Abstract
The energy demand generated by fossil fuels is increasing day by day, and it has drastically increased after the COVID-19 pandemic as industries and household utilities rejuvenate. Renewable sources are thus becoming more essential as easily available, alternative methods of low-cost energy generation. Among these renewables, solar energy, i.e., solar power, is a promising energy source and can be used for solar-based H2 evolution because H2 technology is a leading source of eco-friendly electricity generation, and most of the worldwide efforts to develop this method involve heterogeneous catalysis for H2 evolution via water splitting and its storage, i.e., using a fuel cell. In the current scenario, there is a need to develop a stable, recyclable, and reusable heterogeneous catalyst system, which is a great challenge. In the current study, we have focused on novel ferrite magnetic nanomaterials for recyclable and reusable robust photocatalysis. Moreover, discussions of the factors contributing to the photocatalytic hydrogen evolution, low-cost synthesis techniques, and prospects for making them ideal photocatalysts are uncommon in the literature. The study will impart possible approaches for the design and development of novel ferrite nanomaterials and their nanocomposites for H2 generation in the forthcoming years.
1. Introduction
Traditionally, non-renewable energy sources termed fossil fuels, including coal, oil, nuclear energy, and natural gas, are used in huge amounts for power generation in industries and domestic settings due to the increased demand for petroleum and automobiles. Attention to renewable energy sources, known as clean energy sources, including wind, solar, water, geothermal, and biomass, is increasing, mainly because of their advantages, such as abundance and negligible cost, whereas non-renewable energy sources are limited due to shortages and high costs [1]. According to the ‘Global Energy Review 2021’ by ‘International Energy Agency’, in the current post-pandemic scenario, non-renewable (oil) energy demand rebounded by 3% due to the global vaccination regime; however, it declined by 4% in the year 2020 compared with 2019 [2]. Carbon dioxide (CO2) emissions and energy demand have increased compared with 2020, surpassing the previous gross domestic product (GDP), with increasing demands on the energy sector; therefore, the COVID-19 pandemic has impacted global energy demand. Developing sustainable and green energy sources is a big challenge for all researchers [3]. Considering all the renewable energy sources, hydrogen (H2) energy is currently becoming a low-cost, highly appreciable, and sustainable energy source, which can be used to store, move, and deliver the energy produced from other resources [4]. Moreover, carbon-free, sustainable, and eco-friendly H2 energy generation is an important prerequisite for future economics, and it also acts as the driving force for innovations in the field of renewable energy. William Robert Grove first invented the H2 fuel cell to store generated hydrogen in the year 1839 [5], establishing the foundation for future H2 technology that can store evolved H2 in its pure form; therefore, researchers worldwide are increasingly considering the advantages and needs of H2 energy [6,7,8].
How Can H2 Be Generated Sustainably and Eco-Friendly?
Renewable energy sources, i.e., wind, biomass, and solar, are used for photocatalytic H2 generation [9]; however, these sources are regional and seasonal. Therefore, H2 can be generated by splitting water in H2 and oxygen (O2) by using sunlight (photocatalysis) [10], thermal and chemical (thermochemical catalysis) [11], and electrical (electrocatalytic) methods [12]. In addition, being a natural and abundant source of sunlight with no carbon dioxide (CO2) gas emissions, solar energy is playing a crucial role in overcoming kinetic barriers during heterogeneous catalysis. State-of-the-art methods involve materials where the catalysts possess higher values for solar-driven hydrogen evolution reactions (HER), and they include noble metals, such as pure platinum (Pt), iridium (Ir), and ruthenium (Ru), as well as noble-metal-free photocatalysts. Many reports are available for Pt-based H2 production using photocatalytic HER due to its high redox activity and zero overpotential [13]. However, the latest report on the approach for avoiding mass transport limitations and achieving the highest turnover frequency (TOF) when using Pt nanoparticles compared with the commercial platinum/carbon (Pt/C) catalysts illustrates that some of the pitfalls for obtaining a high-value TOF relate to measurement issues, such as the need for potential scale calibration, the choice of an incorrect counter electrode, and a lack of H2 saturation [14] during solar photocatalytic HER. Similarly, as reported by Koo et al., platinum nanocubes synthesized using an aqueous colloidal route exhibited a promising photocurrent density of 1.77 A/mg at −100 mV [15]. Heterogeneous photocatalysis using oxide-based nanomaterials is becoming a pioneering research area, leading to prominent H2 evolution results both in combination with, and without, noble metals [16,17,18], with Pt and Pt-group members being used with other inexpensive metal oxides to form alloys [19]. Therefore, Pt is the best catalyst in the field of catalysis to date and has also been explored for H2 production using wastewater compounds [20]; however, the production of large amounts of H2 is limited due to the cost of Pt and Pt-based commercial catalysts, high agglomeration rates, poor stability, and low removable efficacy. Low-cost, noble-metal-free photocatalysts are explored by Thakur et.al for efficient H2 evolution (2531 μmol/g) based on a phosphorus-doped graphitic carbon nitride-P25 (TiO2) composite and TiO2/g-C3N4/p-g-C3N4 nanocomposite [21]. The optical properties of titanium nitride were enhanced using red phosphor, meaning the resulting nanocomposite could evolve the 0.5 μmol/g/h [22] of H2. Sergei Poskunov et al. designed a novel photocatalyst, for which a single atom of gold, silver, and copper was deposited on the surface of TiO2, and analyzed its electronic properties using real-time, time-dependent density functional theory (RT-TD-DFT) [23]. Conclusively, the wider research community has explored new emerging magnetic and non-magnetic nanomaterials that are based on noble- and non-noble-metal-based photocatalysts for eco-friendly H2 generation [23,24,25].
2. Role of Removable Photocatalysts
Considering the many innovations achieved in the field of solar photocatalytic H2 evolution, much less attention has been given to the byproducts generated after completing the reaction, which can cause a hazard to the environment [26]. Therefore, the first step towards sustainable and eco-friendly H2 generation using novel nanomaterials is to find their dissociation mechanism and removal efficacy. Magnetic nano-catalysts such as Fe2O3 (hematite) and Fe3O4 (maghemite) are prominent and well established, with inherent or non-inherent magnetic properties, which allow them to be easily separated from an aqueous solution. Furthermore, spinel ferrite nanomaterials are novel types of magnetic nanomaterials that can be removed easily after the overall completion of HER. They are composed of an AB2O4 formula, where A and B are the divalent and trivalent cations coordinated with negatively charged oxygens or anions. Many compositions of spinel ferrites are possible due to the Earth’s abundance of metals, non-metals, and metalloids. Because nano-sized ferrites are an efficient photocatalyst, they are robust, and are characterized by thermal, chemical, and photostability; ease of production; a small band gap; tunable size; and higher levels of visible light absorption with appropriate positioning of the conduction band (CB) and valence band (VB); therefore, they are considered for photocatalytic HER. However, according to the available literature, until the year 2022, research on spinel ferrites for H2 production has been limited. In the year 2019, Pu et al. developed a 1D recyclable p-n junction of a nanocomposite based on CoFe2O4/Cd0.9Zn0.1S, which was separated multiple times from the solution by using a proposed H2 evolution mechanism [27]. Some other experimental reports are also available for the removal and recovery of ferrite-based nanomaterials [28,29]; however, they are limited and do not meet efficiency criteria compared with well-established nanomaterials, such as porous metal–organic frameworks (MOFs) [30]. Only 7% of publications before 2021 included material separation after the reaction [31]. The ab initio methods, i.e., density function theory (DFT), are extremely useful for understanding and exploring spinel ferrite nanomaterials, especially their fundamental properties related to electronic band structures and charge transfer dynamics/kinetics, as well as their experimental limitations. Using these ab initio methods, we can determine their electronic transitions, which are structures between most of the crystal structures, as well as their tetrahedral and octahedral cations (spinel ferrites) [32]. The structural, elastic, electronic, and thermodynamic properties of spinel ferrites nanomaterials derived using periodic ab initio CRYSTAL14 code based on the LCAO (linear combination of atomic orbitals) method with local Gaussian-type basis sets (BSs) have been reported in detail [33].
3. The Mechanism for H2 Evolution
The photocatalytic hydrogen evolution reaction (HER) is only possible when the photocatalyst can absorb the energy provided by a light source; this is necessary for the excitation of electrons from the valence band (VB) to the conduction band (CB), a process that leaves behind a hole. Similarly, in the case of spinel ferrites, the absorption of visible light leads to the excitation of electrons from the VB to the CB, which causes a hole formation in the VB, because of their narrow band gap energy values, which are capable of absorbing most of the visible light. Initially, an excited electron in the CB of spinel ferrite contributes to the breaking of bonds in adsorbed H2O molecules and governs the classic theory called Volmer, Heyrovsky, and Tafel reactions.
The Volmer step contributes to the dissociation of adsorbed water molecules:
H2O + e− → H* + OH
Heyrovsky step and Tafel step contribute to the production of molecular H2
H* + e− + H2O → H2 + OH
2H* + 2e− → H2
Similarly in the case of AB2O4 (ferrites) as photocatalysts, the reaction mechanism, it involves,
AB2O4 + 2H2O → AB2O4–H + H2O
AB2O4–H + H3O +e− → AB2O4–H + H3O
H3O+ + e− +AB2O4–H → AB2O4 + H2 + H2O
AB2O4–H + AB2O4–H → 2 AB2O4 + H2
4. Contributing Factors
Many factors affect H2 evolution during photocatalysis, and they are discussed extensively with a focus on the development of effective photocatalysts. An illustration of the factors that contribute to H2 evolution is given in Figure 1.
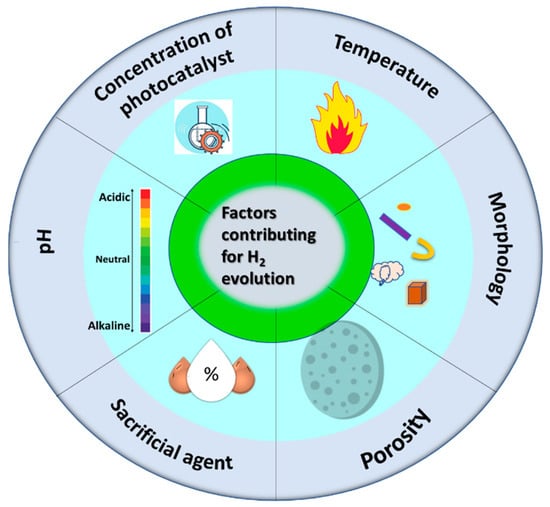
Figure 1.
Illustration of different factors contributing to H2 evolution.
4.1. Morphology
Nanomaterials have high functionality and diverse physicochemical properties that are comprehensively related to their photocatalytic properties [34,35]. Pure and hybrid oxide-based nanomaterials have been employed as efficient photocatalysts for water splitting and can produce and store hydrogen (H2) following hydrogen evolution reactions (HER). Zero-dimensional (0D) nanomaterials, such as CdS, CdSe, and carbon quantum dots, are employed for visible light H2 evolution; however, they are limited in efficiency due to their high-corrosion and charge-recombination rates. Addressing this, Li. et al. reported the photo deposition of metal oxides on quantum dots and alleviated the drawbacks [36]. Bimetallic plasmonic nanomaterials such as Ag@Au, i.e., a core–shell structure with an Au core and Ag shell, have emerged as next-generation photocatalysts for H2 generation [37]. One-dimensional (1D) nanomaterials consist of nanorods (NRs) [38], nanowires (NWs) [39], nanotubes (NTs) [40], and nanofibers (NFs), contributing to their superior properties, which are suitable for photocatalysis for H2 evolution. Their extremely large surface-to-volume ratio is favorable for photogenerated charge carriers and their ballistic transport. Zn2GeO4 is a type of 1D NRs that produces the highest rate of H2, namely, 0.6 mmol/h in basic conditions [41]. The morphological characteristics of 1D porous [42] TiO2 NTs are effectively utilized for photocatalytic activity because their inner diameter supports internal reflections of photons and results in a higher photocurrent density. TiO2 NTs are broadly explored in pure and doped form for solar-based photocatalysis and H2 generation, where doped TiO2 NTs can cover the entire solar spectrum, hence increasing the H2 production efficiency to 17.39 μmol h−1 cm−2. Carbon NTs and NFs have extraordinary mechanical and thermal stability. Hence, Jong-Beom Baek et al. anchored Ru nanoparticles on multiwalled CNTs (MWCNTs) to provide catalysis-active sites that are capable of H2 production of 4194 μ mol V−1, which is 15% greater than Pt/C catalysts [43]. Two-dimensional (2D) nanomaterials are basically in the form of thin films that are established for H2 evolution due to their large surface area compared with zero- and one-dimensional nanomaterials. Because the overall water-splitting phenomenon depends on the amount of light absorbed by the catalysis, and because there is a high chance of recombination of separated charges, scaling up the amount of photocatalyst in aqueous dispersion also affects the rate of H2 evolution. Therefore, thin-film-based photocatalysts are explored in academia with novel nanomaterials such as transition metal dichalcogenides (TMDs) [44], graphene oxide [45], and graphitic carbon nitride (g-C3N4) [46]. The issues regarding the low performance of the catalysis and H2 evolution in the particulate form are overcome by the deposition of thin films using spin-coating, hydrothermal, chemical bath (CBD), and electrostatic spray pyrolysis (ESP) deposition methods, where the experimental parameters are varied to obtain the perfect VB and CB positions required for water splitting [47]. Thin films of metal oxides such as TiO2 and Fe2O3 showed better H2 evolution rates than that of the powder formed [48]. The porosity photocatalysts play a vital role in providing the active sites for H2 production and the water adsorption required for catalysis. The 1D, 2D, and 3D metal–organic frameworks (MOFs) have been widely studied regarding the overall pH range for H2 evolution reactions, among which 2D MOFs with a sheet-like morphology became high-potential candidates [49,50]. Cobalt dithiolene is one of the best photocatalysts based on the lowest overpotential values for H2 evolution, which can also be derived from MOFs [51]. Transition-metal-doped MOFs, such as transition metal phosphides (TMPs), are currently trending 2D nanomaterials for heterogeneous catalysis. However, neither the stability of MOFs during the overall water splitting process nor the degradation and removal mechanisms are elaborated upon in the literature; nor are the associated problems and limitations for scaling up H2 evolution.
4.2. pH and Sacrificial Agents
The acidic pH of the solution contributes to HER, where the H+ ion concentration is high. Diluted acid such as hydrogen sulfate (H2SO4) is used for providing acidic conditions. However, hole scavengers, such as ethanol and methanol/glycerol reaction media, have a basic pH. Different types of scavengers such as methanol, glycerol, formic acid, lactic acid, ethylenediamine (EDTA), triethanolamine, and sodium sulfate (Na2SO4) assist in controlling the charge recombination rate. Likuta et al. have undertaken a kinetic study of pH dependent H2 production [52]. The hydrogen ion concentration or protons are known as the pH of the solution, which also affects the other chemical interactions among the catalyst and substrate during photocatalysis, such as adsorption and the agglomeration of particulates. The greater aggregation, the more the photocatalyst will be sedimented, with more effort needed for their stable suspension. Essentially, measurements of H2 evolution are carried out in an environment where the dissociation of the photocatalyst without light is prevented, with the medium acting as a hole scavenger. Therefore, H2 evolution also depends on the concentration of the sacrificial agent used during photocatalysis, where the stability of photocatalysts plays a considerable role [53].
4.3. Temperature
Temperature is a key factor that contributes to an increased rate of H2 evolution. Recently, an increased rate of about 38.0 mmol·g−1 h−1 at 60 °C was achieved by Núñez et al. via HER, two times greater than room temperature [54]. The catalytic performance of TiO2 nanoparticles can also be enhanced by deposition on the SiO2 substrate, thereby increasing the temperature of the water-splitting reaction and lowering the overpotential and reaction time [55]. The temperature of the photocatalytic reaction setup can also be increased automatically via a high-energy sunlight source. Collectively, the elevated temperature causes an increased rate of carrier mobility and effective charge transfer during visible light photocatalysis. After a certain saturation temperature, the activity can be decreased depending on the stability of the photocatalyst at higher temperatures and carrier recombination. Therefore, the temperature range is optimized for each different nanomaterial. Achieving high photocatalytic activity is a crucial challenge and using transition-metal-based oxide materials is favorable. Moreover, Xu and Li. et al. synthesized graphitic carbon nitride (g-C3N4)-layered microstructures combined with a Pt co-catalyst, with which they could obtain a H2 evolution of 800 μmol−1 g−1 at a lower temperature (10 °C) [56].
4.4. Concentration of Photocatalyst
An aqueous solution of a particulate-form photocatalyst should be prepared such that an optimal surface will be available to water molecules for an adsorption phenomenon called chemisorption. The higher the concentration of the photocatalyst, the more the adsorption of water molecules takes place, and the higher the rate of H2 evolution. The effect of varied concentrations of copper/zinc sulfide/CoFe2O4 (Cu/ZnS/COF) core–shell photocatalysts with 0.1 g/L to 0.6 g/L was studied by Wu et al. They observed that 0.3 g/L was the optimal concentration, where the maximum H2 evolution was observed and then decreased [55].
Table 1 illustrates the contributing factors, such as temperature, pH, sacrificial agent, and amount/concentration of photocatalysts, on photocatalytic H2 evolution via spinel ferrite nanomaterials. Most of the HER are feasible at room temperature; however, an increase in the reaction temperature to 50 °C is needed for some photocatalysts. Considering the pH of the medium, HER are favorable for acidic media, where the reports also suggest that, with an appropriate sacrificial agent, the H2 evolution rate could be increased at a basic pH compared with an acidic or neutral pH [57]. Similarly, the effect of dosage/concentration of photocatalysts on H2 evolution showed that optimal concentration needs to be checked during the experiments. However, the presence of sacrificial agents increased the rate of H2 evolution compared with the medium containing no sacrificial agent.

Table 1.
Factors contributing to H2 evolution based on spinel ferrites photocatalysts.
5. Synthesis Approaches
The process of H2 evolution is generally carried out with a particulate or thin films (anode/cathode) as photocatalysts. In particulate-form H2 production, an amount of the powder-form photocatalyst is subjected to solar power while being continuously stirred; the thin-film forms of the photocatalysts are prepared by depositing them on the conducting substrate before they are placed in a hanging condition in the reactor vessel/tube. Particulate-form H2 production limits the solar-to-hydrogen (STH) efficiency due to the scattering effects being larger than absorption, the small surface area, and the increased charge recombination, although factors limiting the efficiency of oxide-based thin films include the lack of composition of different types of oxide materials, enhancing charge diffusion and separation to actual redox sites. Cobalt and nickel ferrite-based graphene nanocomposites are well explored, and their electrochemical performance showed their aptness for HER [66]. Thin-film deposition methods for spinel ferrites as solar-based photocatalysts include spin coating, spray coating, vacuum deposition, laser deposition, and sputtering, where the particulate from spinel ferrites can be synthesized using the sol–gel method, hydrothermal method, ball milling, etc. Many other researchers reported the synthesis of thin films using chemical methods of synthesis, as shown in Table 2. These methods also limit the uniform deposition and adherent thin films compared with physical methods of deposition. The properties of photocatalysts also depend on the synthesis condition or deposition technique, and in the case of TiO2, the phase varies with the chemical and physical deposition technique of the thin film and holds different strengths and weaknesses. Chemical deposition methods for thin films include successive ionic layer adsorption and reaction (SILAR), hydrothermal coating, electrodeposition, electrospinning, etc. These methods can deposit thin films with different morphologies with proper optimization steps at a low cost. Spinel ferrite Co-ZnFe2O4 thin-film nanostructures developed using hydrothermal reactions possess good photocatalytic activity and could produce H2 with 0.0088 μmol/cm2 min. Chen et al. synthesized CdS-sensitized ZnFe2O4/ZnIn2S4 nanosheets using ionic layer adsorption reactions and could evolute the H2 by about 79 μmol/h. Moreover, the physicochemical properties of spinel ferrites are sensitive to the synthesis strategies because transition and non-transition ions possess different oxidation states, which consequently lead to normal and inverse spinel structures. Normal spinel is a type of ferrite where A and B sites form complete tetrahedral and octahedral coordination with metal ions, respectively, e.g., i = 1, and for the inverse spinel inversion degree, i = 0–1. An A site ion has comparably smaller Shannon ionic radii than a B site and can develop a number of combinations depending on the application required. Moreover, maintaining stoichiometry is the most crucial task for obtaining the desired phases of ferrite nanomaterials. Researchers have reported different types of physical, chemical, and biological methods for the synthesis of spinel ferrites.
Table 2 elaborates on the synthesis strategies reported in the years 2010–2022, the morphology and amount of H2-generated ferrite-based catalysts, and some co-catalysts thereof. The need for porous spinel ferrite nanostructures for enhanced efficiency of H2 generation and CO2 reduction is also fulfilled by many researchers. Xiaoxing Xu et al. reported enhanced H2 performance with Ga-doped ZnFe2O4, where Ga doping created trap states between the energy levels, hence increasing charge migration and band gap energy compared with the parent ZnFe2O4 [67]. There is a strong correlation between catalytic activity and crystal structures along with all the physicochemical properties [68]. Moreover, the high-temperature calcination required for the pure-phase formation of spinel ferrite can eliminate the trapping sites for electrons and holes and hence can be avoided to decrease the recombination rates. Interestingly, the size of magnetic nanomaterials plays a very important role in the determination of their magnetic properties, and after a certain critical size, they become superparamagnetic and single-domain particles that are important for the easy removal of ferrite nanoparticles after H2 evolution in an aqueous medium. Figure 2 illustrates (a) the synthesis methods of ferrite nanomaterials as elaborated in Table 2; (b) their mechanism for solar-based H2 evolution as explained previously in Section 3; (c) removal and recyclability; and (d) applications for fuel cell technology.
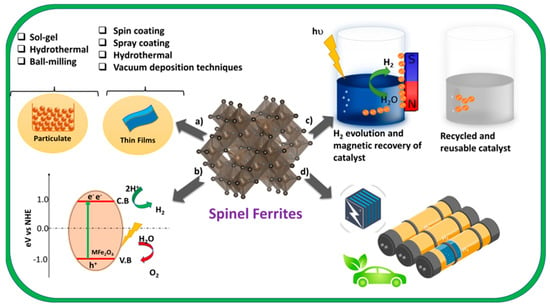
Figure 2.
Showing (a) methods for the synthesis of particulate and thin films of ferrite nanomaterials (b) mechanism of H2 evolution (c) removal and recycling of ferrite photocatalysts (d) applications of H2 energy of vehicles and fuel cells.

Table 2.
Available literature for spinel ferrites photocatalysts.
Table 2.
Available literature for spinel ferrites photocatalysts.
| Year of Publishing | Photocatalyst | Synthesis Method | Morphology and Particle Size (nm) Obtained | Amount of H2 Evolved | Light Intensity | Ref. |
|---|---|---|---|---|---|---|
| 2011 | Co0.85Fe2.15O4 | Atomic Layer deposition | Non-uniform spheres of <10 | 40 μmol/S/g | [69] | |
| 2012 | Fe3O4 | Co-Precipitation | 10 Spherical | 8275 μmol/h/g | 400 W | [18] |
| 2012 | ZnFe2O4 | Microwave irradiation method | Agglomerated spheres of 50 nm | 133.5 μmol/g | 300 W | [63] |
| 2012 | NiFe2O4 | Co-precipitation | 17.8 nm Agglomerated particles | 15.45 μmol/g | 250 W | [70] |
| 2012 | 1. CO3O4 2. Fe3O4 | Sol-Gel | 12.42 nm Coral like | (1) 2000 μmol/h/g (2) 8275 μmol/h/g | 200 W | [71] |
| 2013 | 1. ZnFe2O4 2. ZnFe2O4:Fe2O3 | Plasma Spraying | >10 nm Porous spherical | (1) 46.3 μmol/h (2) 99 μmol/h | 1000 W/m2 | [72] |
| 2013 | CuFe2O4/g-C3N4 with Pt catalyst | Sol-gel | 15–25 nm Particles on g-C3N4 sheets | 76 μmol/h | 300 W | [73] |
| 2013 | NiFe2O4@TiO2 | Sol-gel | 100–300 nm Spherical | 18.5 mL | 18 W/cm2 | [74] |
| 2014 | CaFe2O4/TiO2 | Sol-Gel | 1–2 μm Spherical | 2111 μmol/h/g | - | [75] |
| 2014 | NiFe2O4 | Aerosol Spray Pyrolysis | 11 nm Spherical porous | 0.09 μmol h−1 | - | [76] |
| 2015 | CoFe2O4 | Co-precipitation and ball milling | 25 nm Agglomerates | 2540 and 3490 μmol/g | 250 W | [77] |
| 2015 | ZnFe2O4 | Microwave synthesis | 35 nm Porous spheres | 218 μmol/h/g | AM 1.5 G (1000 W/m2) | [78] |
| 2015 | 1. g-C3N4-CoFe2O4 | Modified Sol-gel method | 67 & 54 nm Irregular shape | 1.55 and 1.24 μmol/g | 300 W | [28] |
| 2. g-C3N4-NiFe2O4 | ||||||
| 2016 | 1. ZnFe2O4 | Co-precipitation | 27 nm | (1) 195 μmol g−1 s−1 | 200 W | [79] |
| 2. Cu0.2Zn0.8Fe2O4 | 20 nm | (2) 9 μmol g−1 s−1 | ||||
| 3. Co0.2Zn0.8Fe2O4 | 18 nm | (3) 675 μmol g−1 s−1 | ||||
| 4. Ni0.2Zn0.8Fe2O4 | 12 nm | (4) 233 μmol g−1 s−1 | ||||
| 2017 | S-NiFe2O4 | Electrodeposition | 2 nm Porous nanoflakes | - | - | [80] |
| 2019 | CoFe2O4/Cd0.9Zn0.1S | Hydrothermal | 20 nm Nanorods | 350.2 μmol/h | AM 1.5 G | [27] |
| 2020 | FeSe2/CoFe2O4 | Hydrothermal | 100 nm rods | - | - | [81] |
6. Conclusions and Future Prospects
To summarize the present work, we have reviewed synthesis approaches for spinel ferrites, factors contributing to H2 evolution, and their need for recoverable and robust photocatalysts. The synthesis techniques for achieving pure phase and good inherent magnetization need calcination processes that lead to low dispersity; therefore, alternative methods for achieving excellent colloidal stability in the desired phase are yet to be explored. The dissociation or stability of spinel ferrite in acidic or basic media is also not yet explored, which should be taken into consideration. H2 evolution using ferrite-based nanomaterials is quite possible at room temperature; however, at elevated temperatures, a study should be carried out for their phase stability and crystallinity because the magnetic properties of spinel ferrites depend on temperature. The choice of a good sacrificial agent for avoiding charge recombination is needed where it can be optimized for ferrite-based nanomaterials. One possible strategy to improve H2 evolution is to improve active sites on spinel ferrites with the design and development of composite nanomaterials and to understand their VB and CB position. Optical properties can be tuned by changing the oxygen concentration and narrowing the band gap of ferrite nanomaterials for greater visible light absorption. Computational analysis can be introduced for the predefined tuning of band gaps using DFT techniques so that the required band edges can be easily inserted in the spinel ferrites required for trapping the electrons during H2 evolution. A novel strategy can be developed based on spinel ferrites–red phosphor (RP) nanocomposites because red phosphors are capable of solar energy harvesting with a band gap of 1.8 eV [22]. Spinel ferrites have a wide optical bandgap and can be combined with different transition and rare-earth elements to be used as phosphors in HER [33]. However, we can use the magnetic ferrite nanomaterials for visible light HER after their successful removal with an external magnetic field, and more efforts should be expended to develop a suitable set-up. On the other hand, Pt/Ir/Ru are the most effective catalysts, and the cost of industry scale-up can be minimized with the development of Pt/Ir/Ru-spinel ferrite nanocomposites for H2 evolution. After the removal of the catalyst, the reusability of the photocatalyst can be checked, which will need successive characterizations. The present overview describing all the aspects of spinel ferrite nanomaterials will be beneficial for the next generation photocatalysts for H2 evolution reactions and could open new routes for their future applications, such as in fuel cells, which are the best photocatalysts. We strongly believe that addressing these research gaps will help to develop efficient, effective, and highly stable, low-cost ferrite-based photocatalysts for sustainable H2 generation and utilization.
Author Contributions
Conceptualization, Y.J. and A.K.; methodology, A.K.; resources, A.K.; data curation, S.W.; writing—original draft preparation, S.W. and Y.J.; writing—review and editing, Y.J. and A.K.; All authors have read and agreed to the published version of the manuscript.
Funding
The author (A.K.) is grateful to Symbiosis International University (SIU) for providing Research Support Fund (RSF).
Data Availability Statement
Data will be made available on request by corresponding authors.
Conflicts of Interest
The authors have no relevant competing interests.
Abbreviations
| Ag@Au | A core-shell structure with the Au core and Ag shell |
| CdS | Cadmium Sulfide |
| CdSe | Cadmium Selenide |
| CO2 | Carbon Dioxide |
| COVID-19 | Coronavirus Disease of 2019 |
| GDP | Gross Domestic Product |
| H2 | Hydrogen |
| Ir | Iridium |
| NRs | Nanorods |
| NWs & NTs | Nanowires & Nanotubes |
| RP | Red Phosphor |
| TiO2/g-C3N4/p-g-C3N4 | metal-free phosphorus doped graphitic carbon nitride-P25 (TiO2) nanocomposite |
References
- Halkos, G.E.; Gkampoura, E.-C. Reviewing Usage, Potentials, and Limitations of Renewable Energy Sources. Energies 2020, 13, 2096. [Google Scholar] [CrossRef]
- Newell, R.; Raimi, D.; Villanueva, S.; Prest, B. Global Energy Outlook 2021: Pathways from Paris; Resources for the future: Washington, DC, USA, 2021; Volume 8. [Google Scholar]
- Eroglu, H. Effects of COVID-19 outbreak on environment and renewable energy sector. Environ. Dev. Sustain. 2021, 23, 4782–4790. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. [Google Scholar] [CrossRef]
- Perry, M.L.; Fuller, T.F. A historical perspective of fuel cell technology in the 20th century. J. Electrochem. Soc. 2002, 149, S59. [Google Scholar]
- Andrei, V.; Ucoski, G.M.; Pornrungroj, C.; Uswachoke, C.; Wang, Q.; Achilleos, D.S.; Kasap, H.; Sokol, K.P.; Jagt, R.A.; Lu, H.; et al. Floating perovskite-BiVO4 devices for scalable solar fuel production. Nature 2022, 608, 518–522. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.W.; Pan, L.; Zhang, X.; Zou, J.J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Malik, M.A.; Narasimharao, K. Pt-Ag/Ag3PO4-WO3 nanocomposites for photocatalytic H2 production from bioethanol. Fuel 2023, 344, 127998. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.; Lingampalli, S.R.; Dey, S.; Roy, A. Solar photochemical and thermochemical splitting of water. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150088. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Zhang, M.; Gou, W.; Zhang, S.; Chen, Z.; Qu, Y.; Ma, Y. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 3502. [Google Scholar] [CrossRef]
- Verma, S.; Sinha-Ray, S.; Sinha-Ray, S. Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review. Polymers 2020, 12, 238. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Morch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Chu, J.; Seo, J.; Jung, G.; Baek, S.H.; Nam, S.W.; Duah, C.; Lee, Y.K.; Jung, W.; Shin, B.J.C. Drop-casted Platinum Nanocube Catalysts for Hydrogen Evolution Reaction with Ultrahigh Mass Activity. ChemSusChem 2021, 14, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Dahanayake, A.; Gunathilake, C.A.; Pallegedara, A.; Jayasinghe, P. Recent Developments in Noble Metal-Free Catalysts for a Photocatalytic Water Splitting Process—A Review. J. Compos. Sci. 2023, 7, 64. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. Recent Advances in Noble Metal (Pt, Ru, and Ir)-Based Electrocatalysts for Efficient Hydrogen Evolution Reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Narasimharao, K.; Malik, M.A. Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials 2022, 12, 2985. [Google Scholar] [CrossRef]
- Mangrulkar, P.A.; Polshettiwar, V.; Labhsetwar, N.K.; Varma, R.S.; Rayalu, S.S. Nano-ferrites for water splitting: Unprecedented high photocatalytic hydrogen production under visible light. Nanoscale 2012, 4, 5202–5209. [Google Scholar] [CrossRef]
- Rioja-Cabanillas, A.; Valdesueiro, D.; Fernández-Ibáñez, P.; Byrne, J.A. Hydrogen from wastewater by photocatalytic and photoelectrochemical treatment. J. Phys. Energy 2020, 3, 012006. [Google Scholar] [CrossRef]
- Wadhai, S.; Jadhav, Y.; Thakur, P. Synthesis of metal-free phosphorus doped graphitic carbon nitride-P25 (TiO2) composite: Characterization, cyclic voltammetry and photocatalytic hydrogen evolution. Sol. Energy Mater. Sol. Cells 2021, 223, 110958. [Google Scholar] [CrossRef]
- Raykar, V.S.; Patil, S.B.; Patil, P.S. Porous TiN/Red Phosphorus Nanocomposite for Photocatalytic Hydrogen Evolution; Macromolecular Symposia, 2020; Wiley Online Library: Hoboken, NJ, USA, 2020; p. 2000003. [Google Scholar]
- Lin, Y.-P.; Bocharov, D.; Kotomin, E.A.; Brik, M.G.; Piskunov, S.J. Influence of Au, Ag, and Cu Adatoms on Optical Properties of TiO2 (110) Surface: Predictions from RT-TDDFT Calculations. Crystals 2022, 12, 452. [Google Scholar] [CrossRef]
- Wei, X.; Naraginti, S.; Yang, X.; Xu, X.; Li, J.; Sun, J.; Liu, Z.; Pei, J. A novel magnetic AgVO3/rGO/CuFe2O4 hybrid catalyst for efficient hydrogen evolution and photocatalytic degradation. Environ. Res. 2023, 229, 115948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, A.; Navakoteswara Rao, V.; Kumar, A.; Shankar, M.V.; Krishnan, V. Defect-Rich MoS2 Ultrathin Nanosheets-Coated Nitrogen-Doped ZnO Nanorod Heterostructures: An Insight into in-Situ-Generated ZnS for Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2019, 2, 5622–5634. [Google Scholar] [CrossRef]
- Najaf, Z.; Nguyen, D.L.T.; Chae, S.Y.; Joo, O.-S.; Shah, A.U.H.A.; Vo, D.-V.N.; Nguyen, V.-H.; Le, Q.V.; Rahman, G. Recent trends in development of hematite (α-Fe2O3) as an efficient photoanode for enhancement of photoelectrochemical hydrogen production by solar water splitting. Int. J. Hydrogen Energy 2021, 46, 23334–23357. [Google Scholar] [CrossRef]
- Shao, Z.; Zeng, T.; He, Y.; Zhang, D.; Pu, X. A novel magnetically separable CoFe2O4/Cd0.9Zn0.1S photocatalyst with remarkably enhanced H2 evolution activity under visible light irradiation. Chem. Eng. J. 2019, 359, 485–495. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Diao, Z.; Wang, M.; Shen, S. Ferrites boosting photocatalytic hydrogen evolution over graphitic carbon nitride: A case study of (Co, Ni)Fe2O4 modification. Sci. Bull. 2016, 61, 292–301. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lee, Z.; Chu, K.-W.; Wei, Y.-H. CoFe2O4@ ZnS core–shell spheres as magnetically recyclable photocatalysts for hydrogen production. J. Taiwan Inst. Chem. Eng. 2016, 66, 386–393. [Google Scholar] [CrossRef]
- Zaman, N.; Noor, T.; Iqbal, N. Recent advances in the metal–organic framework-based electrocatalysts for the hydrogen evolution reaction in water splitting: A review. RSC Adv. 2021, 11, 21904–21925. [Google Scholar] [CrossRef] [PubMed]
- Bielan, Z.; Dudziak, S.; Kubiak, A.; Kowalska, E. Application of spinel and hexagonal ferrites in heterogeneous photocatalysis. Appl. Sci. 2021, 11, 10160. [Google Scholar] [CrossRef]
- Granone, L.I.; Ulpe, A.C.; Robben, L.; Klimke, S.; Jahns, M.; Renz, F.; Gesing, T.M.; Bredow, T.; Dillert, R.; Bahnemann, D.W. Effect of the degree of inversion on optical properties of spinel ZnFe2O4. Phys. Chem. Chem. Phys. 2018, 20, 28267–28278. [Google Scholar] [CrossRef]
- Brik, M.G.; Ma, C.-G.; Yamamoto, T.; Piasecki, M.; Popov, A.I. First-Principles Methods as a Powerful Tool for Fundamental and Applied Research in the Field of Optical Materials. In Phosphor Handbook; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–26. [Google Scholar]
- Adán, C.; Marugán, J.; Sánchez, E.; Pablos, C.; van Grieken, R. Understanding the effect of morphology on the photocatalytic activity of TiO2 nanotube array electrodes. Electrochim. Acta 2016, 191, 521–529. [Google Scholar] [CrossRef]
- Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation. Energies 2021, 14, 7223. [Google Scholar] [CrossRef]
- Zhuge, K.; Chen, Z.; Yang, Y.; Wang, J.; Shi, Y.; Li, Z. In-suit photodeposition of MoS2 onto CdS quantum dots for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2021, 539, 148234. [Google Scholar] [CrossRef]
- Ezendam, S.; Herran, M.; Nan, L.; Gruber, C.; Kang, Y.; Gröbmeyer, F.; Lin, R.; Gargiulo, J.; Sousa-Castillo, A.; Cortés, E. Hybrid plasmonic nanomaterials for hydrogen generation and carbon dioxide reduction. ACS Energy Lett. 2022, 7, 778–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Reddy, D.A.; Kim, Y.; Chun, S.Y.; Ma, R.; Kumar, D.P.; Song, J.K.; Kim, T.K. Drastic Improvement of 1D-CdS Solar-Driven Photocatalytic Hydrogen Evolution Rate by Integrating with NiFe Layered Double Hydroxide Nanosheets Synthesized by Liquid-Phase Pulsed-Laser Ablation. ACS Sustain. Chem. Eng. 2018, 6, 16734–16743. [Google Scholar] [CrossRef]
- Galdámez-Martínez, A.; Bai, Y.; Santana, G.; Sprick, R.S.; Dutt, A. Photocatalytic hydrogen production performance of 1-D ZnO nanostructures: Role of structural properties. Int. J. Hydrogen Energy 2020, 45, 31942–31951. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Liang, J.; Xu, J.; Gu, Q.; Zhou, Y.; Huang, C.; Lin, H.; Wang, X. A novel Zn2GeO4 superstructure for effective photocatalytic hydrogen generation. J. Mater. Chem. A 2013, 1, 7798–7805. [Google Scholar] [CrossRef]
- Qin, N.; Xiong, J.; Liang, R.; Liu, Y.; Zhang, S.; Li, Y.; Li, Z.; Wu, L. Highly efficient photocatalytic H2 evolution over MoS2/CdS-TiO2 nanofibers prepared by an electrospinning mediated photodeposition method. Appl. Catal. B Environ. 2017, 202, 374–380. [Google Scholar] [CrossRef]
- Kweon, D.H.; Okyay, M.S.; Kim, S.J.; Jeon, J.P.; Noh, H.J.; Park, N.; Mahmood, J.; Baek, J.B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11, 1278. [Google Scholar] [CrossRef]
- Bozheyev, F.; Ellmer, K.J. Thin film transition metal dichalcogenide hotoelectrodes for solar hydrogen evolution: A review. J. Mater. Chem. A 2022, 10, 9327–9347. [Google Scholar] [CrossRef]
- Sim, Y.; John, J.; Moon, J.; Sim, U. Photo-Assisted Hydrogen Evolution with Reduced Graphene Oxide Catalyst on Silicon Nanowire Photocathode. Appl. Sci. 2018, 8, 2046. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Z.; Qiu, B.; Xing, M.; Zhang, J. Emerging Cocatalysts on g-C3N4 for Photocatalytic Hydrogen Evolution. Small 2021, 17, e2101070. [Google Scholar] [CrossRef]
- Gaikwad, R.S.; Chae, S.-Y.; Mane, R.S.; Han, S.-H.; Joo, O.-S. Cobalt Ferrite Nanocrystallites for Sustainable Hydrogen Production Application. Int. J. Electrochem. 2011, 2011, 729141. [Google Scholar] [CrossRef]
- Shwetharani, R.; Chandan, H.R.; Sakar, M.; Balakrishna, G.R.; Reddy, K.R.; Raghu, A.V. Photocatalytic semiconductor thin films for hydrogen production and environmental applications. Int. J. Hydrogen Energy 2020, 45, 18289–18308. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Peh, S.B.; Zhai, L.; Ying, Y.; Liu, G.; Cheng, Y.; Zhao, D. Dimensional Impact of Metal–Organic Frameworks in Catalyzing Photoinduced Hydrogen Evolution and Cyanosilylation Reactions. ACS Appl. Energy Mater. 2018, 2, 298–304. [Google Scholar] [CrossRef]
- Solis, B.H.; Hammes-Schiffer, S. Computational study of anomalous reduction potentials for hydrogen evolution catalyzed by cobalt dithiolene complexes. J. Am. Chem. Soc. 2012, 134, 15253–15256. [Google Scholar] [CrossRef] [PubMed]
- Karimi Estahbanati, M.R.; Mahinpey, N.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Kinetic study of the effects of pH on the photocatalytic hydrogen production from alcohols. Int. J. Hydrogen Energy 2019, 44, 32030–32041. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Fernández-González, R.; Díaz, L.; Pulido Melián, E.; Rodríguez, V.D.; Núñez, P. Effect of reaction temperature and sacrificial agent on the photocatalytic H2-production of Pt-TiO2. J. Alloys Compd. 2017, 721, 405–410. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y.H. Highly efficient temperature-induced visible light photocatalytic hydrogen production from water. J. Phys. Chem. C 2015, 119, 18927–18934. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Yang, H.-J.; Liu, Y.; Gurusamy, L.; Karuppasamy, L.; Wu, J.J. Photocatalytic Hydrogen Evolution from Water Splitting Using Core-Shell Structured Cu/ZnS/COF Composites. Nanomaterials 2021, 11, 3380. [Google Scholar] [CrossRef]
- Huang, S.; Ge, F.; Yan, J.; Li, H.; Zhu, X.; Xu, Y.; Xu, H.; Li, H. Synthesis of carbon nitride in moist environments: A defect engineering strategy toward superior photocatalytic hydrogen evolution reaction. J. Energy Chem. 2021, 54, 403–413. [Google Scholar] [CrossRef]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/graphitic carbon nitride heterojunctions for high-efficiency photocatalytic and electrocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2022, 14, 44317–44329. [Google Scholar] [CrossRef]
- Li, C.; Che, H.; Huo, P.; Yan, Y.; Liu, C.; Dong, H. Confinement of ultrasmall CoFe2O4 nanoparticles in hierarchical ZnIn2S4 microspheres with enhanced interfacial charge separation for photocatalytic H2 evolution. J. Colloid Interface Sci. 2021, 581, 764–773. [Google Scholar] [CrossRef]
- Soto-Arreola, A.; Huerta-Flores, A.M.; Mora-Hernández, J.; Torres-Martínez, L.M. Comparative study of the photocatalytic activity for hydrogen evolution of MFe2O4 (M = Cu, Ni) prepared by three different methods. J. Photochem. Photobiol. A Chem. 2018, 357, 20–29. [Google Scholar] [CrossRef]
- Kezzim, A.; Nasrallah, N.; Abdi, A.; Trari, M. Management, Visible light induced hydrogen on the novel hetero-system CuFe2O4/TiO2. Energy Convers. Manag. 2011, 52, 2800–2806. [Google Scholar] [CrossRef]
- Benamira, M.; Lahmar, H.; Messaadia, L.; Rekhila, G.; Akika, F.-Z.; Himrane, M.; Trari, M. Hydrogen production on the new hetero-system Pr2NiO4/SnO2 under visible light irradiation. Int. J. Hydrogen Energy 2020, 45, 1719–1728. [Google Scholar]
- Boukhemikhem, Z.; Brahimi, R.; Rekhila, G.; Fortas, G.; Boudjellal, L.; Trari, M. The photocatalytic hydrogen formation and NO2− oxidation on the hetero-junction Ag/NiFe2O4 prepared by chemical route. Renew. Energy 2020, 145, 2615–2620. [Google Scholar] [CrossRef]
- Dom, R.; Subasri, R.; Hebalkar, N.Y.; Chary, A.S.; Borse, P.H. Synthesis of a hydrogen producing nanocrystalline ZnFe2O4 visible light photocatalyst using a rapid microwave irradiation method. RSC Adv. 2012, 2, 12782–12791. [Google Scholar] [CrossRef]
- Yu, F.; Gong, F.; Yang, Q.; Wang, Y. Fabrication of a magnetic retrievable dual Z-scheme g-C3N4/BiVO4/CoFe2O4 composite photocatalyst with significantly enhanced activity for the degradation of rhodamine B and hydrogen evolution under visible light. Diam. Relat. Mater. 2022, 125, 109004. [Google Scholar] [CrossRef]
- Atacan, K.; Güy, N.; Boutra, B.; Özacar, M. Enhancement of photoelectrochemical hydrogen production by using a novel ternary Ag2CrO4/GO/MnFe2O4 photocatalyst. Int. J. Hydrogen Energy 2020, 45, 17453–17467. [Google Scholar] [CrossRef]
- Nivetha, R.; Chella, S.; Kollu, P.; Jeong, S.K.; Bhatnagar, A.; Andrews, N.G. Cobalt and nickel ferrites based graphene nanocomposites for electrochemical hydrogen evolution. J. Magn. Magn. Mater. 2018, 448, 165–171. [Google Scholar] [CrossRef]
- Xu, X.; Azad, A.K.; Irvine, J.T.S. Photocatalytic H2 generation from spinels ZnFe2O4, ZnFeGaO4 and ZnGa2O4. Catal. Today 2013, 199, 22–26. [Google Scholar] [CrossRef]
- Malik, A.Q.; Singh, H.; Kumar, A.; Aepuru, R.; Kumar, D.; Mir, T.u.G.; Ain, Q.u.; Bhat, A.A.; Mubayi, A. An Overview on Magnetic Separable Spinel as a Promising Materials for Photocatalysis and Waste Water Treatment. ES Energy Environ. 2022, 19, 744. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Allendorf, M.D.; Coker, E.N.; Jacobs, B.W.; McDaniel, A.H.; Weimer, A.W. Hydrogen Production via Chemical Looping Redox Cycles Using Atomic Layer Deposition-Synthesized Iron Oxide and Cobalt Ferrites. Chem. Mater. 2011, 23, 2030–2038. [Google Scholar] [CrossRef]
- Peng, T.; Zhang, X.; Lv, H.; Zan, L. Preparation of NiFe2O4 nanoparticles and its visible-light-driven photoactivity for hydrogen production. Catal. Commun. 2012, 28, 116–119. [Google Scholar] [CrossRef]
- Mangrulkar, P.A.; Joshi, M.M.; Tijare, S.N.; Polshettiwar, V.; Labhsetwar, N.K.; Rayalu, S.S. Nano cobalt oxides for photocatalytic hydrogen production. Int. J. Hydrogen Energy 2012, 37, 10462–10466. [Google Scholar] [CrossRef]
- Dom, R.; Kumar, G.S.; Hebalkar, N.Y.; Joshi, S.V.; Borse, P.H. Eco-friendly ferrite nanocomposite photoelectrode for improved solar hydrogen generation. RSC Adv. 2013, 3, 15217–15224. [Google Scholar] [CrossRef]
- Cheng, R.; Fan, X.; Wang, M.; Li, M.; Tian, J.; Zhang, L. Facile construction of CuFe2O4/g-C3N4 photocatalyst for enhanced visible-light hydrogen evolution. RSC Adv. 2016, 6, 18990–18995. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.; Kwak, B.S.; Han, G.B.; Um, M.-H.; Kang, M. Synthesis of magnetically separable core@shell structured NiFe2O4@TiO2 nanomaterial and its use for photocatalytic hydrogen production by methanol/water splitting. Chem. Eng. J. 2014, 243, 272–279. [Google Scholar] [CrossRef]
- Police, A.K.R.; Basavaraju, S.; Valluri, D.K.; Muthukonda, V.S.; Machiraju, S.; Lee, J.S. CaFe2O4 sensitized hierarchical TiO2 photo composite for hydrogen production under solar light irradiation. Chem. Eng. J. 2014, 247, 152–160. [Google Scholar] [CrossRef]
- Hong, D.; Yamada, Y.; Sheehan, M.; Shikano, S.; Kuo, C.-H.; Tian, M.; Tsung, C.-K.; Fukuzumi, S. Mesoporous Nickel Ferrites with Spinel Structure Prepared by an Aerosol Spray Pyrolysis Method for Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2014, 2, 2588–2594. [Google Scholar] [CrossRef]
- Ortega López, Y.; Medina Vázquez, H.; Salinas Gutiérrez, J.; Guzmán Velderrain, V.; López Ortiz, A.; Collins Martínez, V. Synthesis Method Effect of CoFe2O4 on Its Photocatalytic Properties for H2 Production from Water and Visible Light. J. Nanomater. 2015, 2015, 76. [Google Scholar] [CrossRef]
- Dom, R.; Chary, A.S.; Subasri, R.; Hebalkar, N.Y.; Borse, P.H. Solar hydrogen generation from spinel ZnFe2O4 photocatalyst: Effect of synthesis methods. Int. J. Energy Res. 2015, 39, 1378–1390. [Google Scholar] [CrossRef]
- Boudjemaa, A.; Popescu, I.; Juzsakova, T.; Kebir, M.; Helaili, N.; Bachari, K.; Marcu, I.-C. M-substituted (M = Co, Ni and Cu) zinc ferrite photo-catalysts for hydrogen production by water photo-reduction. Int. J. Hydrogen Energy 2016, 41, 11108–11118. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Ling, T.; Vasileff, A.; Qiao, S.-Z. S-NiFe2O4 ultra-small nanoparticle built nanosheets for efficient water splitting in alkaline and neutral pH. Nano Energy 2017, 40, 264–273. [Google Scholar] [CrossRef]
- Zhang, H.; Nengzi, L.-c.; Li, B.; Cheng, Q.; Gou, J.; Cheng, X. Successfully synthesis of FeSe2/CoFe2O4 heterojunction with high performance for hydrogen evolution reaction. Renew. Energy 2020, 155, 717–724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).