Abstract
Energy cane is a genotype derived from species of sugarcane (Saccharum officinarum and Saccharum spontaneum) with a lower sucrose content and higher fiber content for bioenergy purposes. It is a rustic plant that demands less fertile soils that do not compete with food crops. In this work, an analysis of energy cane bagasse pyrolysis products was performed, assessing the effect of reaction temperature and kinetic and thermodynamic parameters. Anhydrosugars, such as D-allose, were the primary compounds derived from the decomposition of energy cane at 500 °C. Methyl vinyl ketone and acetic acid were favored at 550 and 600 °C. At 650 °C, methyl glyoxal, acetaldehyde and hydrocarbons were favored. Among the hydrocarbons observed, butane, toluene and olefins such as 1-decene, 1-undecene, 1-tridecene and 1-tetradecene were the most produced. The Friedman isoconversional method was able to determine the average activation energies in the ranges 113.7−149.4, 119.9−168.0, 149.3−196.4 and 170.1−2913.9 kJ mol−1 for the decomposition of, respectively, pseudo-extractives, pseudo-hemicellulose, pseudo-cellulose and pseudo-lignin. The thermodynamic parameters of activation were determined within the ranges of 131.0 to 507.6 kJ mol−1 for ΔH, 153.7 to 215.2 kJ mol−1 for ΔG and −35.5 to 508.8 J mol−1 K−1 for ΔS. This study is very encouraging for the cultivation and use of high-fiber-content energy cane bagasse, after sucrose extraction, to produce biofuels as an alternative to the current method of conversion into electricity by low-efficiency burning.
1. Introduction
Increasing populations and economies have led to an increased demand for energy. This increased demand for the consumption of fossil fuels has, at the same time, contributed to high greenhouse gas emissions and a consequent increase in extreme weather events [1]. One alternative is the use of renewable energy, such as solar, wind and biofuels. In Brazil, first-generation liquid biofuels for vehicular transport come from food crops, such as ethanol from sugarcane [2] and biodiesel from vegetable oils [3]. This market, however, competes with the use of land and water for food production. The high cost of production requires government subsidies to enable competition with the production price of fossil gasoline and diesel. Biomass is considered the fourth leading energy source in the world, representing approximately 9% of total sources [4]. An alternative route to using raw materials without competing with food is second-generation biofuels made from waste biomass [5].
Pyrolysis is an efficient route for the thermochemical conversion of waste; it occurs at temperatures from 450 to 650 °C under an inert atmosphere [6,7]. There are several types of biomasses that can be harnessed on a large scale for second-generation biofuels via pyrolysis, such as waste vegetable oils [8,9], waste animal fat [10], brewer’s spent grain [11], cattle manure [12], microalgae [13], nut residues [14], off-spec biodiesel [15] and grease traps [16]. The pyrolysis process of lignocellulosic biomass produces bio-gas, bio-oil and bio-char [17,18]. Bio-oil needs to go through a hydrorefining process to obtain hydrocarbons for drop-in biofuels [19].
In Brazil, sugarcane residues, such as bagasse and straw, represent an abundant source of resources for the sustainable generation of renewable energy. In the 2021/2022 crop year, Brazil produced 586 million tons of sugarcane on 8300 hectares of land, with an estimated generation of 160 million tons of bagasse residue and 99 million tons of straw [20]. Only 30% of the energy content of sugarcane is utilized as sucrose for sugar and alcohol production, with most of the energy being in the bagasse, which is used for the production of electricity from low-efficiency burning with 50% moisture. The pyrolysis of sugarcane residues has been reported. Schmitt et al. (2020) [21] evaluated the pyrolysis of sugarcane bagasse on a pilot scale at 500 °C, observing a yield of 60.1% in bio-oil production. Sohaib et al. (2017) [22] studied the rapid bench-scale pyrolysis of sugarcane bagasse, obtaining at 500 °C the maximum bio-oil yield (~60.4%). At 600 °C the yield was lower, but the calorific value of the bio-oil was (~24.7 MJ kg−1) higher than that of the original biomass. Barros et al. (2017) [23] studied the pyrolysis of bagasse and straw from four sugarcane species, one common species (Saccharum sp.) and three genetically modified species, aiming to obtain a higher energy content. The bagasse showed higher bio-oil yields (~51.0%) than the straw (~41.5%), with the Erianthus sp species being the most efficient. The bio-oil from the Miscanthius sp. species showed a higher volume of hydrocarbons. Durange et al. (2013) [24] carried out the pyrolysis of sugarcane in a fluidized bed pilot plant under air at 500 °C, obtaining a 40% bio-oil yield, with low iron (~27 ppm) and potassium (1 ppm) contents. The bio-oil obtained from sugarcane straw showed high metal contamination, with 807 ppm of iron and 123 ppm of potassium. The bio-oils presented mostly phenolic compounds in their composition.
The term energy cane refers to modified sugarcane species that provide a higher fiber supply (20–40 wt.%) compared to the common species used for sucrose production (5–15 wt.%) [25]. Energy cane is a more resistant and rustic plant that can be grown in less fertile soils and with less use of fertilizers, therefore not competing with food crops [26]. Its biomass productivity is 2.5 times higher than conventional sugarcane; besides having high fiber content, these species produce a juice containing sucrose that can be used in the production of sugar [27,28]. There are two types of energy cane depending on the composition: type I has a low sucrose content (10–14%) and high fiber content (15–20%); type II has a lower sucrose content (<10%) and high fiber content (>20%) [29]. In Brazil, energy cane is obtained from the genetic crossing of the sugarcane species S. officinarum and S. Spontaneum. Henkel et al. (2016) [30] studied the pyrolysis of energy cane bagasse in a laboratory-scale inductively heated reactor at 500–700 °C, with maximum bio-oil production (~49%) at 550 °C. At this temperature, the bio-oil obtained was rich in oxygenated compounds, such as phenols (~70%), carboxylic acids (~15%), furans (~2.3%), ketones (~2.3%) and aldehydes (~2.4%). To our knowledge, Henkel’s study is the only report on the pyrolysis of energy cane with an analysis of the reaction products. The study, however, left some gaps in the detailing of the products obtained, types of hydrocarbons formed and reaction mechanisms.
Besides the detailed analysis of the reaction products, another important aspect is the development of kinetic models to obtain activation energy parameters and pre-exponential factors, aimed at the reactor design for scaling-up to an industrial process [31,32]. Thermogravimetric analysis (TGA) has been used to study the chemical kinetics involved in biomass pyrolysis process [33]. Several models can be employed to determine the kinetic parameters of the rate law, such as single-step, parallel reactions, distributed activation energy, and isoconversional models, such as Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) [34,35]. Peres et al. (2021) [36] studied the pyrolysis kinetics of sugarcane bagasse using the FWO and KAS isoconversional models, obtaining activation energy values between 15 and 73 kJ mol−1 in the region of 75 to 95% conversion. Van Nam and co-workers [37] studied the kinetic modeling of the pyrolysis of sugarcane bagasse using the FWO and KAS models, obtaining activation energy values of 177 kJ mol−1 in the 25 to 75% conversion range.
Due to the complexity of lignocellulosic biomass, the thermogravimetric curves in the derivative form (DTG) present overlapping mass losses that can be separated by the process of mathematical deconvolution, obtaining pseudo-component data. In this case, isoconversional kinetic models enable the calculation of the activation energy for each mass loss component, so that their values are approximately constant as a function of conversion, as recommended by ICTAC [38]. Following this recommendation, Guimaraes et al. (2021) [39] studied the pyrolysis kinetics of the Saccharum spontaneum energy cane, fitting the data to the FWO model. The activation energy of 111 kJ mol−1 was obtained for the decomposition of extractives, 118 kJ mol−1 for hemicellulose, 195 kJ mol−1 for cellulose and 51 kJ mol−1 for lignin. De Carvalho and Tannous (2017) [25] studied the thermal decomposition kinetics of the energy cane species Saccharum robustum, obtaining activation energies assigned to each mass loss: 108–143 kJ mol−1 for extractives, 143–180 kJ mol−1 for hemicellulose and 164–183 kJ mol−1 for cellulose. Oliveira et al. (2022) [40] studied the thermal decomposition of energy cane, using the FWO model for estimating kinetic activation energies of 130, 104, 174, and 63 kJ mol−1, respectively, assigned to extractives, hemicellulose, cellulose and lignin. This variation in activation energy values for each biomass component indicates the complexity of this biomass and requires calculations of the thermodynamic parameters ΔH, ΔG and ΔS to better understand the decomposition of each biomass fraction. Thermodynamic parameter calculations associated with kinetic parameters have been successfully reported for the pyrolysis of other types of biomass [33,35,41]. These thermodynamic parameters, however, have not yet been reported for energy cane pyrolysis. The objective of this work, then, was to make a detailed analysis of energy cane pyrolysis products as a function of temperature, correlate this with their physicochemical characteristics, and construct multi-component models to estimate kinetic and thermodynamic parameters for the decomposition of each energy cane fraction.
2. Materials and Methods
2.1. Biomass Preparation and Characterization
The energy cane bagasse used in this work (genotype RB127048) came from the Federal University of Viçosa, MG, Brazil. This bagasse contained 23.1% fibers and 17.0% Brix. The sample was dried at 105 °C for 24 h and fragmented in a cutting mill (TECNAL, Willye TE-648, Brazil) to obtain particles under 0.425 mm. Biomass characterization was performed via proximate analysis from thermogravimetric curves, following ASTM D7582-15 standard [42]. Biochemical analysis was performed to obtain the content of the solvent extractives, lignin and holocellulose (cellulose + hemicellulose) (Equation (1)), according to TAPPI standards [43,44,45].
The carbon, hydrogen and nitrogen contents were measured using an elemental analyzer model 2400 series II (Perkin Elmer, Waltham, MA, USA) based on the Preg–Dumas method. The sulfur content was obtained by plasma optical emission spectrometry (Spectro, Arcos model with radial vision, Kleve, Germany). The oxygen content was calculated by difference. The low calorific value (LHV) of the energy cane was calculated using Equation (2) [46].
Thermogravimetric analysis (TG/DTG) (NETZSCH, STA 449 F3 Jupiter, Waldkraiburg, Germany) of energy cane bagasse was performed at four heating rates β = 5, 10, 15 and 20 °C·min−1. In each experiment, 5 mg of biomass were used and subjected to heating from 25 to 700 °C under nitrogen (50 mL·min−1).
2.2. Pyrolysis of Energy Cane Bagasse
Pyrolysis experiments of the energy cane bagasse were performed using a Frontier Rx-3050TR, Japan, instrument connected to a gas chromatograph coupled to a mass spectrometer Shimadzu, GC/MS QP 2020 plus, Japan [47]. For each experiment, 110 ± 5 µg of biomass were placed in an inert surface stainless steel crucible, then covered with quartz wool to prevent the unwanted escape of material during pyrolysis. The crucible was injected into the furnace under inert atmosphere (helium, 84 mL·min−1). The pyrolysis gases passed through an interface at 300 °C before entering the chromatograph injector at 250 °C, split ratio of 50:1. The products were separated using an SH-RTx-5 capillary column (60 m × 0.25 mm × 0.25 μm) following the temperature program: 40 °C for 2 min; heating up to 270 °C at 5 °C·min−1; heating up to 280 °C at 10 °C·min−1. The mass spectrometer interface and ion source temperatures were 300 and 250 °C, respectively, with data acquisition in scan mode in the 40–400 m/z range. The peaks of the total ion chromatograms (TICs) were identified using the NIST-USA library, with similarity equal to or greater than 85%. All pyrolysis experiments were performed in duplicate at temperatures of 550, 600 and 650 °C.
2.3. Kinetic Modeling of Energy Cane Bagasse Pyrolysis
In this work, the multi-component kinetic study considered that in the pyrolysis of energy cane bagasse, four parallel and independent reactions occur, in which extractives, hemicellulose, cellulose and lignin are converted into volatiles and bio-char (Equation (3)).
This approach has been previously considered in pyrolysis kinetic studies of lignocellulosic biomasses [33,40,41] and is recommended by the Committee of the International Confederation for Thermal Analysis and Calorimetry [38]. The Asymmetric Double Sigmoidal function (Asym2Sig) (Equation (4)) was used to deconvolute the DTG peaks from the thermogravimetric analyses at the four heating rates [48].
Four adjustable parameters, namely the maximum amplitude of the curve (θ), curve width (w1), and two shape parameters (w2 and w3), were used to characterize each pseudo-component [49]. The optimal values of these four parameters that minimized the objective function were determined.
The residual sum of squares (RSS) was used to calculate the difference between experimental and deconvoluted conversion rates, with the objective of minimizing its value. The objective function in Equation (5) is constrained by w1 > 0, w2 > 0 and w3 > 0, corresponding to the curve width and two shape parameters, respectively.
where M is the number of experimental data, (dα/dT)exp is the experimental conversion rate and (dα/dT)dec is the deconvoluted conversion rate. The conversion is described by α = (mo − mT)/(m0 − mf), where m0 is the initial mass, mT is the mass at temperature T and mf is the final mass.
The kinetic parameters’ activation energy (Ea—kJ mol−1) and pre-exponential factor (A—min−1) represent, respectively, the minimum energy required to initiate a reaction and the vibration frequency of chemical bonds during thermal decomposition. To obtain these parameters, the modeling starts from the thermal degradation rate equation (Equation (6)). This integral form (Equation (7)) has no analytical solution.
in which β = dT/dt is the rate of change of temperature, R (8.314 J mol−1 K−1 is the universal gas constant and f(α) is the reaction model [50].
Isoconversional methods, which do not require prior assumptions of a reaction model, can be categorized into differential and integral methods. The conversion curves were analyzed using the most common differential method, the Friedman (FR) method. For comparison purposes, the results of three other classical integral methods, namely the Flynn−Wall−Ozawa (FWO), Kissinger−Akahira−Sunose (KAS) and Starink (STK), hare presented in the supplementary material [51,52]. The differential isoconversional method proposed by Friedman is based on the direct differentiation of Equation (6) with the natural logarithm, resulting in Equation (8) [53].
The activation energy (Ea) was determined for a constant conversion degree by plotting ln(dα/dt) vs. (1/T) for the FR method. A detailed background of the derivation of this equation can be found in the literature [50,51].
For calculating the pre-exponential factor of the Arrhenius equation, ICTAC recommends using the kinetic compensation effect (KCE) method, which gives a linear behavior (Equation (9)) between the activation energy and the natural logarithm of A [38].
where a and b are the compensation coefficients obtained, respectively, by the angular and linear coefficients of the straight line described in Equation (9).
A graphical artifice was used to determine the proper form of the reaction model, which involved comparing experimental master plot curves with theoretical ones that considered several reaction models. Applying an appropriate reaction model equates the experimental curve in the form of p(x)/p(x0.5) to the theoretically calculated curve in the form of g(α)/g(0.5), as indicated by Equation (10) [31].
In Equation (10), p(x)/p(x0.5) represents the experimental master plot obtained by normalizing experimental kinetic curves with respect to the reference conversion degree (α = 0.5), and g(α)/g(0.5) represents the theoretical master plot constructed for each of the seventeen candidate reaction models.
The thermodynamic parameters, including the change in enthalpy (ΔH), change in Gibbs free energy (ΔG), and the change in entropy (ΔS), can be calculated through the activated complex theory published by Eyring, given that the kinetic parameters are known [52,54]. The equations established by the Eyring theory were used to calculate the thermodynamic parameters, as follows [54,55]:
where Tm is the DTG peak temperature, kB is the Boltzmann constant (1.381 × 10−23 J K−1) and h is the Planck constant (6.626 × 10−34 J s).
3. Results and Discussion
3.1. Energy Cane Bagasse Characterization
Table 1 shows the results of the proximate, ultimate, biochemical and heating values of the energy cane bagasse used in this work, compared with data available in the literature [25,40].

Table 1.
Proximate, ultimate, biochemical and heating value analyses of energy cane bagasse.
The high content of volatile materials (~74.0 wt.%) combined with the low fixed carbon content (~8.4 wt.%) indicate that the pyrolysis of energy cane bagasse can have high bio-oil yields. Furthermore, the possible technical problems of the fouling of inorganic compounds in the pyrolysis process are reduced due to the moderate ash content of 8.4 wt.%. Elemental analysis shows that energy cane bagasse is rich in carbon and oxygen and contains little nitrogen (0.5 wt.%) and sulfur (0.2 wt.%), indicating the possible low formation of nitrogen and sulfur compounds in bio-oil; the burning of this bio-oil would result in very low emissions of nitrogen and sulfur oxides. The estimated lower heating value was 18.5 MJ·kg−1, which is higher than those of the energy cane species reported [25,40].
As for the lignocellulosic constituents, the energy cane bagasse presented 11.2 wt.% of light extractives, 70.4 wt.% of holocellulose and 18.4 wt.% of lignin. These values suggest that the bio-oil from energy cane can present a high content of oxygenated compounds, which are the products of the decomposition of hemicelluloses, cellulose and lignin [30,56].
The TG and DTG profiles (Figure 1) of energy cane bagasse for the heating rates β = 5, 10, 15 and 20 °C min−1 showed three mass loss events. The first one (25–120 °C) showed an average mass loss of 4.8 wt.%, attributed to moisture loss or the volatilization of light extractives. The second event (150–415 °C) occurred in the active pyrolysis zone, the region where the highest mass loss was observed, around 65 wt.%, mainly related to the degradation of hemicellulose and cellulose. The third event (380–600 °C) occurred in the passive pyrolysis zone, with a lower mass loss rate, giving an average mass loss of 9 wt.%, a region where mainly lignin decomposition occurs. These loss percentages corroborate the values obtained for the content of holocellulose (70 wt.%) and lignin (18.4 wt.%). The presence of a shoulder in the DTG curve indicates an overlap of the degradation events of hemicellulose, cellulose and lignin. This observation motivated the use of the multi-component kinetics approach, where the occurrence of three parallel and independent thermal decomposition reactions could be considered. Since the degradation of most of the volatile materials occurred between 150 and 600 °C, the pyrolysis experiments were carried out at 500, 550, 600 and 650 °C. Similar behavior has been observed for the thermal degradation of energy cane bagasse [25,39].
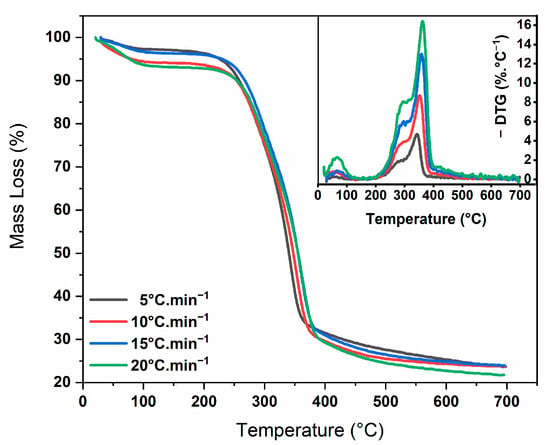
Figure 1.
Thermogravimetric curves (TG/DTG) of energy cane bagasse under an inert atmosphere (N2, 50 mL·min−1) at heating rates of 5, 10, 15 and 20 °C min−1.
3.2. Products of Energy Cane Bagasse Pyrolysis
Figure 2 presents pyrograms of the energy cane bagasse pyrolysis at the four temperatures studied, with the identification of the main compounds in Table 2. The high values of CO2 are evidence of the occurrence of deoxygenation reactions. Additionally, the compounds significantly identified at all temperatures were glyoxal, acetone, methyl vinyl ketone, furfural, phenol and 2,3-dihydro-benzofuran. With an increase in temperature, the formation of the following decreased: acetaldehyde, acetic acid, benzene, 1,3-propanediol, 1,2-cyclopentanedione, 2-methoxy-4-vinyl-phenol, 2,6-dimethoxy-phenol and sucrose. More complex molecules and anhydrosugars such as D-allose were only identified at the lowest pyrolysis temperature, 500 °C, indicating that anhydrosugars are the primary biomass decomposition compounds. At the higher pyrolysis temperatures (600 and 650 °C), unsaturated hydrocarbons such as 1-decene, 1-undecene, 1-tridecene and 1-tetradecene were identified.
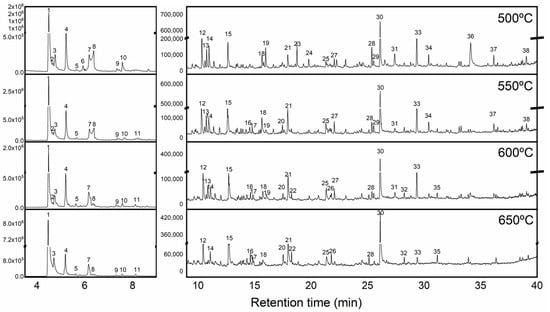
Figure 2.
Total ion chromatograms (TICs) of the pyrolysis of energy cane bagasse at 500, 550, 600 and 650 °C. For compound identification number see Table 2.

Table 2.
Main compounds from TIC of the fast pyrolysis of energy cane bagasse.
To explore the effect of temperature on pyrolysis products, the compounds were initially grouped into CO2, oxygenated, hydrocarbon, unidentified (similarity less than 85%) and others (nitrogen and sulfur compounds) (Figure 3). The increase in temperature decreased the formation of oxygenated compounds and increased the production of CO2 and hydrocarbons, indicating the occurrence of decarboxylation reactions [57].
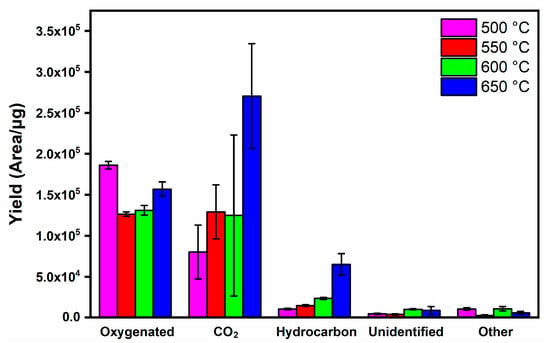
Figure 3.
Product distribution from the pyrolysis of energy cane bagasse.
The high yield of oxygenated compounds is due to the high oxygen content in the composition of energy cane (~44.2%). These compounds are grouped according to their organic functions in Figure 4, as a result of the degradation of holocellulose (hemicellulose + cellulose) (~70.4 wt.%) and lignin (~18.4 wt.%).
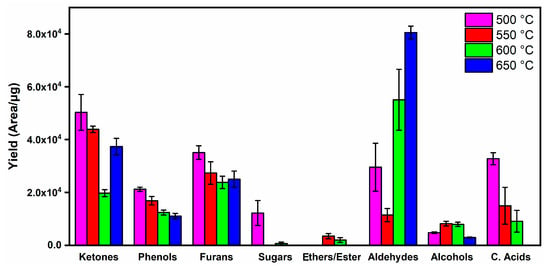
Figure 4.
Product distribution of oxygenates from the pyrolysis of energy cane bagasse.
The increase in temperature decreased the formation of sugars, ketones, furans, carboxylic acids and phenols and increased the production of esters/ethers, aldehydes and alcohols. The decomposition of holocellulose at low temperatures resulted in non-volatile anhydrosugars. However, increasing temperature favors bond breaking and ring openings, resulting in furanic compounds, carboxylic acids and other monomeric sugars. A further temperature increase favored secondary decompositions or decarbonylation and deoxygenation reactions that resulted in light aldehydes, ketones and hydrocarbons [56]. Phenolic compounds mostly result from the degradation of lignin. The low yield of these products is associated with the low lignin content (~18.4 wt.%) in energy cane bagasse.
The effect of temperature on the distribution of the products was assessed using principal component analysis (PCA), shown in Figure 5. The two principal components (PCs) together explained 94% of the variance of the data, with PC1 directly linked to temperature and explaining around 77% of the data variation. Figure 5 shows that the pyrolysis at 500 °C differed from the others in having high yields of D-allose, 2,3-butanedione and acetic acid methyl ester, which are compounds resulting from the degradation of holocellulose at low temperatures. The pyrolysis performed at 550 and 600 °C were similar to each other and favored the formation of methyl vinyl ketone, acetone and acetic acid, products resulting from secondary reactions of the holocellulose. Hydrocarbons such as toluene were also favored. In the pyrolysis at 650 °C, the favored products were methyl glyoxal, methylolacetone, acetaldehyde and hydrocarbons such as butane and toluene.
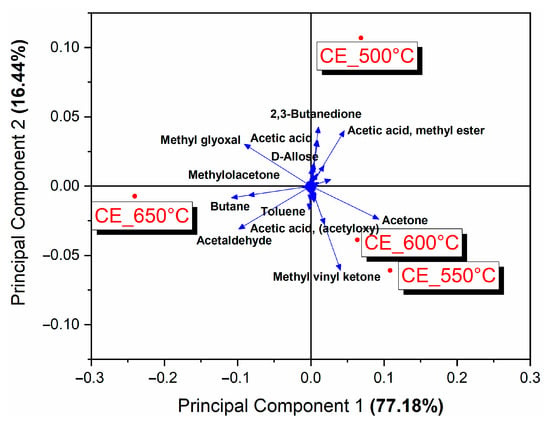
Figure 5.
Biplot (loading + score) of the principal component analysis of the products from the pyrolysis of energy cane bagasse.
3.3. Multi-Component Kinetic Modeling
3.3.1. Results for the Four Deconvoluted Independent Devolatilization Reactions
The Asym2Sig fitting function was used to perform a multi-peak differentiation on the DTG curves. This yielded four independent devolatilization reactions (pseudo-extractives, pseudo-hemicellulose, pseudo-cellulose and pseudo-lignin), as shown in Figure 6. The excellent adjustment obtained with coefficients of determination (R2) greater than 0.99 for all heating rates confirmed that the pyrolysis behavior of energy cane bagasse was well characterized by these four independent devolatilization reactions. A qualitative similarity was observed between the profiles of the four individual devolatilization reactions identified in this study and those reported by Da Silva et al. [48] for the pyrolysis of sugarcane bagasse, which employed a four-component model. The fractional contribution (Ci) of each independent devolatilization reaction in the pyrolysis behavior of energy cane bagasse was determined as follows: 0.0410 for pseudo-extractives, 0.2773 for pseudo-hemicellulose, 0.5760 for pseudo-cellulose and 0.1057 for pseudo-lignin.
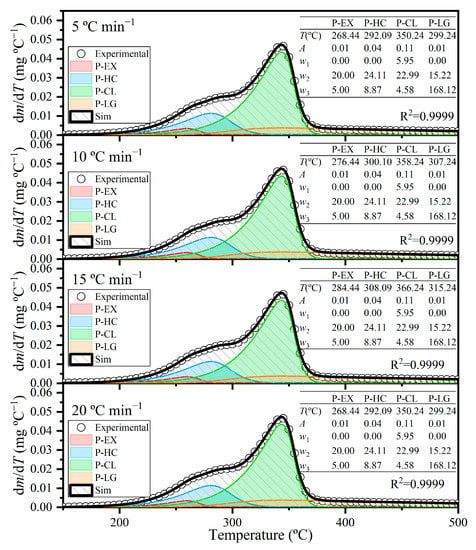
Figure 6.
Deconvolution of the DTG peaks for energy cane bagasse pyrolysis via Asym2Sig function for each pseudo-component at heating rates of 5, 10, 15 and 20 K min−1.
3.3.2. Results for Activation Energy
Figure 7 illustrates the dependence of the activation energy on the extent of conversion for each of the pseudo-components by the FR method. The average values of Ea were 135.6, 147.4, 179.2 and 512.4 kJ mol−1 for the decomposition of, respectively, pseudo-extractives, pseudo-hemicellulose, pseudo-cellulose and pseudo-lignin. The calculations were carried out within a conversion range of 0.05–0.95 at 0.001 intervals. The average values of the coefficient of determination were all higher than 0.96, indicating reasonably credible calculations for obtaining the dependence of activation energy on the conversion extent through the four isoconversional methods.
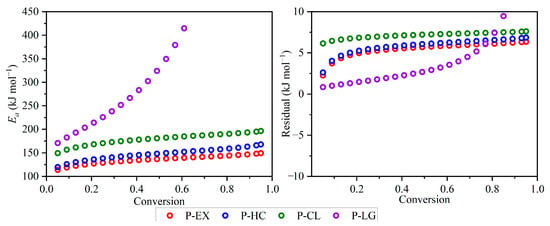
Figure 7.
Dependence of the activation energy and residual plot on the extent of conversion for the pyrolysis of energy cane bagasse with four independent parallel reactions using Friedman method.
The FR isoconversional method yielded average activation energies in the range of 113.7–149.4 kJ mol−1 and 119.9–168.0 kJ mol−1 for the devolatilization of pseudo-extractives and pseudo-hemicellulose, respectively. These values align with literature studies applying similar methodologies and reporting average activation energies in the range of 107.5–142.7 kJ mol−1 and 142.7–180.2 kJ mol−1 for the devolatilization of pseudo-extractives and pseudo-hemicellulose, respectively [25]. The activation energy of pseudo-cellulose (in the range of 149.3–196.4 kJ mol−1) was higher than that of pseudo-hemicellulose, which is consistent with the expectation that the long glucose polymer of the cellulose is more difficult to decompose when compared to the amorphous structure of hemicellulose [49,58]. A remarkable similarity was observed when comparing the activation energy values of energy cane bagasse evaluated in this study to those values reported in the literature for the devolatilization of pseudo-cellulose (164.2–183.0 kJ mol−1) [25]. The activation energy average values for the devolatilization reaction of pseudo-lignin estimated by the FR isoconversional method was 512.4 kJ mol−1. The reported values were consistent with previous studies on pyrolysis kinetics, which have found that the devolatilization of lignin exhibits activation energy values greater than 400 kJ mol−1 [59,60,61,62,63,64,65]. Additionally, in a previous study, De Carvalho and Tannous [25] reported that determining the activation energy of the devolatilization of lignin using isoconversional methods is challenging during the pyrolysis of energy cane stalks because its devolatilization does not exhibit a specific peak, unlike the other components.
The activation energy obtained from the FR isoconversional method suggests that the energy required to decompose the energy cane bagasse into volatiles and bio-char follows the order of pseudo-lignin > pseudo-cellulose > pseudo-hemicellulose > pseudo-extractives. This sequence indicates that pseudo-lignin is less prone to thermal decomposition than the other pseudo-components. The high energy required to break and decompose the long carbon chains in lignin’s structure can be attributed to the presence of a three-dimensional aromatic structure [31].
Information on the minimum energy required to initiate a reaction can be obtained from the activation energy (Ea) values. A lower Ea indicates higher economic feasibility for biomass-to-biofuel conversion through pyrolysis. Consequently, energy cane bagasse exhibits potential as a feedstock for bioenergy/biofuel production, with comparable activation energy values to those of other lignocellulosic biomasses proposed as bioenergy feedstocks in the literature, such as palm kernel shell (227.0 to 545.0 kJ mol−1) [59], fruit peel wastes (150.0 to 550.0 kJ mol−1) [65], ponkan peel waste (74.6 to 605.0 kJ mol−1) [66] and cocoa shell (87.8 to 463.6 kJ mol−1) [67].
Activation energy results were also obtained from the FWO, KAS and STK isoconversional methods (Table S1). The activation energy dependence on the extent of conversion for the three integral methods showed a similar trend with considerable overlap (Figure S1). These methods exhibited slight deviations attributed to the use of different temperature integral approximations. However, the activation energy values for the FR differential method varied with the degree of conversion, similar to the integral isoconversional methods. Additionally, the FR isoconversional method, which is free of mathematical approximations and utilizes numerical differentiation of non-isothermal thermogravimetry data, is highly sensitive to experimental noise [58,59]. The literature indicates that this phenomenon affects activation energy calculations, resulting in the FR isoconversional method typically yielding higher activation energy values than classical integral methods [60,61]. The values obtained from the FR method were utilized in subsequent computations that required the activation energy, such as pre-exponential factors, reaction models, numerical simulations and thermodynamic parameters.
3.3.3. Results for Pre-Exponential Factor of Energy Cane Bagasse
As discussed earlier, the pyrolysis of energy cane bagasse consists of four parallel devolatilization reactions. A strong linear relationship was found between the activation energy, determined through the Friedman isoconversional method, and the pre-exponential factor (in its natural logarithmic form—ln(A)) for each pseudo-component. This relationship was supported by a high correlation coefficient (R2 > 0.96 for all devolatilization reactions), as presented in Figure 8a. The occurrence of the compensation effect in the pyrolysis of energy cane bagasse supports the reliability of this method in estimating the pre-exponential factors that represent the kinetics of pyrolysis reactions.
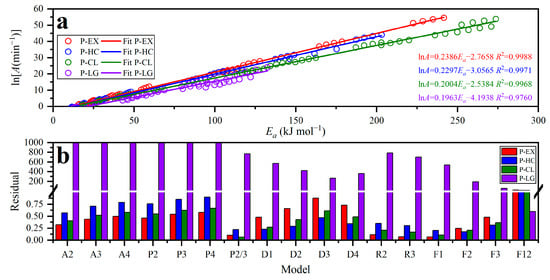
Figure 8.
(a) Linear relationship between ln A and Ea and (b) the relative error values for each of the seventeen candidate reaction models used to determine the most reliable reaction model for the pyrolysis of energy cane bagasse with four independent parallel reactions.
The compensation effect method provided pre-exponential factors of 1.12 × 1012 min−1 for pseudo-extractives, 3.66 × 1012 min−1 for pseudo-hemicellulose, 4.60 × 1013 min−1 for pseudo-cellulose and 1.36 × 1037 min−1 for pseudo-lignin. These magnitude orders fall within the range of pre-exponential factors reported for the pyrolysis of the Invasive Reed Canary (from 107 to 1037 min−1) [68] and Helianthus tuberosus (from 101 to 1047 min−1) [69]. High pre-exponential factor values (exceeding 109 min−1) indicate a highly reactive pyrolysis conversion, likely taking place in the form of a simple complex [70].
3.3.4. Results for Reaction Model
The integral master plot technique was employed to differentiate each pseudo-component’s most plausible devolatilization reaction model associated with the pyrolysis of energy cane bagasse. This was accomplished by examining both experimental and theoretical curves as a function of the conversion degree. Figure 8b illustrates the relative error values for each of the seventeen candidate reaction models used to determine the most reliable reaction model for the pyrolysis of energy cane bagasse. The outcomes of the master plot analysis revealed diverse reaction mechanisms for the pyrolysis of energy cane bagasse, underscoring its multi-component nature. As in another lignocellulosic biomass pyrolysis, numerous parallel reactions that follow distinct mechanisms were observed [71,72].
Alignment with the first-order reaction model (F1) was observed for the devolatilization of pseudo-extractives and pseudo-cellulose. The second-order reaction model (F2) aligned well with the devolatilization reaction of pseudo-hemicellulose, while the twelfth-order reaction model (F12) was ascribed to the devolatilization reaction of pseudo-lignin. The F1, F2 and F12 reaction models refer to the n-order reaction mechanism, which suggests that the thermal decomposition rate was linked to the concentration of reactants raised to a specific power (reaction order). This concept is outlined in Khawam and Flanagan [73]. De Carvalho and Tannous [25] conducted a previous study and verified that the predominant reaction mechanism for the pyrolysis of energy cane stalks is an n-order reaction mechanism, which is consistent with the findings of the current study. Particularly, the devolatilization of pseudo-lignin (n = 12) exhibited a notably high reaction order, which is consistent with previously reported results on lignin devolatilization, where the reaction orders ranged from 11 to 12 [74,75]. The existing literature provides compelling evidence to suggest that the pyrolysis of lignin is governed by the combined effect of different mechanisms such as nucleation, diffusion, geometrical contraction and the power law [75,76]. The observed higher-order reaction model during the devolatilization of pseudo-lignin can thus be justified by this phenomenon.
3.3.5. Results for Verification Step of Summative Kinetic Expression
The multi-component kinetic analysis enabled the determination of one kinetic triplet for each pseudo-component, resulting in the overall kinetic expression (Equation (14)) for practical applications, such as reconstructing the experimental pyrolysis behavior. In order to verify the precision of the overall kinetic expression, the reconstructed curves obtained from it were compared to the experimental conversion and conversion rate curves, as per the guidelines proposed by the ICTAC kinetics committee for analyzing multi-step kinetics from overlapping reactions [38]. This verification is crucial to ensure the usability of the obtained overall kinetic expression in the design and simulation of the pyrolysis of energy cane bagasse.
Figure 9 depicts the reconstructed and experimental pyrolysis behavior of energy cane bagasse under dynamic conditions at varying heating rates (5, 10, 15 and 20 °C min−1). In this figure, experimental data are denoted by open circles while reconstructed curves, defined by the overall pyrolysis rate expression, are represented by continuous lines. The reconstructed curves obtained from the overall kinetic expression showed an acceptable match with the experimental pyrolysis behavior. The correlation coefficient was satisfactory (R2 > 0.94), together with a reasonable adjustment (Fit > 94.8%), for the tested heating rates. Therefore, this dependable and representative kinetic expression could serve as a crucial input for large-scale pyrolysis reactors in their use of energy cane bagasse, which is currently burned to produce electricity with low efficiency.
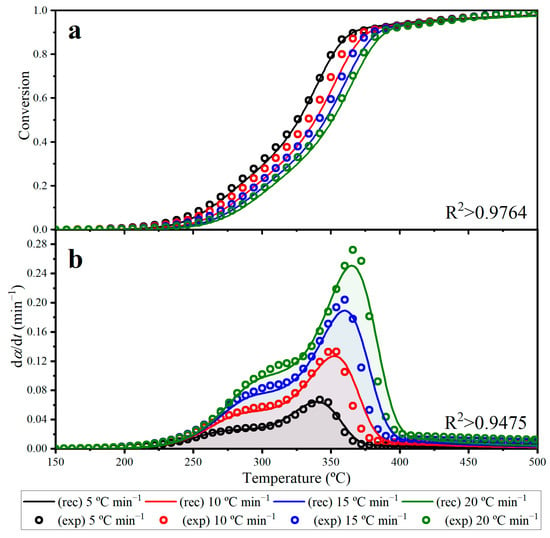
Figure 9.
Plots for (a) conversion (α) and (b) conversion rate (dα/dt) curves against increasing temperature at heating rates of 5, 10, 15 and 20 °C min−1 for the pyrolysis of energy cane bagasse.
3.3.6. Results for Thermodynamic Parameters
This section aims to present the thermodynamic parameters (ΔH, ΔG and ΔS) obtained for the pyrolysis of energy cane bagasse and discuss their implications. Table S2 displays the calculated thermodynamic parameters with respect to progressive conversions. Thermodynamic parameters provide relevant criteria for assessing the energy demands and feasibility of the pyrolysis process [55,77].
All four independent devolatilization reactions showed positive values for ΔH, indicating that the pyrolysis of energy cane bagasse is an endothermic process in which the molecules absorb thermal energy to decompose into bioenergy and renewable chemicals. The potential energy barrier, which is the difference between the average values of Ea and ΔH, can offer insights into the feasibility of the occurrence of a pyrolysis reaction [37]. A smaller difference indicates a higher favorability for a reagent molecule to overcome the barrier for activated complex formation, while a larger difference suggests the opposite. The study results suggest a low energy barrier (below 7.8 kJ mol−1) for the conversion of energy cane bagasse to bioenergy and renewable chemicals via pyrolysis, indicating that the process can be energetically viable with a small amount of required energy.
The available bioenergy that could potentially be recovered from biomass by the pyrolysis process can be depicted by the thermodynamic parameter changes expressed in the Gibbs free energy (ΔG) [55,78]. The average values obtained for energy cane bagasse (ranging from 128.05 to 207.60 kJ mol−1) are comparable and favorable for pyrolytic conversion, compared with ΔG results previously obtained for canola residue (ranging from 158.30 to 212.10 kJ mol−1) [79], pistachio shell (ranging from 179.34 to 184.11 kJ mol−1) [55], and corn cob (ranging from 174.20 to 180.20 kJ mol−1) [78]. This indicates the potential for energy cane bagasse to be converted into bioenergy and renewable chemicals. Positive values of ΔH and ΔG obtained for the pyrolysis of energy cane bagasse suggest the occurrence of a non-spontaneous conversion process. An individual analysis of each pseudo-component revealed a minimal impact of the increase in conversion on the ΔH and ΔG values, indicating a requirement of constant energy over the entire range of the conversion (0.05 < α < 0.95).
The state function known as the change in entropy (ΔS) can be used to measure the degree of disorder in a pyrolysis reaction. Knowledge of ΔS helps distinguish between slow and fast reactions, with positive ΔS values indicating fast reactions and negative values indicating slow ones [80]. A negative entropy change indicates a greater degree of disorder in the reactants compared to the products of the reaction [54]. The negative ΔS values for the pyrolysis of the devolatilization reactions of pseudo-extractives, pseudo-hemicellulose and pseudo-cellulose indicate a lower reactivity and suggest that the conversion process is governed by “slow” reactions. Conversely, the positive ΔS value for the devolatilization reaction of pseudo-lignin implies a rapid formation of the activated complex resulting from volatile release and molecular rearrangement.
The observation of negative and positive values of ΔS indicates that the pyrolysis conversion of energy cane bagasse into bioenergy and renewable chemicals is a complex reaction. The behavior exhibited similarity to previously reported findings [55,79]. Based on thermodynamic findings and explanations, it can be inferred that energy cane bagasse possesses a substantial potential for use as a feedstock for the pyrolysis process aimed at producing bioenergy and renewable chemicals.
4. Conclusions
The characterizations of energy cane bagasse presented high contents of fibers (~23%) and volatile materials (~74%), indicating possible high yields of pyrolysis bio-oil. The increase in reaction temperature favored the production of hydrocarbons and decreased the yield of oxygenated compounds such as ketones, phenols, furans, sugars and carboxylic acids. The effect of pyrolysis temperature also showed that anhydrosugars are the primary compounds resulting from the decomposition of holocellulose at 500 °C. Pyrolysis at 550 and 600 °C favored products such as methyl vinyl ketone and acetic acid. Methyl glyoxal, methylolacetone, and acetaldehyde products were favored at 650 °C, along with hydrocarbons such as butane, toluene, and olefins such as 1-decene, 1-undecene, 1-tridecene and 1-tetradecene. The overlapping events of the DTG curves indicated the decomposition of multi-component fractions, such as hemicellulose, cellulose and lignin. The pyrolysis of energy cane bagasse can be a promising process for bioenergy purposes, being a more efficient alternative to its current use by burning to produce electricity with low efficiency. The results of the activation energy obtained using four isoconversional methods indicate that energy cane bagasse possesses favorable characteristics for conversion into bioenergy and renewable chemicals through the pyrolysis process. The pre-exponential factors for the four pseudo-components during the pyrolysis of energy cane bagasse were found to be greater than 109 min−1, indicating the involvement of simpler chemical reactions. The experimental kinetic curves were analyzed using the integral master plot method. The four independent devolatilization reactions observed followed nth-order-based reaction models. Future research can be directed towards the proposal of molecular-scale reaction pathways for the conversion of energy cane bagasse into bioenergy and bio-based chemicals via the pyrolysis process. The small difference between the change in enthalpy and activation energy for energy cane bagasse (less than 7.8 kJ mol−1) suggests that the pyrolysis process is energetically feasible for producing bioenergy and renewable chemicals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16155669/s1, Figure S1: Dependence of the activation energy and residual plots on the extent of conversion for the pyrolysis of energy cane bagasse with four independent parallel reactions for the four isoconvertional methods FR, FWO, KAS ans STK. Table S1: Main compounds from TIC chromatogram of the fast pyrolysis of energy cane bagasse; Table S2: Thermodynamic parameters with respect to progressing conversions and their respective average values for the pyrolysis of energy cane bagasse with four independent parallel reactions. Units: ΔH and ΔG (kJ mol−1); ∆S (J mol−1 K−1).
Author Contributions
Conceptualization: J.G.A.P. and D.O.L.; methodology: D.O.L., S.A., G.D.M., J.L.F.A. and J.C.G.d.S.; software: J.C.G.d.S.; formal analysis: J.L.F.A., J.G.A.P. and J.C.G.d.S.; investigation: J.L.F.A. and J.G.A.P.; resources: J.G.A.P., D.A.S. and F.R.C.; data curation: D.O.L., S.A., G.D.M., J.C.G.d.S., J.L.F.A., J.G.A.P. and J.F.G.; writing—original draft preparation: D.O.L. and J.F.G.; writing—review and editing: D.A.S., F.R.C., R.R.S., C.M.B.M.B., J.M.F.S. and J.G.A.P.; visualization: D.A.S., F.R.C., R.R.S., C.M.B.M.B., J.M.F.S. and J.G.A.P.; supervision: J.M.F.S. and J.G.A.P.; project administration, J.G.A.P., D.A.S., R.R.S. and C.M.B.M.B.; funding acquisition: J.G.A.P., D.A.S., R.R.S. and C.M.B.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FACEPE (projects IBPG-0395-3.06/19 and BCT-0378-3.06/17), Brazil’s Coordination for the Improvement of Higher Education Personnel (CAPES), CAPES/PNPD (project 88882.306335/2018-01), National Council for Scientific and Technological Development, CNPq (projects 430921/2016-0, 312387/2022-9 and 152245/2020–0), PETROBRAS-ANP-LITPEG (contract 0050.0078506.12.9); ANP-PRH30.1.
Data Availability Statement
Not applicable.
Acknowledgments
Funders above mentioned and English revision by Sidney Pratt, Canadian, BA, MAT (The Johns Hopkins University), RSAdip (TEFL, Cambridge University).
Conflicts of Interest
The authors declare no conflict of interest.
Notation
| Notation | Definition |
| α | conversion |
| dα/dT | conversion rate |
| maximum amplitude of the curve | |
| T | temperature |
| Tp | peak temperature |
| w1 | curve width |
| w2 and w3 | shape parameters |
| M | total number of experimental data points |
| dα/dTexp | experimentally measured conversion rate |
| dα/dTdec | conversion rate deconvoluted |
| rate of change of temperature | |
| pre-exponential factor | |
| activation energy | |
| universal gas constant | |
| reaction model | |
| a and b | compensation coefficients |
| p(x)/p(x0.5) | represents the experimental master plot |
| ΔH | change in enthalpy |
| ΔG | change in Gibbs free energy |
| ΔS | change in entropy |
| kB | Boltzmann constant (1.381 × 10−23 J K−1) |
| h | Planck constant (6.626 × 10−34 J s) |
| Tm | DTG peak temperature |
References
- De Bhowmick, G.; Sarmaha, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.M.C.L.; Souza, T.P.C.; Elihimas, D.R.M.; Silva, J.P.; Albuquerque, A.A.; Pacheco, J.G.A.; Silva, J.M.F. Ethanol dehydration by absorption and biodiesel production by reactive distillation: An innovative integrated process. Biomass Bioenergy 2021, 154, 106263. [Google Scholar] [CrossRef]
- Souza, T.P.C.; Silva, R.J.M.C.L.; Melo, J.C.; Tschoeke, I.C.P.; Silva, J.P.; Pacheco, J.G.A.; Silva, J.M.F. Kinetic modeling of cottonseed oil transesterification with ethanol. React. Kinet. Mech. Catal. 2019, 128, 707–722. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Balances. Overview. Available online: https://www.iea.org/data-and-statistics/data-browser?country=WORLD&fuel=Energy%20supply&indicator=TESbySource (accessed on 30 June 2022).
- Das, P.; Gundimeda, H. Is biofuel expansion in developing countries reasonable? A review of empirical evidence of food and land use impacts. J. Clean. Prod. 2022, 372, 133501. [Google Scholar] [CrossRef]
- Caldeira, V.P.S.; Santos, A.G.D.; Oliveira, D.S.; Lima, R.B.; Souza, L.D. Polyethylene catalytic cracking by thermogravimetric analysis: Effects of zeolitic properties and homogenization process. J. Therm. Anal. Calorim. 2017, 130, 1939–1951. [Google Scholar] [CrossRef]
- Silva, J.B.; Cabral, G.G.; Araujo, M.D.S.; Caldeira, V.P.S.; Coriolano, A.C.F.; Fernandes, V.J., Jr. Catalytic pyrolysis of atmospheric residue of petroleum using pillared interlayed clay containing lanthanum and aluminum polyhydroxications (LaAl13-PILC). Pet. Sci. Technol. 2021, 39, 704–717. [Google Scholar] [CrossRef]
- Melo, J.A.; de Sá, M.S.; Moral, A.; Bimbela, F.; Gandía, L.M.; Wisniewski, A., Jr. Renewable Hydrocarbon Production from Waste Cottonseed Oil Pyrolysis and Catalytic Upgrading of Vapors with Mo-Co and Mo-Ni Catalysts Supported on γ-Al2O3. Nanomaterials 2021, 11, 1659. [Google Scholar] [CrossRef]
- Arias, S.; Vascocelos, D.P.; de Oliveira Libório, D.; Gonzalez, J.F.; Câmara, A.G.; Barbosa, C.M.; Pacheco, J.G.A. Hydrogen-free deoxygenation of industrial vegetable oil waste using Ce, Zr-NiAl catalysts for second-generation biofuels production. Mol. Catal. 2022, 529, 112554. [Google Scholar] [CrossRef]
- Wisniewski Jr, A.; Wosniak, L.; Scharf, D.R.; Wiggers, V.R.; Meier, H.F.; Simionatto, E.L. Upgrade of biofuels obtained from waste fish oil pyrolysis by reactive distillation. J. Braz. Chem. Soc. 2015, 26, 224–232. [Google Scholar] [CrossRef]
- Borel, L.D.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Santana, K.V.R.; Apolônio, F.C.S.O.; Wisniewski, A., Jr. Valorization of cattle manure by thermoconversion process in a rotary kiln reactor to produce environmentally friendly products. Bioenergy Res. 2020, 13, 605–617. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Rossi, R.A.S.; Andrade, L.A.; Barrozo, M.A.S.; Vieira, L.G.M. A study of optimization of solar pyrolysis and catalyst recovery and reuse. Energy Convers. Manag. 2021, 237, 114094. [Google Scholar] [CrossRef]
- Santos, V.O.; Araujo, R.O.; Ribeiro, F.C.; Queiroz, L.S.; Guimarães, M.N.; Colpani, D.; de Souza, L.K. Non-isothermal kinetics evaluation of buriti and inaja seed biomass waste for pyrolysis thermochemical conversion technology. Biomass Convers. Biorefin. 2021, 1–17. [Google Scholar] [CrossRef]
- Padilha, J.F.; Frety, R.; Santos, A.P.; Pontes, L.A.M.; Santos, M.R.; Arias, S.; Pacheco, J.G.A. Deoxygenation of Oleic Acid Methyl Ester in FCC Process Conditions Over Protonated and Sodium Exchanged Y and ZSM-5 Zeolites. Waste Biomass Valorization 2022, 13, 185–194. [Google Scholar] [CrossRef]
- Amaral, A.R.; Bernar, L.P.; Ferreira, C.C.; de Oliveira, R.M.; Pereira, A.M.; Pereira, L.M.; Santos, M.C.; Assunção, F.P.d.C.; Bezerra, K.C.A.; Almeida, H.d.S.; et al. Economic Feasibility Assessment of the Thermal Catalytic Process of Wastes: Açaí Seeds (Euterpe oleracea) and Scum from Grease Traps. Energies 2022, 15, 7718. [Google Scholar] [CrossRef]
- De Rocha, C.D.A.; da Silva, R.H.J.; Hamoy, G.L.H.; Pinto, B.L.; Jonatan, B.S.; Costa, S.M.; Teixeira, M.N. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- De Almeida, R.P.; Aciole, R.C.G.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Pacheco, J.G.A.; Barros, I.C.L. Residue-based activated carbon from passion fruit seed as support to H3PW12O40 for the esterification of oleic acid. J. Clean. Prod. 2021, 282, 124477. [Google Scholar] [CrossRef]
- do Nascimento, L.A.; Peçanha, S.R.S.; Arias, S.; Santos, B.S.; Pacheco, J.G.A.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Barros, I.D.C.L. NiAlCe mixed oxides obtained from layered double hydroxides applied to anisole hydrodeoxygenation. Catal. Today 2022, 394–396, 282–294. [Google Scholar] [CrossRef]
- CONAB. Companhia Nacional de Abastecimento. Boletin de Safra de Cana-de-açúCar, Published on 27 April 2022. Available online: https://www.conab.gov.br/info-agro/safras/cana (accessed on 30 June 2022).
- Schmitt, C.C.; Moreira, R.; Neves, R.C.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.; Dahmena, N. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process. Technol. 2020, 197, 106199–106215. [Google Scholar] [CrossRef]
- Sohaib, Q.; Muhammad, A.; Younas, M. Fast pyrolysis of sugarcane bagasse: Effect of pyrolysis conditions on final product distribution and properties. Energ. Source Part A 2017, 39, 184–190. [Google Scholar] [CrossRef]
- Barros, J.A.S.; Krause, M.C.; Lazzari, E.; Bjerk, T.R.; do Amaral, A.L.; Caramão, E.B.; Krause, L.C. Chromatographic characterization of bio-oils from fast pyrolysis of sugar cane residues (straw and bagasse) from four genotypes of the Saccharum Complex. Microchem. J. 2017, 137, 30–36. [Google Scholar] [CrossRef]
- Durange, J.A.C.; Santos, M.R.L.; Pereira, M.M.; Fernandes, L.A.P., Jr.; Souza, M.N.; Mendes, A.N.; Mesa, L.M.; Sánchez, C.G.; Sanchez, E.M.S.; Pérez, J.M.M.; et al. Physicochemical Properties of Pyrolysis Bio-Oil from Sugarcane Straw and Sugarcane in Natura. J. Biomater. Nanobiotechnol. 2013, 4, 10–19. [Google Scholar] [CrossRef]
- De Carvalho, V.S.; Tannous, K. Thermal decomposition kinetics modeling of energy cane Saccharum robustum. Thermochim. Acta 2017, 657, 56–65. [Google Scholar] [CrossRef]
- Carvalho-Netto, O.V.; Bressiani, J.A.; Soriano, H.L.; Fiori, C.S.; Santos, J.M.; Barbosa, G.V.S.; Xavier, M.A.; Landell, M. GA and Pereira, G. AG. The potential of the energy cane as the main biomass crop for the cellulosic industry. Chem. Biol. Technol. Agric. 2014, 1, 20. [Google Scholar] [CrossRef]
- de Abreu, L.G.F.; Grassia, M.C.B.; de Carvalho, L.M.; da Silva, J.J.B.; Oliveira, J.V.C.; Bressiani, J.A.; Pereira, G.A.G. Energy cane Vs sugarcane: Watching the race in plant development. Ind. Crops Prod. 2020, 156, 112868. [Google Scholar] [CrossRef]
- Hoffstadt, K.; Pohen, G.D.; Dicke, M.D.; Paulsen, S.; Krafft, S.; Zang, J.W.; da Fonseca-Zang, W.A.; Leite, A.; Kuperjans, I. Challenges and Prospects of Biogas from Energy Cane as Supplement to Bioethanol Production. Agronomy 2020, 10, 821. [Google Scholar] [CrossRef]
- Pokhrel, P.; Rajan, N.; Jifon, J.; Roonoey, W.; Jessup, R.; da Silva, J.; Enciso, J.; Attia, A. Evaluation of the DSSAT-CANEGRO model for simulating the growth of energy cane (Saccharum spp.), a biofuel feedstock crop. Crop Sci. 2021, 62, 466–478. [Google Scholar] [CrossRef]
- Henkel, C.; Muley, P.D.; Abdollahi, K.K.; Marculescu, C.; Boldor, D. Pyrolysis of energy cane bagasse and invasive Chinese tallow tree (Triadica sebifera, L.) biomass in an inductively heated reactor. Energy Convers. Manag. 2016, 109, 175–183. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting pecan nutshell pyrolysis as a source of bioenergy and bio-based chemicals using multicomponent kinetic modeling, thermodynamic parameters estimation, and Py-GC/MS analysis. Renew. Sust. Energ. Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Bruce, E.D.; Frety, R.; Pacheco, J.G.A.; Teixeira, C.M.; Barbosa, C.B.M. Thermocatalytic cracking kinetics of myristic acid adsorbed on catalysts with different acidity. Catal. Today 2017, 289, 280–288. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Prospection of catole coconut (Syagrus cearensis) as a new bioenergy feedstock: Insights from physicochemical characterization, pyrolysis kinetics, and thermodynamics parameters. Renew. Energ 2022, 181, 207–218. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Di Domenico, M.; Bolzan, A.; Machado, R.A.F.; Marangoni, C. Evaluating the bioenergy potential of cupuassu shell through pyrolysis kinetics, thermodynamic parameters of activation, and evolved gas analysis with TG/FTIR technique. Thermochim. Acta 2022, 711, 179187. [Google Scholar] [CrossRef]
- Peres, C.B.; Rosa, A.H.; de Morais, L.C. Investigation of pyrolysis kinetics parameters and thermal behavior of thermochemically modified bagasse for bioenergy potential. SN Appl. Sci. 2021, 3, 337. [Google Scholar] [CrossRef]
- Van-Nam, H.; Tam, T.T.; Tho, V.D.S. Kinetic Modeling of thermal decomposition of sugarcane bagasse in the inert gas environment. Vietnam. J. Chem. 2019, 57, 574–580. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Guimarães, H.R.; Tannous, K. Influence of torrefaction on the pyrolysis of energy cane. J. Therm. Anal. Calorim. 2020, 139, 2221–2233. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Tannous, K.; de Lima, E.C.T. Pyrolysis of the hybrid energy cane: Thermal decomposition and kinetic modeling using non-isotermal thermogravimetric analysis. J. Therm. Anal. Calorim. 2022, 147, 7431–7448. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Investigation on prospective bioenergy from pyrolysis of butia seed waste using TGA-FTIR: Assessment of kinetic triplet, thermodynamic parameters and evolved volatiles. Renew. Energ. 2022, 191, 238–250. [Google Scholar] [CrossRef]
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- TAPPI T 204 cm-97; Solvent Extractives of Wood and Pulp. TAPPI Test Methods. Technical Association of the Pulp and Paper Industry TAPPI: Peachtree Corners, GA, USA, 1997.
- TAPPI T222 om-02; Acid-Insoluble Lignin in Wood and Pulp. TAPPI Test Methods. Technical Association of the Pulp and Paper Industry TAPPI: Peachtree Corners, GA, USA, 2002.
- TAPPI T 203 cm-99; Alpha-, Beta-and Gamma-Cellulose in Pulp. TAPPI Test Methods. Technical Association of the Pulp and Paper Industry TAPPI: Peachtree Corners, GA, USA, 2009.
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Arias, S.; González, J.F.; Sousa, L.V.; Barbosa, C.B.M.; Silva, A.O.S.; Fréty, R.; Pacheco, J.G.A. Influence of Ni/Al ratio on the fast pyrolysis of myristic acid when adsorbed on unsupported mixed oxides derived from layered double hydroxides. Catal. Today 2021, 381, 181–191. [Google Scholar] [CrossRef]
- da Silva, J.C.G.; de Albuquerque, J.G.; Galdino, W.V.d.A.; de Sena, R.F.; Andersen, S.L.F. Single-step and multi-step thermokinetic study—Deconvolution method as a simple pathway for describe properly the biomass pyrolysis for energy conversion. Energy Convers Manag. 2020, 209, 112653. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Alves, R.F.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Potential of macauba endocarp (Acrocomia aculeate) for bioenergy production: Multi-component kinetic study and estimation of thermodynamic parameters of activation. Thermochim. Acta 2022, 708, 179134. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Liu, C.-G.; Nawaz, M.; Tawab, A.; Shen, X.; Shen, B.; Mehmood, M.A. Elucidating the pyrolysis reaction mechanism of Calotropis procera and analysis of pyrolysis products to evaluate its potential for bioenergy and chemicals. Bioresour. Technol. 2021, 322, 124545. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Thermal degradation characteristics, kinetics, thermodynamic, and reaction mechanism analysis of pistachio shell pyrolysis for its bioenergy potential. Biomass Convers. Biorefinery 2020, 12, 4847–4861. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Lup, A.N.K.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on reaction mechanisms of metal-catalyzed deoxygenation process in bio-oil model compounds. Appl. Catal. A-Gen. 2017, 541, 87–106. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Moine, E.C.; Groune, K.; El Hamidi, A.; Khachani, M.; Halim, M.; Arsalane, S. Multistep process kinetics of the non-isothermal pyrolysis of Moroccan Rif oil shale. Energy 2016, 115, 931–941. [Google Scholar] [CrossRef]
- Mishra, G.; Kumar, J.; Bhaskar, T. Kinetic studies on the pyrolysis of pinewood. Bioresour. Technol. 2015, 182, 282–288. [Google Scholar] [CrossRef]
- Yao, Z.; Cai, D.; Chen, X.; Sun, Y.; Jin, M.; Qi, W.; Ding, J. Thermal behavior and kinetic study on the co-pyrolysis of biomass with polymer waste. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Z.; Zheng, Z.; Li, W.; Tan, H.; Huang, Y.; Zhang, Y. Exploring how lignin promoting the co-pyrolysis with polylactic acid: Artificial neural network modeling, kinetic analysis and product distribution. Sustain. Mater. Technol. 2023, 35, e00549. [Google Scholar] [CrossRef]
- Jin, W.; Singh, K.; Zondlo, J. Pyrolysis kinetics of physical components of wood and wood-polymers using isoconversion method. Agriculture 2013, 3, 12–32. [Google Scholar] [CrossRef]
- Arenas, C.N.; Navarro, M.V.; Martínez, J.D. Pyrolysis kinetics of biomass wastes using isoconversional methods and the distributed activation energy model. Bioresour. Technol. 2019, 288, 121485. [Google Scholar] [CrossRef]
- da Silva, J.C.G.; Andersen, S.L.F.; Costa, R.L.; Moreira, R.d.F.P.M.; José, H.J. Bioenergetic potential of Ponkan peel waste (Citrus reticulata) pyrolysis by kinetic modelling and product characterization. Biomass Bioenergy 2019, 131, 105401. [Google Scholar] [CrossRef]
- Sangaré, D.; Bostyn, S.; Moscosa-Santillan, M.; García-Alamilla, P.; Belandria, V.; Gökalp, I. Comparative pyrolysis studies of lignocellulosic biomasses: Online gas quantification, kinetics triplets, and thermodynamic parameters of the process. Bioresour. Technol. 2021, 346, 126598. [Google Scholar] [CrossRef] [PubMed]
- Alhumade, H.; da Silva, J.C.G.; Ahmad, M.S.; Çakman, G.; Yıldız, A.; Ceylan, S.; Elkamel, A. Investigation of pyrolysis kinetics and thermal behavior of Invasive Reed Canary (Phalaris arundinacea) for bioenergy potential. J. Anal. Appl. Pyrolysis 2019, 140, 385–392. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ahmad, M.S.; Liu, Q.; Liu, C.-G.; Tahir, M.H.; Aloqbi, A.A.; Tarbiah, N.I.; Alsufiani, H.M.; Gull, M. Helianthus tuberosus as a promising feedstock for bioenergy and chemicals appraised through pyrolysis, kinetics, and TG-FTIR-MS based study. Energy Convers. Manag. 2019, 194, 37–45. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Pinzi, S.; Buratti, C.; Bartocci, P.; Marseglia, G.; Fantozzi, F.; Barbanera, M. A simplified method for kinetic modeling of coffee silver skin pyrolysis by coupling pseudo-components peaks deconvolution analysis and model free-isoconversional methods. Fuel 2020, 278, 118260. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, O.P.; Diwan, P.K. Non-isothermal kinetics of pseudo-components of waste biomass. Fuel 2019, 253, 1149–1161. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Romero Millán, L.M.; Sierra Vargas, F.E.; Nzihou, A. Kinetic Analysis of Tropical Lignocellulosic Agrowaste Pyrolysis. BioEnergy Res. 2017, 10, 832–845. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Hu, W.; Chen, Z.; Liu, S.; Hu, Z.; Xiao, B. Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics. Bioresour. Technol. 2016, 219, 510–520. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chai, M.; Li, C.; Yellezuome, D.; Liu, R. Pyrolysis kinetics and thermodynamic parameters of bamboo residues and its three main components using thermogravimetric analysis. Biomass Bioenergy 2023, 170, 106705. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Kinetics and thermodynamic analysis of maize cob pyrolysis for its bioenergy potential using thermogravimetric analyzer. J. Therm. Anal. Calorim. 2019, 137, 1431–1441. [Google Scholar] [CrossRef]
- Tahir, M.H.; Çakman, G.; Goldfarb, J.L.; Topcu, Y.; Naqvi, S.R.; Ceylan, S. Demonstrating the suitability of canola residue biomass to biofuel conversion via pyrolysis through reaction kinetics, thermodynamics and evolved gas analyses. Bioresour. Technol. 2019, 279, 67–73. [Google Scholar] [CrossRef]
- Boonchom, B. Kinetics and Thermodynamic Properties of the Thermal Decomposition of Manganese Dihydrogenphosphate Dihydrate. J. Chem. Eng. Data 2008, 53, 1533–1538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).