Experimental Activities on a Hydrogen-Powered Solid Oxide Fuel Cell System and Guidelines for Its Implementation in Aviation and Maritime Sectors
Abstract
1. Introduction
2. Materials and Methods
3. Layout of the SOFC Test Station
- measurement and control instruments; i.e., manometers for measuring pressure, automatic and manual systems for regulating pressure, mass flow controllers for regulating flow rates from the terminal, thermocouples for detecting temperatures in key stations;
- a depot for gas stocks (hydrogen, nitrogen), with a high degree of purity (about 99.99%) and a maximum pressure of 250 bar;
- a working fluid supply and distribution system, managed by a control unit that operates on the numerous valves of the test bench;
- an air volumetric compressor that stores the air in an auxiliary tank at 12 bar, with a two-stage purification system, to eliminate impurities from the air;
- a reforming section for the fuel processing of carbon-based gases, in particular methane, converted into hydrogen-rich gaseous streams;
- a reformate section to prevent steam to condensate;
- a SOFC setup system, the heart of the test bench, which contains the fuel cell stack;
- a furnace, useful for heating the system and maintaining it at the set nominal temperature;
- a control system, for data acquisition, detection/collection, transmission, control, and processing activities;
- a gas exhaust system, which discharges the exhaust gas deriving from the stack;
- a safety system (not shown in Figure 1) made up of the sensors, the gaseous composition control unit, and the hood and forced gas suction system.
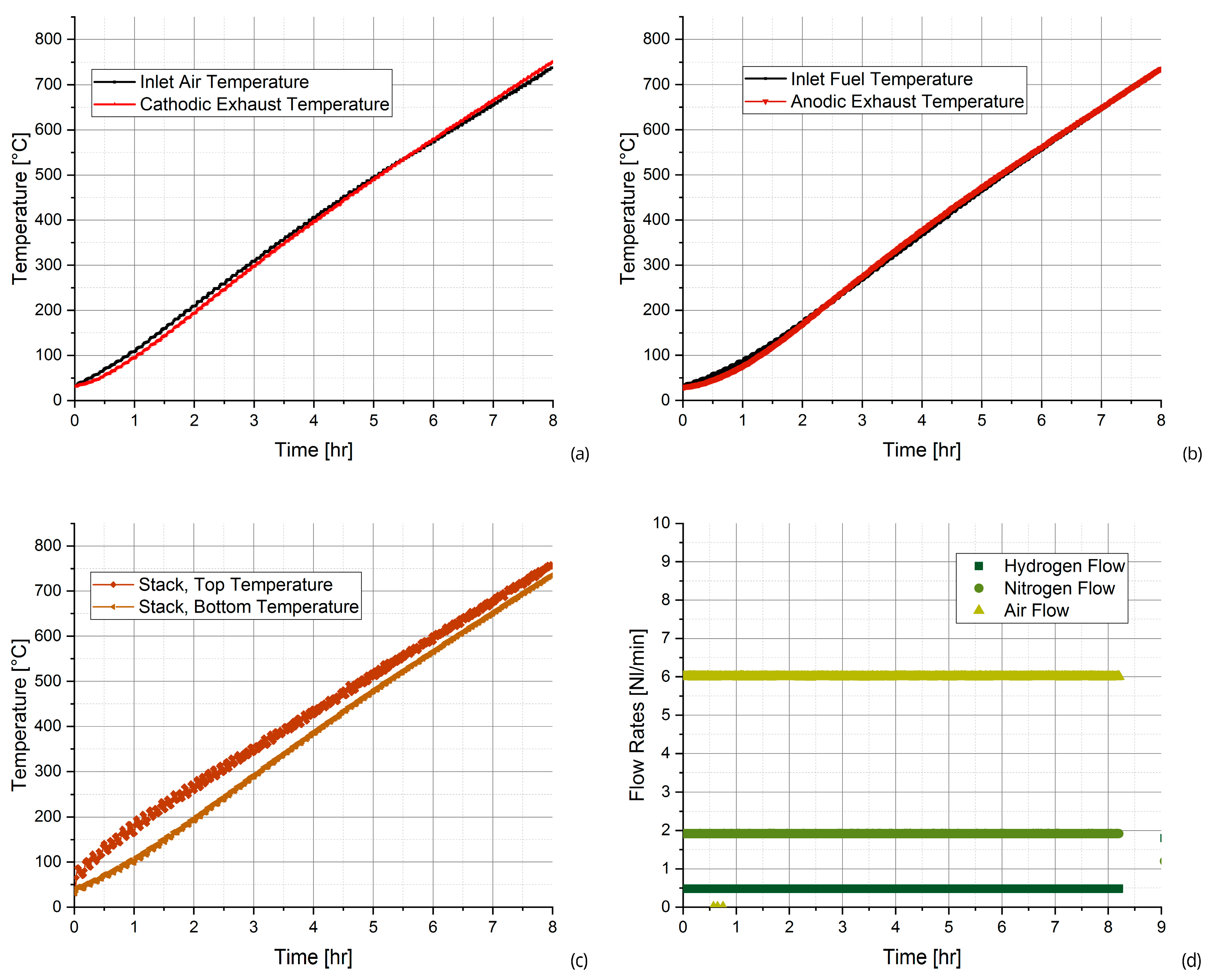
- The first stage is required to heat the SOFC system, which is led from ambient temperature to approximately 750 °C, the nominal temperature, thanks to the furnace present in the test bench. The input flows, namely hydrogen, air, and nitrogen flows, are maintained constant and the heating is performed with a temperature ramp of 100 °C h−1; therefore, more than 7 h are needed.
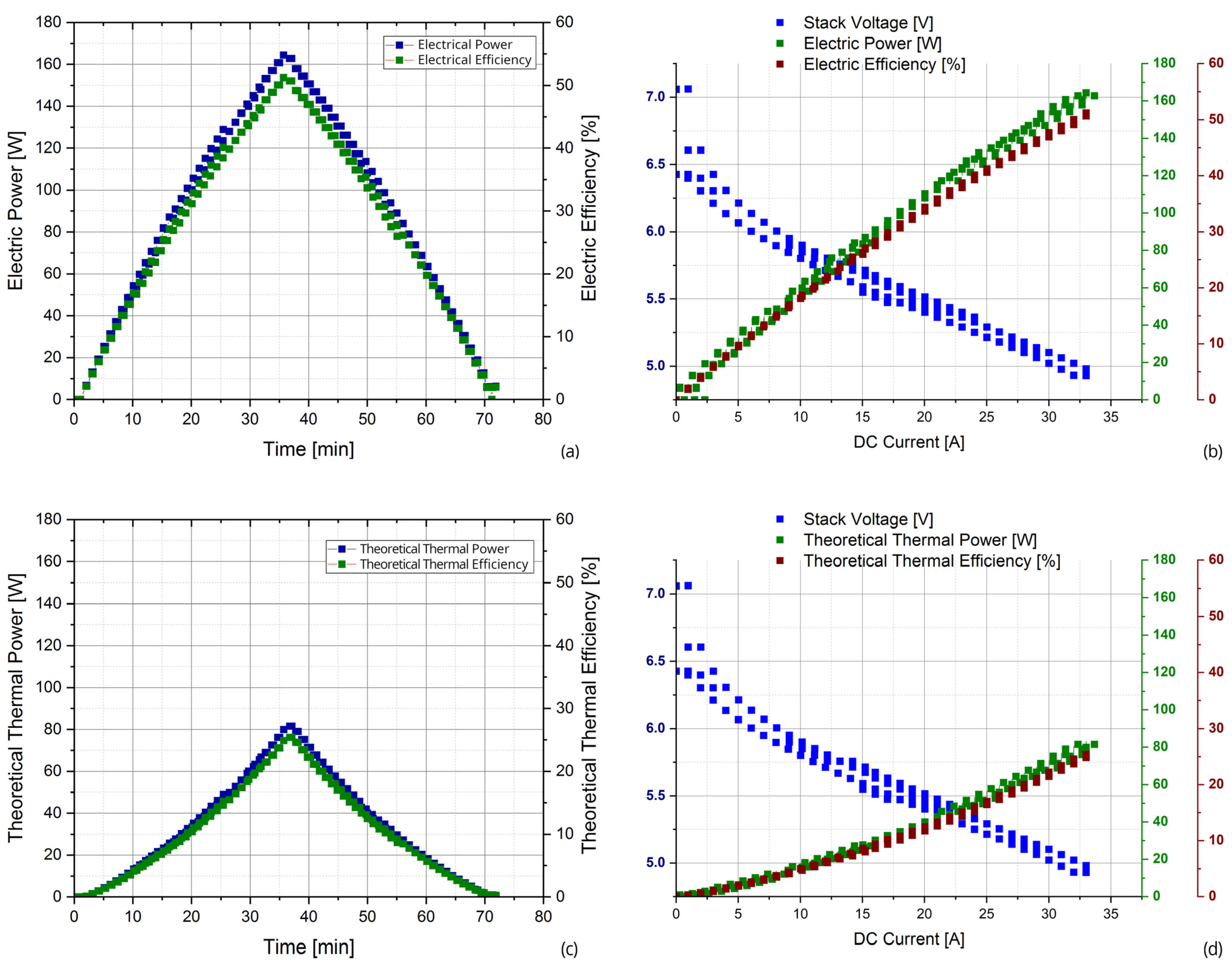
- During the test stage, instead, the SOFC system is at the nominal temperature, and the experimental tests are conducted changing the DC load, from the minimum to the maximum current (from 0 A to 33 A) and vice versa but maintaining constant the input flows. The current load step is imposed to 1 A; the current is varied with a time step of 1 min, intending to overcome the SOFC system dynamic oscillations.
4. Results
5. Insights for SOFC Applications in Sustainable Mobility
5.1. Automotive Sector
5.2. Maritime Sector
5.2.1. Technical Challenges
- Humidity: SOFC performance can be impacted by high humidity in two different ways. The fuel and oxidant gases may be diluted by the water vapor, which may result in a drop in performance. Second, too much moisture can result in the fuel cell becoming liquid water, which can cause flooding and obstruct the flow of gases. However, compared to fuel cells that operate at lower temperatures, such as PEM fuel cells, the effects of humidity are typically less noticeable with SOFCs due to their high working temperatures [75].
- Salinity: Marine air’s high salinity can cause corrosion problems. The fuel cell could malfunction if salt deposits on its components and interacts with the materials, causing corrosion. These effects can be lessened by choosing the right materials and applying protective coatings.
- Temperature Fluctuations: The maritime environment is subject to severe temperature changes, ranging from extremely cold temperatures in northern regions to high temperatures in tropical places. The thermal management system and the fuel cell’s functionality may be impacted by these temperature fluctuations. Additionally, due to variations in thermal expansion, thermal cycling (heating up and cooling down) can cause mechanical stresses in the SOFC materials, perhaps resulting in degradation and failure.
5.2.2. Fuel Storage, Regulations, and Refueling Procedure
5.2.3. Drive-Cycle Requests and Features
5.3. Aviation SECTOR
5.3.1. Technical Challenges
5.3.2. Fuel Storage, Regulations, and Refueling Procedure
5.3.3. Drive-Cycle Requests and Features
- Taxi: low-power operation, ground movement of an aircraft
- Takeoff: requires the most power to lift the aircraft off the ground
- Climb: demanding a lot of power to reach cruising altitude
- Cruise: moderate, constant power demand for maintaining altitude and speed
- Descent and landing: low power demand, altitude reduction, and landing preparation
- Taxi: return to low-power operation
6. Guidelines for SOFC System Scale-Up
7. Targets and Remarks
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- European Commission. A European Strategy for Low-Emission Mobility; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Schlüter, A.; Genovese, M.; Fragiacomo, P. Preparing for More Sustainable Mobility. In Sustainable and Smart Energy Systems for Europe’s Cities and Rural Areas; Carl Hanser Verlag GmbH & Co. KG, Ed.; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2022; pp. 197–216. ISBN 978-3-446-47294-5. [Google Scholar]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, Technological Status, and Fundamentals of PEM Fuel Cells—A Review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Bessekon, Y.; Zielke, P.; Wulff, A.C.; Hagen, A. Simulation of a SOFC/Battery powered vehicle. Int. J. Hydrogen Energy 2019, 44, 1905–1918. [Google Scholar] [CrossRef]
- Qin, X.; Cao, J.; Geng, G.; Li, Y.; Zheng, Y.; Zhang, W.; Yu, B. Solid oxide fuel cell system for automobiles. Int. J. Green Energy 2022, 1–10. [Google Scholar] [CrossRef]
- Botti, J.; Speck, C.E. Solid Oxide Fuel Cell Power Unit for Hybrid Vehicles and Electric Vehicles—SAE Technical Paper 2005-24-094; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- Ji, Z.; Qin, J.; Cheng, K.; Liu, H.; Zhang, S.; Dong, P. Design and Performance of a Compact Air-Breathing Jet Hybrid-Electric Engine Coupled with Solid Oxide Fuel Cells. Front. Energy Res. 2021, 8, 613205. [Google Scholar] [CrossRef]
- Lindahl, P.; Moog, E.; Shaw, S.R. Simulation, Design and Validation of a UAV SOFC Propulsion System. In Proceedings of the 2009 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2009; pp. 1–8. [Google Scholar]
- Rostami, M.; Manshadi, M.D.; Farajollahi, A.H.; Marefati, M. Introducing and evaluation of a new propulsion system composed of solid oxide fuel cell and downstream cycles; usage in Unmanned Aerial Vehicles. Int. J. Hydrogen Energy 2022, 47, 13693–13709. [Google Scholar] [CrossRef]
- Ahn, A.; Stone Welles, T.; Akih-Kumgeh, B.; Milcarek, R.J. Experimental Investigation of a Novel Combined Rapid Compression-Ignition Combustion and Solid Oxide Fuel Cell System Format Operating on Diesel. In Proceedings of the ASME 2021 Power Conference, Virtual, 20–22 July 2021; American Society of Mechanical Engineers: New York, NY, USA, 2021. [Google Scholar]
- Sun, Y.; Anwar, M.; Hassan, N.M.S.; Spiryagin, M.; Cole, C. A review of hydrogen technologies and engineering solutions for railway vehicle design and operations. Railw. Eng. Sci. 2021, 29, 212–232. [Google Scholar] [CrossRef]
- Piraino, F.; Fragiacomo, P. A multi-method control strategy for numerically testing a fuel cell-battery-supercapacitor tramway. Energy Convers. Manag. 2020, 225, 113481. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Piraino, F. Fuel cell hybrid powertrains for use in Southern Italian railways. Int. J. Hydrogen Energy 2019, 44, 27930–27946. [Google Scholar] [CrossRef]
- Piraino, F.; Genovese, M.; Pagnotta, L.; Caposciutti, M.; Flaccomio Nardi Dei, L.; Fragiacomo, P. Integrated hydrogen and battery energy systems as emergency backup in electric trains. Energy Convers. Manag. X 2023, 18, 100382. [Google Scholar] [CrossRef]
- Martinez, A.S.; Brouwer, J.; Samuelsen, G.S. Feasibility study for SOFC-GT hybrid locomotive power part II. System packaging and operating route simulation. J. Power Sources 2012, 213, 358–374. [Google Scholar] [CrossRef]
- Ahrend, P.; Azizi, A.; Brouwer, J.; Samuelsen, G.S. A Solid Oxide Fuel Cell-Gas Turbine Hybrid System for a Freight Rail Application. In Proceedings of the ASME 2019 13th International Conference on Energy Sustainability, Bellevue, WA, USA, 15–18 July 2019; American Society of Mechanical Engineers: New York, NY, USA, 2019. [Google Scholar]
- Schroeder, D.J.; Majumdar, P. Feasibility analysis for solid oxide fuel cells as a power source for railroad road locomotives. Int. J. Hydrogen Energy 2010, 35, 11308–11314. [Google Scholar] [CrossRef]
- Kistner, L.; Bensmann, A.; Hanke-Rauschenbach, R. Optimal Design of a Distributed Ship Power System with Solid Oxide Fuel Cells under the Consideration of Component Malfunctions. Appl. Energy 2022, 316, 119052. [Google Scholar] [CrossRef]
- van Veldhuizen, B.; van Biert, L.; Aravind, P.V.; Visser, K. Solid Oxide Fuel Cells for Marine Applications. Int. J. Energy Res. 2023, 2023, 5163448. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Han, M. Industrial Development Status and Prospects of the Marine Fuel Cell: A Review. J. Mar. Sci. Eng. 2023, 11, 238. [Google Scholar] [CrossRef]
- IndustryandEnergy. EU World’s First Cruise Ship Powered by Solid Oxide Fuel Cell. Available online: https://www.industryandenergy.eu/electrification/worlds-first-cruise-ship-powered-by-solid-oxide-fuel-cell/ (accessed on 12 June 2023).
- Udomsilp, D.; Rechberger, J.; Neubauer, R.; Bischof, C.; Thaler, F.; Schafbauer, W.; Menzler, N.H.; de Haart, L.G.J.; Nenning, A.; Opitz, A.K.; et al. Metal-Supported Solid Oxide Fuel Cells with Exceptionally High Power Density for Range Extender Systems. Cell Rep. Phys. Sci. 2020, 1, 100072. [Google Scholar] [CrossRef]
- Boldrin, P.; Brandon, N.P. Progress and outlook for solid oxide fuel cells for transportation applications. Nat. Catal. 2019, 2, 571–577. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Leah, R.T.; Bone, A.; Hammer, E.; Selcuk, A.; Rahman, M.; Clare, A.; Mukerjee, S.; Selby, M. Development Progress on the Ceres Power Steel Cell Technology Platform: Further Progress towards Commercialization. ECS Trans. 2017, 78, 87–95. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, L.; Mushtaq, N.; Raza, R.; Sajid, M.; Wu, Y.; Lin, W.; Kim, J.-S.; Lund, P.D.; Yun, S. Semiconductor Electrochemistry for Clean Energy Conversion and Storage. Electrochem. Energy Rev. 2021, 4, 757–792. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, Y.; Li, L.; Shi, Q.; Shi, J.; Yun, S.; Akbar, M.; Wang, B.; Kim, J.-S.; Zhu, B. Proton Shuttles in CeO2/CeO2−δ Core–Shell Structure. ACS Energy Lett. 2019, 4, 2601–2607. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, B.; Yun, S.; Zhang, W.; Xia, C.; Afzal, M.; Cai, Y.; Liu, Y.; Wang, Y.; Wang, H. Fast ionic conduction in semiconductor CeO2−δ electrolyte fuel cells. NPG Asia Mater. 2019, 11, 51. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Wu, Y.; Gao, J.; Zhang, Q. Advances in metal–organic frameworks and their derivatives for diverse electrocatalytic applications. Electrochem. Commun. 2021, 126, 107024. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, H.; Wang, X.; Zhang, L.Y.; Jing, M.; Yuan, W.; Li, C.M. Dynamically self-assembled adenine-mediated synthesis of pristine graphene-supported clean Pd nanoparticles with superior electrocatalytic performance toward formic acid oxidation. J. Colloid Interface Sci. 2022, 613, 515–523. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Wang, R.; Zhang, W.; Yun, S.; Wang, B. Advances in lithium-ion battery materials for ceramic fuel cells. Energy Mater. 2022, 2, 200041. [Google Scholar] [CrossRef]
- Raza, T.; Yang, J.; Wang, R.; Xia, C.; Raza, R.; Zhu, B.; Yun, S. Recent advance in physical description and material development for single component SOFC: A mini-review. Chem. Eng. J. 2022, 444, 136533. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, B.; Shi, J.; Yun, S. Advanced low-temperature solid oxide fuel cells based on a built-in electric field. Energy Mater. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the Technology of Solid Oxide Fuel Cell (SOFC) Energy Systems for Stationary Power Generation: A Review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Leah, R.T.; Bone, A.; Lankin, M.; Selcuk, A.; Rahman, M.; Clare, A.; Rees, L.; Phillip, S.; Mukerjee, S.; Selby, M. Ceres Power Steel Cell Technology: Rapid Progress towards a Truly Commercially Viable SOFC. ECS Trans. 2015, 68, 95–107. [Google Scholar] [CrossRef]
- Geipel, C.; Hauptmeier, K.; Herbrig, K.; Mittmann, F.; Münch, M.; Pötschke, M.; Reichel, L.; Strohbach, T.; Seidel, T.; Surrey, A.; et al. Stack Development and Industrial Scale-Up. ECS Trans. 2019, 91, 123–132. [Google Scholar] [CrossRef]
- Wehrle, L.; Wang, Y.; Boldrin, P.; Brandon, N.P.; Deutschmann, O.; Banerjee, A. Optimizing Solid Oxide Fuel Cell Performance to Re-evaluate Its Role in the Mobility Sector. ACS Environ. Au 2021, 2, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Kerviel, A.; Pesyridis, A.; Mohammed, A.; Chalet, D. An Evaluation of Turbocharging and Supercharging Options for High-Efficiency Fuel Cell Electric Vehicles. Appl. Sci. 2018, 8, 2474. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, C.; Lyu, Z.; Han, M.; Wu, J.; Sun, Z.; Iguchi, F.; Yashiro, K.; Kawada, T. Performance and stability analysis of SOFC containing thin and dense gadolinium-doped ceria interlayer sintered at low temperature. J. Mater. 2022, 8, 347–357. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Stevenson, J.W.; Choi, J.-P. Long-term evaluation of solid oxide fuel cell candidate materials in a 3-cell generic short stack fixture, part I: Test fixture, sealing, and electrochemical performance. J. Power Sources 2014, 255, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Z.; Han, M. Optimization of Methane Reforming for High Efficiency and Stable Operation of SOFC Stacks. ECS Trans. 2021, 103, 201–209. [Google Scholar] [CrossRef]
- Del Zotto, L.; Monforti Ferrario, A.; Hatunoglu, A.; Dell’era, A.; McPhail, S.; Bocci, E. Experimental Procedures & First Results of an Innovative Solid Oxide Fuel Cell Test Rig: Parametric Analysis and Stability Test. Energies 2021, 14, 2038. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Corigliano, O.; De Lorenzo, G.; Mirandola, F.A. Experimental Activity on a 100-W IT-SOFC Test Bench Fed by Simulated Syngas. J. Energy Eng. 2018, 144, 04018006. [Google Scholar] [CrossRef]
- Fragiacomo, P.; De Lorenzo, G.; Corigliano, O. Performance Analysis of an Intermediate Temperature Solid Oxide Electrolyzer Test Bench under a CO2-H2O Feed Stream. Energies 2018, 11, 2276. [Google Scholar] [CrossRef]
- Fragiacomo, P.; De Lorenzo, G.; Corigliano, O. Design of an SOFC/SOE station: Experimental test campaigns. Energy Procedia 2018, 148, 543–550. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Corigliano, O.; De Lorenzo, G. Design of an SOFC/SOE experimental station: Planning of simulation tests. Energy Procedia 2018, 148, 535–542. [Google Scholar] [CrossRef]
- Corigliano, O.; Fragiacomo, P. Extensive analysis of SOFC fed by direct syngas at different anodic compositions by using two numerical approaches. Energy Convers. Manag. 2020, 209, 112664. [Google Scholar] [CrossRef]
- Genovese, M.; Schlüter, A.; Scionti, E.; Piraino, F.; Corigliano, O.; Fragiacomo, P. Power-to-hydrogen and hydrogen-to-X energy systems for the industry of the future in Europe. Int. J. Hydrogen Energy 2023, 48, 16545–16568. [Google Scholar] [CrossRef]
- Li, H.; Garcia, T.; Lee, M.H. Chapter 17—Solid Oxide Fuel Cells for Vehicles. In Fuel Cells for Transportation; Das, P.K., Jiao, K., Wang, Y., Frano, B., Li, X., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 547–573. ISBN 978-0-323-99485-9. [Google Scholar]
- Baldi, F.; Moret, S.; Tammi, K.; Maréchal, F. The role of solid oxide fuel cells in future ship energy systems. Energy 2020, 194, 116811. [Google Scholar] [CrossRef]
- Henne, R.H.; Friedrich, K.A. APPLICATIONS—TRANSPORTATION|Auxiliary Power Units: Fuel Cells. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 157–173. ISBN 978-0-444-52745-5. [Google Scholar]
- Kumar, S.S.; Aruna, S.T. Hydrocarbon Compatible SOFC Anode Catalysts and Their Syntheses: A Review. Sustain. Chem. 2021, 2, 707–763. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Ma, S.; Lin, M.; Lin, T.-E.; Lan, T.; Liao, X.; Maréchal, F.; Van Herle, J.; Yang, Y.; Dong, C.; Wang, L. Fuel cell-battery hybrid systems for mobility and off-grid applications: A review. Renew. Sustain. Energy Rev. 2020, 135, 110119. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Genovese, M.; Piraino, F.; Corigliano, O.; De Lorenzo, G. Hydrogen-Fuel Cell Hybrid Powertrain: Conceptual Layouts and Current Applications. Machines 2022, 10, 1121. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Piraino, F.; Genovese, M.; Corigliano, O.; De Lorenzo, G. Strategic Overview on Fuel Cell-Based Systems for Mobility and Electrolytic Cells for Hydrogen Production. Procedia Comput. Sci. 2022, 200, 1254–1263. [Google Scholar] [CrossRef]
- Vargas, J.E.V.; Seabra, J.E.A. Fuel-cell technologies for private vehicles in Brazil: Environmental mirage or prospective romance? A comparative life cycle assessment of PEMFC and SOFC light-duty vehicles. Sci. Total. Environ. 2021, 798, 149265. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, Z.; Maréchal, F. Environomic design for electric vehicles with an integrated solid oxide fuel cell (SOFC) unit as a range extender. Renew. Energy 2017, 112, 124–142. [Google Scholar] [CrossRef]
- Delphi demos SOFC tech for truck APU. Fuel Cells Bull. 2010, 3, 2010. [CrossRef]
- Markowski, J.; Pielecha, I. The Potential of Fuel Cells as a Drive Source of Maritime Transport. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on the Sustainable Energy and Environmental Development, Krakow, Poland, 14–17 November 2017; Institute of Physics Publishing: Bristol, UK, 2019; Volume 214, p. 214. [Google Scholar]
- Elkafas, A.G.; Rivarolo, M.; Gadducci, E.; Magistri, L.; Massardo, A.F. Fuel Cell Systems for Maritime: A Review of Research Development, Commercial Products, Applications, and Perspectives. Processes 2023, 11, 97. [Google Scholar] [CrossRef]
- Di Micco, S.; Mastropasqua, L.; Cigolotti, V.; Minutillo, M.; Brouwer, J. A framework for the replacement analysis of a hydrogen-based polymer electrolyte membrane fuel cell technology on board ships: A step towards decarbonization in the maritime sector. Energy Convers. Manag. 2022, 267, 115893. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Sapra, H.; Stam, J.; Reurings, J.; van Biert, L.; van Sluijs, W.; de Vos, P.; Visser, K.; Vellayani, A.P.; Hopman, H. Integration of solid oxide fuel cell and internal combustion engine for maritime applications. Appl. Energy 2021, 281, 115854. [Google Scholar] [CrossRef]
- International Energy Agency IEA. International Shipping; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Micoli, L.; Coppola, T.; Turco, M. A Case Study of a Solid Oxide Fuel Cell Plant on Board a Cruise Ship. J. Mar. Sci. Appl. 2021, 20, 524–533. [Google Scholar] [CrossRef]
- Minh, N.Q. 8—Cell and Stack Design, Fabrication and Performance. In High-Temperature Solid Oxide Fuel Cells for the 21st Century, 2nd ed.; Kendall, K., Kendall, M., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 255–282. ISBN 978-0-12-410453-2. [Google Scholar]
- Minh, N.Q. Reversible Solid Oxide Cell Technology. In Encyclopedia of Energy Storage; Cabeza, L.F., Ed.; Elsevier: Oxford, UK, 2022; pp. 338–343. ISBN 978-0-12-819730-1. [Google Scholar]
- Aicher, T.; Lenz, B.; Gschnell, F.; Groos, U.; Federici, F.; Caprile, L.; Parodi, L. Fuel processors for fuel cell APU applications. J. Power Sources 2006, 154, 503–508. [Google Scholar] [CrossRef]
- Fallah Vostakola, M.; Amini Horri, B. Progress in Material Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Eichhorn Colombo, K.W.; Kharton, V.V. Start-Up of a Solid Oxide Fuel Cell System with a View to Materials Science-Related Aspects, Control and Thermo-Mechanical Stresses. Crystals 2021, 11, 732. [Google Scholar] [CrossRef]
- Huang, Y.L.; Pellegrinelli, C.; Wachsman, E.D. Fundamental Impact of Humidity on SOFC Cathode ORR. J. Electrochem. Soc. 2016, 163, F171–F182. [Google Scholar] [CrossRef]
- IMO. IMO—International Maritime Organization. Available online: https://www.imo.org/ (accessed on 9 June 2023).
- Fu, Z.; Lu, L.; Zhang, C.; Xu, Q.; Zhang, X.; Gao, Z.; Li, J. Fuel cell and hydrogen in maritime application: A review on aspects of technology, cost and regulations. Sustain. Energy Technol. Assess. 2023, 57, 103181. [Google Scholar] [CrossRef]
- Pomaska, L.; Acciaro, M. Bridging the Maritime-Hydrogen Cost-Gap: Real options analysis of policy alternatives. Transp. Res. Part D Transp. Environ. 2022, 107, 103283. [Google Scholar] [CrossRef]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Alternative fuel options for low carbon maritime transportation: Pathways to 2050. J. Clean. Prod. 2021, 297, 126651. [Google Scholar] [CrossRef]
- Zanobetti, F.; Pio, G.; Jafarzadeh, S.; Ortiz, M.M.; Cozzani, V. Inherent safety of clean fuels for maritime transport. Process. Saf. Environ. Prot. 2023, 174, 1044–1055. [Google Scholar] [CrossRef]
- Molkov, V. 4.04—Hydrogen Safety Engineering and Standards. In Comprehensive Renewable Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 86–118. ISBN 978-0-12-819734-9. [Google Scholar]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Hydrogen Logistics: Safety and Risks Issues. In Hydrogen Infrastructure for Energy Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in Gas Sensor Technology for Hydrogen Safety. Proc. Int. J. Hydrog. Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrogen Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Duong, P.A.; Ryu, B.; Kim, C.; Lee, J.; Kang, H. Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels. Energies 2022, 15, 3331. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Cinti, G. Operation of a Solid Oxide Fuel Cell Based Power System with Ammonia as a Fuel: Experimental Test and System Design. Energies 2020, 13, 6173. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Turnock, S.R.; Hudson, D.A. Route to zero emission shipping: Hydrogen, ammonia or methanol? Int. J. Hydrogen Energy 2021, 46, 28282–28297. [Google Scholar] [CrossRef]
- Genovese, M.; Cigolotti, V.; Jannelli, E.; Fragiacomo, P. Hydrogen Refueling Process: Theory, Modeling, and In-Force Applications. Energies 2023, 16, 2890. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Liu, Z.; Liu, J. Review of researches on important components of hydrogen supply systems and rapid hydrogen refueling processes. Int. J. Hydrogen Energy 2023, 48, 1904–1929. [Google Scholar] [CrossRef]
- Alkhaledi, A.N.; Sampath, S.; Pilidis, P. Propulsion of a hydrogen-fuelled LH2 tanker ship. Int. J. Hydrogen Energy 2022, 47, 17407–17422. [Google Scholar] [CrossRef]
- Baldi, F.; Ahlgren, F.; Nguyen, T.-V.; Thern, M.; Andersson, K. Energy and Exergy Analysis of a Cruise Ship. Energies 2018, 11, 2508. [Google Scholar] [CrossRef]
- Soriani, S.; Bertazzon, S.; DI Cesare, F.; Rech, G. Cruising in the Mediterranean: Structural aspects and evolutionary trends. Marit. Policy Manag. 2009, 36, 235–251. [Google Scholar] [CrossRef]
- Borén, C.; Castells-Sanabra, M.; Grifoll, M. Ship emissions reduction using weather ship routing optimisation. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2022, 236, 856–867. [Google Scholar] [CrossRef]
- Ruf, Y.; Kaufmann, M.; Lange, S.; Pfister, J.; Heieck, F.; Endres Brussels, A. Fuel Cells and Hydrogen Applications for Regions and Cities. Eur. Union Fuel Cells Hydrogen 2017, 1, 108–123. [Google Scholar]
- Ramasubramanian, B.; Rao, R.P.; Chellappan, V.; Ramakrishna, S. Towards Sustainable Fuel Cells and Batteries with an AI Perspective. Sustainability 2022, 14, 16001. [Google Scholar] [CrossRef]
- Benet, Á.; Villalba-Herreros, A.; D’amore-Domenech, R.; Leo, T.J. Knowledge gaps in fuel cell-based maritime hybrid power plants and alternative fuels. J. Power Sources 2022, 548, 232066. [Google Scholar] [CrossRef]
- Chehrmonavari, H.; Kakaee, A.; Hosseini, S.E.; Desideri, U.; Tsatsaronis, G.; Floerchinger, G.; Braun, R.; Paykani, A. Hybridizing solid oxide fuel cells with internal combustion engines for power and propulsion systems: A review. Renew. Sustain. Energy Rev. 2023, 171, 112982. [Google Scholar] [CrossRef]
- Haseltalab, A.; van Biert, L.; Sapra, H.; Mestemaker, B.; Negenborn, R.R. Component sizing and energy management for SOFC-based ship power systems. Energy Convers. Manag. 2021, 245, 114625. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Ma, Y.; Xia, R.; Han, F. Performance Analysis and Design of Direct Ammonia Fuel Tubular Solid Oxide Fuel Cell for Shipborne Unmanned Aerial Vehicles. Aerospace 2023, 10, 397. [Google Scholar] [CrossRef]
- Hosseini, S.E. 4.12—Hydrogen and Fuel Cells in Transport Road, Rail, Air, and Sea. In Comprehensive Renewable Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 317–342. ISBN 978-0-12-819734-9. [Google Scholar]
- Afonso, F.; Sohst, M.; Diogo, C.M.; Rodrigues, S.S.; Ferreira, A.; Ribeiro, I.; Marques, R.; Rego, F.F.; Sohouli, A.; Portugal-Pereira, J.; et al. Strategies towards a more sustainable aviation: A systematic review. Prog. Aerosp. Sci. 2023, 137, 100878. [Google Scholar] [CrossRef]
- Müller-Casseres, E.; Szklo, A.; Fonte, C.; Carvalho, F.; Portugal-Pereira, J.; Baptista, L.B.; Maia, P.; Rochedo, P.R.; Draeger, R.; Schaeffer, R. Are there synergies in the decarbonization of aviation and shipping? An integrated perspective for the case of Brazil. iScience 2022, 25, 105248. [Google Scholar] [CrossRef] [PubMed]
- Airbus announces plans to decarbonise air travel. Fuel Cells Bull. 2021, 2021, 10. [CrossRef]
- Airbus studies fuel cell pods for future aircraft. Fuel Cells Bull. 2021, 2021, 6. [CrossRef]
- Fernandes; Andrade, S.T.D.P.; Bistritzki, V.N.; Fonseca, R.M.; Zacarias, L.G.; Gonçalves, H.N.C.; de Castro, A.F.; Domingues, R.Z.; Matencio, T. SOFC-APU systems for aircraft: A review. Int. J. Hydrogen Energy 2018, 43, 16311–16333. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Coutinho, M.; Bento, D.; Souza, A.; Cruz, R.; Afonso, F.; Lau, F.; Suleman, A.; Barbosa, F.R.; Gandolfi, R.; Affonso, W.; et al. A review on the recent developments in thermal management systems for hybrid-electric aircraft. Appl. Therm. Eng. 2023, 227, 120427. [Google Scholar] [CrossRef]
- Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393–419. [Google Scholar] [CrossRef]
- FAA Federal Aviation Administration. Available online: https://www.faa.gov/ (accessed on 9 June 2023).
- EASA European Union Aviation Safety Agency. Available online: https://www.easa.europa.eu/en/regulations (accessed on 9 June 2023).
- Prewitz, M.; Schwärzer, J.; Bardenhagen, A. Potential analysis of hydrogen storage systems in aircraft design. Int. J. Hydrogen Energy 2023, 48, 25538–25548. [Google Scholar] [CrossRef]
- Genovese, M.; Fragiacomo, P. Hydrogen refueling station: Overview of the technological status and research enhancement. J. Energy Storage 2023, 61, 106758. [Google Scholar] [CrossRef]
- Genovese, M.; Cigolotti, V.; Jannelli, E.; Fragiacomo, P. Current standards and configurations for the permitting and operation of hydrogen refueling stations. Int. J. Hydrogen Energy 2023, 48, 19357–19371. [Google Scholar] [CrossRef]
- Collins, J.M.; McLarty, D. All-electric commercial aviation with solid oxide fuel cell-gas turbine-battery hybrids. Appl. Energy 2020, 265, 114787. [Google Scholar] [CrossRef]
- Gray, N.; McDonagh, S.; O’Shea, R.; Smyth, B.; Murphy, J.D. Decarbonising ships, planes and trucks: An analysis of suitable low-carbon fuels for the maritime, aviation and haulage sectors. Adv. Appl. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Ji, Z.; Guo, F.; Dong, P. Study on the performance comparison of three configurations of aviation fuel cell gas turbine hybrid power generation system. J. Power Sources 2021, 501, 230007. [Google Scholar] [CrossRef]
- Bahari, M.; Rostami, M.; Entezari, A.; Ghahremani, S.; Etminan, M. A comparative analysis and optimization of two supersonic hybrid SOFC and turbine-less jet engine propulsion system for UAV. Fuel 2022, 319, 123796. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Xiu, X.; Ha, C.; Dong, P. Comparative study of fuel types on solid oxide fuel cell—Gas turbine hybrid system for electric propulsion aircraft. Fuel 2023, 347, 128426. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Li, C.; Guo, F.; Dong, P. Influence of fuel reforming with low water to carbon ratio on thermodynamic performance of aviation solid oxide fuel cell—Gas turbine hybrid system. J. Power Sources 2022, 546, 231978. [Google Scholar] [CrossRef]
- Li, C.; Cheng, K.; Ma, S.; Liu, H.; Ji, Z.; Qin, J. Performance analysis of solid oxide fuel cell/piston engine hybrid system for aviation. Appl. Therm. Eng. 2022, 214, 118797. [Google Scholar] [CrossRef]
- Cui, L.; Huo, H.; Xie, G.; Xu, J.; Kuang, X.; Dong, Z. Long-Term Degradation Trend Prediction and Remaining Useful Life Estimation for Solid Oxide Fuel Cells. Sustainability 2022, 14, 9069. [Google Scholar] [CrossRef]
- Staffolani, A.; Baldinelli, A.; Barelli, L.; Bidini, G.; Nobili, F. Early-Stage Detection of Solid Oxide Cells Anode Degradation by Operando Impedance Analysis. Processes 2021, 9, 848. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, D.; Xu, Y.; Wu, X.; Li, X. Comprehensive Analysis of Solid Oxide Fuel Cell Performance Degradation Mechanism, Prediction, and Optimization Studies. Energies 2023, 16, 788. [Google Scholar] [CrossRef]
- Ryu, B.R.; Duong, P.A.; Kang, H. Comparative analysis of the thermodynamic performances of solid oxide fuel cell–gas turbine integrated systems for marine vessels using ammonia and hydrogen as fuels. Int. J. Nav. Arch. Ocean Eng. 2023, 15, 100524. [Google Scholar] [CrossRef]
- Rivarolo, M.; Rattazzi, D.; Magistri, L. Best operative strategy for energy management of a cruise ship employing different distributed generation technologies. Int. J. Hydrogen Energy 2018, 43, 23500–23510. [Google Scholar] [CrossRef]
- Gianni, M.; Pietra, A.; Coraddu, A.; Taccani, R. Impact of SOFC Power Generation Plant on Carbon Intensity Index (CII) Calculation for Cruise Ships. J. Mar. Sci. Eng. 2022, 10, 1478. [Google Scholar] [CrossRef]
- Salim, K.M.A.; Maelah, R.; Hishamuddin, H.; Amir, A.M.; Ab Rahman, M.N. Two Decades of Life Cycle Sustainability Assessment of Solid Oxide Fuel Cells (SOFCs): A Review. Sustainability 2022, 14, 12380. [Google Scholar] [CrossRef]
- Perčić, M.; Vladimir, N.; Jovanović, I.; Koričan, M. Application of fuel cells with zero-carbon fuels in short-sea shipping. Appl. Energy 2022, 309, 118463. [Google Scholar] [CrossRef]
- Wang, Y.; Maidment, H.; Boccolini, V.; Wright, L. Life cycle assessment of alternative marine fuels for super yacht. Reg. Stud. Mar. Sci. 2022, 55, 102525. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Q.; Liu, L.; Wu, Y.; Liu, H.; Gu, Z.; Zhu, C. A review of low and zero carbon fuel technologies: Achieving ship carbon reduction targets. Sustain. Energy Technol. Assess. 2022, 54, 102762. [Google Scholar] [CrossRef]
- Clean Hydrogen Joint Undertaking Strategic Research and Innovation Agenda 2021–2027; Clean Hydrogen Partnership: Brussels, Belgium, 2022; Available online: https://www.clean-hydrogen.europa.eu/about-us/key-documents/strategic-research-and-innovation-agenda_en (accessed on 12 June 2023).



| Fuel Cell Active Area\cm2 | 80 |
|---|---|
| Anode | Ni/YSZ |
| Electrolyte | 8YSZ |
| Cathode | (La,Sr)(Co,Fe)O3/Gd2O3-CeO2 |
| Pros | Cons and Restrictions |
|---|---|
|
|
|
| Features | Maritime |
|---|---|
| Power required | <10 kW–50 MW [91] |
| Average payload | 3000 Passengers [92] |
| Driving range | <1200 km [93] |
| Current main propulsion | Diesel, methane, LNG [94] |
| Pros | Cons and Restrictions |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragiacomo, P.; Piraino, F.; Genovese, M.; Corigliano, O.; De Lorenzo, G. Experimental Activities on a Hydrogen-Powered Solid Oxide Fuel Cell System and Guidelines for Its Implementation in Aviation and Maritime Sectors. Energies 2023, 16, 5671. https://doi.org/10.3390/en16155671
Fragiacomo P, Piraino F, Genovese M, Corigliano O, De Lorenzo G. Experimental Activities on a Hydrogen-Powered Solid Oxide Fuel Cell System and Guidelines for Its Implementation in Aviation and Maritime Sectors. Energies. 2023; 16(15):5671. https://doi.org/10.3390/en16155671
Chicago/Turabian StyleFragiacomo, Petronilla, Francesco Piraino, Matteo Genovese, Orlando Corigliano, and Giuseppe De Lorenzo. 2023. "Experimental Activities on a Hydrogen-Powered Solid Oxide Fuel Cell System and Guidelines for Its Implementation in Aviation and Maritime Sectors" Energies 16, no. 15: 5671. https://doi.org/10.3390/en16155671
APA StyleFragiacomo, P., Piraino, F., Genovese, M., Corigliano, O., & De Lorenzo, G. (2023). Experimental Activities on a Hydrogen-Powered Solid Oxide Fuel Cell System and Guidelines for Its Implementation in Aviation and Maritime Sectors. Energies, 16(15), 5671. https://doi.org/10.3390/en16155671








