Comparative Evaluation of PSA, PVSA, and Twin PSA Processes for Biogas Upgrading: The Purity, Recovery, and Energy Consumption Dilemma
Abstract
1. Introduction
2. Materials and Methods
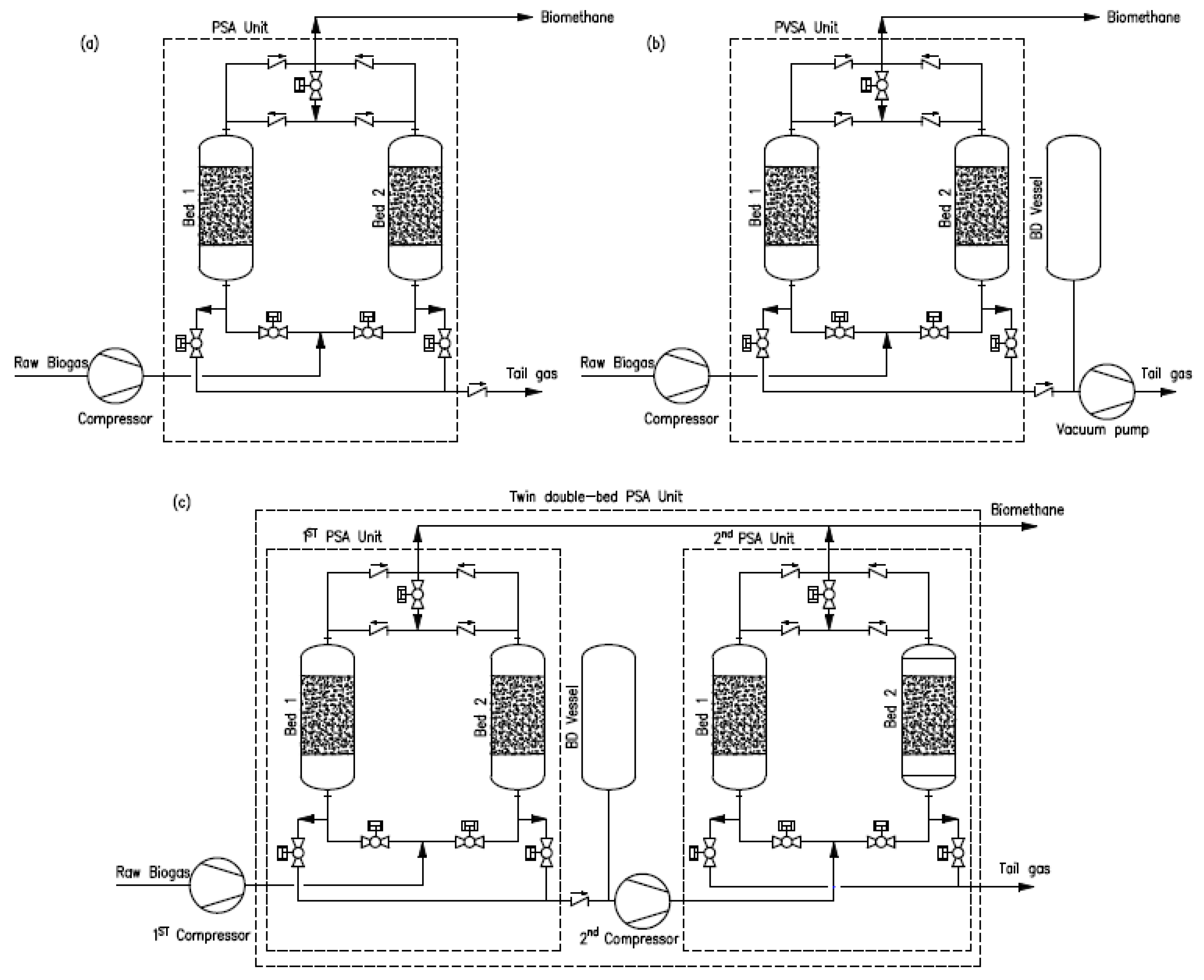
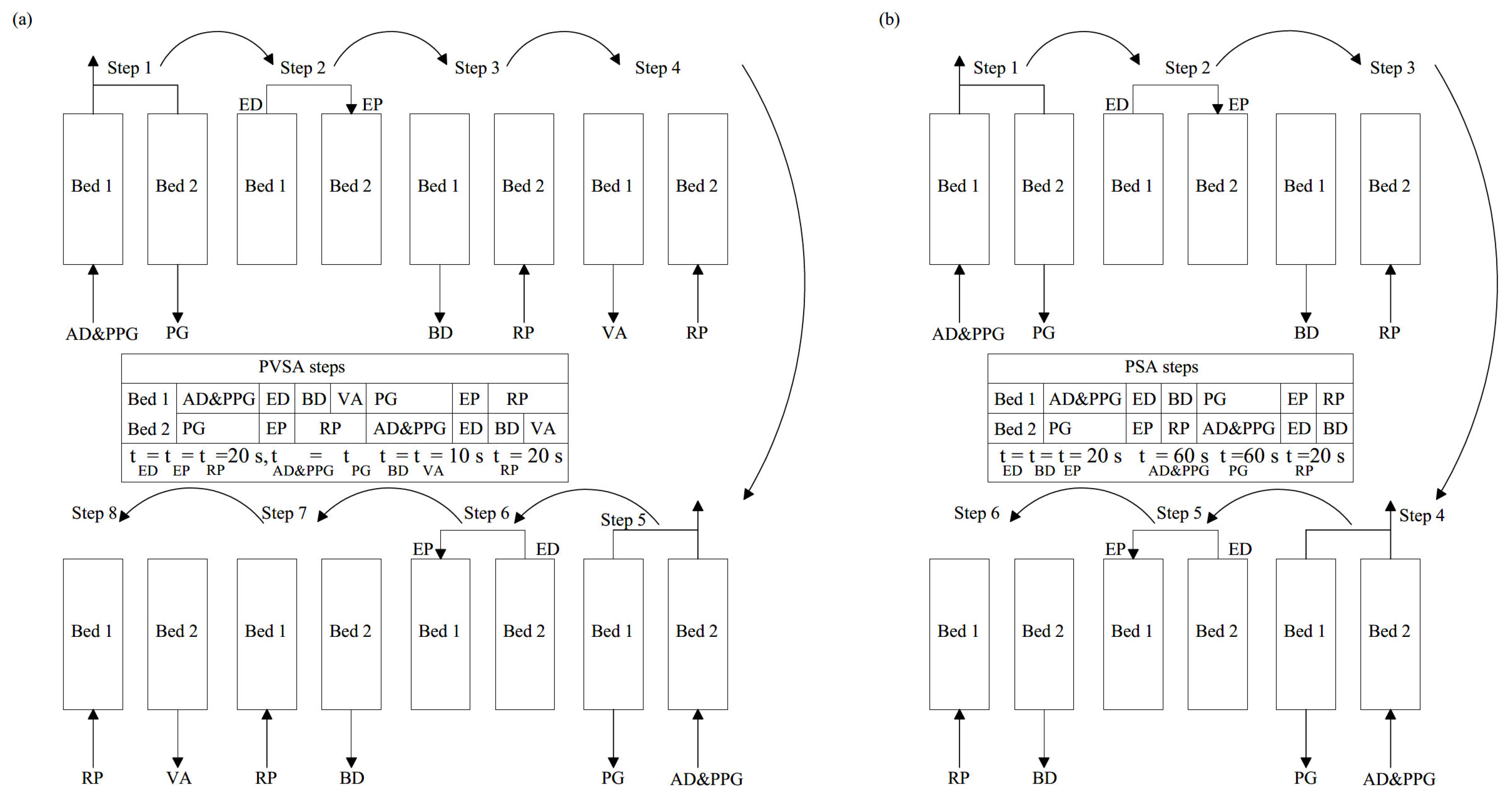
2.1. Process Description
2.2. Mathematical Model
3. Results and Discussion
3.1. Effect of Purge to Feed Ratio
3.2. Effect of Adsorption Time
3.3. Effect of Temperature
3.4. Comparison of PSA, PVSA, and Twin Double-Bed PSA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EurObserv’ER Consortium. The State of Renewable Energies in Europe, 2019 ed.; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Biomethane: An Easy Answer to the Complex Equation of Transport Decarbonization. 2020. Available online: https://www.ngva.eu/wp-content/uploads/2020/05/NGVA-Europe_Biomethane_May2020.pdf (accessed on 20 April 2022).
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the Biomethane Market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Wadi, B.; Manovic, V. Advances, challenges, and perspectives of biogas cleaning, upgrading, and utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology—A review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of biogas upgrading technologies and future perspectives: A review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Augelletti, R.; Conti, M.; Annesini, M.C. Pressure swing adsorption for biogas upgrading. A new process configuration for the separation of biomethane and carbon dioxide. J. Clean. Prod. 2017, 140, 1390–1398. [Google Scholar] [CrossRef]
- Santos, M.P.S.; Grande, C.A.; Rodrigues, A.E. Pressure Swing Adsorption for Biogas Upgrading. Effect of Recycling Streams in Pressure Swing Adsorption Design. Ind. Eng. Chem. Res. 2011, 50, 974–985. [Google Scholar] [CrossRef]
- Grande, C.A.; Rodrigues, A.E. Biogas to Fuel by Vacuum Pressure Swing Adsorption I. Behavior of Equilibrium and Kinetic-Based Adsorbents. Ind. Eng. Chem. Res. 2007, 46, 4595–4605. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Xu, Y.; Bao, D.; Zhang, S. Assessment of the energy consumption of the biogas upgrading process with pressure swing adsorption using novel adsorbents. J. Clean. Prod. 2015, 101, 251–261. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Helwani, Z. Production of ultrapure biomethane from stratified bed in non-adiabatic and non-isothermal plate pressure swing adsorption. Chem. Eng. Res. Des. 2023, 190, 335–352. [Google Scholar] [CrossRef]
- Rzepka, P.; Jasso-Salcedo, A.B.; Janicevs, A.; Vasiliev, P.; Hedin, N. Upgrading of raw biogas into biomethane with structured nano-sized zeolite |NaK|-A adsorbents in a PVSA unit. Energy Procedia 2019, 158, 6715–6722. [Google Scholar] [CrossRef]
- Durán, I.; Rubiera, F.; Pevida, C. Modeling a biogas upgrading PSA unit with a sustainable activated carbon derived from pine sawdust. Sensitivity analysis on the adsorption of CO2 and CH4 mixtures. Chem. Eng. J. 2022, 428, 132564. [Google Scholar] [CrossRef]
- Petracchini, F.; Paolini, V.; Liotta, F.; Paciucci, L.; Facci, E. Vacuum swing adsorption on natural zeolites from tuffs in a prototype plant. Environ. Prog. Sustain. Energy 2017, 36, 887–894. [Google Scholar] [CrossRef]
- Sonnleitner, E.; Schöny, G.; Hofbauer, H. Assessment of zeolite 13X and Lewatit® VP OC 1065 for application in a continuous temperature swing adsorption process for biogas upgrading. Biomass Convers. Biorefinery 2018, 8, 379–395. [Google Scholar] [CrossRef]
- Golmakani, A.; Fatemi, S.; Tamnanloo, J. Investigating PSA, VSA, and TSA methods in SMR unit of refineries for hydrogen production with fuel cell specification. Sep. Purif. Technol. 2017, 176, 73–91. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Manović, V. Production of negative-emission biomethane by twin double-bed pressure swing adsorption with tail gas sequestration. Chem. Eng. J. 2021, 408, 127312. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, W.; Zhang, D.; Na, P.; Fu, B. The removal and capture of CO2 from biogas by vacuum pressure swing process using silica gel. J. CO2 Util. 2018, 27, 259–271. [Google Scholar] [CrossRef]
- Santos, M.P.S.; Grande, C.A.; Rodrigues, A.E. Dynamic Study of the Pressure Swing Adsorption Process for Biogas Upgrading and Its Responses to Feed Disturbances. Ind. Eng. Chem. Res. 2013, 52, 5445–5454. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Upgrade of Methane from Landfill Gas by Pressure Swing Adsorption. Energy Fuels 2005, 19, 2545–2555. [Google Scholar] [CrossRef]
- Grande, C.A.; Rodrigues, A.E. Layered Vacuum Pressure-Swing Adsorption for Biogas Upgrading. Ind. Eng. Chem. Res. 2007, 46, 7844–7848. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids; McGraw Hill: New York, NY, USA, 1987. [Google Scholar]
- Bird, R.B. Transport phenomena. Appl. Mech. Rev. 2002, 55, R1–R4. [Google Scholar] [CrossRef]
- Dantas, T.L.; Luna, F.M.T.; Silva, I.J.; de Azevedo, D.C.; Grande, C.A.; Rodrigues, A.E.; Moreira, R.F. Carbon dioxide–nitrogen separation through adsorption on activated carbon in a fixed bed. Chem. Eng. J. 2011, 169, 11–19. [Google Scholar] [CrossRef]
- Dantas, T.L.P.; Amorim, S.M.; Luna, F.M.T.; Silva, I.J.; de Azevedo, D.C.S.; Rodrigues, A.E.; Moreira, R.F.P.M. Adsorption of Carbon Dioxide onto Activated Carbon and Nitrogen-Enriched Activated Carbon: Surface Changes, Equilibrium, and Modeling of Fixed-Bed Adsorption. Sep. Sci. Technol. 2009, 45, 73–84. [Google Scholar] [CrossRef]
- Wakao, N.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to-fluid mass transfer coefficients in packed beds: Correlation of sherwood numbers. Chem. Eng. Sci. 1978, 33, 1375–1384. [Google Scholar] [CrossRef]
- Yang, R. Gas Separation by Adsorption Processes; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Vliet, G.C. Natural Convection Local Heat Transfer on Constant-Heat-Flux Inclined Surfaces. J. Heat Transf. 1969, 91, 511–516. [Google Scholar] [CrossRef]
- Churchill, S.W.; Chu, H.H. Correlating equations for laminar and turbulent free convection from a horizontal cylinder. Int. J. Heat Mass Transf. 1975, 18, 1049–1053. [Google Scholar] [CrossRef]
- Wakao, N.; Kaguei, S.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to-fluid heat transfer coefficients in packed beds: Correlation of nusselt numbers. Chem. Eng. Sci. 1979, 34, 325–336. [Google Scholar] [CrossRef]
- Yang, J.; Lee, C.-H. Adsorption dynamics of a layered bed PSA for H2 recovery from coke oven gas. AIChE J. 1998, 44, 1325–1334. [Google Scholar] [CrossRef]
- Sereno, C.; Rodrigues, A. Can steady-state momentum equations be used in modelling pressurization of adsorption beds? Gas Sep. Purif. 1993, 7, 167–174. [Google Scholar] [CrossRef]
- A.P.T. Code. Performance Test Code on Compressors and Exhausters; The American Society of Mechanical Engineers: New York, NY, USA, 1997. [Google Scholar]
- Golmakani, A.; Fatemi, S.; Tamnanloo, J. CO2 Capture from the Tail Gas of Hydrogen Purification Unit by Vacuum Swing Adsorption Process, Using SAPO.34. Ind. Eng. Chem. Res. 2016, 55, 334–350. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, J.-Y.; Kang, Y.-T. Experimental Study on PSA Process for High Purity CH4Recovery from Biogas. Korean J. Air Cond. Refrig. Eng. 2011, 23, 281–286. [Google Scholar] [CrossRef][Green Version]
- De Witte, N.; Denayer, J.F.M.; van Assche, T.R.C. Effect of Adsorption Duration and Purge Flowrate on Pressure Swing Ad-sorption Performance. Ind. Eng. Chem. Res. 2021, 60, 13684–13691. [Google Scholar] [CrossRef]
- Shokroo, E.J.; Shahcheraghi, M.; Farniaei, M. Study of feed temperature effects on performance of a domestic industrial PSA plant. Appl. Petrochem. Res. 2014, 4, 317–323. [Google Scholar] [CrossRef]
- Sarker, A.I.; Aroonwilas, A.; Veawab, A. Equilibrium and Kinetic Behaviour of CO2 Adsorption onto Zeolites, Carbon Molecular Sieve and Activated Carbons. Energy Procedia 2017, 114, 2450–2459. [Google Scholar] [CrossRef]





| Description | Formulation | |
|---|---|---|
| Gas phase mass balance for component i | (1) | |
| Linear driving force (LDF) model for mass transfer | (2) | |
| The heat balance formula for bed wall | (3) | |
| The heat balance formula for solid adsorbent | (4) | |
| The heat balance formula for gas phase | (5) | |
| Formula for heat transfer from gas phase to bed [25,26,27,28] | (6) | |
| Formula for effective axial heat dispersion coefficient [29,30] | (7) | |
| Formula for heat transfer from bed wall to atmosphere [31,32] | (8) | |
| Formula for heat transfer from solid adsorbent to gas phase [29,33] | (9) | |
| Pressure drop calculation by Ergun equation [34,35] | (10) |
| Case No. | PG/F (%) | CH4 Purity (%) | CH4 Recovery (%) |
|---|---|---|---|
| PG1 | 20 | 98.8 | 49.6 |
| PG2 | 15 | 97.9 | 55.3 |
| PG3 | 10 | 95.5 | 61.1 |
| PG4 | 5 | 92.4 | 70.2 |
| Case No. | tads (s) | CH4 Purity (%) | CH4 Recovery (%) | Feed Compressor (kJ/kg CH4) | Vacuum Compressor (kJ/kg CH4) | Total Energy (kJ/kg CH4) |
|---|---|---|---|---|---|---|
| t1 | 45 | 99.96 | 58.78 | 1168 | 475 | 1643 |
| t2 | 65 | 99.81 | 67.24 | 1009 | 386 | 1395 |
| t3 | 85 | 98.99 | 72.79 | 928 | 384 | 1312 |
| t4 | 105 | 95.35 | 76.18 | 920 | 355 | 1275 |
| t5 | 115 | 92.06 | 78.13 | 868 | 346 | 1214 |
| t6 | 125 | 89.05 | 79.58 | 852 | 339 | 1191 |
| t7 | 145 | 83.99 | 81.87 | 828 | 308 | 1136 |
| Case No. | tads (s) | Temp. (°C) | CH4 Purity (%) | CH4 Recovery (%) |
|---|---|---|---|---|
| T1 | 45 | 25 | 92.5 | 70.2 |
| T2 | 45 | 35 | 83.96 | 60.2 |
| T3 | 40 | 35 | 87.39 | 57.5 |
| T4 | 35 | 35 | 91.17 | 54.3 |
| T5 | 33 | 35 | 93.32 | 47.8 |
| Case No. | tads (s) | Type | PVA (bar) | CH4 Purity (%) | CH4 Recovery (%) | 1st Compressor (kJ/kg CH4) | 2nd Compressor (kJ/kg CH4) | Total Energy (kJ/kg CH4) |
|---|---|---|---|---|---|---|---|---|
| V1 | 45 | PSA | 1 | 92.5 | 70.2 | 869 | 869 | |
| V2 | 45 | TD PSA | 1 | 87 | 90 | 869 | 34 | 903 |
| V3 | 55 | PVSA | 0.6 | 93.1 | 71.2 | 948 | 56.5 | 1005 |
| V4 | 55 | PVSA | 0.5 | 95.4 | 67.5 | 1008 | 83 | 1091 |
| V5 | 65 | PVSA | 0.5 | 93.1 | 71.1 | 958 | 82 | 1040 |
| V6 | 85 | PVSA | 0.5 | 87.8 | 75.6 | 897 | 77 | 974 |
| V7 | 90 | PVSA | 0.5 | 86.5 | 76.6 | 886 | 75 | 961 |
| V8 | 55 | PVSA | 0.1 | 99.9 | 63.5 | 1074 | 387 | 1461 |
| V9 | 65 | PVSA | 0.1 | 99.8 | 67.2 | 1009 | 386 | 1395 |
| V10 | 85 | PVSA | 0.1 | 98.9 | 72.8 | 928 | 384 | 1312 |
| V11 | 105 | PVSA | 0.1 | 95.3 | 76.2 | 920 | 355 | 1275 |
| V12 | 115 | PVSA | 0.1 | 92.0 | 78.1 | 868 | 346 | 1214 |
| V13 | 125 | PVSA | 0.1 | 89.0 | 79.6 | 852 | 339 | 1191 |
| V14 | 132 | PVSA | 0.1 | 87.0 | 80.4 | 842 | 327 | 1169 |
| V15 | 132 | PVSA | 0.01 | 87.9 | 80.4 | 842 | 874 | 1716 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golmakani, A.; Wadi, B.; Manović, V.; Nabavi, S.A. Comparative Evaluation of PSA, PVSA, and Twin PSA Processes for Biogas Upgrading: The Purity, Recovery, and Energy Consumption Dilemma. Energies 2023, 16, 6840. https://doi.org/10.3390/en16196840
Golmakani A, Wadi B, Manović V, Nabavi SA. Comparative Evaluation of PSA, PVSA, and Twin PSA Processes for Biogas Upgrading: The Purity, Recovery, and Energy Consumption Dilemma. Energies. 2023; 16(19):6840. https://doi.org/10.3390/en16196840
Chicago/Turabian StyleGolmakani, Ayub, Basil Wadi, Vasilije Manović, and Seyed Ali Nabavi. 2023. "Comparative Evaluation of PSA, PVSA, and Twin PSA Processes for Biogas Upgrading: The Purity, Recovery, and Energy Consumption Dilemma" Energies 16, no. 19: 6840. https://doi.org/10.3390/en16196840
APA StyleGolmakani, A., Wadi, B., Manović, V., & Nabavi, S. A. (2023). Comparative Evaluation of PSA, PVSA, and Twin PSA Processes for Biogas Upgrading: The Purity, Recovery, and Energy Consumption Dilemma. Energies, 16(19), 6840. https://doi.org/10.3390/en16196840








