, thus increasing toxicity [30]. The O2 and CO2 concentration curves during LIB pack combustion are shown in Figure 10. The concentration of O2 was closely related to HRR and negatively associated with CO2 concentration. The intense combustion of the batteries caused a decrease in O2 concentration (decrease of 1.26%) and an increase in CO2 concentration (increase of 0.94%).

Figure 10.
Concentrations of O2 and CO2 as a function of time during LIB pack combustion.
Considerable research has been carried out to investigate the harmful gas generation of the TR process [21,31]. As shown in Figure 3, the batteries also generated a large amount of smoke during the combustion or TR process, which was overlooked in previous studies. The electrolytes and active materials inside the battery were burned, carbonized, and erupted, which was the main reason for smoke release. SPR is one of the critical parameters for characterizing the smoke-releasing process. By integrating the SPR curve, TSP was calculated. Their curves are shown in Figure 11. The SPR curve contained five peaks, of which the values were 0.6, 1.9, 1.9, 1.9, and 2.3 m2/s, respectively. The peaks of SPR corresponded to the peaks of HRR. At the initial combustion stage, smoke diffused in the space to form a small SPR peak. With the increase in HRR, SPR continuously increased. However, the flame strength was not the only reason for the rise in SPR. The highest value of SPR appeared at the fifth peak position, mainly caused by TR propagation. Due to heat dissipation in the process of TR propagation, the LFP battery combusted incompletely, which caused an increase in SPR [8,32,33]. After the combustion of the LIB pack, the space was still filled with a large amount of smoke and the SPR value was 1.5 m2/s. At 4000 s, the value of TSP reached 4074 m2.

Figure 11.
SPR and TSP during the LIB pack combustion process.
The initial mass was measured and then subtracted from subsequent measurements to determine the mass loss [34]. As shown in Table 1, the amount of mass loss per Ah during LIB pack TR (6.94 g) was much larger than that of a single LFP battery (6.12 or 4.86 g) and much less than that of an NCM battery (8.87 g). Mass loss was related to the degree of burning of the battery [32]. NCM batteries combust more violently than LFP batteries [5,11], leading to higher mass loss. In the LIB pack, the accumulation of TR and combustion energy caused more violent burning than that of a single battery, so the mass loss per Ah of the LFP battery pack was higher than that of a single LFP battery. Figure 12 shows a photograph of the LIB pack after the combustion test. After the pack was burned, the safety valves were broken and the deformation of the batteries was small due to the restriction of the steel plates. The electrolytes and active materials were burned and ejected from the batteries, which was the main reason for the mass loss.

Table 1.
Mass loss after the TR process.

Figure 12.
Photograph of the LIB pack after the combustion test.
3.5. Hazards Analysis of LIB Pack Combustion
Table 2 compares this study’s combustion features of fully charged LIBs with previous research. With the increased battery capacity for the TR process of a single LFP battery, the combustion time, normalized THR, and normalized mass loss were reduced, indicating that the degree of combustion for LFP batteries would decrease. However, the heat release was more concentrated and the internal heat was more challenging to release, thus increasing the maximum temperature (Tmax) and HRR (HRRmax). The TR temperature and normalized mass loss of LFP batteries are much lower than those of NCM batteries. For the TR process of the LFP LIB pack, there will be more than one battery TR at the same time, which leads to a higher HHRmax value (more than eight times that of a single 60 Ah battery) under heating conditions. The heat dissipation of the battery pack is worse and more energy will accumulate inside the battery pack, which leads to higher Tmax, normalized THR, and normalized mass loss. Therefore, the thermal and flame hazards of each battery in the LFP LIB pack during TR are higher than those of a single battery. This suggests that it is necessary to strengthen heat dissipation to reduce the TR hazard of each battery in a pack.

Table 2.
Combustion hazards of LIBs.
4. Conclusions
This work conducted a combustion test on an LIB pack consisting of 24 large-format prismatic LiFePO4 LIBs. The fire behavior, temperature, HRR, SPR, and mass loss were analyzed to help more deeply reveal the burning features and hazards of LIB packs. The TR propagation process of the LIB pack was shown from these two perspectives: propagation route and influencing factors. The main conclusions are as follows:
The combustion of the LIB pack was similar to that of a single battery, with four elements: safety valve rupture, stable combustion, jet fire, and gradual extinction. The difference is that the LIB pack had multiple jet fires during combustion, the final combustion time reached 2787 s, and the height of the jet flame was more than 1.2 m. The safety valve rupture process of the LIB pack was divided into two stages according to the linear fitting results: the rapid propagation stage affected by propane flame and the slow propagation stage affected by heat transfer between batteries.
The combustion of the LIB pack had five HRR peaks with values of 166, 314, 288, 170, and 190 kW, respectively. These five HRR peaks corresponded to the five stages of the LIB pack combustion. In stage 1, the combustion of the batteries was caused by the heat from the external heat source; the heat from the external heat source and heat transfer between batteries caused TR and combustion in stages 2 and 3. It was mainly the heat transfer between batteries that led to battery TR in stages 4 and 5. The TR propagation of the LIB pack included two modes, the first was from the outside to the inside, and the second was from the middle to both sides. These two modes were affected by the heating area of the external heat source and heat transfer between the batteries.
The combustion of the LIB pack caused an increase in CO2 concentration, a decrease in O2 concentration, and produced much smoke. The highest SPR (2.3 m2/s) was reached during stage 5 of combustion and the TSP was 4074 m2. The mass loss analysis found that the LIB pack had a higher normalized mass loss (6.94 g/Ah) than a single LFP battery.
Author Contributions
Conceptualization, H.C., Y.L. (Youwei Liu) and M.Z.; Data curation, H.C.; Formal analysis, H.C.; Funding acquisition, K.Y.; Investigation, H.C.; Methodology, H.C., Y.L. (Youwei Liu), M.Z. and Y.L. (Yilin Lai); Supervision, K.Y.; Visualization, K.Y., H.L. and J.L.; Writing—original draft, H.C.; Writing—review & editing, K.Y., Y.L. (Youwei Liu), H.L. and Z.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the special program for independent research of state key laboratory of operation and control of renewable energy & storage systems (grant number: DG71-22-017).
Data Availability Statement
Not applicable.
Conflicts of Interest
No conflict of interest exists in the submission of this manuscript, and the manuscript has been approved by all authors for publication.
References
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Xu, C.; Jin, C.; Li, K.; Du, Z.; Wang, Q.; Ouyang, M.; Feng, X. Dynamic thermophysical modeling of thermal runaway propagation and parametric sensitivity analysis for large format lithium-ion battery modules. J. Power Sources 2022, 520, 230724–230742. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhao, Z.; Wang, Q.; Jin, C.; Li, Y.; Sheng, J.; Li, K.; Du, Z.; Xu, C.; et al. An experimental analysis on thermal runaway and its propagation in Cell-to-Pack lithium-ion batteries. Appl. Therm. Eng. 2022, 211, 118418–118433. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Rui, X.; Wang, S.; Jin, C.; He, L.; Zhang, F.; Wang, Q.; Feng, X. A comparative analysis on thermal runaway behavior of Li (NixCoyMnz)O2 battery with different nickel contents at cell and module level. J. Hazard. Mater. 2020, 393, 122361–122380. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Zhao, C.; Chen, H.; Wang, Q.; Sun, J. Experimental and modeling analysis of jet flow and fire dynamics of 18650-type lithium-ion battery. Appl. Energ. 2021, 281, 116054–116064. [Google Scholar] [CrossRef]
- Qin, P.; Jia, Z.; Wu, J.; Jin, K.; Duan, Q.; Jiang, L.; Sun, J.; Ding, J.; Shi, C.; Wang, Q. The thermal runaway analysis on LiFePO4 electrical energy storage packs with different venting areas and void volumes. Appl. Energy 2022, 313, 118767–118778. [Google Scholar] [CrossRef]
- Peiyan, Q.I.; Jie, Z.M.; Da, J.; Kai, Y.; Jianling, L.; Yilin, L.; Fei, G.; Hao, L. Combustion characteristics of lithium–iron–phosphate batteries with different combustion states. eTransportation 2022, 11, 100148–100156. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Wang, B.; Liew, K.M.; Yang, L. Experimentally exploring thermal runaway propagation and prevention in the prismatic lithium-ion battery with different connections. Process Saf. Environ. Prot. 2022, 164, 517–527. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Ke, W.; Wang, W.; Zhang, S.; Zhou, B. Effect of lithium-ion battery diameter on thermal runaway propagation rate under one-dimensional linear arrangement. Therm. Sci. Eng. Prog. 2022, 31, 101301–101312. [Google Scholar] [CrossRef]
- Zhai, H.; Li, H.; Ping, P.; Huang, Z.; Wang, Q. An experimental-based Domino prediction model of thermal runaway propagation in 18,650 lithium-ion battery modules. Int. J. Heat Mass Transfer 2021, 181, 122024–122034. [Google Scholar] [CrossRef]
- Mao, B.; Liu, C.; Yang, K.; Li, S.; Liu, P.; Zhang, M.; Meng, X.; Gao, F.; Duan, Q.; Wang, Q.; et al. Thermal runaway and fire behaviors of a 300 Ah lithium ion battery with LiFePO4 as cathode. Renew. Sust. Energ. Rev. 2021, 139, 110717–110731. [Google Scholar] [CrossRef]
- Zhou, Z.; Ju, X.; Zhou, X.; Yang, L.; Cao, B. A comprehensive study on the impact of heating position on thermal runaway of prismatic lithium-ion batteries. J. Power Sources 2022, 520, 230919–230930. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Zhu, J.; Chen, S.; Han, G.; Dai, H. Revealing the failure mechanisms of lithium-ion batteries during dynamic overcharge. J. Power Sources 2022, 543, 231867–231882. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Zhang, M.; Li, S.; Gao, F.; Duan, Q.; Sun, J.; Wang, Q. The efficiency and toxicity of dodecafluoro-2-methylpentan-3-one in suppressing lithium-ion battery fire. J. Energy Chem. 2022, 65, 532–540. [Google Scholar] [CrossRef]
- Sun, X.; Tang, F.; Lu, K.; Ren, F.; Shi, C.; Merci, B.; Hu, L. Fundamentals of window-ejected fire plumes from under-ventilated compartment fires: Recent progresses and perspectives. Prog. Energy Combust. Sci. 2023, 94, 101039–101076. [Google Scholar] [CrossRef]
- Jin, C.; Sun, Y.; Wang, H.; Lai, X.; Wang, S.; Chen, S.; Rui, X.; Zheng, Y.; Feng, X.; Wang, H.; et al. Model and experiments to investigate thermal runaway characterization of lithium-ion batteries induced by external heating method. J. Power Sources 2021, 504, 230065–230076. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, T.; Wang, Q. Experimental study on the influence of different heating methods on thermal runaway of lithium-ion battery. J. Energy Storage 2021, 42, 103063–103072. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Duan, Q.; Chen, M.; Xu, J.; Zhao, C.; Sun, J.; Wang, Q. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801–101811. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, P.; Duan, Q.; Zhao, C.; Wang, Q. Experimental investigation on the cooling and suppression effects of liquid nitrogen on the thermal runaway of lithium ion battery. J. Power Sources 2021, 495, 229795–229806. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, W.; Qin, P.; Duan, Q.; Wang, Q. Numerical modeling on thermal runaway triggered by local overheating for lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116928–116937. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302–104314. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Peng, Y.; Zhang, F.; Ren, D.; Liu, X.; Lu, L.; Nitta, Y.; Wang, L.; Ouyang, M. Reductive gas manipulation at early self-heating stage enables controllable battery thermal failure. Joule 2022, 6, 2810–2820. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Mao, B.; Chen, M.; Huang, Z.; Wang, Q. Experimental study on thermal runaway and fire behaviors of large format lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116949–116962. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, J.; Chen, M.; Weng, J.; Wang, J. An Experimental Study on the Thermal Failure Propagation in Lithium-Ion Battery Pack. J. Electrochem. Soc. 2018, 165, A2184–A2193. [Google Scholar] [CrossRef]
- Gao, S.; Feng, X.; Lu, L.; Kamyab, N.; Du, J.; Coman, P.; White, R.E.; Ouyang, M. An experimental and analytical study of thermal runaway propagation in a large format lithium ion battery module with NCM pouch-cells in parallel. Int. J. Heat Mass Transfer 2019, 135, 93–103. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Xu, C.; Wu, W.; Zhang, W.; Hou, J.; Rui, X.; Chen, Y.; Fan, L.; Feng, X.; et al. Multi-objective optimization of side plates in a large format battery module to mitigate thermal runaway propagation. Int. J. Heat Mass Transfer 2022, 186, 122395–122413. [Google Scholar] [CrossRef]
- Li, K.; Xu, C.; Wang, H.; Jin, C.; Rui, X.; Chen, S.; Feng, X.; Fan, L.; Ouyang, M. Investigation for the effect of side plates on thermal runaway propagation characteristics in battery modules. Appl. Therm. Eng. 2022, 201, 117774–117794. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257–227270. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A comprehensive investigation on the thermal and toxic hazards of large format lithium-ion batteries with LiFePO4 cathode. J. Hazard. Mater. 2020, 381, 120916–120927. [Google Scholar] [CrossRef]
- Takagishi, Y.; Tozuka, Y.; Yamanaka, T.; Yamaue, T. Heating simulation of a Li-ion battery cylindrical cell and module with consideration of gas ejection. Energy Rep. 2022, 8, 3176–3188. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, Q.; Duan, Q.; Li, Y.; Li, L.; Wang, Q. Experimental investigation on thermal runaway propagation of large format lithium ion battery modules with two cathodes. Int. J. Heat Mass Transfer 2021, 172, 121077–121091. [Google Scholar] [CrossRef]
- Lai, X.; Wang, S.; Wang, H.; Zheng, Y.; Feng, X. Investigation of thermal runaway propagation characteristics of lithium-ion battery modules under different trigger modes. Int. J. Heat Mass Transfer 2021, 171, 121080–121095. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Meng, N.; Yu, Z.; Yang, H. Study of thermal runaway and the combustion behavior of lithium-ion batteries overcharged with high current rates. Thermochim. Acta 2022, 715, 179276–179288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).