Application of Cement-Based Materials as a Component of an Engineered Barrier System at Geological Disposal Facilities for Radioactive Waste—A Review
Abstract
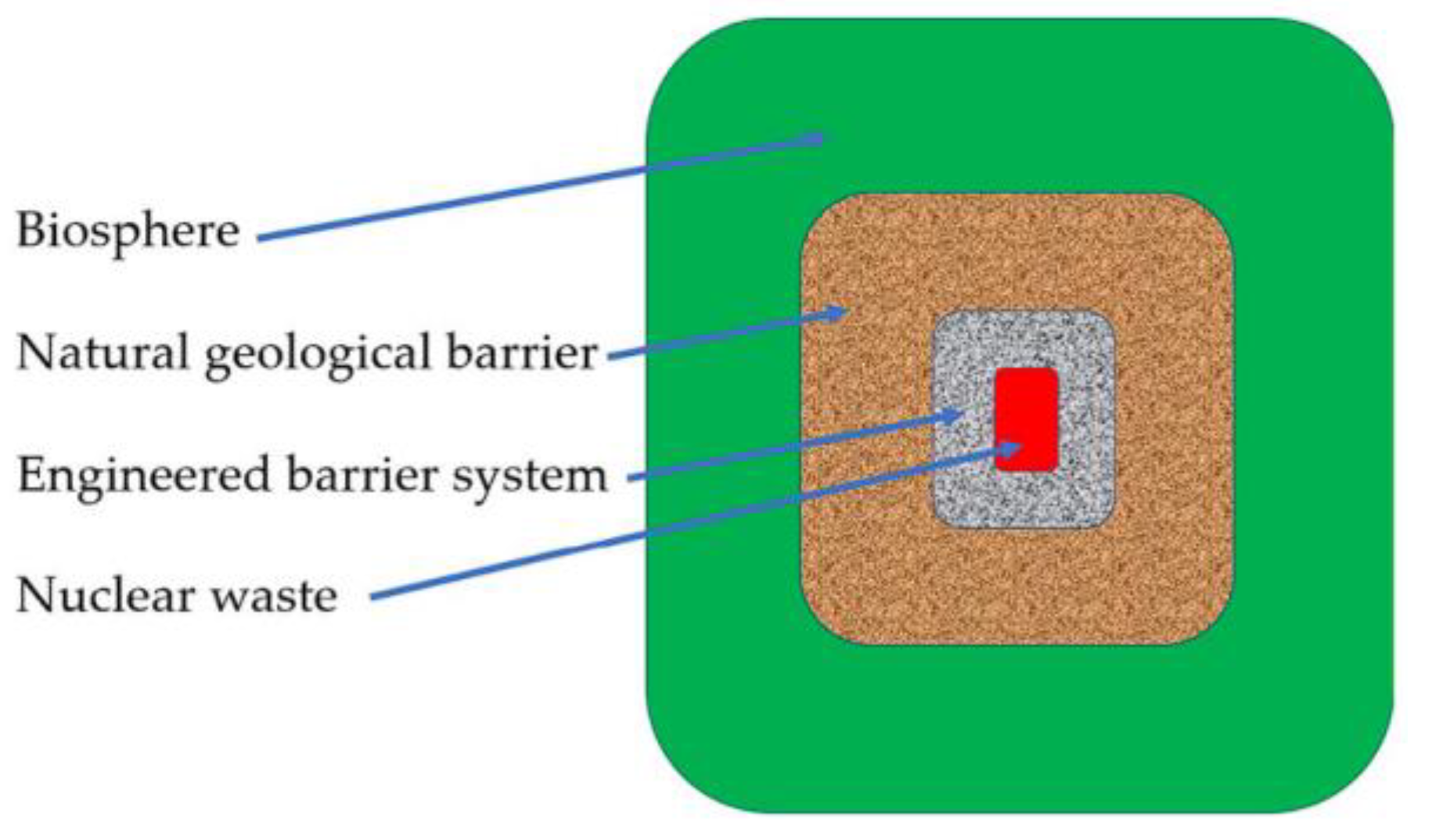
1. Introduction
2. International Cement-Based Materials Application in Design Concepts and Operating Facilities of RW Deposition
2.1. Cement as Matrix for LLW and ILW
- Cementing directly in the barrel/container. During this process, the components, including radioactive waste, are mixed in a standard 200 L steel barrel using a single or interchangeable mixing element. After the cement is grasped and solidified, the barrel with the resultant compound is closed with a lid and sent to a near-surface disposal facility.
- Cementing by a mixing plant. In this method, cement and waste are mixed to a homogeneous state and poured into standard 200-L steel drums or containers. The barrel/container shall be locked and, once the strength has been established, disposed of in the near-surface facility.
- Cementing in situ. Radioactive waste cementing is often carried out on a large scale. For example, in India, 4 m3 tanks are used for radioactive waste conditioning, which are placed in a concrete ditch on a vermiculite layer. The air-conditioning tanks are interconnected at a certain level to avoid overflow. They are also equipped with one-time mixing mechanisms and a cement injection channel. All the tanks in the reinforced concrete trench are connected to an exhaust system equipped with a cyclone separator, filters and a blower. Since immobilization in cement is made inside the conditioning vats, this process is defined as «cementing in situ». This process is also used in the United States, where vats and bunkers of up to several million liters are filled with cemented radioactive waste.
2.2. Cement as a Buffer Material for Radioactive Waste Disposal
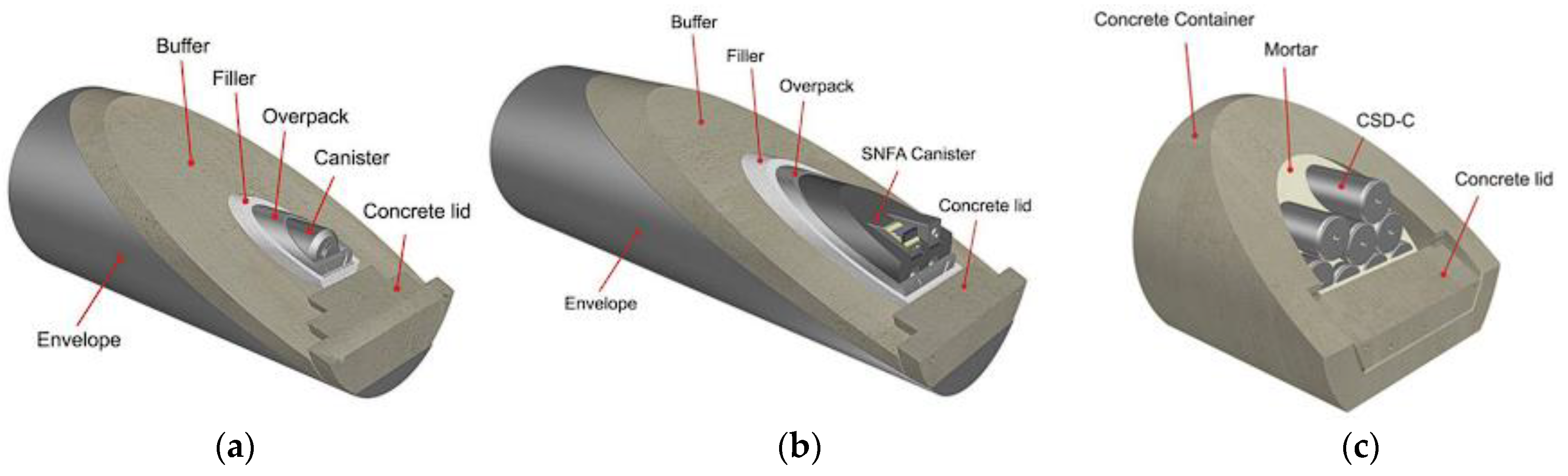
2.2.1. Cement as a Backfill Material in the Deep Geological Repository Project for Long-Lived (LL) High-, Intermediate-, Low- and Short-Lived (SL) High-Level Waste (Belgium)
2.2.2. Cement Buffer NRVB (Nirex Reference Vault Backfill) in the Concept of Intermediate-Level Radioactive Waste Isolation in Geological Disposal Facility (United Kingdom)
- The high hydrogen index of the medium (pH) provided by the dissolution of the various mineral components of the cement buffer by underground water, which makes it possible to significantly reduce the mobility of many radionuclides;
- High porosity and, consequently, permeability to ensure homogeneous chemical conditions, allowing for the removal of gaseous products resulting from corrosion of metal components of radioactive waste (H2), activity of micro-organisms in aerobic and anaerobic conditions when they decompose organic radioactive waste (CH4, CO2), as well as a number of radioactive gases (containing isotopes 3H, 14CO2, 14CH4, 222Rn) [27,28] and the creation of a high specific surface area for the sorption of radionuclides;
2.2.3. Cement Mortar as a Filler for Concrete Containers with Short-Lived Low- and Intermediate-Level Waste in Geological Repository in Bátaapáti (Hungary)
2.2.4. Cement as a Component of the Backfilling Material in the Geological Repository of Low- and Intermediate-Level Waste at Morsleben (Germany)
2.2.5. Cement Filling of Containers with Low-Level Waste of Geological Disposal Facility Cigéo (France)
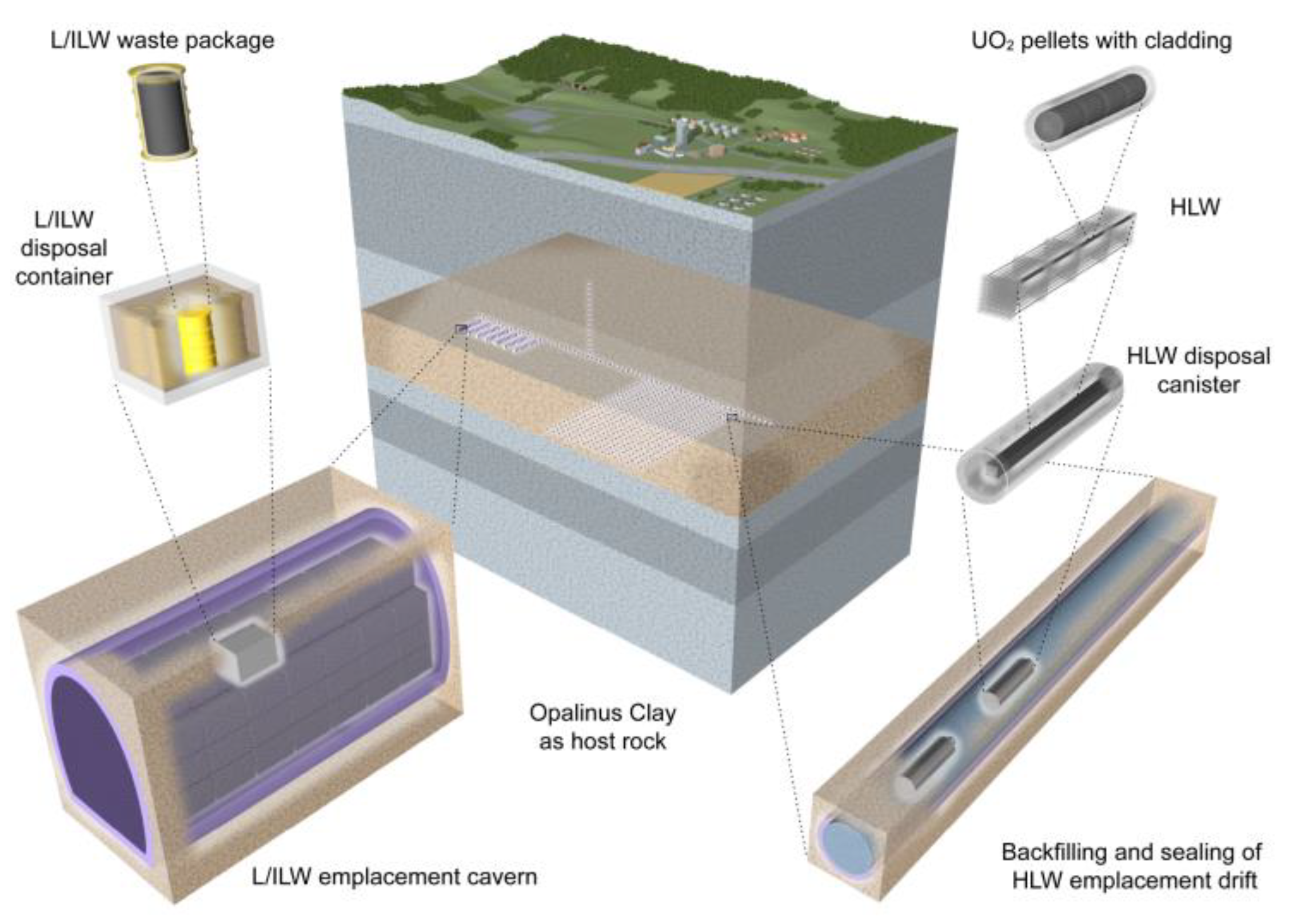
2.2.6. Special Cement Composition as Radioactive Waste Chamber filling Material for Low- and Intermediate-Level Waste in the Geological Disposal Facility Project (Switzerland)
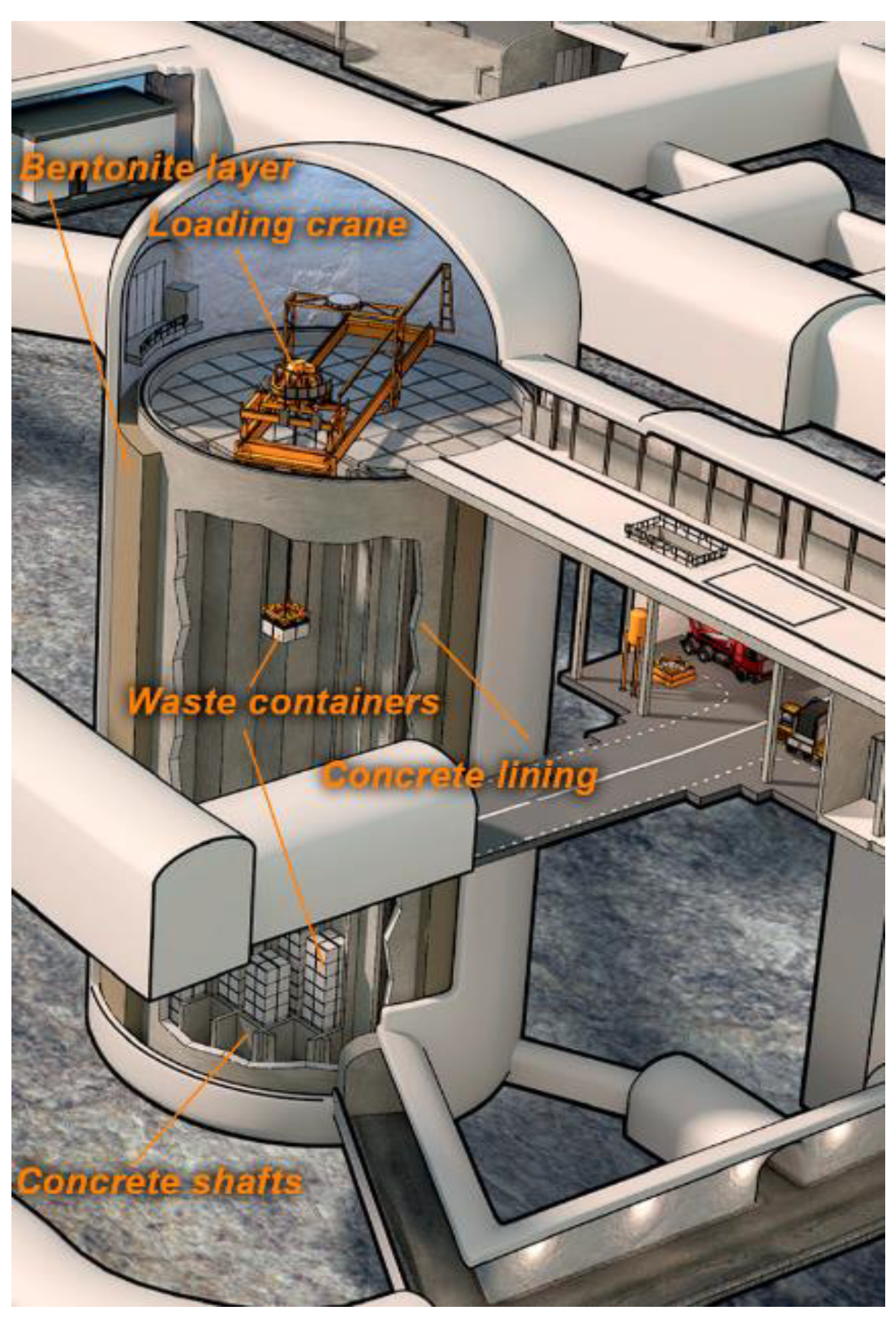
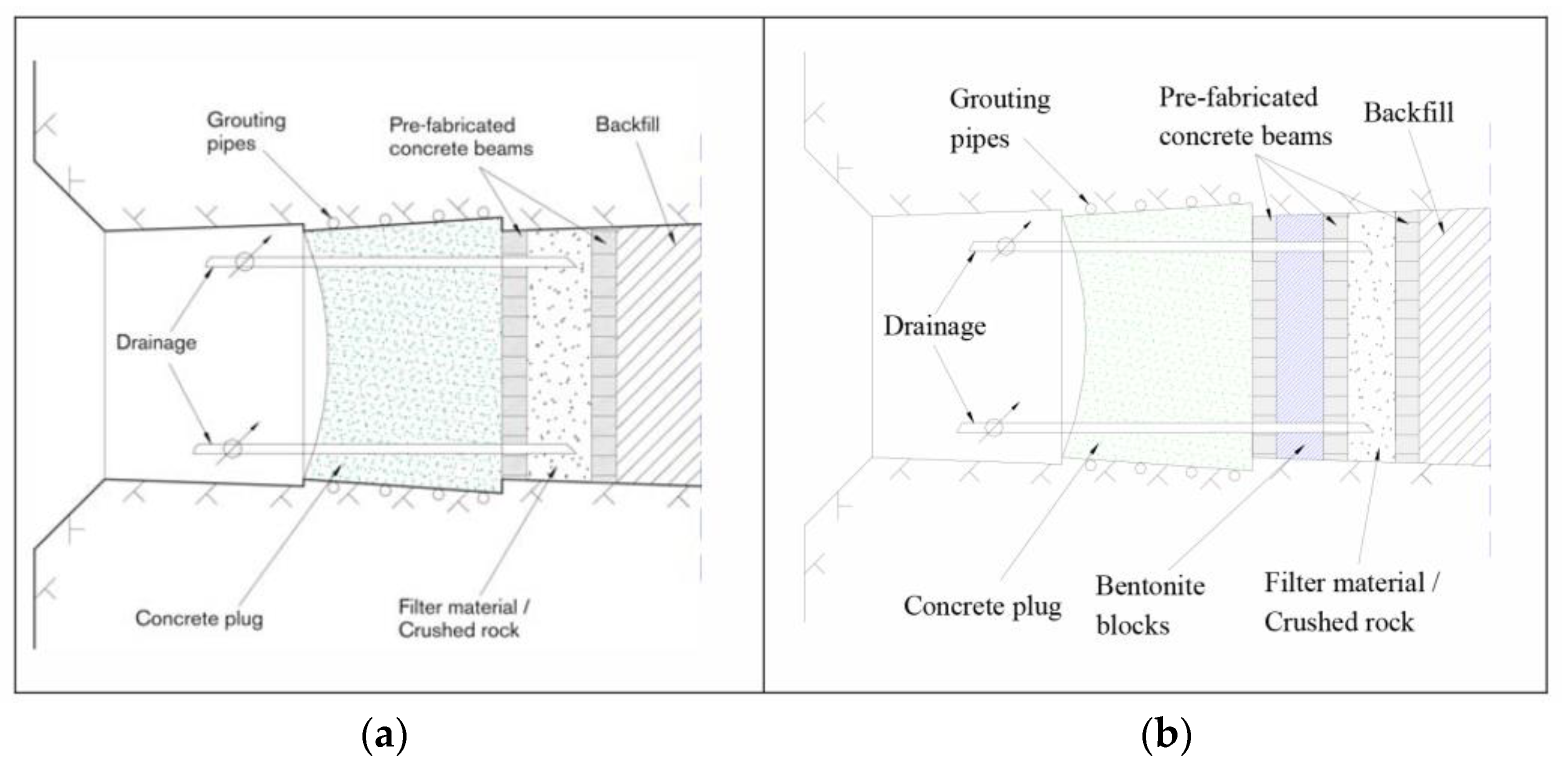
2.2.7. Cement Backfilling as One of the Options to Fill the Gaps between Radioactive Waste Containers in the SFL Geological Repository Project for Long-Lived Low- and Intermediate-Level Waste (Sweden)
2.3. Concrete as a Material for Lining and Isolation of Tunnels for Radioactive Waste in Geological Repository/Disposal Facility
2.3.1. Concrete as Material for Tunnel Lining in the Host Rock of Bátaapáti National Radioactive Waste Repository (Hungary)
2.3.2. Concrete as a Drift Lining Material in High-Level Waste and Spent Nuclear Fuel Deep Geological Repository Project (Spain)
2.3.3. Concrete as a Material of Tunnel Plugging, Isolation of Waste Chambers in the Low- and Intermediate-Level Waste Repository Project in the Municipality of Kincardine (Canada)
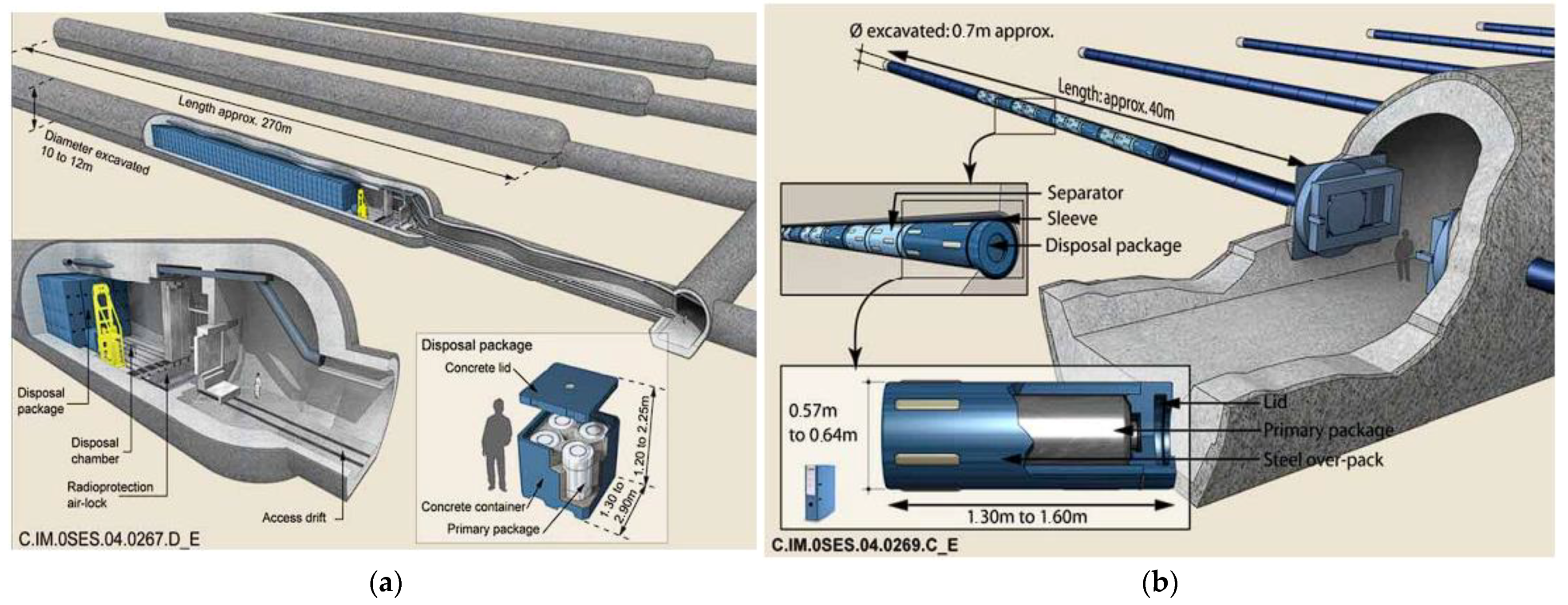
2.3.4. Concrete as a Lining and Plugging of Tunnels Material with Intermediate- and High-Level Waste in the Project of Geological Disposal Facility Cigéo (France)
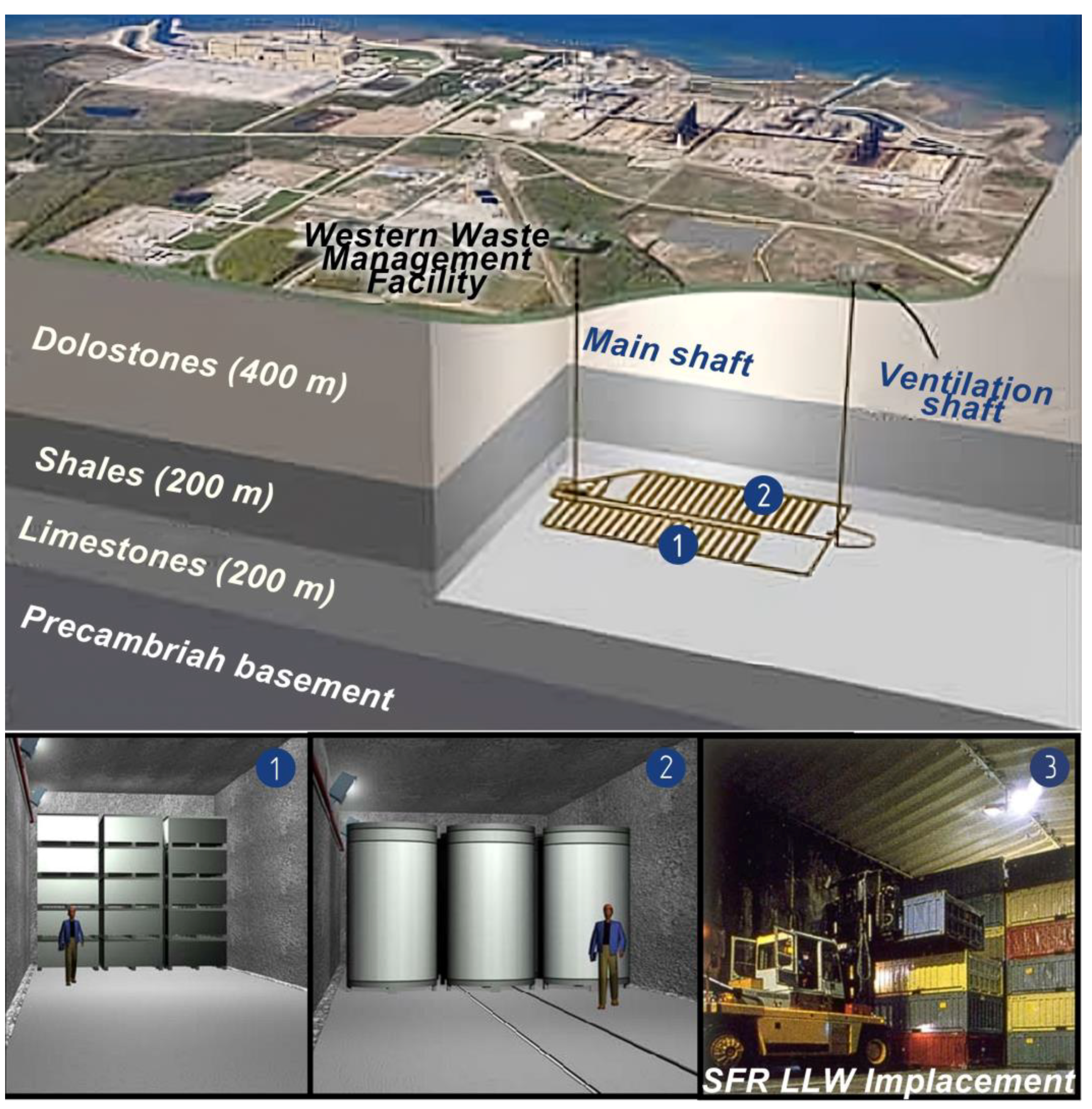
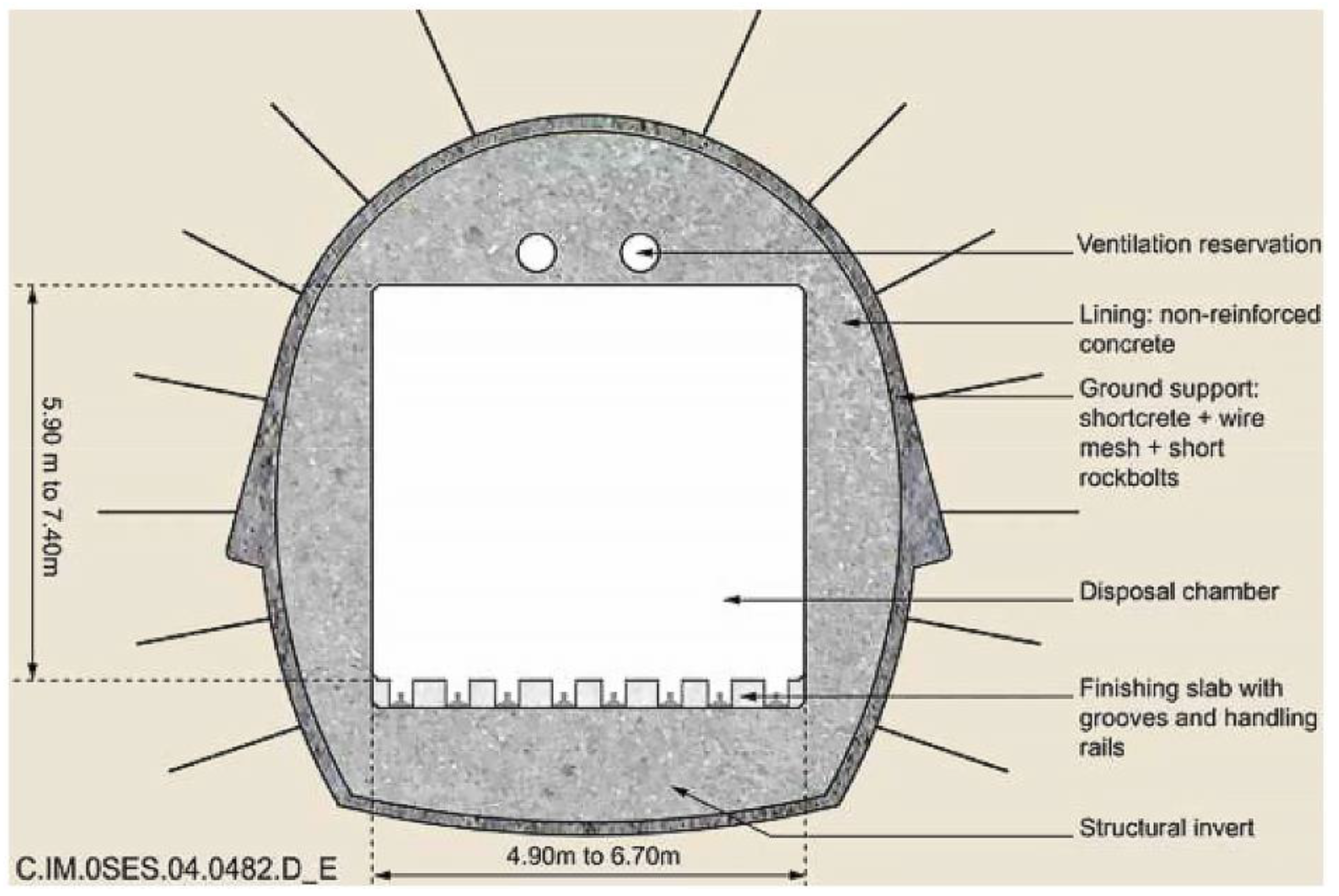
2.3.5. Concrete as Bunker Lining Material of the Short-Lived Low- and Intermediate-Level Waste Geological Repository SFR (Sweden)
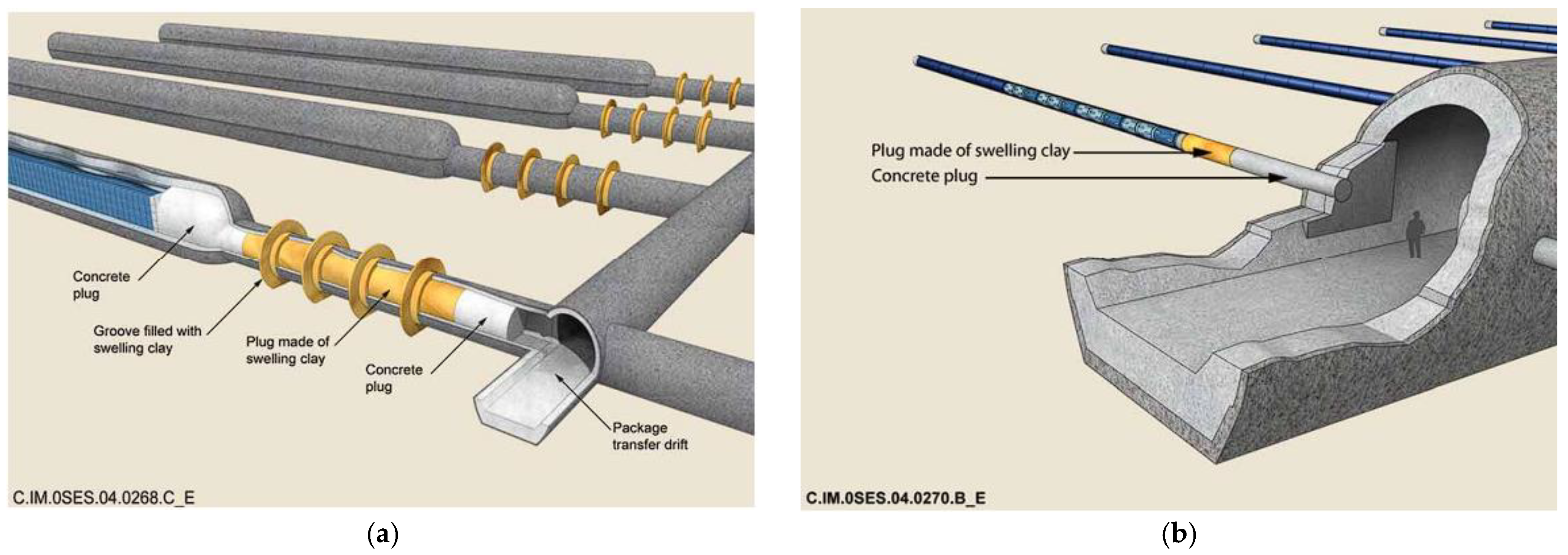
2.3.6. Low-pH Concrete as Tunnel Plug Material in Design Concepts for High-Level Waste and Spent Nuclear Fuel Deep Geological Repository KBS-3 (Sweden, Finland)
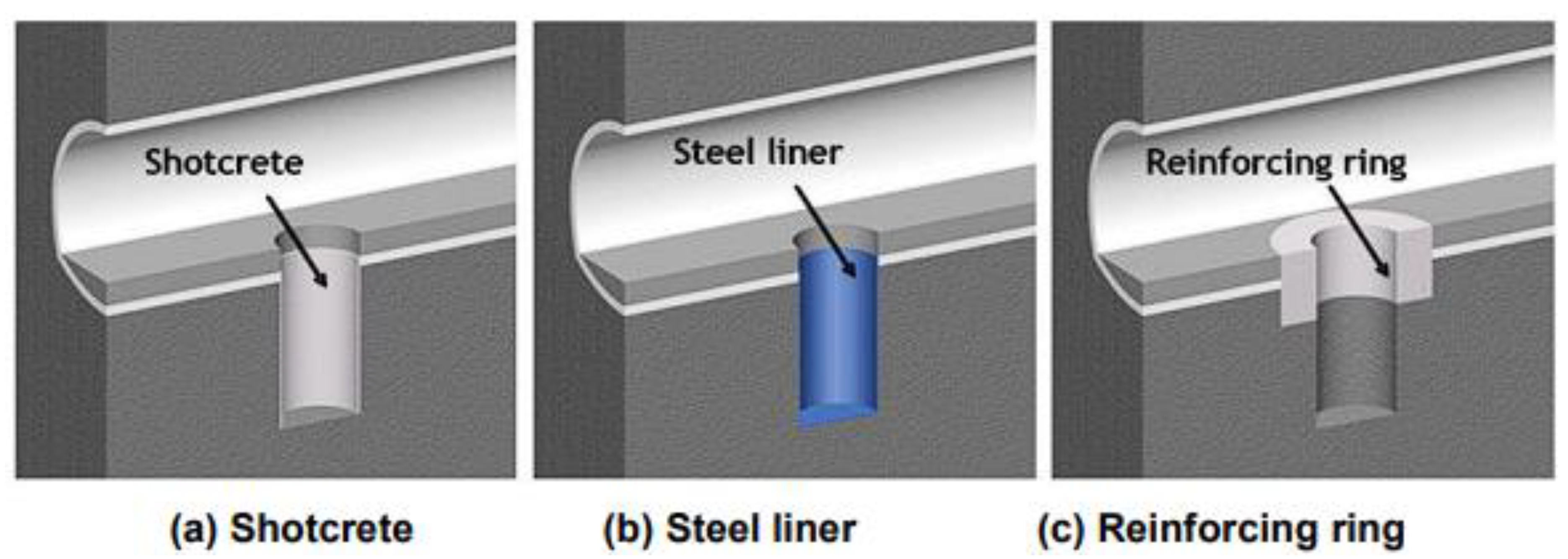
2.3.7. Concrete as Lining and Plugging Material in the Geological Disposal Facility Design Concept of Disposal High-Level Waste and Transuranic Spent Nuclear Fuel (Japan)
2.4. Conclusions
- Radioactive waste contained in cement matrices inside steel drums;
- The material of radioactive waste conditioned container and/or its buffer;
- Buffer material between radioactive waste container and storage walls;
- The material of the lining of the walls of the storage;
- Material isolating tunnels with placed radioactive waste packaging structures (tunnel plugs, walls and seals).
3. Changes in Concrete Engineered Barrier by Means of External Factors of Geological Disposal Facility
3.1. Concrete Changes Due to Elevated Temperatures (above 100 °C) Typical for RW Class 1 Packages
- Compressive strength decreases by about 25% at the specified maximum temperature of the operating geological disposal facility of 300 °C and continues to decrease with greater force when this limit is exceeded. Strength degradation tends to increase when cement dries.
- Modulus of elasticity decreases in volume than strength and is a more sensitive parameter to external influences. The relative modulus tends to decrease linearly from 100 °C to almost zero at 800 °C but fluctuates strongly at 300 °C—the relative value is in the range of 40 to 90%. However, this significant reduction of the elastic module does not endanger the integrity of the geological disposal facility barriers. Maintaining a high modulus of elasticity is critical if the strain under the load is limited. In the geological disposal facility, the primary load at elevated temperatures will be the result of limiting temperature and shrinkage strains. Under these conditions, achieving a low elastic modulus value is useful because it reduces the stresses caused by limiting deformation and therefore reduces the risk of cracking.
- Temperature expansion coefficient of concrete is approximately constant at the maximum operating temperature of 300 °C, with a value of 10 ± 2·10−6 mm/(mm·°C). For cement mortar, deformation at elevated temperatures can be reinforced by shrinkage during drying, so the final deformation is caused by shrinkage.
- Thermal conductivity of concrete decreases approximately linearly over the entire range of 20 to 300 °C, with a relative coefficient of thermal conductivity around 0.4.
- Hydraulic permeability of concrete increases with temperature. This conclusion is based on a limited amount of data on the change of porosity of concrete at elevated temperatures and using information on the permeability to porosity ratio, its estimated value, assumed for the maximum operating temperature of 300 °C may be 10 times higher than for the standard temperature. This could result in a high flow rate of groundwater through the geological disposal facility barriers, even though the effect of changes in cement materials would be relatively small and comparable to cracking.
3.2. Changes in Concrete Due to Groundwater in Geological Disposal Facilities
3.3. Effects of HLW Irradiation on Concrete Properties
3.4. Influence of Biogenic Processes on Cement Material Properties
4. Influence of Concrete on Individual Component of Engineered Barrier System under Exposure of Conditions of Geological Disposal Facility
4.1. Changes of Bentonite Clay Buffer Properties under the Exposure of Model High-Alkali Conditions of Cement/Concrete
4.2. Interaction of Engineered Barrier Materials in the System «Concrete-Steel» under the Exposure of Groundwater
4.2.1. Influence of Low Oxygen Content and High Pore Cement Solution pH on the Integrity of Steel Packages after Closure of Geological Disposal Facility
4.2.2. Pitting Corrosion and Stress Corrosion of Steel Super Container Material under the Action of Cement Pore Solution with pH~13.6
4.2.3. Formation of Iron Sulphides on the Surface of a Steel Container in Contact with Groundwater with Low-Alkali Cement and Related Corrosion Mechanisms
4.3. Influence of Cement Materials on the Solubility Rate of Vitrified RW Class 1
4.3.1. Dissolution of Borosilicate Glass, a Material of Vitrified HLW in Air Conditioning Practice, under the Action of Groundwater Contacting Cement, as a Material of Engineered Safety Barrier
4.3.2. Dissolution of Sodium-Aluminophosphate Glass, Which Is the Material of Vitrified HLW under the Exposure of Groundwater Contacting Cement, as a Material of Engineered Safety Barrier
5. Mutual Transformations of Concrete-Bentonite under Exposure of Granitic Host Rock Groundwater
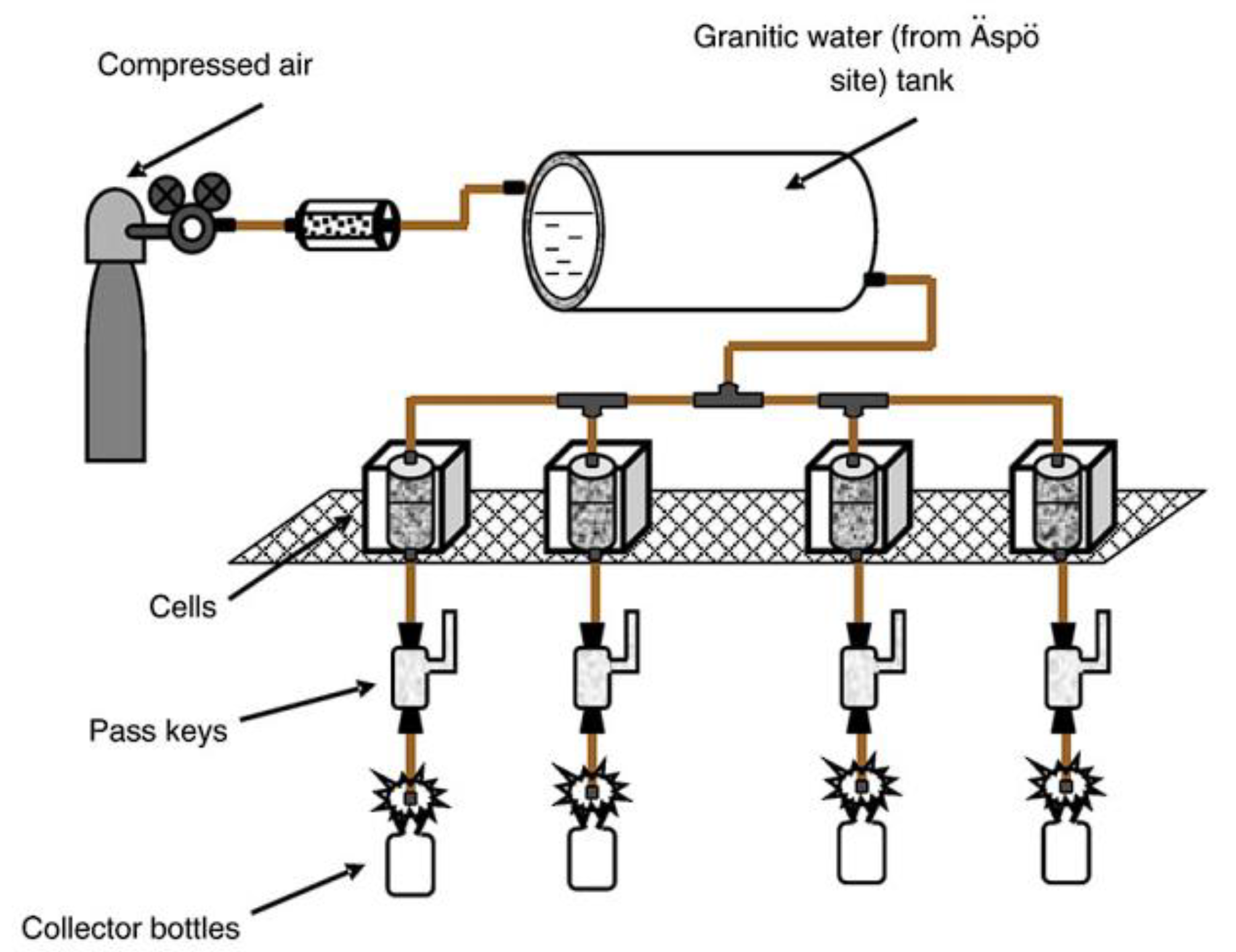
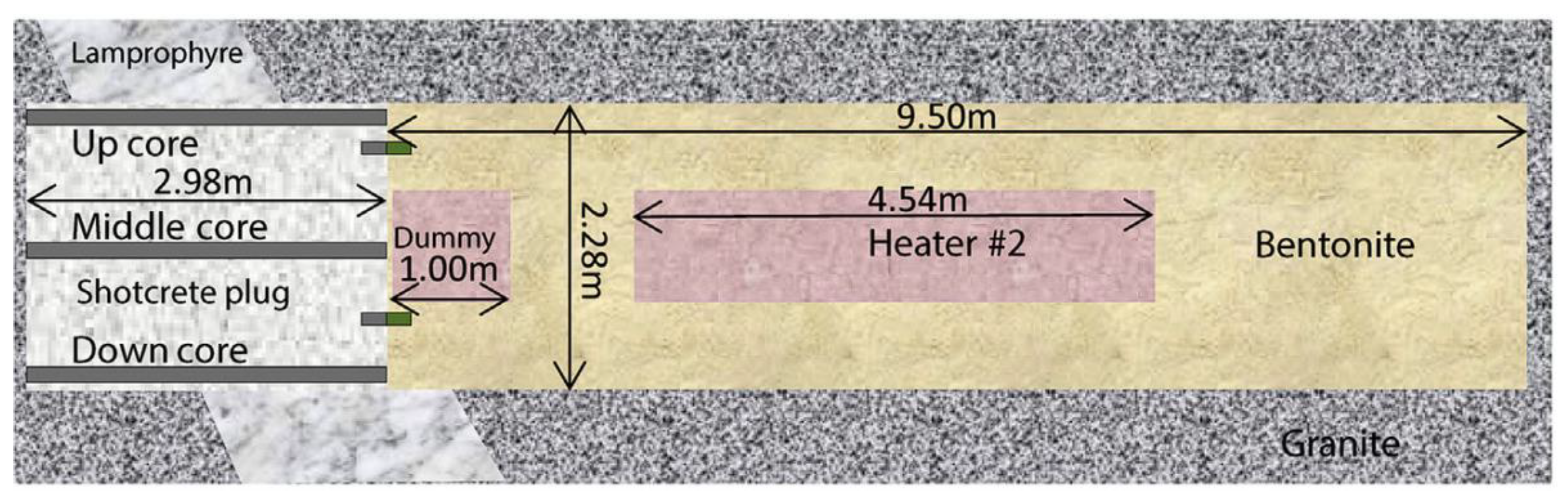
5.1. Mutual Influence of Engineered Barrier Materials in the «Concrete-Bentonite» System under the Exposure of Groundwater in the Long Term Experiments
5.1.1. Concrete-Bentonite Mineral Surfaces Passivation and Formation of Crystalline Phases in the Concrete Pores by Means of Groundwater Exposure
5.1.2. Transport of Ions in the Concrete-Bentonite Interface (Transfer of Mg2+ Ions, SO42− from Bentonite to Concrete and Ca2+, Na+, K+ from Concrete to Bentonite) under the Action of Groundwater (Transfer of Cl− and HCO3− Ions Dissolved in Groundwater)
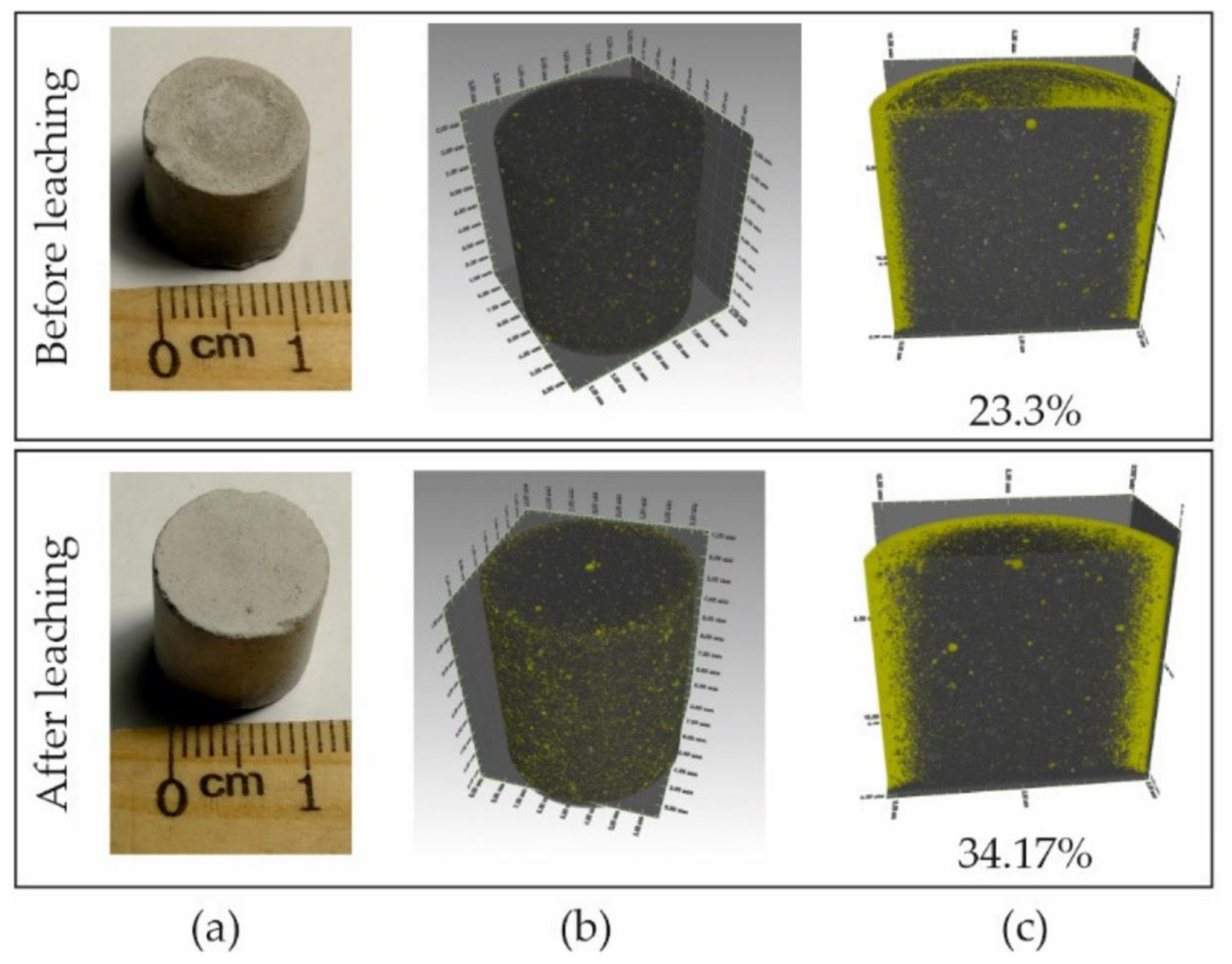
5.1.3. Alteration of the Mechanical Properties of Concrete at Concrete/Bentonite Interface Due to Groundwater Characteristic of the Host Rock
5.2. Mutual Influence of Engineered Barrier Materials in the «Concrete-Bentonite» System under the Exposure of Synthetic Groundwater in the S Term Experiments
- Decalcification of C-S-H in a medium in which portlandite dissolves and progressive hydration of anhydrous phases occurs.
- Development of a film of calcite on the interface of concrete with bentonite and dispersed precipitation of calcite in localized zones of bentonites.
- The beginning of the development of Mg-containing components on the interface of bentonite with concrete, associated with the formation of Mg-clay 2:1 sheet silicates as the main neogenic phases expected in the long term.
- Formation of secondary ettringite in concrete at the interface with bentonite.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| µ-CT | Computer microtomography |
| C/B | Concrete-Bentonite interface |
| C/W | Concrete-Water interface |
| CEC | Cation Exchange Capacity |
| DGR | Deep Geologic Repository |
| EBS | Engineered Barrier System |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| ESB | Engineered Safety Barrier |
| FEBEX | Full-Scale Engineered Barrier Experiment |
| GTS | Grimsel Test Site |
| GW | Groundwater |
| HLW | High-Level Waste |
| IUPAC | International Union of Pure and Applied Chemistry |
| LL | Long-Lived |
| LLW | Low-Level Waste |
| LRW | Liquid Radioactive Waste |
| NPP | Nuclear Power Plant |
| NRVB | Nirex Reference Vault Backfill |
| NSDF | Near-Surface Disposal Facility |
| NSR | Near-Surface Repository |
| OPC | Ordinary Portland Cement |
| PEEK | Polyetheretherketone |
| pH | Hydrogen index |
| RW | Radioactive Waste |
| SEM | Scanning Electron Microscopy |
| SL | Short-Lived |
| SNF | Spent Nuclear Fuel |
| SNFA | Spent Nuclear Fuel Assembly |
| SSA | Specific Surface Area |
| STIMAN | Structural Image Analysis |
| TEM | Transmission Electron Microscopy |
| TRU | Transuranic |
| W/C | Water-Cement ratio |
References
- Ojovan, M.I.; Steinmetz, H.J. Approaches to Disposal of Nuclear Waste. Energies 2022, 15, 7804. [Google Scholar] [CrossRef]
- Olszewska, W.; Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G.; Lankof, L.; Pająk, L. Multibarrier system preventing migration of radionuclides from radioactive waste repository. Nukleonika 2015, 60, 557–563. [Google Scholar] [CrossRef]
- Disposal of Radioactive Waste for Protecting People and the Environment. No. SSR-5 Specific Safety Requirements. Available online: https://www-pub.iaea.org/MTCD/publications/PDF/Pub1449_web.pdf (accessed on 26 October 2022).
- Geological Disposal Facilities for Radioactive Waste for Protecting People and the Environment. No. SSG-14 Specific Safety Guide. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1483_web.pdf (accessed on 26 October 2022).
- Kozlov, P.V.; Gorbunova, O.A. Cementing as a Method of Radioactive Waste Immobilization; Mayak PA: Ozersk, Russia, 2011; 143p, ISBN 978-5-903159-30-7. [Google Scholar]
- Alonso, M.C.; Calvo, J.L.G.; Cuevas, J.; Turrero, M.J.; Fernández, R.; Torres, E.; Ruiz, A.I. Interaction processes at the concrete-bentonite interface after 13 years of FEBEX-Plug operation. Part I: Concrete alteration. Phys. Chem. Earth 2017, 99, 38–48. [Google Scholar] [CrossRef]
- Margeanu, C.A. Overview on the Multinational Collaborative Waste Storage and Disposal Solutions. 2012, p. 404. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:45099892 (accessed on 26 October 2022).
- Drace, Z.; Mele, I.; Ojovan, M.I.; Rahman, R.A. An Overview of Research Activities on Cementitious Materials for Radioactive Waste Management; Cambridge University Press: Cambridge, UK, 2012; Volume 1475. [Google Scholar]
- IAEA. Performance of Engineered Barrier Materials in Near Surface Disposal Facilities for Radioactive Waste; TECDOC-1255; IAEA: Vienna, Austria, 2001; Available online: https://www-pub.iaea.org/MTCD/publications/PDF/te_1255_prn.pdf (accessed on 26 October 2022).
- Drace, Z.; Ojovan, M.I. The Behaviors of Cementitious Materials in Long Term Storage and Disposal: An Overview of the Results of the IAEA Ordinated Research Project; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
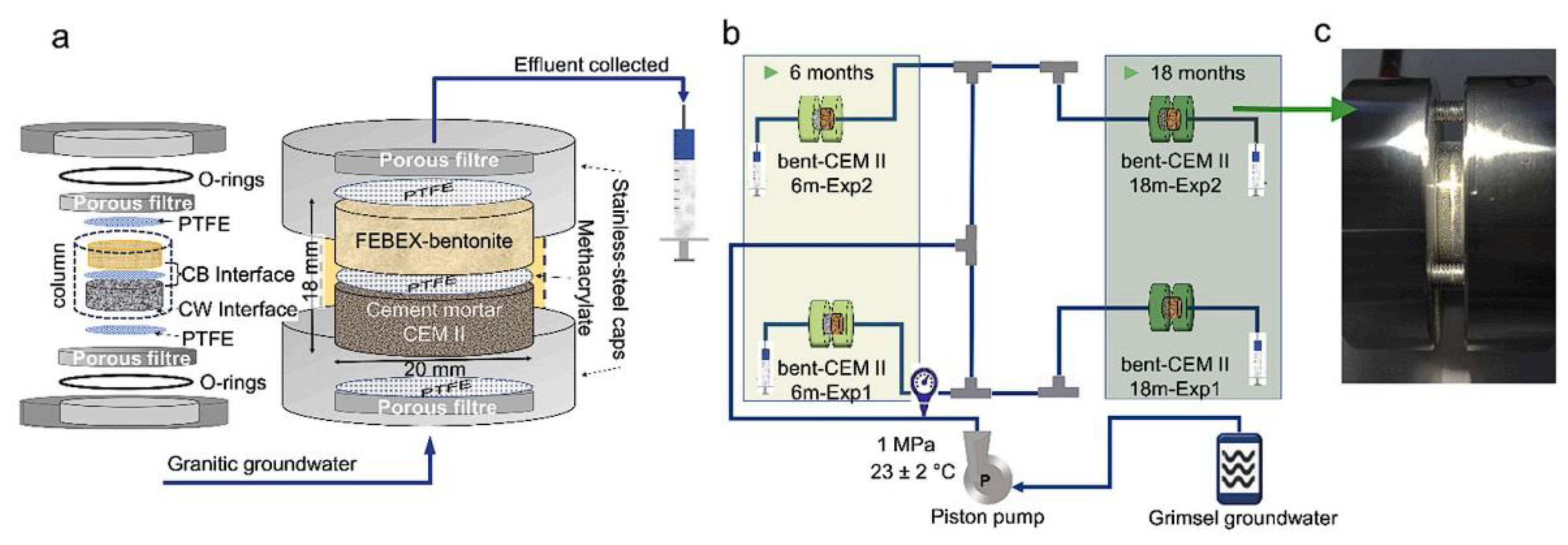
- González-Santamaria, D.E.; Fernández, R.; Ruiz, A.I.; Ortega, A.; Cuevas, J. Bentonite/CEM-II cement mortar interface experiments: A proxy to in situ deep geological repository engineered barrier system surface reactivity. Appl. Geochem. 2020, 117, 104599. [Google Scholar] [CrossRef]
- González-Santamaria, D.E.; Fernández, R.; Ruiz, A.I.; Ortega, A.; Cuevas, J. High-pH/low pH ordinary Portland cement mortars impacts on compacted bentonite surfaces: Application to clay barriers performance. Appl. Clay Sci. 2020, 193, 105672. [Google Scholar] [CrossRef]
- Fernández, R.; Torres, E.; Ruiz, A.I.; Cuevas, J.; Alonso, M.C.; Calvo, J.L.G.; Rodríguez, E.; Turrero, M.J. Interaction processes at the concrete-bentonite interface after 13 years of FEBEX-Plug operation. Part II: Bentonite contact. Phys. Chem. Earth Parts A/B/C 2017, 99, 49–63. [Google Scholar] [CrossRef]
- Johannesson, L.E.; Börgesson, L.; Goudarzi, R.; Sandén, T.; Gunnarsson, D.; Svemar, C. Prototype repository: A full scale experiment at Äspö HRL. Phys. Chem. Earth Parts A/B/C 2007, 32, 58–76. [Google Scholar] [CrossRef]
- Johansson, E.; Siren, T.; Hakala, M.; Kantia, P. ONKALO POSE Experiment. Phase 1 and 2: Execution and Monitoring. 2014. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:46094985 (accessed on 9 September 2021).
- Drace, Z.; Ojovan, M.I. The Behaviors of Cementitious Materials in Long Term Storage and Disposal of Radioactive Waste Results of a Coordinated Research Project; International Atomic Energy Agency: Vienna, Austria, 2013; Available online: https://www.cambridge.org/core/journals/mrs-online-proceedings-library-archive/article/abs/behaviours-of-cementitious-materials-in-long-term-storage-and-disposal-an-overview-of-results-of-the-iaea-coordinated-research-project/7F7F418E4419269494BD049B1DD6606A (accessed on 9 September 2021).
- Cementing Liquid Radioactive Waste. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/36/030/36030790.pdf (accessed on 9 September 2021).
- International Atomic Energy Agency. Conditioning of Low- and Intermediate-Level Radioactive Wastes; International Atomic Energy Agency (IAEA): Vienna, Austria, 1983. [Google Scholar]
- International Atomic Energy Agency. Improved Cement Solidification of Low and Intermediate Level Radioactive Wastes; Technical Reports Series No. 350; IAEA: Vienna, Vienna, 1993. [Google Scholar]
- Varlakov, A.P.; Zherebtsov, A.A.; Germanov, A.V.; Maryahkin, M.A.; Krapivina, M.K. Raw Conditioning Using Special Cements And Hot Pressing Method. In Proceedings of the X Russian Conference with International Participation “Radiochemistry-2022”, Saint-Petersburg, Russia, 26–30 September 2022; p. 401. [Google Scholar]
- Varlakov, A.P.; Germanov, A.V.; Maryakhin, M.A.; Varlakova, G.A.; Krapivina, M.K.; Zherebtsov, A.A.; Petrov, V.G.; Kalmykov, S.N. Influence of irradiation on the properties of the cement compound as a matrix for curing high-level wastes. At. Energy 2021, 130, 218–222. [Google Scholar] [CrossRef]
- Zherebtsov, A.A.; Kapustin, V.V.; Varlakova, G.A.; Varlakov, A.P.; Petrov, V.G.; Vlasova, I.E.; Kharitonov, I.D.; Kalmykov, S.N. Chemical resistance and structural characteristics of cement compounds with radioactive waste simulators after exposure to ionizing radiation. At. Energy 2019, 127, 328–331. [Google Scholar]
- Sorokin, V.T.; Gataullin, R.M.; Sviridov, N.V.; Pavlov, D.I. Longevity of NZK-150-1.5P type reinforced concrete containers for class 2 radioactive waste disposal. Radioact. Waste 2022, 3, 37–49. [Google Scholar] [CrossRef]
- Preparatory Safety Assessment: Conceptual Model Description of the Reference Case, External Report, sck CEN-ER-215, 12/Ewe/P-42, September 2012. Available online: https://researchportal.sckcen.be/en/publications/preparatory-safety-assessment-conceptual-model-description-of-the (accessed on 15 October 2022).
- Tsebakovskaya, N.S.; Utkin, S.S.; Kapyrin, I.V.; Medyantsev, N.V.; Shamina, A.V. Review of Foreign Practices of SNF and RW Disposal; Linge, I.I., Yu, D., Polyakov, M., Eds.; Publishing House “Komtechprint”: Moscow, Russia, 2015; 208p. [Google Scholar]
- Corkhill, C.; Hyatt, N. Nuclear Waste Management; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Collier, N.C.; Heyes, D.W.; Butcher, E.J.; Borwick, J.; Milodowski, A.E.; Field, L.P.; Kemp, S.J.; Mounteney, I.; Bernal, S.A.; Corkhill, C.L.; et al. Gaseous carbonation of cementitious backfill for geological disposal of radioactive waste: Nirex Reference Vault Backfill. Appl. Geochem. 2019, 106, 120–133. [Google Scholar] [CrossRef]
- Vasconcelos, R.G.W.; Beaudoin, N.; Hamilton, A.; Hyatt, N.C.; Provis, J.L.; Corkhill, C.L. Characterization of a high pH cement backfill for the geological disposal of nuclear waste: The Nirex Reference Vault Backfill. Appl. Geochem. 2018, 89, 180–189. [Google Scholar] [CrossRef]
- Crossland, I.G.; Vines, S.P. Why a Cementitious Repository? United Kingdom Nirex Limited 2001, Report N/034. 2001; 19p. Available online: https://rwm.nda.gov.uk/publication/why-a-cementitious-repository-nirex-report-n034-2001/ (accessed on 15 October 2022).
- Bamforth, P.B.; Baston, G.M.N.; Berry, J.A.; Glasser, F.P.; Heath, T.G.; Jackson, C.P.; Savage, D.; Swanton, S.W. Cement Materials for Use as Backfill, Sealing and Structural Materials in Geological Disposal Concepts. A Review of Current Status. Serco, United Kingdom, RP0618-252A. 2012. Available online: https://www.researchgate.net/profile/David-Savage-9/publication/261471551_Cement_materials_for_use_as_backfill_sealing_and_structural_materials_in_geological_disposal_concepts_A_review_of_current_status/links/00463534542beaa80b000000/Cement-materials-for-use-as-backfill-sealing-and-structural-materials-in-geological-disposal-concepts-A-review-of-current-status.pdf (accessed on 15 October 2022).
- Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Watson, S.; Metcalfe, R.; Paulley, A.; McEwen, T.; Michie, U. Identification of How Aspects of Nirex PGRC Would Differ If Adapted to Alternative Geologies. Quintessa Report QRS-1338A-1. 2007. Available online: https://www.researchgate.net/profile/Tim-Mcewen/publication/265260401_Identification_of_How_Aspects_of_the_Nirex_PGRC_Would_Differ_if_Adapted_to_Alternative_Geologies/links/54de823d0cf2510fcee3e219/Identification-of-How-Aspects-of-the-Nirex-PGRC-Would-Differ-if-Adapted-to-Alternative-Geologies.pdf (accessed on 15 October 2022).
- Bogatov, S.A.; Kryuchkov, D.V.; Pavlov, D.I.; Sychenko, D.V. Analysis of Various Concepts for RW Class 1 Disposal in Crystalline Rocks. Radioact. Waste 2020, 3, 66–77. [Google Scholar] [CrossRef]
- Hungary National Report: Sixth Report Prepared within the Framework of the Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management. Available online: https://www.iaea.org/sites/default/files/jc6thnationalreporthungary.pdf (accessed on 9 December 2022).
- Waste Acceptance System For Bátaapáti National Radioactive Waste Repository. Available online: https://gnssn.iaea.org/RTWS/general/Shared%20Documents/Waste%20Management/Feb%202015%20WS%20on%20LLW%20disposal/Day%202)%20Hungary.pdf (accessed on 15 October 2022).
- Morsleben–BGE. Available online: https://www.bge.de/en/morsleben/ (accessed on 13 September 2021).
- Safety: Anticipating the Risks|ANDRA. Available online: https://international.andra.fr/projects/cigeo/safety-anticipating-risks (accessed on 13 September 2021).
- Andra, D. Argile: Tome Architecture and Management of a Geological Repository; Andra Report C.RP.ADP.04.0001; Andra: Malabry, France, 2005. [Google Scholar]
- Types of Radioactive—NAGRA. Available online: https://www.nagra.ch/en/types.htm (accessed on 13 September 2021).
- NAGRA. Technical Report 21-01E. Waste Management Program 2021 of the Waste Producers. Available online: https://nagra.ch/wp-content/uploads/2022/08/NTB-21-01E.pdf (accessed on 25 October 2021).
- Nõs, B.; Molnár, P.; Baksay, A. Disposal of low and intermediate level waste in Hungary/Odlaganje otpada niskog i srednjeg stupnja radioaktivnsti u mađarskoj. Rud.-Geol.-Naft. Zb. 2012, 24, 81–85. [Google Scholar]
- High Level Waste—ENRESA. Available online: https://www.enresa.es/eng/index/activities-and-projects/cts (accessed on 14 September 2021).
- Gierszewski, P. Overview of Ontario Power Generation’s Proposed Deep Geologic Repository for Low & Intermediate Level Waste at the Bruce Site, Ontario, Canada-8517. 2008. Available online: https://archivedproceedings.econference.io/wmsym/2008/pdfs/8517.pdf (accessed on 15 October 2022).
- King, F.K. OPG’s deep geological repository for low and intermediate level waste-recent progress. In Proceedings of the TOPSEAL 2006 international topical meeting, Olkiliuoto, Finland, 17–20 September 2006. [Google Scholar]
- SFR—Final Repository for Short-Lived Radioactive Waste|SKB. Available online: https://skb.se/upload/publications/pdf/SFR_folder_engelsk.pdf (accessed on 15 September 2021).
- KBS-3H Seminar Was —Topics -NUMO Web Site. Available online: https://www.numo.or.jp/en/what/topics_170911.html (accessed on 15 October 2021).
- Loukusa, H.; Nordman, H. Feasibility of KBS-3 spent fuel disposal concept for Norwegian spent fuel. AINS Group Espoo. 2020. Available online: https://www.norskdekommisjonering.no/wp-content/uploads/2020/07/Technical-report-Feasability-of-KBS-3-spent-fuel-disposal-concept-for-Norwegian-fuel-20200615.pdf (accessed on 15 October 2022).
- Siren, T.; Hakala, M.; Valli, J.; Kantia, P.; Hudson, J.A.; Johansson, E. In situ strength and failure mechanisms of migmatitic gneiss and pegmatitic granite at the nuclear waste disposal site in Olkiluoto, Western Finland. Int. J. Rock Mech. Min. Sci. 2015, 79, 135–148. [Google Scholar] [CrossRef]
- Pettersson, S.; Loennerberg, B. Final Repository for Spent Nuclear Fuel in Granite-the KBS-3V Concept in Sweden and Finland. 2008. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:41025036 (accessed on 15 October 2022).
- Börgesson, L. KBS-3H Summary Report: Design of Buffer and Filling Components; Svensk Kärnbränslehantering AB—Swedish Nuclear Fuel and Waste Management Company: Stockholm, Sweden, 2017. [Google Scholar]
- Mathern, A.; Flansbjer, M.; Löfgren, I.; Magnusson, J. Experimental study of time-dependent properties of a low-pH concrete for deposition tunnels. In Proceedings of the International Federation for Structural Concrete 5th International Fib Congress, Melbourne, Australia, 7–11 October 2018; Volume 7, p. 11. [Google Scholar]
- Hakanen, M.; Ervanne, H. The Influence of Organic Cement Additives on Radionuclide Mobility A Literature Survey (POSIVA-WR--06-06), Finland. 2006. Available online: https://www.semanticscholar.org/paper/The-Influence-of-Organic-Cement-Additives-on-Hakanen-Ervanne/7367ec0c3d801844195fddde6af769b1360636da (accessed on 15 October 2022).
- García, D.; Grivé, M.; Duro, L.; Brassinnes, S.; de Pablo, J. The potential role of the degradation products of cement superplasticizers on the mobility of radionuclides. Appl. Geochem. 2018, 98, 1–9. [Google Scholar] [CrossRef]
- Vogt, C. Full-Scale Test of the Dome Plug for KBS-3V Deposition Tunnels—Concrete Properties; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2019. [Google Scholar]
- Malm, R.; Enzell, J.; Hassanzadeh, M. Full-Scale Test of the Dome Plug for KBS-3V Deposition Tunnels–Structural Response of the Concrete Dome; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2019. [Google Scholar]
- Enzell, J.; Malm, R. Full-Scale Test of the Dome Plug for KBS-3V Deposition Tunnels; Project Summary and Evaluation of the Final Results; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2019. [Google Scholar]
- Geological Disposal Concepts—Operation—NUMO Website. Available online: https://www.numo.or.jp/en/jigyou/geological.html (accessed on 15 September 2021).
- NUMO. The NUMO Pre-Siting SDM-Based Safety Case; Report NUMO-TR-21-01; Nuclear Waste Management Organization of Japan: Tokyo, Japan, 2021. [Google Scholar]
- Blokhin, A.I.; Blokhin, P.A.; Kazieva, S.T. Application of the TRACT calculation code for assessment of radionuclide compositions and radiation characteristics of SNF and RW of class 1. Radioact. Waste 2020, 13, 99–111. [Google Scholar] [CrossRef]
- Tyupina, E.A.; Pryadko, A.V.; Merkushkin, A.O. Method for obtaining a silver-containing sorbent based on bentonite for fixing radioiodine compounds. Sorpt. Chromatogr. Process. 2021, 21, 26–32. [Google Scholar] [CrossRef]
- Bogatov, S.A. HLW Disposal of in Verticale Deposition Holes with Cement Backfill—Pro and Contra with Regard to Long Term Safety of Geological Disposal Facility. Radioact. Waste 2018, 1, 21–33. (In Russian) [Google Scholar]
- Dickinson, M.; Holton, D.; Bamforth Ph Cairns, M. Development of Understanding of Disposability of High-Heat-Generating Wastes in the UK. WMS J. 2015, 1, 13. [Google Scholar]
- Khoury, G.A. Effect of fire on concrete and concrete structures. Prog. Struct. Eng. Mater. 2000, 2, 429–447. [Google Scholar] [CrossRef]
- Naus, D.J. A Compilation of Elevated Temperature Concrete Material Property Data and Information for Use in Assessments of Nuclear Power Plant Reinforced Concrete Structures; Report ORNL/TM-20009/175; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2010; 328p. [Google Scholar]
- Calvo, J.L.G.; Hidalgo, A.; Alonso, C.; Luco, L.F. Development of low-pH cementitious materials for HLRW repositories: Resistance against ground water aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef]
- Morozov, I.; Zakusin, S.; Kozlov, P.; Zakusina, O.; Roshchin, M.; Chernov, M.; Boldyrev, K.; Zaitseva, T.; Tyupina, E.; Krupskaya, V. Bentonite–Concrete Interactions in Engineered Barrier Systems during the Isolation of Radioactive Waste Based on the Results of Short-Term Laboratory Experiments. Appl. Sci. 2022, 12, 3074. [Google Scholar] [CrossRef]
- Rozov, K.B.; Rumynin, V.G.; Nikulenkov, A.M.; Leskova, P.G. Sorption of 137Cs, 90Sr, Se, 99Tc, 152 (154) Eu, 239 (240) Pu on fractured rocks of the Yeniseysky site (Nizhne-Kansky massif, Krasnoyarsk region, Russia). J. Environ. Radioact. 2018, 192, 513–523. [Google Scholar] [CrossRef]
- Meleshyn, A.Y.; Zakusin, S.V.; Krupskaya, V.V. Swelling pressure and permeability of compacted bentonite from 10th khutor deposit (Russia). Minerals 2021, 11, 742. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.R.; Lindgård, J.; Duchesne, J.; Fournier, B.; De Weerdt, K. Determination of the pH and the free alkali metal content in the pore solution of concrete: Review and experimental comparison. Cem. Concr. Res. 2017, 96, 13–26. [Google Scholar] [CrossRef]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The pore solution of blended cements: A review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Duchesne, J.; Reardon, E.J. Measurement and prediction of portlandite solubility in alkali solutions. Cem. Concr. Res. 1995, 25, 1043–1053. [Google Scholar] [CrossRef]
- Jenni, A.; Mäder, U.; Lerouge, C.; Gaboreau, S.; Schwyn, B. In situ interaction between different concretes and Opalinus Clay. Phys. Chem. Earth Parts A/B/C 2014, 70–71, 71–83. [Google Scholar] [CrossRef]
- Varlakov, A.P.; Kapustin, V.V.; Varlakova, G.A.; Zherebtsov, A.A.; Petrov, V.G.; Shirshin, E.A.; Kalmykov, S.N. The Effect of Radiation Doses Typical for High-level Waste on the Poperties of the Cement Matrix. Radioact. Waste 2018, 1, 63–68. [Google Scholar]
- GOST R 50926-96. Highly Active Solidified Waste. General Technical Requirements. 18 July 1996. Available online: https://www.russiangost.com/p-48099-gost-r-50926-96.aspx (accessed on 15 October 2022).
- Safonov A, V.; Boldyrev, K.A. URL In the Nizhnekanskiy Massif: Studying Biogenic Processes Under HLW Disposal Project. Radioact. Waste 2019, 2, 92–100. [Google Scholar] [CrossRef]
- Safonov, A.V.; Gorbunova, O.A.; German, K.E.; Zakharova, E.V.; Tregubova, V.E.; Ershov, B.G.; Nazina, T.N. Microbiological Aspects of Radioactive Waste Storage. Radiatsionnaia Biol. Radioecol. 2015, 55, 293–301. [Google Scholar] [CrossRef]
- Gorbunova, O.A. Prevention of Biogenic Destruction and Improvement of the Quality of the Cement Matrix Immobilizing Radioactive Waste: Abstract of the Thesis. Dis. Doctor of Technical Sciences: 05.17.11, 25.00.36/Gorbunova Olga Anatolyevna; FSUE “Radon”. 2012. 32p. Available online: https://static.freereferats.ru/_avtoreferats/01005089405.pdf (accessed on 20 October 2022).
- Gorbunova, O.A. Influence of microbiological destruction of the cement matrix on the safety of long-term storage of conditioned radioactive waste. Fiz. Khim. Obrab. Mate 2011, 4, 98–106. [Google Scholar]
- Ershov, B.G.; Safonov, A.V.; Nazina, T.N.; Gorbunova, O.A. Influence of Microbiological Processes on the Safety of Radioactive Waste Management. Safety of Nuclear Technologies and the Environment No. 1 2012: “NFC Closure”. Available online: http://www.atomic-energy.ru/technology/39922 (accessed on 20 October 2022).
- Kamorny, D.A.; Safonov, A.V.; Boldyrev, K.A.; Abramova, E.S.; Tyupina, E.A.; Gorbunova, O.A. Modification of the Cement Matrix with Organic Additives for Stabilizing Pertechnetate Ions. J. Nucl. Mater. 2021, 557, 153295. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Lekhov, V.A.; Dorzhieva, O.V.; Belousov, P.E.; Tyupina, E.A. Buffer properties of bentonite barrier systems for radioactive waste isolation in geological repository in the Nizhnekanskiy Massif. Radioact. Waste 2020, 1, 35–55. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive–waste management—A review. Clay Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Dixon, D.; Sandén, T.; Jonsson, E.; Hansen, J. Backfilling of Deposition Tunnels: Use of Bentonite Pellets. SKB P-11-44. 2011. 46p. Available online: http://www.skb.com/publication/2308782/P-11-44.pdf (accessed on 20 October 2022).
- Boerjesson, L.; Gunnarsson, D.; Johannesson, L.E.; Jonsson, E. Design, Production and Initial State of the Buffer; Technical Report, SKB 2010. TR-10-15; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2010; 89p. [Google Scholar]
- Wersin, P.; Johnson, L.H.; McKinley, I.G. Performance of the bentonite barrier at temperatures beyond 100 C: A critical review. Phys. Chem. Earth Parts A/B/C 2007, 32, 780–788. [Google Scholar] [CrossRef]
- Fries, T.; Claudel, A.; Weber, H.; Johnson, L.; Leupin, O. The Swiss concept for the disposal of spent fuel and vitrified HLW. Intern. In Proceedings of the Underground Disposal Unit Design & Emplacement Processes for a Deep Geological Repository, Prague, Czech Republic, 16–18 June 2008; Available online: http://library.sinap.ac.cn/db/fangshexing201103//41025019.pdf (accessed on 20 October 2022).
- Kaufhold, S.; Dohrmann, R. Distinguishing between more and less suitable bentonites for storage of high-level radioactive waste. Clay Miner. 2016, 51, 289–302. [Google Scholar] [CrossRef]
- Kale, R.C.; Ravi, K. Influence of thermal loading on index and physicochemical properties of Barmer bentonite. Appl. Clay Sci. 2018, 165, 22–39. [Google Scholar] [CrossRef]
- Pusch, R. Permeability of Highly Compacted Bentonite; no. SKBF/KBS-TR--80-16; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 1980. [Google Scholar]
- Pusch, R. Swelling Pressure of Highly COMPACTED bentonite; no. SKBF/KBS-TR--80-13; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 1980. [Google Scholar]
- Pusch, R. Use of bentonite for isolation of radioactive waste products. Clay Miner. 1992, 27, 353–361. [Google Scholar] [CrossRef]
- Komine, H.; Ogata, N. Prediction for swelling characteristics of compacted bentonite. Can. Geotech. J. 1996, 33, 11–22. [Google Scholar] [CrossRef]
- Lloret, A.; Villar, M.V.; Sanchez, M.; Gens, A.; Pintado, X.; Alonso, E.E. Mechanical behavior of heavily compacted bentonite under high suction changes. Géotechnique 2003, 53, 27–40. [Google Scholar] [CrossRef]
- Tan, Ö.; Yılmaz, L.; Zaimoğlu, A.S. Variation of some engineering properties of clays with heat treatment. Mater. Lett. 2004, 58, 1176–1179. [Google Scholar] [CrossRef]
- Garcia Garcia, S. The Impact of Groundwater Chemistry on the Stability of Bentonite Colloids. Ph.D. Thesis, KTH, Stockholm, Sweden, 2007. [Google Scholar]
- García-García, S.; Jonsson, M.; Wold, S. Temperature effect on the stability of bentonite colloids in water. J. Colloid Interface Sci. 2006, 298, 694–705. [Google Scholar] [CrossRef]
- Geneste, P.; Raynal, M.; Atabek, R.; Dardaine, M.; Oliver, J. Characterization of a French clay barrier and outline of the experimental program. Eng. Geol. 1990, 28, 443–454. [Google Scholar] [CrossRef]
- Alonso, E.E.; Vaunat, J.; Gens, A. Modeling the mechanical behavior of expansive clays. Eng. Geol. 1999, 54, 173–183. [Google Scholar] [CrossRef]
- Cui, Y.J.; Yahia-Aissa, M.; Delage, P. A model for the volume change behavior of heavily compacted swelling clays. Eng. Geol. 2002, 64, 233–250. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Chernov, M.S.; Bychkova, Y.V. Transformation of structure and adsorption properties of montmorillonite under thermochemical treatment. Geochem. Int. 2019, 57, 314–330. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Nekrasova, N.A.; Belousov, P.E.; Krupskaya, V.V. Sorption of Radionuclides 137Cs, 90Sr, and 233U on Various Natural Sorbents. Radiochemistry 2021, 63, 741–746. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Biryukov, D.V.; Belousov, P.E.; Lekhov, V.A.; Romanchuk, A.Y.; Kalmykov, S.N. Use of natural clay materials to increase nuclear and radiation safety of nuclear legacy facilities. Radioact. Waste 2018, 2, 30–43. [Google Scholar]
- Torstenfelt, B.; Allard, B.; Andersson, K.; Kipatsi, H.; Eliasson, L.; Olofsson, U.; Persson, H. Radionuclide Diffusion and Mobility in Compacted Bentonite; no. SKBF-KBS-TR--83-34; Swedish Nuclear Fuel Supply Co.: Göteborg, Sweden, 1983. [Google Scholar]
- Karnland, O.; Olsson, S.; Nilsson, U. Mineralogy and Sealing Properties of Various Bentonites and Smectite-Rich Clay Materials; Technical Report TR 06-30; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2006; 112p, Available online: http://www.skb.se/upload/publications/pdf/TR-06-30.pdf (accessed on 20 October 2022).
- Marty, N.C.M.; Claret, F.; Lassin, A.; Tremosa, J.; Blanc, P.; Made, B.; Giffaut, E.; Cochepin, B.; Tournassat, C. A database of dissolution and precipitation rates for clay-rocks minerals. Appl. Geochem. 2015, 55, 108–118. [Google Scholar] [CrossRef]
- Rozalén, M.L.; Huertas, F.J.; Brady, P.V.; Cama, J.; Garcia-Palma, S.; Linares, J. Experimental study of the effect of pH on the kinetics of montmorillonite dissolution at 25 °C. Geochim. Cosmochim. Acta 2008, 72, 4224–4253. [Google Scholar] [CrossRef]
- Huertas, F.J.; Caballero, E.; Jimenez de Cisneros, C.; Huertas, F.; Linares, J. Kinetics of montmorillonite dissolution in granitic solutions. Appl. Geochem. 2001, 16, 397–407. [Google Scholar] [CrossRef]
- Pryadko, A.V.; Tyupina, E.A.; Zakusin, S.V. Sorption properties of bentonites with respect to strontium and cesium under conditions of near-surface radioactive waste storage facilities. Uspikhi V Khimii I Khimicheskoi Tekhnologii 2021, 35, 72–74. [Google Scholar]
- Pryadko, A.V.; Tyupina, E.A.; Zakusin, S.V. Influence of acidic and alkaline effects on the structure, sorption and surface properties of bentonites. Adv. Chem. Chem. Technol. 2020, 34, 17–19. [Google Scholar]
- Tyupina, E.A.; Krupskaya, V.V. The use of bentonite as a buffer material in radioactive waste storage. In Proceedings of the Second International Scientific and Practical Conference Dedicated to the 60th Anniversary of Fsue “Radon” “Environmental Protection and management of Radioactive Waste Scientificly—Industrial Centers. Nrhf Decommissioning”, Sergiev Posad, Russia, 23–24 September 2020; pp. 129–132. [Google Scholar]
- Karnland, O.; Olsson, S.; Nilsson, U.; Sellin, P. Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys. Chem. Earth Parts A/B/C 2007, 32, 275–286. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.G.; Ye, W.M.; Cui, Y.J.; Wang, Q. Swelling deformation of Gaomiaozi bentonite under alkaline chemical conditions in a repository. Eng. Geol. 2020, 279, 105891. [Google Scholar] [CrossRef]
- Bao, C.; Guo, J.; Zhang, H. Alteration of compacted GMZ bentonite by infiltration of alkaline solution. Clay Miner. 2016, 51, 237–247. [Google Scholar] [CrossRef]
- Ye, W.M.; Zheng, Z.J.; Chen, B.; Chen, Y.G.; Cui, Y.J.; Wang, J. Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Appl. Clay Sci. 2014, 101, 192–198. [Google Scholar] [CrossRef]
- The Scientific Foundations of Deep Geological Disposal; Report No: N/016; United Kingdom Nirex Limited: Harwell, UK, 2001; 118p.
- Kursten, B.; Macdonald, D.D.; Smart, N.R.; Gaggiano, R. Corrosion issues of carbon steel radioactive waste packages exposed to cementitious materials with respect to the Belgian supercontainer concept. Corros. Eng. Sci. Technol. 2017, 52, 11–16. [Google Scholar] [CrossRef]
- Robineau, M.; Deydier, V.; Crusset, D.; Bellefleur, A.; Neff, D.; Vega, E.; Sabot, R.; Jeannin, M.; Refait, P. Formation of Iron Sulfides on Carbon Steel in a Specific Cement Grout Designed for Radioactive Waste Repository and Associated Corrosion Mechanisms. Materials 2021, 14, 3563. [Google Scholar] [CrossRef] [PubMed]
- Gin, S.; Frugier, P. SON68 Glass Dissolution Kinetics at High Reaction Progress: Experimental Evidence of the Residual Rate. Mater. Res. Soc. Symp. Proc. 2003, 807, 175–182. [Google Scholar] [CrossRef]
- Knaus, K.G.; Bourcier, W.L.; McKeegan, K.D.; Merzbacher, C.I.; Nguyen, S.N.; Ryerson, F.J.; Smith, D.K.; Weed, H.C.; Newton, L. Dissolution Kinetics of a Simple Analogue Nuclear Waste Glass as a Function of Time, pH and Temperature. Mater. Res. Soc. Symp. Proc. 1989, 176, 371–381. [Google Scholar] [CrossRef]
- Gin, S.; Jollivet, P.; Fournier, M.; Berthon, C.; Wang, Z.; Mitroshkov, A.; Zhu, Z.; Ryan, J.V. The Fate of Silicon During Glass Corrosion under Alkaline Conditions: A Mechanistic and Kinetic Study with the International Simple Glass. Geochmica Et Cosmochim. Acta 2015, 151, 68–85. [Google Scholar] [CrossRef]
- Cassingham, N.; Corkhill, C.L.; Backhouse, D.J.; Hand, R.J.; Ryan, J.V.; Vienna, J.D.; Hyatt, N.C. The Initial Dissolution Rates of Simulated UK MagnoxTh ORP Blend Nuclear Waste Glass as a Function of pH, Temperature and Waste Loading. Mineral. Mag. 2015, 79, 1529–1542. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Livens, F.R.; Monteith, J.E.; Small, J.S.; Trivedi, D.P.; Vaughan, D.J.; Wogelius, R.A. The kinetics and mechanisms of simulated British Magnox waste glass dissolution as a function of pH, silicic acid activity and time in low temperature aqueous systems. Appl. Geochem. 2000, 15, 1399–1416. [Google Scholar] [CrossRef]
- Fournier, M.; Frugier, P.; Gin, S. Resumption of Alteration at High Temperature and pH: Rates Measurements and Comparison with Initial Rates. Procedia Mater. Sci. 2014, 7C, 202–208. Available online: https://www.sciencedirect.com/science/article/pii/S2211812814010694 (accessed on 30 July 2017). [CrossRef]








| Location | Type of RW | Process | Productivity by Initial Waste |
|---|---|---|---|
| Belgium, NPP (three) | Concentrates, slurries | Stirring in drums | 2 m3/day |
| UK, Hinckley Point, NPP * | Sludge of spent fuel holding basins | Stirring in drums | 12 drums 80-L volume per day |
| India, Tarapur NPP | Sludge | Cementing at disposal site | 500 m3/day |
| India, The Bhabha Atomic Research Centre (BARC) | Sludge | Cementing at disposal site | 225 m3/day |
| Netherlands, Center of Nuclear Research in Petten | Sludge, liquid waste | Pre-stirring, final in drums | 5 m3/day 0.5 m3/day |
| USA, Los Alamos National Laboratory | Concentrates of ILW | Stirring in drums | 4 m3/day |
| USA, Brookhaven National Laboratory | Evaporation concentrates | Addition of concentrate to cement mixture with vermiculite (1:3) in concrete containers with a capacity of 4.2 m3 | 15 m3 per 6 months |
| France, Marcoule, NPP | Solid and liquid RW | Stirring in concrete containers | 6 m3/day 3–5 m3/day |
| France, Nuclear Research Centre at Fontaine-au-Rose | Evaporation concentrates | Stirring in the drum (cement + vermiculite) | 0.3 m3/day |
| France, Centre for Nuclear Research in Saclay | Sludge | Pre-mixing and discharge into concrete containers | 100–300 kg/day |
| France, Nuclear Research Centre in Cadarache | Evaporation concentrates | Stirring in concrete containers | 1.7 m3/day |
| France, Waste Management Centre in La Manche | Compacted solid waste | Cementing in drums | 20 m3/day |
| Germany, NPP | Evaporation concentrates, sludge | Stirring in drums | 2–7 m3/shift |
| Germany, Center for Nuclear Research in Jülich | Concentrates of LLW | Stirring in drums | 50 L/day |
| Germany, Nuclear Research Centre in Karlsruhe | Concentrates of ILW | Stirring in drums | 3–4 m3/shift |
| Switzerland, NPP (two) | Evaporation concentrates, sludge, ion-exchange resins | Stirring in drums | 10–25 drums/day |
| Sweden, Ringhals NPP, Oskarshamn NPP | Evaporation concentrated, ion-exchange resins | Stirring in 1 m3 concrete containers | 2–5 containers/day |
| Component | Mass Content, % |
|---|---|
| OPC (CEM I 52,5N) | 26 |
| Calcium carbonate (CaCO3) | 29 |
| Hydrated lime (Ca(OH)2) | 10 |
| Water | 35 |
| Component | Amount, kg/m3 | Mass Content *, % |
|---|---|---|
| Sulphate resistant Portland cement CEM I 42,5 N SR3 MH/LA, produced by Anläggningscement Degerhamn, Sweden | 120 | 5.14 |
| Silica fume or silica fume slurry ** (SiO2) | 80 | 3.43 |
| Limestone Limus 25 (CaCO3) | 369 | 15.80 |
| Water | 165 | 7.06 |
| Sand 0-8 mm (natural, from Äspö site) | 1037 | 44.41 |
| Gravel (natural or crushed) | 558 | 23.90 |
| Superplasticizer Glenium 51 | 6 | 0.26 |
| Total | 2335 | 100.00 |
| Country | Repository/Disposal Facility | Type of Engineered Barrier |
|---|---|---|
| Belgium | DGR project | Drift backfill/One of supercontainer barriers |
| Canada | DGR project at Bruce cite | Concrete walls isolating RW containing chambers/Concrete caps isolating access shafts |
| Finland | GDR project (KBS-3V) | Concrete plugs and seals |
| France | Cigéo GDF project | Concrete plugs/Tunnel buffer mixed with bentonite/Concrete lining |
| Germany | DGR Morsleben | Mine backfill |
| Hungary | DGR Bátaapáti | RW matrix/Container filling/Drift lining |
| Spain | DGR project | Concrete lining |
| Sweden | SFL DGR project for ILW/LLW disposal | Buffer |
| SFR DGR for ILW/LLW disposal | Shaft lining | |
| DGR project (KBS-3V) | Concrete plugs and seals | |
| Switzerland | GDF project for ILW/LLW | Cement matrix/Container filling/Buffer |
| United Kingdom | GDF project (ceased) | Cement matrix/Buffer/Container filling |
| Japan | GDF project for HLW and SNF | Container filling/Concrete lining/Concrete plugs |
| Radionuclide | Specific Activity, Bq/t U | |
|---|---|---|
| VVER-440 (T = 7 Years) | BN-600 (T = 14 Years) | |
| 14C | 5.6 × 109 | 5.24 × 1010 |
| 79Se | 8.58 × 108 | 2.38 × 109 |
| 99Tc | 7.50 × 1011 | 1.44 × 1012 |
| 129I | 1.03 × 109 | 2.29 × 109 |
| 135Cs | 1.85 × 1010 | 1.39 × 1011 |
| 234U | 3.23 × 109 | 1.94 × 1010 |
| 235U | 3.62 × 108 | 1.34 × 1010 |
| 237Np | 5.56 × 109 | 2.87 × 1010 |
| 238Pu | 1.20 × 1013 | 5.75 × 1013 |
| 239Pu | 2.14 × 1013 | 7.19 × 1013 |
| 240Pu | 2.65 × 1013 | 1.15 × 1013 |
| 241Pu | 1.11 × 1014 | 7.60 × 1013 |
| 242Pu | 6.08 × 1010 | 1.52 × 108 |
| 241Am | 9.83 × 1013 | 2.48 × 1012 |
| 243Am | 1.14 × 1011 | 1.24 × 108 |
| 245Cm | 5.53 × 107 | 7.15 × 104 |
| Aqueous Phase | Na+ | Al3+ | Si4+ | K+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| Model solution before experiment | 8.87 | - | - | 1.15 | 4.95 | 12.2 | 25.6 | 4.95 | 8.87 | 6.1 |
| After experiment | 67.8 | 0.01938 | 0.3877 | 119.82 | 0.000411 | 0.8034 | - | - | - | 12.1 |
| Cement/from Cement Exposure | Changes in Concrete/from Concrete Side Barrier |
|---|---|
| Temperatures characteristic of RW Class 1 and 2 packages | Reduced concrete compressive strength by 25% |
| Groundwater flow through pores of concrete | Dissolving Portlandite, cement C-S-H gel, increasing porosity and reducing strength. The hydraulic permeability of concrete with silica fume admixture is at the level of permeability of granite rocks, which meets the requirement for material for deep repositories. |
| Influence of HLF irradiation on concrete properties | No significant changes of compressive strength, no visible damage of concrete samples and sufficient changes in structure under absorbed dose of high-level waste up to 108 Gy. The maximum radiolytic hydrogen release does not exceed 10−3 mol/(g of the sample) at 108 Gy. |
| Biogenic processes | Carbonization and neutralization of basic cement stone minerals, resulting in their leaching and reduced strength (for nitrate-containing cement matrices with radioactive waste). It is assumed that the cement materials in the geological disposal facility at Yeniseysky site will contact the indigenous and extraterrestrial microbiota, as well as intensification of microbial processes under the influence of heat emission and radiolytic gases. |
| Cement/from Cement Exposure | Changes in Concrete/from Concrete Side Barrier |
|---|---|
| Effect of alkaline concrete pore solution on compacted bentonite buffer | No significant changes in cation exchange capacity, swelling pressure and hydraulic permeability of compacted bentonite at pH 12.4 which is common for hardened concrete. At values of pH typical for fresh cement mortar (13.3), compacted bentonite buffer shows a decrease in cation exchange capacity, swelling pressure, and an increase in hydraulic permeability. |
| Influence on steel container from alkaline pore solution of concrete | Under anaerobic conditions, geological disposal facility ensures the integrity of the steel packaging structure (corrosion rate 0.03 µm/year) |
| Influence on steel container from alkaline pore solution of concrete at temperature 80 °C | Increased corrosion rate of 3–6 μm/year in anaerobic geological disposal facility conditions with occurrence of local corrosion hotspots with thickness of 10 μm. |
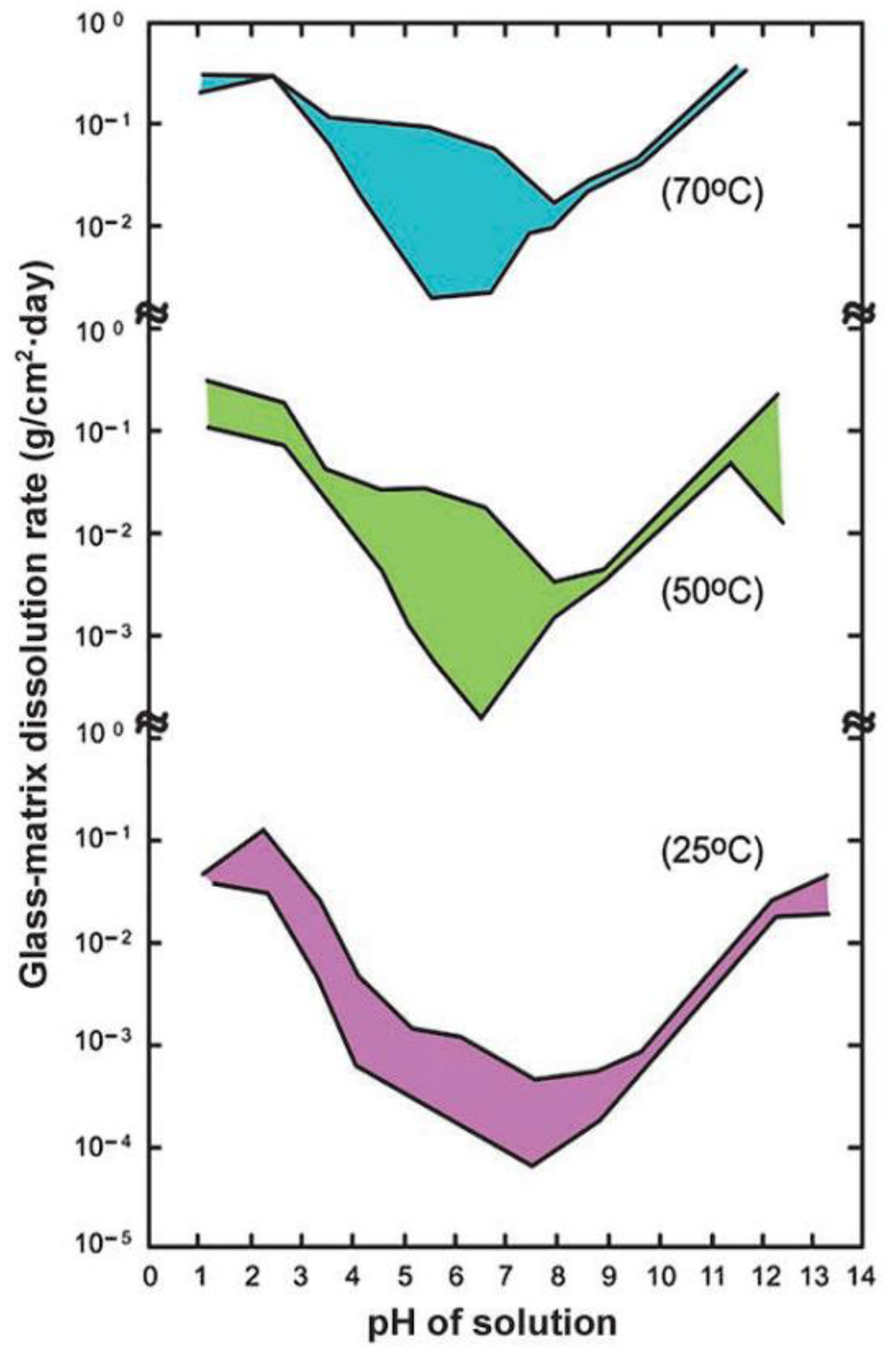
| Effect of alkaline pore solution of concrete on borosilicate and aluminophosphate glass matrices | With increased pH, the dissolution rate of glass matrices increases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyupina, E.A.; Kozlov, P.P.; Krupskaya, V.V. Application of Cement-Based Materials as a Component of an Engineered Barrier System at Geological Disposal Facilities for Radioactive Waste—A Review. Energies 2023, 16, 605. https://doi.org/10.3390/en16020605
Tyupina EA, Kozlov PP, Krupskaya VV. Application of Cement-Based Materials as a Component of an Engineered Barrier System at Geological Disposal Facilities for Radioactive Waste—A Review. Energies. 2023; 16(2):605. https://doi.org/10.3390/en16020605
Chicago/Turabian StyleTyupina, Ekaterina Aleksandrovna, Pavel Pavlovich Kozlov, and Victoria Valerievna Krupskaya. 2023. "Application of Cement-Based Materials as a Component of an Engineered Barrier System at Geological Disposal Facilities for Radioactive Waste—A Review" Energies 16, no. 2: 605. https://doi.org/10.3390/en16020605
APA StyleTyupina, E. A., Kozlov, P. P., & Krupskaya, V. V. (2023). Application of Cement-Based Materials as a Component of an Engineered Barrier System at Geological Disposal Facilities for Radioactive Waste—A Review. Energies, 16(2), 605. https://doi.org/10.3390/en16020605







