Abstract
Using a wide range of organic substrates in the methane fermentation process enables efficient biogas production. Nonetheless, in many cases, the efficiency of electricity generation in biogas plant cogeneration systems is much lower than expected, close to the calorific value of the applied feedstock. This paper analyses the energy conversion efficiency in a 1 MWel agricultural biogas plant fed with corn silage or vegetable waste and pig slurry as a feedstock dilution agent, depending on the season and availability. Biomass conversion studies were carried out for 12 months, during which substrate samples were taken once a month. The total primary energy in the substrates was estimated in laboratory conditions by measuring the released heat (17,760 MWh·year−1), and, in the case of pig slurry, biochemical methane potential (BMP, (201.88 ± 3.21 m3·Mg VS−1). Further, the substrates were analysed in terms of their chemical composition, from protein, sugar and fat content to mineral matter determination, among other things. The results obtained during the study were averaged. Based on such things as the volume of the biogas, the amount of chemical (secondary) energy contained in methane as a product of biomass conversion (10,633 MWh·year−1) was calculated. Considering the results obtained from the analyses, as well as the calculated values of the relevant parameters, the biomass conversion efficiency was determined as the ratio of the chemical energy in methane to the (primary) energy in the substrates, which was 59.87%, as well as the electricity production efficiency, as the ratio of the electricity produced (4913 MWh·year−1) to the primary energy, with a 35% cogeneration system efficiency. The full energy conversion efficiency, related to electricity production, reached a low value of 27.66%. This article provides an insightful, unique analysis of energy conversion in an active biogas plant as an open thermodynamic system.
1. Introduction
The energy carriers used in global transportation today are primarily derived from fossil fuels. This causes an increase in greenhouse gas emissions [1,2,3,4]. Europe is striving to become a greenhouse-gas-neutral continent with policies oriented towards a modern economy. Accordingly, the European Union has for many decades supported the intensification of renewable energy sources (RES). In Poland, the organic waste energy recovery sector, in principle, has been developing since around 2010 [5,6,7].
Currently, the main feedstock for biogas production for energy purposes is waste from agricultural and food production, including livestock production, as well as corn silage [8,9]. Microorganisms transform the organic compounds contained in waste substrates in anaerobic conditions [10,11,12]. By means of specific fermentation or anaerobic respiration, they produce biogas, which consists mainly of methane (50–65%), carbon dioxide (30–45%) and other gases in small quantities, including ammonia and hydrogen sulphide [13,14,15]. From a biochemical point of view, anaerobic processes break down sugars, protein and fat.
Equation (1) illustrates the decomposition of an organic compound in methane fermentation. The subscripts c, h, o, n, s, y and x denote the number of atoms present in the chemical compound molecule and/or involved in the anaerobic biodegradation reaction [14,16].
CcHhOoNnSs + yH2O → xCH4 + (c − x)CO2 + nNH3 + sH2S
It must be noted, however, that despite the high potential of the Polish market in terms of waste and agricultural substrate availability, investors are still keen to grow maize for energy purposes. In times of a global energy crisis, energy carriers must be conserved, and low-cost alternatives to maize must be used. Anaerobic technologies offer high potential in the management of bio-organic wastes [17,18]. Vegetable waste is mainly hulls, oil cake or whole plants that do not meet the quality requirements. Due to its composition, including mainly simple and complex sugars, this material should be processed as feedstock in anaerobic digestion. Plants that use agricultural and food production waste as feedstock operate both in Poland and elsewhere across the world [19,20]. Such solutions enable the optimum use of the plant resources harvested.
Biogas is typically produced continuously under suitable environmental conditions at a pH of around 7 [21,22]. Its composition depends on the kind of chemical compounds undergoing biodegradation. This process is based on a one- or two-stage system, separating hydrolysis and acid fermentation from methanogenesis in a varying number of digesters, depending on the biogas plant capacity. To efficiently carry out anaerobic digestion, it is imperative to prepare suitable feedstock for the plant and to create the correct environmental conditions [23]. Using a continuous process when processing large volumes of waste is more advantageous. Further, a process carried out at temperatures suitable for thermophilic microflora runs faster and enables the use of smaller reactor volumes [24]. Yet, it should be mentioned that the increased biochemical reaction rate, which follows an increase in temperature, does not comply with the Arrhenius rule or Van’t Hoff rule. Thus, the transition from mesophilic to thermophilic conditions should not be expected to bring a two- or three-fold increase in process speed.
This paper analyses the efficiency of primary to secondary energy conversion in an anaerobic digestion process. Primary energy is a naturally occurring energy form that has not undergone any man-made conversion process. It exists as non-renewable energy (fuel chemical energy) and renewable energy (solar, hydro, geothermal and biomass, including organic waste, among other things). Secondary energy is the result of converting primary energy into carrier form [25,26,27]. In the anaerobic degradation process, the intermediate secondary energy carrier is methane [28,29]. Both forms are secondary energy. Losses occur at every stage of the energy chain [30]. The next stage in the chain, which involves converting chemical energy contained in methane into electricity, proceeds relatively poorly. When biogas is burned in a CHP engine, the generated heat can be recovered to provide additional energy. When heat and losses are not managed, the efficiency is estimated to be around 35% [31].
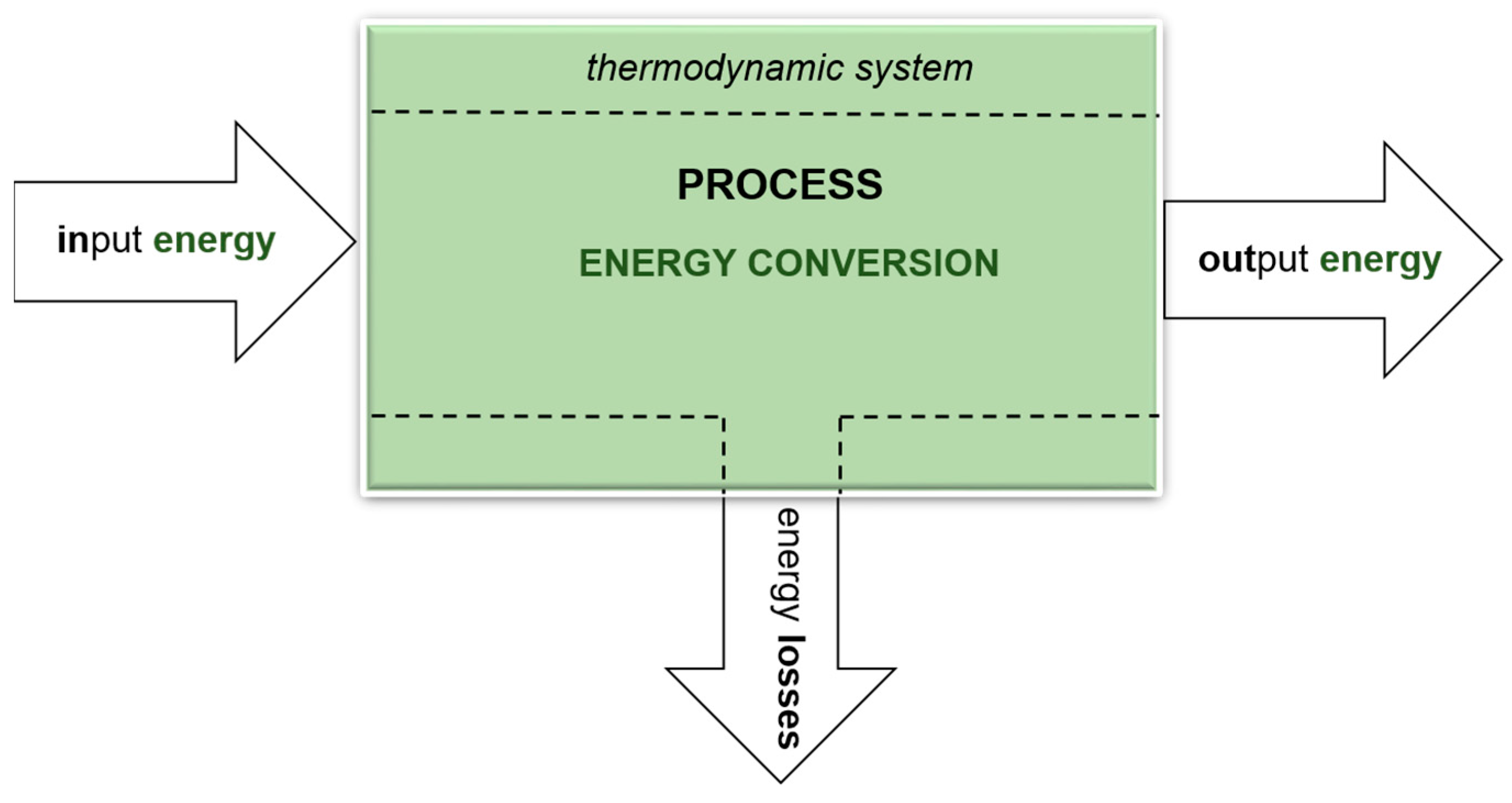
Figure 1 shows a simple diagram of the conversion of one energy type into another, as occurring in a thermodynamic system. A biogas installation is an open thermodynamic system in which both matter and energy are exchanged with the environment. Substrates are the carriers of primary energy. The output (secondary) energy is always less than the input (primary) energy. This means that the energy efficiency, as the degree of energy conversion in a process, is always less than 1, which is associated with the occurrence of losses and reduced fuel efficiency [32,33].
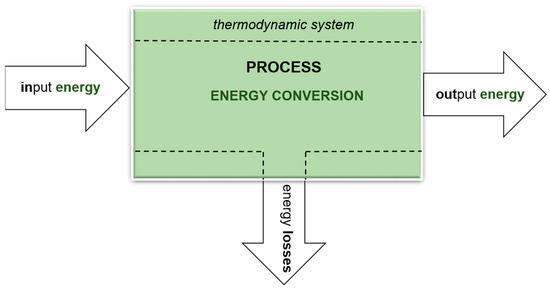
Figure 1.
General scheme of energy conversion in a thermodynamic system, in a specific process (author’s own scheme).
This study aimed to analyse the energy transformation efficiency in methane fermentation carried out at a technical scale using substrates in the form of maize silage and agricultural and food waste. The final stage involved estimating the electricity generation efficiency as the efficiency of the full energy conversion of the biogas plant.
2. Materials and Methods
2.1. Substrates
The analysed biogas installation, located on a farm in Poland’s Wielkopolskie Voivodeship, mainly used maize silage (MS) as the feedstock. For economic reasons and depending on the season and availability, vegetable waste was used as an MS substitute at 50% of the total solids stream. The remaining feedstock was MS. The waste stream included onion (ONI), carrot (CAR), potatoes (POT), celery (CEL), leek (LE) and parsley (PAR). The maize silage used at the plant was produced “on site”—on the farm—while the vegetable waste was supplied from a nearby production facility. Further, the plant was fed with pig slurry (PS), sourced from the same farm, whose function was to hydrate the feedstock. Thus, from a logistical and economic perspective, the most favourable solutions were used.
Table 1 shows the percentage of each feedstock fraction. The percentage content was determined based on the weight of waste raw materials applied to the digesters.

Table 1.
Percentage of each fraction in the feedstock.
2.2. Physicochemical and Chemical Analysis of Materials
The energy value (EV) of the test materials (except for pig manure, which is explained later in this section) was determined by burning dried and crushed samples of the test substrates using a CB 370 ballistic bomb calorimeter (Gallenkamp, Cambridge, UK) in a specialised laboratory. The bomb calorimeter consisted of a sealed vessel made of acid-resistant stainless steel with reinforced walls, making it possible to burn the fuel placed inside it. The vessel was placed in a calorimeter, which was used to measure the amount of heat released from the initiation of the reaction until thermal equilibrium. The bomb used in the experiment to measure the heat of solids combustion was equipped with a bottom that enabled the burnt sample to be placed inside the bomb, as well as a valve for introducing oxygen and contact electrodes. The combustion heat was measured based on the volume and temperature of the air escaping from the calorimeter (kJ·100 g−1).
Substrate and sample physicochemical analyses were carried out using the following methods: pH—potentiometric analysis (Elmetron CP-215, Zabrze, Poland); total solids, TS—weight analysis (a Zalmed SML dryer, Zalmed, Łomianki, Poland), which was the method used to simultaneously determine the water content of the materials tested; volatile solids, VS—gravimetric analysis (combustion at 550 °C) (MS Spectrum PAF 110/6 oven, Warsaw, Poland) [1,2].
The quantification of protein, fat, minerals (insoluble ash), as well as starch and total dietary fibre, was carried out according to the procedures described below.
- Protein—estimated from total Kjeldahl nitrogen; AOAC 920.87 [34].
- Fat—extracted using a Soxhlet apparatus, model Büchi B-811, (Büchi Labortechnik AG, Flawil, Switzerland), AOAC 920.85 [35].
- Mineral matter—ash, gravimetric analysis (RADWAG electronic laboratory scale AS R2 PLUS, RADWAG, Radom, Poland) [36];
- Starch—Luff–Schoorl titration method; the determination principle is based on the reduction reaction of Cu+2 ions contained in the Luff fluid by the reducing saccharides present in the solution tested. The reaction takes place in an alkaline environment (pH of about 9.5), at boiling point. The Luff fluid consists of copper (II) sulphate (VI), sodium carbonate and citric acid [37];
- Dietary fibre method—determined in the undigested fraction with the use of 0.25 N H2SO4 and 0.25 N NaOH, AOAC 962.09 [38].
A gas chromatography method (GC-2014 gas chromatograph, Shimadzu, Kyoto, Japan) was used to determine the glucose, fructose and sucrose content. To this end, non-volatile saccharides were converted into more volatile derivatives, such as trimethylsilyl. Once the column had been appropriately selected using chromatography and separated into individual sugars, the saccharides were identified and their quantitative analysis was conducted based on the chromatographic peak areas.
Table 2 shows the analytical results for protein, fat and total sugars, as well as ash, total solids and volatile solids, for all substrates except pig manure.

Table 2.
Selected parameters of the materials tested, in relation to 100 g fresh weight.
Table 3 summarises the results concerning the different types of sugars—including glucose, fructose, sucrose, starch and dietary fibre—for the same substrates.

Table 3.
Content of the different sugar types in the substrates tested, relative to 100 g fresh weight.
The micronutrient and macronutrient content of the materials used during the study (see Table 4) was analysed using atomic absorption spectrometry, ASA (ZA3300 ASA spectrometer, Hitachi, Tokyo, Japan).

Table 4.
Selected minerals in the substrates tested, relative to 100 g fresh weight.
In the case of the pig manure, only the necessary parameters were determined due to the different methodologies for determining the energy value compared to most of the substrates tested (see Table 5).

Table 5.
Physicochemical parameters of the slurry used.
2.3. Biogas Production at a Laboratory Scale
Pig slurry, used in biogas plant operations as a feedstock diluting agent, was the only substrate for which the biochemical methane potential (BMP) was determined at a laboratory scale. In this case, the BMP is an intermediate parameter in estimating a material’s energy value. Determining the energy value by measuring the combustion heat using the calorimetric bomb was impossible due to the low solids content of the pig slurry. The most important physicochemical properties of the pig slurry are presented in Table 4. The fermenting mixture from the analysed biogas plant was used as the inoculum in the experiment. The content of feedstock and inoculum in the batches was consistent with the standard [39] where the amount of total solids in the inoculum ranged from 1.5% to 2%, whereas in the fermenting mixture, they did not exceed 10%.
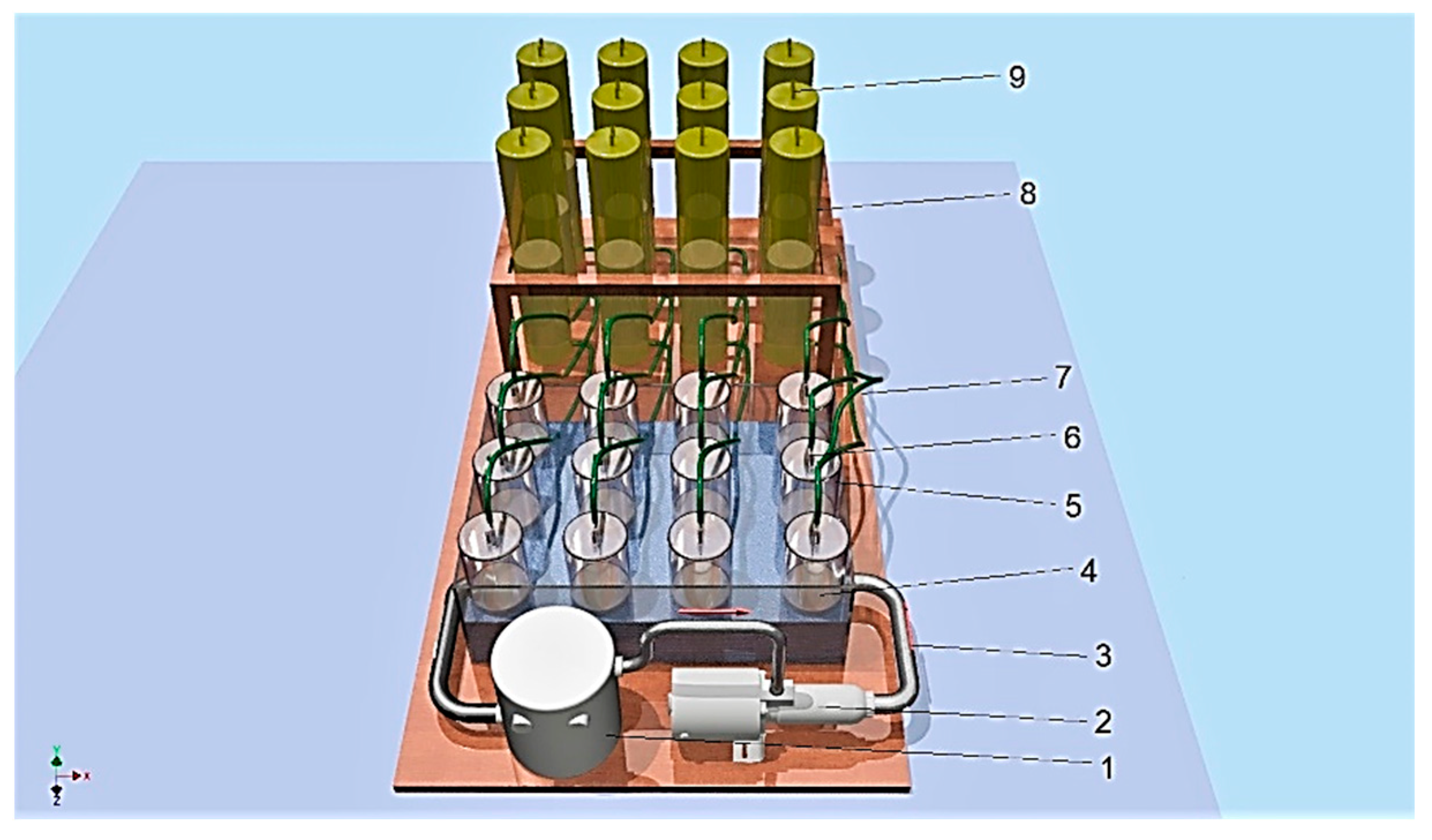
The slurry’s biochemical methanogenic potential was determined in an anaerobic bioreactor working in batch mode (see Figure 2), under mesophilic conditions (38 °C). The biofermentors were placed in a water jacket (4) and connected to a heater (1) [9,20]. The gas produced in the process flowed (7) to the tanks (8), where it was stored. The HRT (hydraulic retention time) was 14 days. Under the DIN 38 414-S8 standard [40], the study was carried out until the daily biogas production of all biofermentors fell below 1% of the total production. The biogas volume obtained from the slurry was measured every 24 h. The methane, carbon dioxide, hydrogen sulphide, ammonia and oxygen concentrations in the biogas were measured using a Geotech GA5000 gas analyser (Geotech, Bydgoszcz, Poland). The efficiency of the biogas production (m3 Mg−1) from the total solids and volatile solids was estimated according to the results of the experiment. The BMP for the pig slurry as a substrate was calculated by subtracting the biogas volume produced by the inoculum alone from the biogas volume produced by the samples [9,20,41].
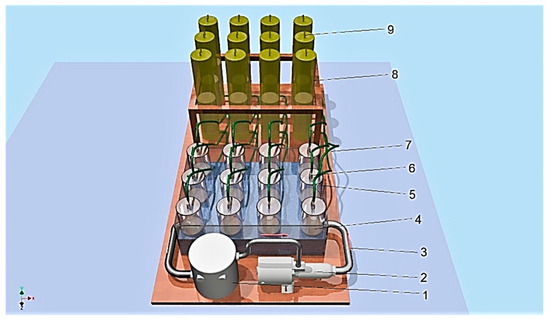
Figure 2.
Anaerobic biodigesters: 1—heater, 2—pump, 3—heating tube, 4—jacket (38 °C), 5—biodigester (1.4 L), 6—slurry valve, 7—pipe, 8—tank, 9—biogas valve.
2.4. Biogas Production at a Technical Scale
A technical-scale production was run for 12 months at a biogas installation in the Wielkopolskie Voivodeship, with a 1 MWel cogeneration system. The plant comprised three digesters (two primary digesters and one secondary digester, which also acted as a digestate tank). The primary digesters contained three paddle agitators with adjustable pitches, operating in interval mode (triggered at specific time intervals). The mixing time, 20 min·h−1 in the case of the biogas plant analysed, was determined based on observations and practical experience, considering the substrate properties, the size of the digester tanks and its propensity to form scum. Notably, the interval mode is by far the most popular in biogas plants due to the high energy intensity of the mixing equipment.
The feedstock—maize or vegetable waste silage—were sampled once a month to determine their energy value (as stated in Section 2.1). At least three samples were taken and tested each time. Analysed for representativeness, the material was then tested. In estimating measurement uncertainty, this study used procedures compliant with Polish and German standards [42,43]. The energy value results obtained for the samples tested over the year were averaged.
As previously reported, the maize silage was replaced by vegetable processing waste. On average, the vegetable processing waste stream replaced 25 Mg of maize silage each year. The initial plans provided for the plant to be fed with 50 Mg of maize silage each day. Hence, half of this amount was successfully replaced by vegetable waste. This approach has made it possible to use waste that is a valuable calorific material for methane fermentation. The amount of biogas produced (average values per day were used for the calculations) was read by the operator using an ST51 thermal gas mass flow meter (Introl, Sp. z o.o., Katowice, Poland), located upstream of the cogeneration unit. The biogas plant in question is a testament to a successful regenerative system that minimises raw material consumption, waste volume, emissions and energy losses by creating a closed process loop (i.e., circular economy).
The data values required to calculate the plant performance parameters (biomass energy conversion efficiency and electricity generation efficiency) specified in the paper’s aim were determined step by step based on well-known chemical and physical relationships, as presented in the text, and are summarised in a logical sequence.
3. Results and Discussion
3.1. Substrate Composition
The starting point of the research carried out in this study was the analysis of the total solids, volatile solids and mass content of biodegradable compounds, including the protein, fat and total carbohydrates in all substrates except pig slurry (Table 2). The confirmed high moisture content of vegetable waste indicates that it is unsuitable for incineration or storage. Yet, as indicated by numerous literature sources [44,45], the composition of vegetables makes them suitable substrates for biogas plants. One limitation of their anaerobic digestion, like in the case of other plant wastes, is the potential for rapid acidification and increased production of VFAs (volatile fatty acids), which reduce the anaerobic reactor’s activity [8,20]. At the biogas plant in question, the pig slurry used as a low-TS and pH-neutral diluting agent (see Table 5), as well as a source of nitrogen [46], also acted as a stabilising buffer for the system. Tests carried out for the same materials on monosaccharides (glucose, fructose and sucrose), as well as on starch and dietary fibre (Table 3), indicate that the simple sugar content (particularly fructose) in vegetables is lower than in fruit [44,47], which significantly limits the risk of an adverse pH drop. Essentially, carbohydrates are the building blocks for methane production, and in the case of vegetables, their sources are mainly dietary fibre and starch (see Table 3). The purpose of conducting a detailed analysis of the sugars in vegetables and other nutrients (Table 2) was to obtain information on the chemical composition of the substrates. On this basis, the owner of the biogas plant should initially estimate the methanogenic potential of the batch introduced into the installation.
As the substrates used in the plant studied, maize silage and potatoes had the highest carbohydrate content: 23.5 ± 0.023 g·100 g−1 and 20.5 ± 0.02 g·100 g−1, respectively (Table 2). The fewest carbohydrates were found in celery (7.7 ± 0.076 g·100 g−1) and leeks (5.7 ± 0.006 g·100 g−1). These values were confirmed by the results of the analyses concerning the individual sugars contained in the raw materials (Table 3). Maize silage contained a significant amount of starch, amounting to 123 ± 0.072 g·100 g−1, as did potatoes, at 6.6 ± 0.097 g·100 g−1. Carrots and onions had the least starch, at 0.002 g·100 g−1 and 0.1 ± 0.001 g·100 g−1, respectively. Celery and parsley had the highest fibre content, at 4.9 ± 0.029 g·100 g−1 and 4.2 ± 0.025 g·100 g−1, respectively. As for the group of simple sugars, the highest amount of sucrose was found in parsley at 4.8 ± 0.028 g·100 g−1. The potato was characterised by a very low simple sugar content. By contrast, onions contained the most glucose, at 1.7 ± 0.01 g·100 g−1, and fructose, at 1.5 ± 0.009 g·100 g−1. The highest amount of protein was found in maize silage—3.7 ± 0.013 g·100 g−1—and parsley—2.6 ± 0.009 g·100 g−1 (Table 2). Fat was also most abundant in maize silage (1.5 ± 0.011 g·100 g−1).
The present study also included an analysis of the micronutrients and macronutrients contained in the substrates (see Table 4). Knowledge of the mineral matter content makes it possible to balance the feedstock nutrients. It is very practical and makes it easier to make decisions regarding the supplementation of biogas plants (minerals are responsible for specific metabolic pathways). In the case of the present study, the mineral content was optimal for the functioning of the bacterial flora without inhibiting the process at the same time [48,49].
As shown by the above analysis, the chemical composition of maize silage is the most favourable compared to the raw materials used in the plant under study. In practice, maize silage remains the most commonly used material in biogas installation due to its widespread availability and high nutrient content. However, as highlighted in the paper’s introduction, alternatives to maize silage—today’s biogas market mainstay—should be sought out due to the need for crop rotation and rising maize silage prices.
The results of the analyses presented in Table 2, Table 3 and Table 4 are intended to illustrate the chemical composition of the individual feedstocks in the substrate stream of the biogas plant in question. Analysis of protein, fat and sugar quantities enabled a theoretical verification of the substrate energy potential, which any professionally operated biogas plant should exploit optimally [10,14]. These data make it possible to estimate the maximum proportion of methane in biogas, considering the stoichiometry of the conversion occurring according to Equation 1. In principle, this study did not aim to identify such data. Nonetheless, when analysing biogas plant operations, including energy transformation efficiency, several pertinent issues must be raised.
Creating up-to-date studies of substrates’ chemical composition and their BMP is a key aspect of biogas plant operation [50]. Any biogas plant should be regarded as a professional plant for the processing of organic matter, including waste, which enables the production of biogas containing biomethane as an energy carrier [1,51]. In practice, the biogas market, including in Poland, tends to overestimate the methane content of the biogas obtained and ignore the above indications. Neglect in terms of biogas plant technological processes is driving many operators involved in renewable energy production to bankruptcy. Due to a lack of competency, Poland is unable to make efficient use of energy carriers (methane) from widely available waste materials, which forces it to import billions of cubic metres of methane.
3.2. Slurry BMP and Calorific Value of Other Substrates
The pig slurry used in the biogas plant analysed was characterised by poor biogas yields from fresh matter (FM) due to its low TS content of 5.6 ± 0.06% (see Table 5). The amount of biogas produced in relation to FM, as indicated by the data collated in Table 6, was 18 ± 0.24 m3·Mg FM−1, while in terms of VS, it was 395.85 ± 5.66 m3·Mg VS−1, which is in line with the literature’s data [46,52]. Since the content of methane in the biogas obtained from PS was 51%, the BMP of this material is 201.88 ± 3.21 m3·Mg VS−1.

Table 6.
Biogas generation efficiency, including methane from pig slurry.
Table 7 shows the energy value (also called calorific value) of the individual fractions of the substrate stream fed daily into the plant. The calorific values (more precisely, combustion heat), obtained for individual samples using the calorimetric bomb and expressed in kJ·kg−1, were converted and presented in useful units (also for further calculations), including kWh·kg−1, MWh·day−1 and MWh·year−1.

Table 7.
Energy value of daily feedstocks (excluding slurry).
The suitability of plant biomass for energy purposes is largely conditioned by the heat of combustion, heating value and chemical composition [52,53]. These features have a major impact on the technological conditions of processing and the quality of the product obtained, and in the methane digestion process under discussion, on the quality of biogas [54]. The main component of biomass is coal, and it is the energy contained in it that is released during combustion [53]. As mentioned earlier in the article, simple and complex sugars are the main source of carbon in plant waste, including vegetable waste.
The first column of Table 7 shows the average daily amounts of substrates fed. The most caloric feedstock turned out to be maize silage. The daily value of energy provided by MS was 33,819 MWh·day−1, while the annual value was 12,344 MWh·day−1. The next substrate in terms of caloric value was potatoes, providing 7708 MWh·day−1 calories daily, and 2814 MWh·year−1 annually. The substrates of the lowest calorific value were celery 0.868 MWh·day−1 and leek 0.672 MWh·day−1 (see Table 7). These results correlate with the results of the carbohydrate content of the mentioned materials (Table 2 and Table 3).
The above analysis of the energy value of the substrates used in the plant in question provides information on the values of primary energy provided by each substrate to the system. The total value of primary energy in the biomass applied to the plant, after summing up the relevant data in Table 7 (columns 6–8), was 48,659 kWh·day−1, 48,659 MWh·day−1 and 17,760 MWh·year−1.
Currently, the dominant source of primary energy on Earth is the chemical energy of fossil fuels [25,26]. However, the prospects of the depletion of these fuels and the threat to the state of the environment intensify interest in RES. Biomass is one of the oldest and most widely used RES today, and as a result, it constitutes the world’s third largest natural energy (primary energy) source [3,55]. The heating value, as the basic energy parameter of biomass, is usually lower than that of conventional fuels. As confirmed by the values shown in Table 7, a characteristic feature of this parameter is the relatively wide dispersion of its values, which is due to the different chemical composition of the materials forming the harvested biomass. Important differentiating factors are plant species, place of growth, weather conditions, growing season and others.
3.3. Efficiency of Methane and Electricity Production in a Biogas Plant: Chemical Energy and Electricity
Secondary energy is the result of converting primary energy into carrier form. In the process of anaerobic degradation, methane is an intermediate carrier of secondary energy, which is part of biogas as the main product of the process. Biogas burned in a cogeneration system becomes a source of heat and electricity in one system based on internal combustion engines: the fuel burns in the engine and activates the generator, which converts mechanical energy into electricity [56,57].
Table 8 summarises the results of biogas production (annual, daily and, due to subsequent calculations, hourly). The amount of biogas produced annually (m3·year−1) was determined taking into account 365 days. The biogas plant operation was assumed to be 8000 h·year−1, thus excluding activities related to plant operation, maintenance, etc. The daily production of biogas from pig slurry and the substrates used was 7420 m3·day−1, the annual production was 2,708,300 m3·year−1 and the hourly production was 338.84 m3·h−1.

Table 8.
Biogas production in the plant under study.
Subsequently, the capacity of the plant was estimated in relation to the biogas output of the given feedstock, in MW units (Table 9). Taking into account the hourly biogas production—82.125 m3·h−1 for pig slurry and 256.41 m3·h−1 for other substrates—as well as the average methane in biogas (52 ± 1%), total chemical energy (bond energy) in m3 of methane (0.009968 MWh) and efficiency of the cogeneration system (35%), the power of the plant obtained from pig slurry was 0.15 MW and from other substrates 0.47 MW. The total power of the plant was 0.62 MW, where the designed capacity of the biogas plant is 1 MW. For the obtained power value, the amount of electricity produced was 4913 MWh·year−1. The above-discussed parameter values are shown in Table 9.

Table 9.
The obtained capacity of the biogas plant (MW) and the electricity produced (MWh·year−1).
In turn, Table 10 presents the results of the efficiency of the process of converting the energy accumulated in the feedstock (primary), which was 17,760 MWh·year−1 (Table 7), into the energy contained in the methane produced (secondary, chemical), amounting to 10,633 MWh·year−1. The value of the energy contained in CH4 was obtained by including the following in the calculation: the annual biogas production of substrates excluding pig slurry (2,051,300 m3·year−1, see Table 8), the average methane content in the biogas (52 ± 1%) and the total chemical energy in 1 m3 of methane (0.009968 MWh). The non-inclusion of pig slurry was due to its very low TS content (Table 5), which meant that it was mainly treated as a dilution factor in the study. The efficiency of biomass conversion in the plant under study (as the ratio of chemical energy in methane to primary energy in substrates, see Equation (2)) was 59.87%.

Table 10.
Efficiency of biomass conversion under anaerobic conditions (excluding pig slurry) and efficiency of electricity production in the cogeneration system.
where:
EF–bc—efficiency of biomass conversion (%),
E methane—chemical energy in methane (secondary), MWh·year−1;
E substrate—energy in substrates (primary), MWh·year−1.
At the last stage of the process, the efficiency of full energy conversion in the plant was determined. The energy efficiency of the biogas installation is the degree of conversion of the primary energy in the biomass introduced into the plant during the year into electricity. Thus, this parameter is to determine the efficiency of use of the fuel accumulated in the substrates. Knowing that the energy efficiency is the ratio of the amount of energy coming out of the process (the amount of electricity produced, estimated from the amount of biogas, including methane, and the capacity of the plant), which is 4913 MWh·year−1 (Table 8 and Table 9), to the amount of energy introduced into the process (the cumulative energy value of the substrates, see Equation (3)), which is 17,760 MWh·year−1, the energy efficiency of full conversion is 27.66%.
where:
E–EF—energy efficiency, %;
Electricity—electricity produced by the CHP system, MWh·year−1;
E substrate—energy in substrates (primary), MWh·year−1.
Taking into account the losses in the conversion of biomass into methane and the low efficiency of the cogeneration system, 35% (Table 9), the obtained low result of the energy efficiency of the plant under study, was considered reasonable and feasible.
Anaerobic digestion relatively efficiently converts the primary energy in the waste into the chemical energy contained in methane. With the methane content in the biogas at a level of 52%, as a result of biomass conversion, the chemical energy in CH4 was obtained at the level of 59.93%. However, steps can be taken to optimise the digestion process itself to increase the decomposion efficiency of organic matter into biogas. To this end, the approach to biogas plants needs to change. They should be considered biochemical industrial plants that require efficient technological supervision, due to the presence of many important factors that affect the efficiency of the plant. These include pH, process temperature, type of mixing system, C:N ratio and others. It is in the interest of biogas plant owners to maximise biogas production, with the highest possible content of methane as an energy carrier [51]. In Poland, it is common to observe the implementation of commercial anaerobic digestion processes at capacities far below their optimum value, due to various irregularities, including, among others, lack of knowledge of the chemical composition of the substrates used and, consequently, poor quantitative and qualitative selection of substrates and co-substrates, or failure to monitor key process stability parameters, etc. [58]. When implementing optimisation measures in the biogas plant under study, it is recommended to pay special attention to the environmental conditions prevailing in the digester, such as pH, buffer capacity or VFA concentration.
If the efficiency of biomass conversion obtained in this work was considered to be slightly underestimated, and the process to be in need of optimisation, the conversion of chemical energy contained in methane into electricity in the plant under study must be assessed as definitely inefficient. The efficiency of the cogeneration system of the biogas plant under study, in terms of electricity production (the efficiency of the internal combustion engine is about 40% minus the efficiency of the generator), is only 35%. Biogas was burned in gas engines driving power generators, but waste heat was not used (except for technological purposes). It is worth emphasising that most biogas plants in Poland operate in this way, which is a major problem that generates energy losses and results in low conversion efficiencies [59,60]. This factor is the direct cause of poor utilisation of the primary chemical energy accumulated in the feedstock. The energy efficiency of the full energy conversion in this study was only 27.66%.
Increasing the efficiency of the cogeneration system in biogas plants requires appropriate strategies, technologies and practices. The first recommendation is to select advanced internal combustion engines, gas turbines or other generators that can significantly improve the efficiency of the system, in addition to upgrading existing plants. Another important recommendation is to adapt the combustion process to the characteristics of biogas. Gas purification, precise regulation of the ratio of mixing gas and air and control of the combustion temperature are key factors in increasing and maintaining the high efficiency of the system. An equally important factor for increasing the efficiency of the system is the effective use of the heat produced, not only in technological processes (to heat digesters, etc.) but also on a larger scale, for drying and heating purposes [61]. However, its transport over long distances is difficult and is accompanied by unavoidable losses. On the other hand, heat storage generates high costs. Therefore, it is important that cogeneration plants are located near places with high heat consumption, because otherwise, heat recovery is neither interesting nor cost-effective. The solution is to build cogeneration units near medium-sized and large cities and enterprises where there is a demand for heat [62]. It would also have to be considered whether it would be more advantageous, in some situations, to burn the biogas in boilers for heating-only purposes. Undoubtedly, the priority in the operation of biogas plants should be obtaining biogas from waste and treating this process as the most environmentally friendly form of waste management.
It is also worth mentioning that the recently changing geopolitical situation and the related global energy crisis have contributed to the increasing role of renewable energy carriers. Large fluctuations in the prices of carriers derived from fossil fuels are observed on world markets. In Poland, policy in this area is more complicated, on the one hand, due to the large number of operating hard coal mines, and, on the other hand, the negative attitude of society towards new technologies. Polish scientists and some politicians are aware that biogas plants can be a sustainable source of energy, but despite public education in this area, a large group of people are against the construction of this type of installation.
4. Conclusions
Based on the results obtained in laboratory conditions (primary chemical energy accumulated in substrates, 17,760 MWh·year−1), the results obtained at a technical scale and the values of estimated parameters (secondary chemical energy contained in methane, 10,633 MWh·year−1), the biomass conversion efficiency was determined as the ratio of the secondary chemical energy of methane to the primary chemical energy of the substrates in this paper.
The obtained value of 59.87% indicated a relatively efficient process of biomass conversion in the process of anaerobic digestion carried out in the plant under study, which, however, requires optimisation measures to increase energy conversion. An important stage of this study was the estimation of the amount of electricity produced, which amounted to 4913 MWh·year−1, and then the efficiency of the full energy conversion in the substrates introduced into the installation. The efficiency of electricity production in relation to the total energy input (feedstock) reached a low value of 27.66%.
This article indicates the factors that reduce the total energy efficiency of the methane fermentation process. The main reason for the very low conversion efficiency of the primary chemical energy of the substrates was the low efficiency of the cogeneration systems of biogas plants operating in Poland, including the failure to utilise heat for broader purposes (beyond technological), including heating or drying. As a conclusion to the issues raised in the study and the results obtained, it was proposed to implement measures to increase the cogeneration efficiency, including the full use of waste heat or the combustion of produced gas in boilers for heating purposes only.
Author Contributions
Conceptualization, K.P.; methodology, K.P. and A.A.P.; software, K.P. and D.S.; validation, K.P. and A.A.P.; formal analysis, A.A.P. and A.K.-W.; investigation, K.P. and A.A.P.; resources, K.P., A.A.P. and A.K.-W.; data curation, K.P., A.A.P. and D.S.; writing—original draft preparation, K.P.; writing—review and editing, A.A.P.; visualization, K.P. and D.S.; supervision, K.P.; project administration, A.A.P. and A.K.-W.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicate.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Durczak, K.; Witaszek, K.; Mioduszewska, N.; Kowalik, I. The efficiency of industrial and laboratory anaerobic digesters of organic substrates: The use of the Biochemical Methane Potential Correction Coefficient. Energies 2020, 13, 1280. [Google Scholar]
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Witaszek, K.; Piekutowska, M.; Idzior-Haufa, M.; Wawrzyniak, A. Degree of biomass conversion in the integrated production of bioethanol and biogas. Energies 2021, 14, 7763. [Google Scholar]
- Masjuki, H.M.; Kalam, M.A. An Overview of Biofuel as a renewable energy source: Development and challenges. Procedia Eng. 2013, 56, 39–53. [Google Scholar]
- United Nations Industrial Development Organization. The European Green Deal: Europe’s New Growth Strategy a Climate-Neutral EU by 2050; UNIDO Liaison Office in Brussels: Bruxelles, Belgium, 2020. [Google Scholar]
- Pietrzak, M.B.; Igliński, B.; Kujawski, W.; Iwański, P. Energy Transition in Poland—Assessment of the Renewable Energy Sector. Energies 2021, 14, 2046. [Google Scholar] [CrossRef]
- Igliński, B.; Piechota, G.; Kiełkowska, U.; Kujawski, W.; Pietrzak, M.B.; Skrzatek, M. The assessment of solar photovoltaic in Poland: The photovoltaics potential, perspectives and development. Clean Technol. Environ. Policy 2023, 25, 281–298. [Google Scholar]
- Igliński, B.; Kiełkowska, U.; Pietrzak, M.; Skrzatek, M.; Kumar, G.; Piechota, G. The regional energy transformation in the context of renewable energy sources potential. Renew. Energy 2023, 218, 119246. [Google Scholar] [CrossRef]
- Witaszek, K.; Pilarski, K.; Niedbała, G.; Pilarska, A.A.; Herkowiak, M. Energy efficiency of comminution and extrusion of maize substrates subjected to methane fermentation. Energies 2020, 13, 1887. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of confectionery waste in biogas production by the anaerobic digestion process. Molecules 2019, 24, 37. [Google Scholar]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar]
- Weiland, P. Biogas Production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects—A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Waliszewska, B.; Zborowska, M.; Witaszek, K.; Waliszewska, H.; Kolasiński, M.; Szwarc-Rzepka, K. Evaluation of bio-methane yields for high-energy organic waste and sewage sludge: Apilot-scale study for a wastewater treatment plant. Environ. Eng. Manag. J. 2019, 18, 2023–2034. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.-Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources, 2nd ed.; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Stazi, V.; Tomei, M.C. Enhancing anaerobic treatment of domestic wastewater: State of the art, innovative technologies and future perspectives. Sci. Total Environ. 2018, 635, 78–91. [Google Scholar] [PubMed]
- Silva dos Santos, I.F.; Braz Vieira, N.D.; Bruni de Nóbrega, L.G.; Barros, R.M.; Tiago Filho, G.L. Assessment of potential biogas production from multiple organic wastes in Brazil: Impact on energy generation, use, and emissions abatement. Resour. Conserv. Recycl. 2018, 131, 54–56. [Google Scholar]
- Bouallagui, H.; Lahdheb, H.; Romdan, E.B.; Rachdi, B.; Rachdi, B.; Hamdi, M. Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J. Environ. Manag. 2009, 90, 1844–1849. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of vegetable dumplings waste from industrial production by anaerobic digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.V.N.; Anjum, H.; Chang, C.K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar]
- Kydes, A.; Cleveland, C.J. Primary energy. In Encyclopedia of Earth; Cleveland, C.J., Ed.; Environmental Information Coalition, National Council for Science and the Environment: Washington, DC, USA, 2006. [Google Scholar]
- Øvergaard, S. Issue Paper: Definition of Primary and Secondary Energy; Prepared as input to Chapter 3: Standard International Energy Classification (SIEC) in the International Recommendation on Energy Statistics (IRES) Norway; Oslo Group on Energy Statistics, Statistics Norway: Oslo, Norway, 2008. [Google Scholar]
- Zhu, B.; Liu, Y.; Liu, Z.; Yu, H. A study on primary energy and secondary Energy price in distributed Energy projects. In Proceedings of the CUE2018-Applied Energy Symposium and Forum 2018: Low Carbon Cities and Urban Energy Systems, Shanghai, China, 5–7 June 2018. [Google Scholar]
- Razmi, A.R.R.; Afshar, H.H.; Pourahmadiyan, A.; Torabi, M. Investigation of a combined heat and power (CHP) system based on biomass and compressed air energy storage (CAES). Sustain. Energy Technol. Assess. 2021, 46, 101253. [Google Scholar]
- Taeyoung, J. The effectiveness of combined heat and power (CHP) plant for carbon mitigation: Evidence from 47 countries using CHP plants. Sustain. Energy Technol. Assess. 2022, 50, 101809. [Google Scholar]
- Wang, C.; Long, W. The economic analyses of cogeneration cooling heating and power on residence application. J. Archit. Sci. 2004, 20, 155–159. [Google Scholar]
- Kabalci, E. Hybrid Renewable Energy Systems and Microgrids; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-821724-5. [Google Scholar]
- Popović, M. Entropy change of open thermodynamic systems in self-organizing processes. Therm. Sci. 2014, 18, 1425–1432. [Google Scholar]
- Luscombe, J. Thermodynamics; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2020; ISBN 978-0-36-757199-3. [Google Scholar]
- AOAC International. Association of Analytical Chemists Official Method 920.87. Protein (Total) in Flour. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- AOAC International. Association of Analytical Chemists Official Method 920.85. Fat (Crude) or Ether Extract of Flour. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- AOAC International. Association of Analytical Chemists Official Method 923.03. Ash of Flour. Direct Method. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- Marrubini, G.; Papetti, A.; Genorini, E.; Ulrici, A. Determination of the Sugar Content in Commercial Plant Milks by Near Infrared Spectroscopy and Luff-Schoorl Total Glucose Titration. Food Anal. Methods 2017, 10, 1556–1567. [Google Scholar]
- AOAC International. Association of Analytical Chemists Official Method 962.09. Fiber (crude) in animal feed and pet food. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- Norm VDI 4630; Fermentation of Organic Materials Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Engineers Club: Düsseldorf, Germany, 2006.
- DIN Guideline 38 414-S8; Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Institute for Standardization: Berlin, Germany, 1985.
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A. Cell immobilization on lignin–polyvinylpyrrolidone material used for anaerobic digestion of waste wafers and sewage sludge. Environ. Eng. Sci. 2019, 36, 478–490. [Google Scholar]
- ISO. Guide to the Expression of Uncertainty in Measurement (GUM); ISO: Geneva, Switzerland, 1993. [Google Scholar]
- Konieczka, P.; Namieśnik, J. Evaluation and Quality Control of Analytical Measurement Results; Scientific-Technical Publishing House WNT: Warsaw, Poland, 2007. [Google Scholar]
- Sitorusa, B.; Sukandarb; Panjaitanc, S.D. Biogas recovery from anaerobic digestion process of mixed fruit-vegetable wastes. Energy Procedia 2013, 32, 176–182. [Google Scholar]
- Butnariua, M.; Butu, A. Chemical composition of vegetables and their products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Santos, A.D.; Silva, J.R.; Castro, L.M.; Quinta-Ferreira, R.M. A biochemical methane potential of pig slurry. Energy Rep. 2022, 8, 153–158. [Google Scholar]
- Morales-Polo, C.; Cledera-Castro, M.M.; Soria, B.Y.M. Biogas production from vegetable and fruit markets waste—Compositional and batch characterizations. Sustainability 2019, 11, 6790. [Google Scholar]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar]
- Bozym, M.; Florczak, I.; Zdanowska, P.; Wojdalski, J.; Klimkiewicz, M. An analysis of metal concentrations in food wastes for biogas production. Renew. Energy 2015, 77, 467–472. [Google Scholar]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar]
- Nsair, A.; Cinar, S.O.; Alassali, A.; Qdais, H.A.; Kuchta, K. Operational parameters of biogas plants: A review and evaluation study. Energies 2020, 13, 3761. [Google Scholar]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Proc. Technol. 1998, 54, 17–46. [Google Scholar]
- Esteves, B.; Sen, U.; Pereira, H. Influence of chemical composition on heating value of biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar]
- Perea-Moreno, M.A.; Samerón-Manzano, E.; Perea-Moreno, A.J. Biomass as renewable energy: Worldwide research trends. Sustainability 2019, 11, 863. [Google Scholar]
- Dalpaz, R.; Konrada, O.; da Silva Cyrne, C.C.; Barzotto, H.P.; Hasan, C.; Filho, M.G. Using biogas for energy cogeneration: An analysis of electric and thermal energy generation from agro-industrial waste. Sustain. Energy Technol. Assess. 2020, 40, 100774. [Google Scholar]
- Luo, L.; Cristofari, C.; Levrey, S. Cogeneration: Another way to increase energy efficiency of hybrid renewable energy hydrogen chain—A review of systems operating in cogeneration and of the energy efficiency assessment through exergy analysis. J. Energy Storage 2023, 66, 107433. [Google Scholar]
- Ciuła, J.; Wiewiórska, I.; Banaś, M.; Pająk, T.; Szewczyk, P. Balance and energy use of biogas in Poland: Prospects and Ddrections of development for the circular economy. Energies 2023, 16, 3910. [Google Scholar]
- Bednarek, A.; Klepacka, A.M.; Siudek, A. Development barriers of agricultural biogas plants in Poland. Econ. Environ. 2023, 1, 229–258. [Google Scholar]
- Chasnyk, O.; Sołowski, G.; Shkarupa, O. Historical, technical and economic aspects of biogas development: Case of Poland and Ukraine. Renew. Sust. Energy Rev. 2015, 52, 227–239. [Google Scholar]
- Holik, M.; Zivić, M.; Virag, Z.; Barac, A.; Vujanović, M.; Avsec, J. Thermo-economic optimization of a Rankine cycle used for waste-heat recovery in biogas cogeneration plants. Energy Convrs. Manag. 2021, 232, 113897. [Google Scholar]
- Apunda, M.O.; Oloo, B. Nyangoye selection of a combined heat and power (CHP), and CHP generation compared to buying of electrical power from the national grid and separate thermal heat production. Open Sci. J. 2017, 2, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).