Abstract
In order to solve the problem of the spontaneous combustion of coal gangue, a coal gangue fire-extinguishing material of gel–foam was developed. The foaming agent was screened by the Waring blender method with varying foam amounts, and the superabsorbent foam stabilizer was synthesized by free radical polymerization. Moreover, the gel–foam was used in a spontaneous combustion of coal gangue mountain field practice. The results showed that when the mass fraction of sodium dodecyl sulfonate and coconut oil amide propyl betaine was 0.6% and 4:6, the foaming amount was as high as 1500 mL. When the mass ratio of chitosan to acrylic acid was 1:6, the neutralization degree was 80%, the cross-linking agent was 0.8%, and the initiator was 0.01%, the water absorption of the synthesized superabsorbent foam stabilizer reached 476 mL/g. The synthesized gel–foam was tested in a spontaneous combustion coal gangue hill in a certain area, and no reburning sign was found within one month.
1. Introduction
Coal is the most commonly used energy source at present; consequently, coal mining brings a large amount of coal gangue emissions. The most commonly used treatment method for coal gangue is natural accumulation, to form a coal gangue mountain. China is a big country in coal production and consumption; according to incomplete statistics, there are nearly 2000 large coal gangue hills, with about 6 billion tons of accumulated coal gangue, covering an area of about 20,000 hectares, and increasing by 150–200 million tons per year [1,2,3,4]. The overall distribution of coal gangue is characterized by “more in the north than in the south, and less in the west than in the east”. The national coal gangue emission distributions are shown in Figure 1. Combined with the occurrence status and regional distribution characteristics of coal resources in China, considering the coal gangue horizon and the coal accumulation basin, coal gangue production areas in China can be roughly divided into five regions: namely, Northeast China, North China, South China, the Southwest, and the Northwest. According to the output of the producing area, they are divided into five grades of red, orange, yellow, green, and blue, as shown in Figure 2 below [5,6].
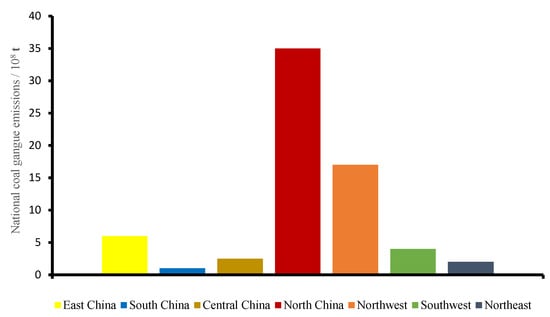
Figure 1.
Cumulative emissions of coal gangue from various regions of the country.
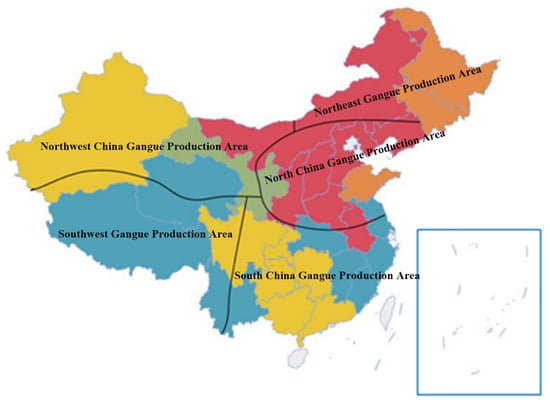
Figure 2.
Distribution of gangue producing areas in China.
Coal gangue contains about 15% of coal and other fuels. Due to the wind blowing and raining all year-round, it is naturally oxidized in a certain environment and generates heat [7,8,9]. Under sufficient oxygen and a good heat storage environment, spontaneous combustion is likely to occur when it reaches an ignition point [10]. At present, more than one third of the 2000 large coal gangue mines in China have experienced, or are even currently experiencing, spontaneous combustion. This produces a large number of toxic and harmful gases such as CO, CO2, SO2 and NOx, which are discharged into the atmospheric environment, and heavy metal elements such as arsenic, mercury, fluorine, lead and selenium are released into the soil and groundwater [11,12,13]. This pollutes the surrounding atmosphere, water and soil, causes serious environmental and ecological pollution problems, and seriously threatens the production safety of mining areas and the health of surrounding personnel [14]. A fire prevention and extinguishing material was prepared for the spontaneous combustion of coal gangue at present.
Domestic scholars have drawn lessons from many fire-extinguishing materials in coal gangue fire prevention and extinguishing, and water-based foam is the most widely used fire-extinguishing material. However, the interface of water-based foam can only be stabilized with the help of surfactants, which are easy to collapse when transported through porous media (such as clots). Therefore, water-based foam is not suitable for long-term fire prevention [15,16]. Compared with water-based foam, three-phase foam has better gas resistance, greater residual resistance and a longer attenuation half-life. However, the stability of three-phase foam is limited by rising temperatures, resulting in fire failure. At present, gel has been used as an external phase to improve the stability of the foam, called gel–foam. After gelation, bubbles are firmly trapped in the high viscosity gel film in porous media, improving its resilience, heat absorption and blocking ability, thereby reducing the flow of oxygen [17]. Gel–foam, using foam as a carrier, can be transported to higher and farther places, as foam itself has a certain sealing performance. A large flow of gel–foam can quickly accumulate, and ignition gel has good cooling and oxygen insulation. In addition, when the foam bursts, the foamed gel can still maintain a porous structure and respond quickly to temperature changes, resulting in rapid water loss at high temperatures. Finally, the gel can encapsulate and seal the high-temperature fire source. High water content can keep coal in a humid environment, and through its cooling, wetting and ability to prevent floating coal from becoming exposed to oxygen, the spontaneous combustion of coal can be avoided. Therefore, the combination of gel fire-extinguishing technology and foam fire-extinguishing technology not only has the advantages of gel fire-extinguishing, but also the low cost of foam fire-extinguishing. The research on gel–foam has attracted more and more attention, and the development of a new gel–foam fire-extinguishing material for preventing the spontaneous combustion of coal gangue has broad application prospects [18].
Gel–foam is a kind of complex mixed system, which is formed by dispersing the polymer in water, adding a foaming agent and foaming under the action of gas. After a period of time, in the foam–liquid film, the polymers crosslink each other to form a three-dimensional network structure, which constitutes the rigid skeleton of gel–foam. Gel–foam has special properties, including not only the properties of gel, but also the characteristics of foam. It has good temperature resistance, fire resistance, plugging and inhibition performance, good film-forming ability, long-term effective coating of coal, and prominent reburning prevention effect. The foam is used as the carrier of gel, and the perfusion of fire-extinguishing materials has been improved to a certain extent. The fire-extinguishing efficiency is high, and the control effect on coal spontaneous combustion in small-scale high-rise areas is obvious [19,20,21].
2. Experimental Part
2.1. Main Experimental Materials
- (1)
- Foaming agent material. Eight representative and economically appropriate surfactants were selected and numbered as 1 #–8 #, respectively. The materials are shown in Table 1;
 Table 1. Types of surfactants.
Table 1. Types of surfactants. - (2)
- Superabsorbent foam-stabilizer monomer: chitosan, acrylic acid;
- (3)
- Crosslinkers and initiators:
Cross-linking agent: N, N′-methylene bisacrylamide;
Initiator: potassium persulfate.
2.2. Synthesis of Superabsorbent Foam Stabilizer
In this experiment, superabsorbent foam stabilizer was synthesized by a hydrothermal method. A certain mass of chitosan was dissolved in 2% glacial acetic acid solution and stirred evenly in a magnetic stirrer, until it became a viscous liquid. Then, a certain amount of potassium persulfate solution was added, and the neutralization degree of acrylic acid reached 80% by neutralizing acrylic acid solution, with 20% sodium hydroxide solution in an ice bath. After the temperature of acrylic acid solution was restored to room temperature, the above solution was transferred into chitosan solution and stirred evenly. Then, a certain mass of cross-linking agent (N, N′-methylene bisacrylamide) was added, and the gel was prepared by heating the magnetic stirrer in a water bath with a heating constant temperature. The reaction temperature was controlled, and the gel was protected by nitrogen. The main test process is shown in Figure 3a.
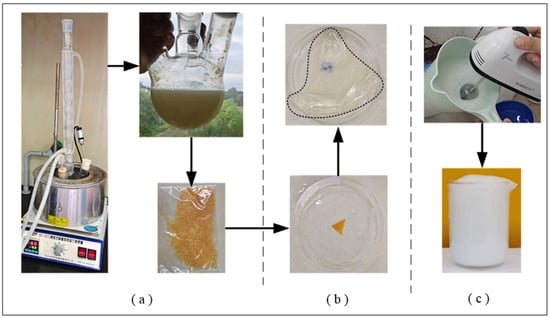
Figure 3.
Schematic diagram of experimental process. (a) preparation of superabsorbent foam stabilizer. (b) water absorption test of foam stabilizer sample. (c) preparation of gel foam.
2.3. Experimental Method
(1) Foaming experiment. The Waring blender stirring–foaming method was used. The rotation speed of the agitator reached 1000 r/min. When the stirring was longer than 60 s, the foam volume number was immediately stopped and read. The numerical value was the foaming capacity of the material. Figure 3c shows the surfactant stirring foaming process;
(2) Water absorption performance test experiment. The natural filtration method was used to determine the water absorption rate. Firstly, 1 g superabsorbent gel sample was accurately weighed, placed in a 1000 mL beaker, and a certain volume (1000 mL) of distilled water was added (this material was used in a coal-mine production practice, so the water-absorption rate test was still carried out with tap water). After its water absorption was saturated, the free water was filtered with 200-mesh nylon cloth, and placed on it for 30 min. Then the sample quality was weighed. It is shown the swelling process of super absorbent foam stabilizer samples in Figure 3b;
(3) Stability experiment. There are two main methods to evaluate foam stability. One is the defoaming half-life, which means the time needed to reduce the foam volume by half, and is called the bursting half-life. The other is the liquid-separation half-life, which means the time needed to separate surfactant solution by half, and is called the liquid separation half-life. In this paper, the defoaming half-life method was used to evaluate the foam stability of the foam stabilizer;
(4) Temperature resistance experiment. Using an electric blast drying oven as a heating source, we set the heating temperature to 200 °C unchanged. The temperature resistance time of different gel–foam ratios was investigated according to the half-life.
3. Experiment Process and Result Analysis
Gel–foam is a dispersion system composed of the average distribution of gas in the gel. It is generally composed of three parts: one is a foaming agent that can produce foam; one is a foam stabilizer that can prolong the foam persistence; the other is a gel forming system that constitutes the foam skeleton structure. In this part, surfactant was selected by a single compounding method, and then the superabsorbent foam stabilizer was synthesized, and the temperature resistance of the gel–foam was tested.
3.1. Selection of Foaming Agent
- (1)
- Selection of single foaming agent
Based on the principle of the comprehensive performance of the foaming agents, eight surfactants were selected for foaming capacity experiments. Firstly, the foaming capacity of a single foaming agent was measured by the Waring blender method, and three materials with the best starting foaming ability were selected for eight materials by the Waring blender stirring method. The standard waring-blender method uses distilled water. Since this product will be used in coal mine production, tap water was used in this experiment. The foaming volume of each foaming agent is shown in Figure 4.
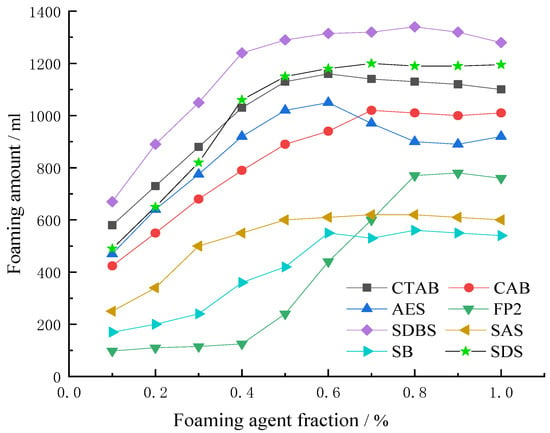
Figure 4.
Foaming multiples of a single foaming agent.
In the Waring blender method, the foam is produced by the liquid under mechanical agitation. However, the factors affecting the foaming capacity of the liquid are more complicated. The concentration of the foaming agent is the most important influencing factor. As can be seen from Figure 4, the foaming volume increases with the increase of surfactant concentration in a certain concentration range, but when the foaming agent concentration reaches a certain value, the trend of the foaming volume tends to level off. This is because, at the turning point, the concentration of foaming agent has reached its critical micelle concentration. If the concentration of foaming agent continues to increase, the foaming agent can only form micelles in the liquid, and cannot continue to reduce the surface tension of the foaming system.
At the same concentration, SDS, SDBS, CAB, AES have stronger foaming abilities, and the maximum foaming volume is more than 1000 mL. This is mainly because the effective surface tension of SDS, SDBS, CAB and AES is lower than that of SAS, SB and FP2. Effective surface tension is characterized by a lower surface tension that is conducive to reducing the surface energy of the liquid, and conducive to increasing the foam volume of the blowing agent. In addition, because water has a certain degree of hardness, the foaming volume of the foaming agent with poor hard-water resistance is small. Four kinds of foaming agents, SDS, SDBS, CAB and AES, which have better foaming properties, were selected for pairwise compounding experiments.
- (2)
- Compounding of foaming.
We combined SDBS, CAB, AES, CAB, four kinds of foaming agents, into pairs; the concentration was determined to be 0.6%. Then, we changed the ratio of the four kinds of foaming agents when paired, and used the Waring blender method to carry out the foaming experiment, and obtained the foam. The volume change is shown in Figure 5.
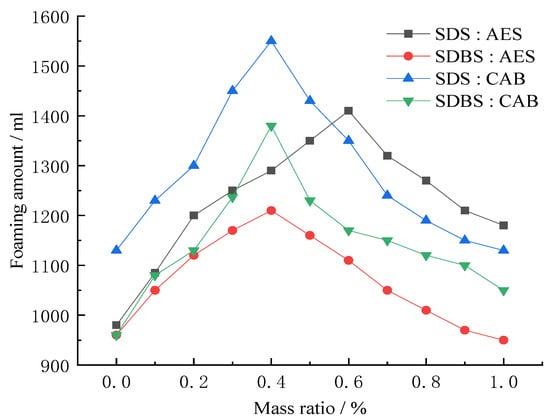
Figure 5.
Foaming multiple of pairwise compounding foaming agent.
As can be seen from Figure 5, the foaming volume of the composite foaming agent increased with the approach of the relative proportion of foaming agents, and was significantly higher than that of the single foaming agent. Overall, a synergistic effect was achieved by using two blowing agents in appropriate proportions. Considering the economic and effective factors, 0.6% volume fraction with SDS:CAB ratio of 4: 6 was chosen as the best.
3.2. Preparation of Foam Stabilizer
- (1)
- Optimization of thermal preparation conditions of chitosan-acrylic acid highly absorbent hydrogels.
The ratio of chitosan and acrylic acid monomer, initiator concentration, cross-linking agent concentration and neutralization degree of acrylic acid in chitosan-grafting acrylic acid have great influence on the water absorption of foam stabilizer. Through orthogonal experiment, the chitosan acrylic acid mass fraction, chitosan concentration, cross-linking agent concentration and neutralization degree of acrylic acid were optimized. The orthogonal factor levels are shown in Table 2, and orthogonal analysis data are shown in Table 3. A is the ratio of volume of acrylic acid to mass of chitosan (mL/g), B is initiator mass concentration (%), C is mass concentration of the cross-linking agent (%), and D is neutralization degree of acrylic acid (%).

Table 2.
Orthogonal level factor table.

Table 3.
Analysis of orthogonal experimental results for synthesis of superabsorbent hydrogel.
Based on the experience of previous studies, we selected the optimal synthesis conditions of 65 °C and a 1 h reaction time as the determined conditions. The article discusses the effects of four main influencing factors—namely, chitosan/acrylic acid concentration ratio, initiator concentration, acrylic acid neutralization degree and crosslinker concentration—on the water absorption properties of the synthesized highly absorbent gels through orthogonal tests. It can be seen from Table 3 that among the four factors affecting the water absorption rate, the degree of acrylic acid neutralization was the most important factor, the ratio of chitosan and acrylic acid monomer was the second most important factor, and the initiator had the least effect on the water absorption rate.
- (2)
- Univariate analysis of the thermal preparation conditions of chitosan-acrylic acid highly absorbent hydrogels.
- (a)
- Effect of chitosan and acrylic monomer ratios on water absorption properties.As can be seen from Figure 6a, when the monomer dosage was low, there were not enough monomers to graft and cross-link with chitosan. The induction period of the polymerization reaction was prolonged, the reaction rate was slow, and the cross-link density was low. The soluble part of the gel increased, so the absorption rate was low. As the monomer dosage increased, more available monomer molecules near the chitosan large radical chain growth site may have been grafted onto the chitosan backbone, which improved the grafting efficiency and the hydrophilicity of the corresponding highly absorbent agent which, in turn, improved the water absorption rate. When the monomer dosage reached a certain level, the monomer concentration was too high, and the increase in this ratio led to the self-polymerization of acrylic acid, and the grafting or linking of chitosan with acrylic acid. When the monomer concentration was too high, the increase of the ratio led to the fierce competition between the self-polymerization of acrylic acid and the grafting or linking of chitosan and acrylic acid, and thus the polymerization process had difficulty in dissipating heat. It was easy to cause explosive polymerization, but the polymerization reaction was not easy to control, and the chance of a self-cross-linking reaction in the specimen increased so that the cross-linking was too large. Thus, the water absorption multiple was reduced. Therefore, a more suitable acrylic acid volume to chitosan mass ratio was 6 mL/g, and the water absorption rate was 476 mL/g.
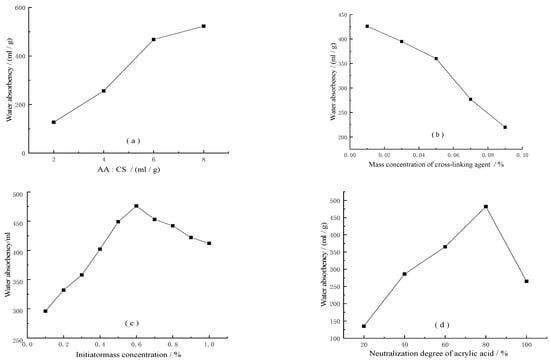 Figure 6. Trends in indicators versus factors, (a) chitosan and acrylic monomer ratios, (b) cross-linking agent dosage, (c) neutralization degree of acrylic acid, (d) neutralization degree of acrylic acid.
Figure 6. Trends in indicators versus factors, (a) chitosan and acrylic monomer ratios, (b) cross-linking agent dosage, (c) neutralization degree of acrylic acid, (d) neutralization degree of acrylic acid. - (b)
- Effect of cross-linking agent dosage on water absorption performance.The amount of cross-linking agent was one of the most important factors affecting the water absorption performance in the process of synthesizing the highly absorbent resins, because the amount of cross-linking agent directly affected the water absorption rate and water retention capacity of the system. As can be seen from Figure 6b, when the amount of cross-linking agent was small, the degree of cross-linking of the resin was small. With the addition of the cross-linking agent, the concentration of the cross-linking degree increased, and the water absorption capacity of the resin also increased. When the amount of cross-linking agent was too large, the higher concentration of cross-linking agent led to more cross-linking points being produced in the polymer network, and the free space between the polymer chains was reduced. The cross-linking points in the cross-linking network structure of the highly absorbent resin increased, and the cross-linking density increased, resulting in the polymer network space becoming smaller, the swelling becoming worse, and the water absorption rate decreasing. Thus, the amount of cross-linking agent should be moderate.
- (c)
- Effect of initiator dosage on water absorption performance.The amount of initiator was an important factor affecting the gel performance. As can be seen from Figure 6c, when the amount of initiator was too small, the reaction system had fewer active centers, slower polymerization, lower cross-linking, and lower water absorption. With the increase of initiator dosage, the degree of cross-linking of the system gradually increased and the water absorption rate also increased; however, when the initiator dosage was too much, the active center of the system increased. This caused the cross-linking reaction: the cross-linking degree increased and the network structure of the system became tight, which is not conducive to water absorption, and thus the water absorption rate decreased. At the same time, when the initiator content was too much, the higher number of free radicals in the reaction system, the faster reaction speed, and more chain termination reactions also reduced the molecular weight of the polymer, and the contraction of the resulting cross-linked network decreased its liquid absorption rate.
- (d)
- Effect of neutralization degree of acrylic acid on water absorption performance.
The effect of neutralization degree on superabsorbent hydrogels was mainly distributed in three aspects. One was the ratio of -COOH and -COONa+ in the system. Second, the neutralization degree also had a great influence on the polymerization rate. When the neutralization degree was low, the polymerization rate is fast. the degree of chain transfer was large, and the reaction was not easy to control. It was easy to burst polymerization, and the chance of self-cross-linking and low molecular weight acrylic resin in the final resin was large, which was not conducive to the improvement of the water absorption multiplier. When the neutralization degree was too high, the polymerization rate was slow and the conversion rate was low. Thirdly, when the neutralization degree exceeded a certain value, chitosan started to hydrolyze, and the cross-linked structure was destroyed. With the gradual increase of the neutralization degree, the degree of destruction increased and the water absorption rate decreased rapidly.
The effect of the degree of neutralization of acrylic acid was also investigated, while the other variables were kept at the following conditions: reaction temperature, 80 °C; reaction time, 1 h. The ratio of the volume of acrylic acid to the mass of chitosan was 3 mL/g. The effects of neutralization degree on the reaction yield and water absorption of the products are shown in Figure 6d. The results showed that the effects of the neutralization degree on the reaction yield and water absorption were similar. The reaction yield and water absorption increased with the increase of neutralization degree, reaching a maximum of 80% neutralization degree, which then decreased with the further increase of the neutralization degree. The reaction yield and water absorption increased with the increase of the neutralization degree, reaching a maximum of 80% neutralization degree, which then decreased with the further increase of neutralization degree. The increase in carboxylic acid anions enhanced the interchain repulsion, and thus the dynamics of the polymer network extension. As a result, the yield and water absorption increased. However, when excess NaOH was added to the reaction solution, the excess Na cations shielded the charge of the carboxylate anions; thus, preventing the effective repulsion between the carboxylate anions and decreasing the yield and water absorption. Therefore, a more suitable degree of acrylic acid neutralization should be 80%.
From the Figure 6, it can be seen that the water absorption multiplier tended to increase with the increase of chitosan mass fraction, and then gradually decrease. The water absorption multiplier tended to decrease with the increase of crosslinker concentration, and the water absorption multiplier tended to increase with the increase of chitosan/acrylic acid concentration ratio and initiator concentration. This is because in the graft copolymerization reaction, the chitosan graft acrylic monomer opportunity increased, to some extent, with the increase of chitosan mass fraction; however, when the chitosan mass fraction increased to a certain value, the water absorption multiplicity decreased with the decrease of the number of hydrophilic groups -COOH and -COO-. When the water absorption multiplier and the cross-link density of the polymer had a great relationship, cross-link density increased. The water absorption multiplier decreased due to the short distance between the cross-link points and the large elastic contraction of the cross-link network. When the amount of initiator additive was small, the decomposition rate was slow. The chain initiation reaction was slow. The grafting rate was low within a certain reaction time, and the water absorption multiplier of the synthesized highly absorbent gel was low.
3.3. Performance Test of Superabsorbent Hydrogel Foam Stabilizer
The above synthesized gel–foam stabilizer and acrylamide conventional foam stabilizer were added to the compound foaming agent (CAB:SDS was 6:4, concentration 0.6%) in a certain ratio, and stirred for 1 min using the Warning blender stirring method. Then, the foam was poured into a 2000 mL measuring cylinder, and the time required to reduce the foam volume by half was recorded for evaluating the foam stability. Experimental results are shown in Table 4 below.

Table 4.
Test results of foam stabilization performance.
As can be seen from the Table 4, The addition of foam stabilizer and the increasing concentration of foam stabilizer have a certain degree of influence on the foaming ability of surfactant. The main manifestation is that the foaming volume decreases to varying degrees compared with that before the addition of foam stabilizer. With the increasing concentration of foam stabilizer, the foam volume decreases. After the addition of two substances, the foaming volume decreased significantly. But it can be seen that the effect of synthetic gel on the foaming capacity of the system was smaller than that of acrylamide, which can be seen. This is because both of them achieve the purpose of stabilizing bubbles by thickening the solution. Increasing the viscosity of the mixed solution enhances the intermolecular force, which is convenient for the contradictory foaming mechanism of surfactant. Therefore, although these two foam stabilizers improve the foam stability to a certain extent, they inhibit the foaming ability of the system. So we need to find the balance between foam stabilization time and foam volume. The foam stabilization time of the synthesized gel is significantly longer than that of acrylamide.
As can be seen from the Figure 7, with the increase in the concentration of stabilizer, the gel–foam system foaming volume was reduced and the precipitation was reduced. The gel–foam system defoaming half-life showed a trend of first increasing, and then decreasing. At the same concentration, the synthesized highly absorbent gel showed a better foam stabilization effect compared with acrylamide, and its concentration of 0.5% showed the longest stabilization time of 1080 min, which was selected as a foam stabilizer for the foam compounding system.

Figure 7.
Gel–foams with different mass fractions of foam stabilizers ((a)—0.1%; (b)—0.3%; (c)—0.5%; (d)—0.7%; (e)—0.9%).
3.4. Heat Resistance Time of Gel–Foam
To study the temperature resistance of the gel–foam at different gel ratios, the experiment fixed the foaming agent (CAB:SDS of 6:4 and concentration of 0.6%) constant and added 2%, 4%, 6%, 8% and 1% of gel–foam, respectively. The half-lives were recorded by putting them into a drying oven at 200 °C.
Table 5 shows that the temperature resistance of gel–foam was higher than that of ordinary two-phase foam, and that it increased significantly with the increase of the mass concentration of stabilizer. This is because the main component of the ordinary foam film was water. As the temperature increased, the solution expanded and the thermal movement of molecules intensified, resulting in weaker intermolecular forces and less hydration. The surfactant molecules were not closely arranged, the solution viscosity was reduced, the drainage rate was accelerated, and the foam stability was reduced; therefore, the temperature resistance time was shorter. Due to the addition of the thickening agent in the gel–foam, the cross-linking reaction between molecules formed the three-dimensional network structure of the foam. The free water was effectively bound and fixed in the formed three-dimensional network structure, greatly improving the stability. As a result, the gel–foam had better temperature resistance.

Table 5.
Temperature resistance time test.
4. Application of Gel–Foam in Coal Gangue Hill Fire Area Treatment
In view of the fire situation of Yuquan coal gangue hill, gel–foam was used to treat a gangue hill.
4.1. Coal Gangue Mountain Measuring Point Arrangement
A coal gangue in Yu County, Yangquan, Shanxi, as shown in Figure 8, of 1 # coal gangue mountain preliminary determination, 1 # surface anomaly area, and 2 # surface anomaly area, as shown in Figure 9, was selected within the length of 35 m, width of 5 m and the length of 55 m, with a width of a 5 m long area for grid division. The arrangement of a high-temperature regional surface temperature and harmful gas detailed measurement points is seen below, with the same direction of each adjacent measurement point spacing for 5 m.
Figure 8.
Coal gangue hills.
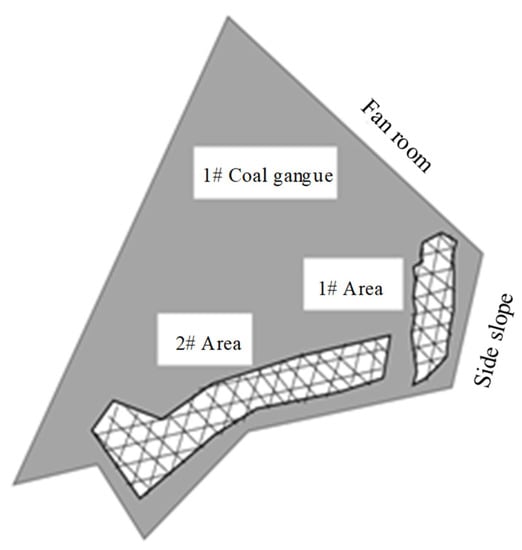
Figure 9.
Grid division of surface anomaly area.
In the coal gangue mountain platform, a vehicle-mounted drilling rig is used for drilling, with a detection drilling depth according to the actual situation at the site. Generally, it is appropriate to see the gangue after continuing to drill 2 m, with a drilling diameter of 75 mm, and any two adjacent drill hole distances between 10 m: the specific layout is shown in Figure 10. There is an area using armored temperature sensors to measure and monitor the temperature of the bottom of the borehole, to record the temperature of each measurement point (°C), as seen in Figure 11, a coal gangue mountain surface temperature isogram.
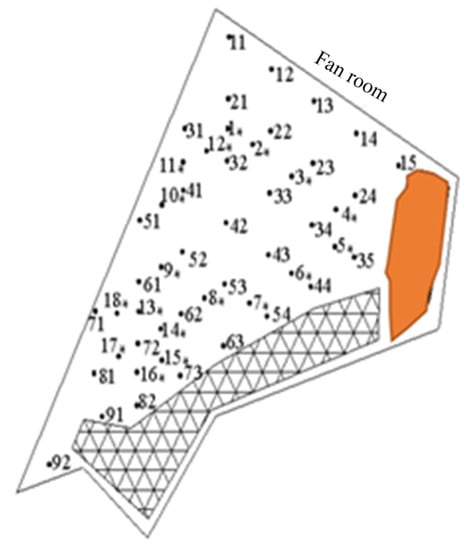
Figure 10.
Distribution map of borehole detection points in coal gangue hill.
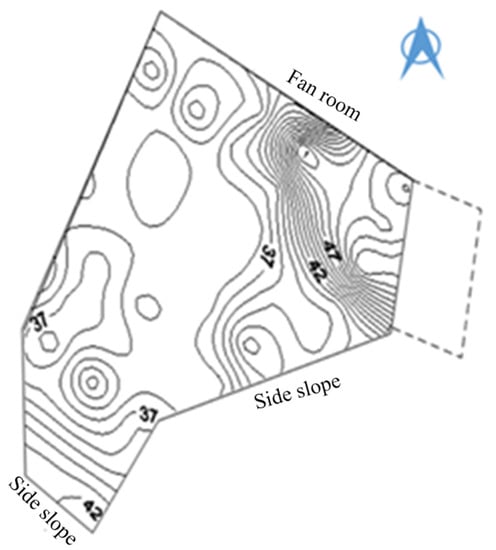
Figure 11.
Surface temperature equivalence of coal gangue hill.
4.2. Gangue Hill Fire Prevention Technology
Using gel–foam fire-extinguishing technology through the construction of boreholes in the gangue mountain natural fire area, composite materials can be injected. These composite materials contain water, can absorb heat, can quickly reduce the temperature, and can effectively play the role of blocking the air leakage channel, achieving the purpose of extinguishing the fire area.
- (1)
- Layout of glue injection borehole.
The arrangement of the grouting borehole is the same as the grouting borehole in Figure 10.
- (2)
- Composite Colloid Materials and Formulas.
The composite colloidal material in the prevention and control of spontaneous combustion of gangue hill was prepared by a foaming agent, water, and a small amount of superabsorbent foam stabilizer.
4.3. Effect Test of Spontaneous Combustion Fire Control
- (1)
- Surface temperature monitoring of gangue hill.
After the fire-extinguishing work, the surface temperature of the gangue hill was measured in a wide range by a portable infrared thermometer. The key monitoring area was the area near the slope, and the treated area. At the same time, the surface temperature around the gangue hill was recorded and compared. Since the surface temperature measurement position was not a scatter distribution, the data are no longer listed here. After 1, 3, 6, 10, 20, 30 days of monitoring, no surface high temperature area was found.
- (2)
- Internal temperature monitoring of gangue hill.
In the process of pre-construction drilling and fire source excavation, a number of temperature measuring points in the internal fire zone were retained, and the positions of these measuring points were counted, as shown in Figure 10. There were 21 random measuring points in total. The internal temperature was measured, as shown in Figure 12.
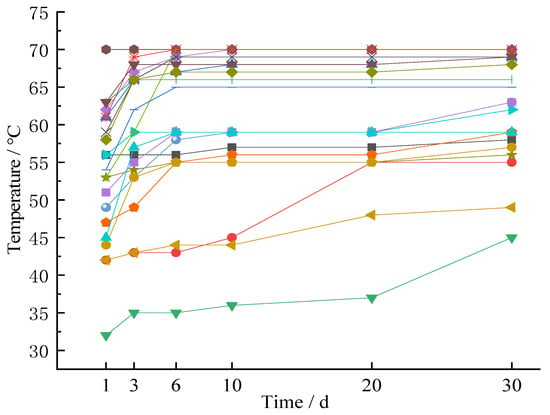
Figure 12.
Monitoring results of internal temperature of gangue hill after fire-extinguishing.
From the above monitoring data, the surface temperature of the gangue hill was consistent with the surface temperature outside the gangue hill, and there was no high temperature area on the surface. The internal temperature of the gangue hill was up to 70 °C, which meets the requirements of coal-mine gangue hill disaster prevention and control norms. It can be said that the fire-extinguishing work of the gangue hill achieved good results. There was no re-ignition phenomenon in a month.
5. Conclusions
(1) With the increase of surfactant concentration, the foaming volume of the foam increased rapidly. When the surfactant concentration increased to a certain extent, the foam volume tended to be flat or slightly reduced;
(2) The foaming volume of the compound system increased, indicating that the compound surfactant had a synergistic effect, and the compound surfactant had better performance than the single surfactant;
(3) Among the four factors affecting the water absorption rate of foam stabilizers, the degree of acrylic acid neutralization was the most important factor, the ratio of chitosan and acrylic acid monomer was the second most important factor, and the initiator had the least effect on the water absorption rate;
(4) With the increase of foam stabilizer concentration, the foaming volume of the gel–foam system decreased, and the defoaming half-life of the gel–foam system first increased, and then decreased. At the same concentration, the synthesized superabsorbent hydrogel showed a good foam stabilization effect. It was selected as foam stabilizer for a foam compound system;
(5) A coal gangue mountain was drilled with gel–foam to extinguish a fire. Field use showed that the foamed gel could extinguish coal ignition effectively.
Author Contributions
Conceptualization, X.Z.; methodology, X.Z.; software, X.Z.; validation, X.Z.; formal analysis, Y.P.; investigation, Y.P.; data curation, Y.P.; writing—original draft preparation, Y.P.; writing—review and editing, X.Z.; project administration, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored by National Natural Science Foundation of China (No51804270), the Natural Science Foundation of Hunan Province (No2020JJ5547) and the Research Fund of Hunan Provincial Department of Education (No19C1745).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, F.-B.; Shi, B.-B.; Cheng, J.-W.; Ma, L.-J. A new approach to control a serious mine fire with using liquid nitrogen as extinguishing media. Fire Technol. 2015, 51, 325–334. [Google Scholar]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.; Zhu, J.; Shi, J. Study on the composition and structure of foamed gel for fire prevention and extinguishing in coal mines. Process Saf. Environ. Prot. 2019, 128, 176–183. [Google Scholar] [CrossRef]
- Wang, G.; Yan, G.; Zhang, X.; Du, W.; Huang, Q.; Sun, L.; Zhang, X. Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J. Loss Prev. Process Ind. 2016, 44, 474–486. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wei, J.; Zhao, Y.; Shen, L. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications. Fuel 2020, 264, 116903. [Google Scholar] [CrossRef]
- Wu, M.; Liang, Y.; Zhao, Y.; Wang, W.; Hu, X.; Tian, F.; He, Z.; Li, Y.; Liu, T. Preparation of new gel foam and evaluation of its fire-extinguishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.; Bai, L. Properties of foamed gel for coal ignition suppression in underground coal mine. Combust. Sci. Technol. 2019, 191, 1294–1308. [Google Scholar] [CrossRef]
- Ren, W.; Guo, Q.; Zuo, B.; Wang, Z. Design and application of device to add powdered gelling agent to pipeline system for fire prevention in coal mines. J. Loss Prev. Process Ind. 2016, 41, 147–153. [Google Scholar] [CrossRef]
- Xi, X.; Shi, Q. Study of the preparation and extinguishment characteristic of the novel high-water-retaining foam for controlling spontaneous combustion of coal. Fuel 2021, 288, 119354. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B. Experimental research on gel-stabilized foam designed to prevent and control spontaneous combustion of coal. Fuel 2019, 254, 115558. [Google Scholar] [CrossRef]
- Li, S.; Zhou, G.; Wang, Y.; Jing, B.; Qu, Y. Synthesis and characteristics of fire-extinguishing gel with high water absorption for coal mines. Process Saf. Environ. Prot. 2019, 125, 207–218. [Google Scholar] [CrossRef]
- Xiao, C.; Balasubramanian, S.N.; Clapp, L.W. Rheology of Viscous CO2 Foams Stabilized by Nanoparticles under High Pressure. Ind. Eng. Chem. Res. 2017, 56, 8340–8348. [Google Scholar] [CrossRef]
- Wang, X.; Mohanty, K. Improved Oil Recovery in Fractured Reservoirs by Strong Foams Stabilized by Nanoparticles. Energy Fuels 2021, 35, 3857–3866. [Google Scholar] [CrossRef]
- Verma, A.; Chauhan, G.; Ojha, K.; Padmanabhan, E. Characterization of Nano-Fe2O3-Stabilized Polymer-Free Foam Fracturing Fluids for Unconventional Gas Reservoirs. Energy Fuels 2019, 33, 10570–10582. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D.; Aimetti, F. Texture and stability of emulsions and suspensions: Role of oscillatory structural forces. Adv. Colloid Interface Sci. 2004, 108–109, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of Nanoparticles on the Interfacial Properties of Liquid/Liquid and Liquid/Air Surface Layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lan, Q.; Liu, Q.; Xu, J.; Sun, D. Aqueous foams stabilized by Laponite and CTAB. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 406–413. [Google Scholar] [CrossRef]
- Shao, Z.L.; Wang, D.M.; Wang, Y.M.; Zhong, X.; Tang, X.; Hu, X. Controlling coal fires using the three-phase foam and water mist techniques in the Anjialing Open Pit Mine, China. Nat. Hazards 2015, 75, 1833–1852. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, X.L.; Li, T. Experimental Investigation on Flame Characterization and Temperature Profile of Single/Multiple Pool Fire in Cross Wind. J. Therm. Sci. 2021, 30, 324–332. [Google Scholar] [CrossRef]
- You, Q.; Wang, H.; Zhang, Y.; Liu, Y.; Fang, J.; Dai, C. Experimental study on spontaneous imbibition of recycled fracturing flow-back fluid to enhance oil recovery in low permeability sandstone reservoirs. J. Petrol. Sci. Eng. 2018, 166, 375–380. [Google Scholar] [CrossRef]
- Wassmuth, F.R.; Hodgins, L.H.; Schramm, L.L.; Kutay, S.M. Screening and coreflood testing of gel foams to control excessive gas production in oil wells. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).