Abstract
The present work aims to evaluate the performance of the constant-volume method by several sets of experiments carried out in three different closed vessels (a sphere and two cylinders) analyzing the obtained results in order to obtain accurate laminar burning velocities. Accurate laminar burning velocities can be used in the development of computational fluid dynamics models in order to design new internal combustion engines with a higher efficiency and lower fuel consumption leading to a lower degree of environmental pollution. The pressure-time histories obtained at various initial pressures from 0.4 to 1.4 bar and ambient initial temperature were analyzed and processed using two different correlations (one implying the cubic low coefficient and the other implying the burnt mass fraction). The laminar burning velocities obtained at various initial pressures are necessary for the realization of a complete kinetic study regarding the combustion reaction and testing the actual reaction mechanisms. Data obtained from measurements were completed and compared with data obtained from runs using two different detailed chemical kinetic mechanisms (GRI 3.0 and Warnatz) and with laminar burning velocities from literature. Our experimental burning velocities ranging from 35.3 cm/s (data from spherical vessel S obtained using the burnt mass fraction) to 37.5 cm/s (data from cylindrical vessel C1 obtained using the cubic law) are inside the interval of confidence as reported by other researchers. From the dependence of the laminar burning velocity on the initial pressure, the baric coefficients were obtained. These coefficients were further used to obtain the overall reaction orders. The baric coefficients (ranging between −0.349 and −0.212) and the overall reaction orders (ranging between 1.42 and 1.50) obtained in this study fall within the reference range of data specific to methane–air mixtures examined at ambient initial temperature.
1. Introduction
Currently, our society depends a lot on the energy achieved by burning fuels. A significant part of this energy involves the combustion of gases. The common used gas is methane which is not only the simpler organic compound but also a valuable fuel and an important raw material in the chemical industry. Methane burning with air, or oxygen-enriched air, was deeply studied in the past using various initial conditions (concentration, pressure, temperature, inert addition) or experimental techniques [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The most important characteristics of methane combustion are the flammability limits; the parameters describing the process of initiation with hot bodies (the ignition temperature, the induction period, the critical ignition energy) or with electrical sparks (the minimum ignition energy, the minimum ignition current); the parameters describing the propagation process (the maximum explosion pressure, the time to reach the maximum explosion pressure, the maximum rates of pressure rise, the severity factor, the propagation speed, the laminar burning velocity, the maximum adiabatic pressure, and the adiabatic flame temperature); the parameters that describe the explosion extinguishing (the quenching distance, the maximum experimental safe gap). Of these characteristics, a small number are intrinsic characteristics of explosive gas mixtures: the laminar burning velocity (a parameter which depends on the overall reaction rate of the flame front), the maximum adiabatic pressure and the adiabatic flame temperature. The rest of the parameters depend strongly on the measurement method and are thus influenced by the size of the used enclosure, by the thermal losses appearing during the propagation process and by the characteristics of the initiation sources [15,16,17,18].
Lately, more attention was paid to the high-accuracy measurement and prediction of laminar burning velocities which help to obtain a detailed examination of combustion properties of a gaseous fuel but also to develop and validate new kinetic models. These aspects are important inputs for operating internal combustion engines, gas turbine combustors, rocket engines, or industrial furnaces. Although various experimental methods have been developed and a lot of data on laminar burning velocity of methane–air mixtures have been reported, small differences still exist. For example, for C1–C4 hydrocarbons, differences of the order of 3–5 cm/s were reported [19]; therefore, accurate data on this parameter are needed.
In the present work focused on the laminar burning velocity, methane was chosen as test fuel being the major constituent of natural gas, mine gas, and biogas. Methane is a fuel which produces less carbon dioxide for each unit of heat released compared to other hydrocarbons. Therefore, methane is important for electricity and power generation, by burning it as a fuel in internal combustion engines, gas turbines, steam generators, rocket engines, and so on. Thus, the study of the combustion properties of methane remains always a topic of interest.
The laminar burning velocities of methane–air mixtures were measured using both constant-pressure methods (experiments using Bunsen flames [20,21,22], flat flames [23,24,25,26,27,28,29], counter-flow twin flames [30,31,32]), or constant-volume methods (experiments using cylindrical vessels [33,34,35,36,37,38,39], spherical vessels [7,33,40,41,42,43,44,45,46], or cubic chambers [47,48]). The measurements were conducted for various equivalence ratios between 0.6 and 1.7 at various initial pressures (up to 70 atm [41]) and temperatures (up to 650 K [33]). Some experiments were conducted in the presence of diluents [7,21,24,28,30,33,41] or in oxygen-enriched atmosphere [21,24]. An overview of some laminar burning velocities of stoichiometric methane–air mixtures at ambient initial conditions reported in literature using different experimental techniques is given in Table 1.

Table 1.
Overview of methane–air laminar burning velocities measurements (for ϕ = 1.0, p0 = 1 bar and T0 = 298 K) reported in literature.
All experimental methods used to determine the laminar burning velocities of methane–air mixtures have advantages and disadvantages. The Bunsen method is a simple method, but the flames are affected by curvature, instability, stretch, and heat loss [49,50]. The flat flames are not affected by the heat loss and are used to measure the laminar burning velocities for mixtures near to the explosion limits. However, the flat flames are affected by radiation, boundary, and catalytic effects due to the burner surface, instability, or flow disturbance due to the burner holes [50,51]. The counter-flow twin flames method is a sensitive method in respect to the changes in the flow characteristics but it is hard to be used at a pressure higher than 5 atm [19,30]. The constant-volume method allows obtaining the laminar burning velocities from measurements of pressure evolution in time with the possibility to use the variation of the initial temperature and pressure simultaneously (in a single experiment). Besides the pressure evolution in time, the flame front position during the combustion can be monitored to obtain more accurate values of the laminar burning velocity [52]. A comprehensive review of these measurement methods to obtain the laminar burning velocities was presented by Konnov et al. [10] and Egolfopoulos et al. [19].
Although the constant-volume method is a simple method that can be applied to obtain the laminar burning velocity at high initial temperatures and pressures such as those found in engines, it has the disadvantage that the flames can develop instabilities [49,52]. To obtain the laminar burning velocity, the pressure-time curves obtained from measurements using the constant-volume method are subsequently processed using different equations. On one hand, there are equations which involve obtaining the burnt mass fraction, and on the other hand, there are the equations which correlate the pressure and temperature with the laminar burning velocity. Another set of equations which allow obtaining the laminar burning velocities are those applied to the incipient period of flame propagation. The equation using the early stage of flame propagation is ideal for obtaining laminar burning velocities at the initial moment of process (i.e., at p0, T0), while the equation based on burnt mass fraction delivers transient laminar burning velocities, which is characteristic for intermediate values of pressure and temperature of the unburnt gas.
In addition to experimental results, the laminar burning velocities of methane–air mixtures were predicted using various detailed kinetic mechanisms such as USC Mech II [4], Konnov Mech [53], Gri Mech 3.0 [7,22,33,39,54], Aramco [39] or San Diego Mech [54].
Although there is a large amount of data on laminar burning velocities of methane–air mixtures, there are discrepancies between the experimental values obtained with the help of different methods and those derived from kinetic modelling even if the same experimental technique, correlations, or mechanism are used. The accuracy with which the laminar burning velocity is determined plays an important role, because (a) the laminar burning velocity is a basic physicochemical parameter of premixed gaseous fuels; (b) the laminar burning velocity is an important parameter in studying the stabilization of flames on burners; (c) the laminar burning velocity contributes at direct determination of the energy released during the combustion processes; (d) the laminar burning velocity is a fundamental parameter that influences the performance and pollutant emissions of some combustion devices; (e) the laminar burning velocity is a property that affects the ignition energy and the induction period; (f) the laminar burning velocity contributes to the understanding of the concepts of laminar flame. In this respect, the present paper aims to evaluate the performance of the constant-volume method by several sets of experiments carried out in three different closed vessels (a sphere and two cylinders) followed by the analysis of the obtained results. For this purpose, two different correlations applied on pressure-time histories to obtain the laminar burning velocity will be used to obtain more accurate values. The first correlation is applied to the incipient stage of the flame propagation and involves obtaining the cubic low coefficient of pressure variation assuming the isothermal compression of the unburned gas ahead of the flame front. The other correlation is applied over an extended domain of conditions achieved during the combustion process and correlates the laminar burning velocity with the burnt mass fraction, taking into account an adjusted adiabatic compression law of burnt gas. The laminar burning velocities from experimental data will be compared with the laminar burning velocities obtained from a detailed numerical modelling of methane–air laminar flames propagating under the same initial conditions (concentration, pressure, and temperature) using two different chemical kinetic models. The two sets of data (experimental and computed) will be analyzed against the initial pressure of the gaseous mixture. Additionally, the results obtained herein will be compared among themselves and against the laminar burning velocities delivered by other researchers. Accurate laminar burning velocities are necessary for computational fluid dynamics models not only to predict the effects of explosions but also to design new internal combustion engines with a higher efficiency and lower fuel consumption which will lead to a lower degree of environmental pollution. Moreover, the experimental laminar burning velocities obtained at various initial pressures are necessary for a complete kinetic study regarding the combustion reaction, and even at elaboration of the reaction mechanisms.
2. Experimental Apparatus and Procedure
In this paper, three explosion vessels with different geometries (a sphere and two cylinders) and volumes were used to examine the combustion of the stoichiometric CH4–air mixture at various initial pressures (within 0.4 and 1.4 bar) and room temperature. At stoichiometric composition of any fuel–oxidizer mixture, a complete fuel and oxidizer consumption occurs. Therefore, we can consider that in our study on a stoichiometric methane–air mixture is assured not only a complete burning, but also the stability of components diffusion to the flame front.
The vessel S is a sphere with a radius of 5 cm. The vessel C1 is a cylinder with a height of 15 cm and a radius of 5 cm. The vessel C2 is a cylinder with a height of 6 cm and a radius of 3 cm. Vessel C2 is equipped with a transparent window of synthetic glass at its top which allows the observation of the flame propagation. All vessels are made to withstand vacuum and pressures up to 40 bar. Each vessel has two stainless steel electrodes for explosion initiation and two ionization probes for flame front monitoring. The spark gap of 3.0 mm is located in the geometrical center of each vessel. The initiation electrodes have a diameter of 1 mm and round tips. They are insulated from the vessel’s mantle by a Teflon sheath. In this study, the energies of the initiation sparks were maintained between 1 and 5 mJ which allow avoidance of any gas turbulence caused by excessive initiation energy. This turbulence can produce disturbances in flame propagation from the very beginning of the propagation process. The tips of the ionization probes were mounted 3–5 mm away from the vessel’s wall and their signals (peaks) were necessary not only to detect the flame front position, but also to know when the flame radius has reached 0.9 from the vessel radius. The information given by the ionization probes was useful in this work especially when the pressure-time curves were processed using the correlation between the laminar burning velocity and the burnt mass fraction in order to obtain the laminar burning velocities. The records of the ionization probes can also be used for obtaining the laminar burning velocities using the expansion coefficients of the burned gases () [55] which will form the subject of another study.
A schematic representation of the experimental vessels used in this study can be found in Figure 1. The use of small-scale vessels for determining the deflagration features has the advantage that the flames propagating inside them cannot develop cellular structures. In addition, a greater number of measurements can be achieved in a shorter time using a small amount of reactants.
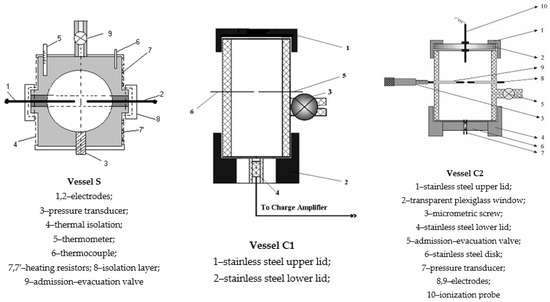
Figure 1.
Schematic representation of experimental vessels.
The experiments were carried out in the following way: (1) the methane–air mixture was prepared by partial pressure method at a total pressure of 5 bar, stored in a 10 L metallic bottle, and left for 48 h to become homogenous prior use; (2) before every test, the explosion vessel was flushed with dry air and then evacuated down to 0.1 mbar in order to avoid the traces of the burned gas which may influence the mixture composition; (3) the methane–air mixture was then introduced, at the desired pressure, and it was allowed to become quiescent to avoid the turbulence due to a fast admission; (4) the explosion was initiated by inductive-capacitive sparks whose energy was adjusted to a minimum value (≤5 mJ) to avoid any disturbing turbulence at initiation; (5) the signals of the acquisition system and ionization probes were captured, stored, and then evaluated.
In order to minimize the operational errors, each experiment was repeated a minimum of three times (in each case using fresh methane–air mixture) then the obtained values were averaged.
During the experiments, the initial pressures of methane–air mixture were measured by a strain gauge manometer (Edwards type EPS-10HM), while the pressure variation was recorded using a piezoelectric pressure transducer (Kistler 601A) connected to a Charge Amplifier (Kistler 5001SN). The signals of the Charge Amplifier and ionization probes were registered with a Digital Oscilloscope (TestLab™ Tektronix 2505), usually at 104 signals per second. In accordance with the manufacturers who provided the above mentioned equipment, theirs accuracy is as follows: the strain gauge manometer has an error less than 0.1%; the piezoelectric pressure transducer has an error less or equal to 0.1%; the charge amplifier has an error less or equal to 0.1%; the DC gain accuracy of the oscilloscope is ±3% for 10 mV/div to 5 V/div (in this work, the data were acquired at 1 V/div). Overall, the errors made in laminar burning velocity evaluation range between 3.0% and 3.5%.
Methane (SIAD-Italy), 99.9% was used without further purification.
Other details can be found in our previous works [7,9,56].
3. Computing Programs
The kinetic modelling of stoichiometric methane–air laminar premixed flames at ambient initial temperature and various initial pressures (0.4–1.4 bar) was made using INSFLA and 1-D COSILAB packages. INSFLA was run using the Warnatz mechanism [57] for combustion of C1–C4 hydrocarbons, which includes 592 elementary reactions and 53 chemical species. The runs were done without taking into account the radiative energy losses. COSILAB was run using the GRI 3.0 mechanism [58] which includes 325 elementary reactions and 53 chemical species. The runs were performed for the isobaric combustion of the premixed laminar free flames. The kinetic modelling of methane–air laminar premixed flames delivered the computed laminar burning velocities used in this study.
More details regarding the kinetic modelling of premixed flames by means of these two packages can be found elsewhere [9,59,60].
4. Data Evaluation
In this study, the laminar burning velocities were obtained using two different methods for processing the pressure-time curves:
- (a)
- Using the cubic law of pressure variation in a closed vessel, after assuming the isothermal compression of the unburned gas ahead of the flame front, in the incipient stage of flame propagation [52]:
In this equation, R is the radius of the explosion vessel; k is the cubic law coefficient of pressure rise; Δpmax is the maximum pressure rise. For each experiment, k was obtained using the following nonlinear regression: Δp = a + k ∙ (t—b)3 in which a and b represent the pressure and time corrections, respectively, necessary to eliminate any possible delays in the signal recording or in the signal shift of pressure transducer. The coefficients a and b were determined using a restricted number of points under the condition p0 ≤ p ≤ 1.5 p0.
- (b)
- Using the correlation between the laminar burning velocity and the burnt mass fraction [61]:
In the present Equation (2), n is the burned gas fraction and rb is the instantaneous flame radius, respectively.
The burned gas fraction was obtained using a method applied over an extended domain of conditions achieved during the constant volume combustion, taking into account an adjusted adiabatic compression law of burnt gas as proposed by Oancea et al. [62].
In both cases, the laminar burning velocities were obtained after the pressure-time curves have been processed. In Figure 2, the pressure-time curves recorded during the explosion of the stoichiometric CH4–air mixture at sub-atmospheric pressure (p0 = 0.6 bar) are represented. Similar curves were obtained for all initial pressures studied in this work.
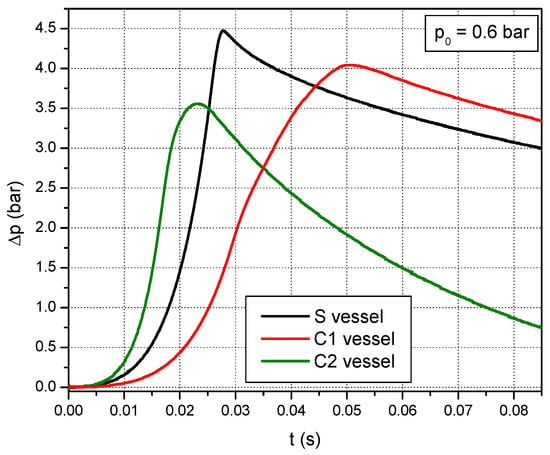
Figure 2.
Pressure-time histories used to obtain laminar burning velocities.
5. Results and Discussion
The main purpose of any combustion research is to provide a deep understanding of the combustion process including the mechanisms of ignition, flame propagation, species distribution, and energy release. The results of such a research are meant to control this process, from both points of view safety and its utilization as a source of energy.
The laminar burning velocity is a specific feature of the combustion process which not only governs the rate of energy delivered in the course of the combustion, but also represents a key parameter regarding the wall quenching process and modelling of the turbulent combustion flames [33]. It is also an important input in design of explosion vessels and relief devices, mitigation of damaging effects of explosions, or optimization of internal combustion engines.
In this study, the laminar burning velocities of stoichiometric methane–air mixtures with various initial pressures have been obtained after examination of pressure-time histories achieved during explosions in three closed vessels. The pressure-time curves were processed using two different correlations. In Figure 3, the laminar burning velocities obtained using these correlations are presented. First, the pressure-time histories were processed using the cubic law of pressure variation to obtain the laminar burning velocities. This is a simple procedure applied to the early stage of flame propagation when the temperature gradients of burned and unburned gas have insignificant values which can be neglected. Despite these advantages, by evaluating only the data from the incipient period of the flame propagation, much valuable information is wasted. Therefore, the same experimental curve was processed using an upgraded equation for the burnt mass fraction over an enlarged period of explosion propagation, until the point when the energy losses become significant. For cylindrical vessels, an “apparent” radius was used instead of the geometrical radius of the vessel. This was obtained using a sphere which has the same volume as the studied cylinder [63].
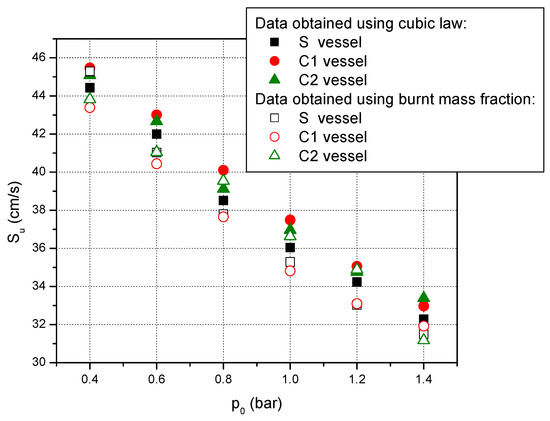
Figure 3.
Laminar burning velocities of stoichiometric methane–air mixtures at various initial pressures and ambient initial temperature obtained using the cubic law of pressure variation and the burnt mass fraction model.
As it can be seen from Figure 3, these two methods of processing the pressure-time curves obtained during the propagation of the explosion in the stoichiometric methane–air mixture lead to quite similar results. When we compare the results obtained in the cylindrical vessels with those obtained in the spherical vessel, one can observe some discrepancies. These discrepancies can be attributed to the fact that in the cylindrical vessels the flame propagates spherically only until its radius reaches the vessel’s radius. After this moment, it suffers deformations due to the geometry of the vessel. Or, one of the assumptions of the constant-volume method is that the flame propagates spherically during the whole explosion and the flame front is smooth and free from diffusion-thermal and hydrodynamic instabilities [41].
A comparison of present data with results available in literature obtained using similar-aspect experimental vessels is given in Table 2. Here, the laminar burning velocities for stoichiometric methane–air mixture at ambient initial conditions are presented.

Table 2.
Comparison between the laminar burning velocities determined in the present study with literature data; ϕ = 1.0, p0 = 1 bar and T0 = 298 K.
One can observe that the laminar burning velocities obtained from the present research come close to the data reported in the literature regardless of whether the cubic law of pressure variation or burnt mass fraction is used, within experimental errors. The disagreements between the present results and literature data can be attributed to systematic differences due to measurement procedures used or their setups. Moreover, as mentioned above, the vessel shape and volume may also lead to differences between the present laminar burning velocities and those reported in the literature due to the fact that in cylindrical cells (and especially in elongated ones) the flame no longer propagates spherically from a certain point onwards.
Bosschaart and de Goey [25] specified that the experimental values of laminar burning velocities of stoichiometric methane–air flames, at ambient initial conditions, are about 36.0 ± 1.0 cm/s. On the other hand, Yan et al. [27] pointed out that the laminar burning velocity of methane–air mixtures, at ambient initial conditions, depend upon the experimental method approached and that, for a stoichiometric mixture, this parameter can vary between 35 cm/s and 45 cm/s. The authors mentioned also that it is necessary to consider the flame stretch due to flame front curvature and/or flow divergence when it is desired to obtain the laminar burning velocity. Our results ranging from 35.3 cm/s (data from spherical vessel S obtained using the burnt mass fraction) to 37.5 cm/s (data from cylindrical vessel C1 obtained using the cubic law) are within the interval of confidence described in above mentioned papers. Therefore, we can consider these values to be reliable even if, as was pointed out in [39], the method of registration of pressure-time evolution does not allow the direct observation of the flame front, and therefore, it is more difficult to analyze the dependence of the laminar burning velocity on the stretch rate, or the examination of the cellular structure of a flame at a given pressure and temperature.
When the initial pressure is varied, it is observed that an increase in initial pressure determines a decrease in laminar burning velocities. As it can be seen from Figure 4, the present results obtained from measurements in the spherical vessel and processed using the two methods chosen for this study agree well with data reported by Iijima et al. [43] and Garforth and Rallis [64] who performed measurements in spherical vessels at various initial pressures within the range of initial pressures studied in this research. Data reported by Sharma et al. [44] at sub-atmospheric pressure are lower compared to our results but agree well at pressures above atmospheric pressure.
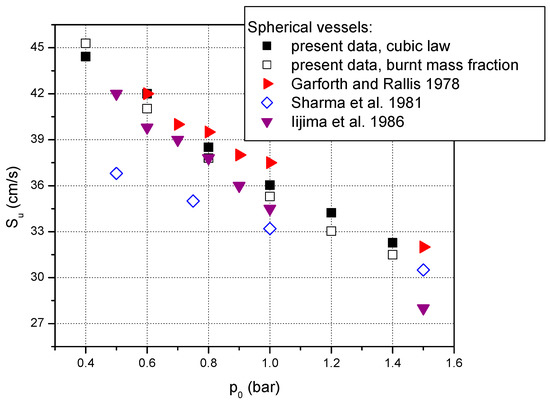
Figure 4.
Laminar burning velocities of stoichiometric methane–air mixture at various initial pressures and ambient initial temperature. Comparison between present measurements and data reported in literature using spherical vessels: ▼ Iijima et al. [43]; ◊ Sharma et al. [44]; ► Garforth and Rallis [64].
Figure 5 depicts the laminar burning velocities obtained with the help of the two cylindrical vessels at ambient initial temperature and various initial pressures. From this figure it is observed that our measurements agree well with data reported in [6].
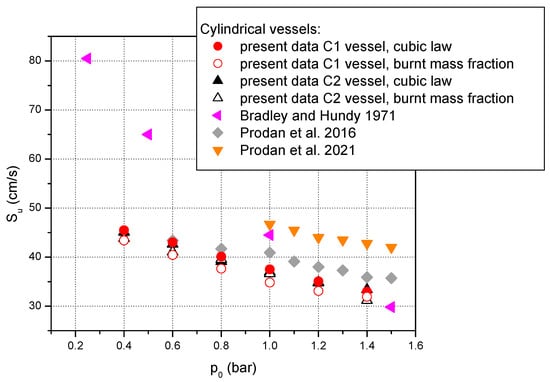
Figure 5.
Laminar burning velocities of stoichiometric methane–air mixture at various initial pressures and ambient initial temperature. Comparison between present measurements and data reported in literature using cylindrical vessels: ♦ Prodan et al. [6]; ◄ Bradley and Hundy [36];▼ Prodan et al. [65].
As it can be seen from Figure 4 and Figure 5 and as it has been pointed out by Faghih and Chen [50], there are wide divergences regarding the experimental values of the laminar burning velocities reported by different groups of researchers. These discrepancies appear even for the same mixture composition and the same initial conditions. One of the possible sources of these divergences is the confinement, especially when we refer to a non-spherical vessel. Burke et al. [66] studied the effect of cylindrical confinement on the determination of the laminar flame speeds using outwardly propagating flames and showed that these non-spherical vessels have often smaller volumes than the spherical vessels (for safety purposes) and the flame speeds are measured for flames near to the vessel’s walls. Burke et al. concluded that, due to the vessel asymmetry, the flame propagation velocity is higher in the axial direction in comparison with the flame propagation velocity in the radial direction. Therefore, to avoid this situation they proposed that a restriction of rb < 0.3 R (R is the cylinder radius) is necessary to obtain accurate laminar burning velocities, mainly for enclosures with large radii. Burke et al. mentioned that, for this part of the data range, the results indicate that the effect of a cylindrical vessel on laminar burning velocities is small. However, regarding our small vessels radii, the restriction proposed in [66] seems to be very rigorous considering that in the present paper the laminar burning velocities were obtained using a restriction of rb < 0.9 R as was recommended in [60].
From the figures presented above it is also observed that the higher values of the laminar burning velocities were obtained from data measured in cylindrical vessels in comparison with those measured in spherical vessel. This is in accord with literature data. For example, Wang et al. [67] conducted a numerical study of geometric effect on laminar burning velocity measurements using the constant volume method. They used three different 2-D numerical models including a spherical and two cylindrical vessels with different diameter to length ratio. Their results showed that the evolution of the explosion pressure and the pressure rise rate are very similar in the considered vessels. They also noted that this is true only before the flames touch the vessel’s wall. Wang et al. observed that in the case of the cylindrical vessels, the flame surface area is changed by cylindrical confinement. This leads to an overestimation of 3.8% in laminar burning velocity achieved in the cylindrical vessels compared to the spherical ones. However, the data achieved in the cylindrical vessels may be utilized to obtain results with almost the same accuracy if these data are restricted to a point before flame impingement and as long as the final pressure is not clearly altered by the heat loss. Therefore, the maximum explosion pressures obtained in cylindrical vessels should be compared with data achieved in spherical vessels under same initial conditions or at least compared with ideal constant volume equilibrium pressure to assess the divergence.
Additionally to data obtained from pressure-time histories processed using the cubic law of pressure variation and burnt mass fraction, two different computing packages (COSILAB and INSFLA) were used to calculate the laminar burning velocities of the studied mixture. The runs with the Cosilab package were made using the GRI 3.0 mechanism. This is a widespread mechanism for 1-D modelling of laminar premixed flames designed to model the combustion of natural gas [68]. The runs with the INSFLA package were made using the Warnatz mechanism which was developed for kinetic modelling of fuel–air flames at various initial conditions [58]. Numerically simulated laminar burning velocities are depicted in Figure 6 together with data acquired in spherical vessel and cylindrical vessel C1. From this figure it can be seen that data obtained with the GRI 3.0 mechanism agree with the experimental results. On the other hand, data computed with the Warnatz mechanism are higher than those computed with the GRI 3.0 mechanism which may be due to the fact that, in the present work, the runs with the INSFLA package were performed without taking into account the radiative energy losses.
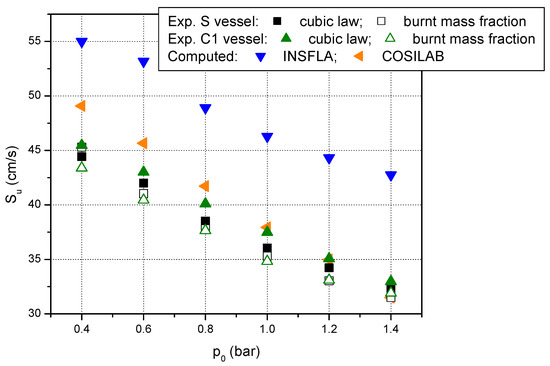
Figure 6.
Laminar burning velocities of stoichiometric methane–air mixture at various initial pressures and ambient initial temperature from experiments and numerical modelling.
Excepting the data reported by Reyes et al. [39], the computed laminar burning velocities obtained using the GRI Mech 3.0 agree well with literature data computed with the same kinetic mechanism, as it results from Table 3 where data referring to the stoichiometric mixture of methane–air at ambient initial conditions are given.

Table 3.
Computed laminar burning velocities for stoichiometric methane–air mixture at p0 = 1 bar and T0 = 298 K.
The agreement is not satisfactory when examining the data obtained with the help of the Warnatz mechanism. In this case, only data reported in [73] agree with the present results. Additionally, as Bosschaart and de Goey observed [25], the Warnatz mechanism was fitted to measurements in which the laminar burning velocities had higher values. Therefore, it leads to laminar burning velocities larger than recent experiments suggest. However, Bosschaart and de Goey do not indicate that this mechanism needs to be set aside, but only suggest that it should be used with caution.
Regarding the accuracy of various kinetic mechanisms, Youan et al. [70] provided a numerical study on water vapor addition to the methane–air mixtures and tested different kinetic mechanisms (among them GRI Mech 3.0) to obtain accurate laminar burning velocity under the engine-relevant conditions. They concluded that the best values of the laminar burning velocities under studied conditions were delivered by the Aramco Mech 1.3. However, Youan et al. specified also that the GRI Mech 3.0 gave reasonable predictions of the laminar burning velocity.
The initial pressure is one of the most important parameters influencing the laminar burning velocity. For a given hydrocarbon–air composition and initial temperature, an increase in initial pressure leads to a decrease in laminar burning velocity. Hu et al. [74] conducted a study on H2–air flames and pointed out that a decrease in laminar burning velocity due to an increase in initial pressure can be understood by the suppression of the overall chemical reaction which takes place due to the decrease of H and OH mole fractions within the flames. Indeed, the authors of the paper [37] observed strong correlations between the laminar burning velocity and the maximum radical concentrations of H and OH radicals in the reaction zone of premixed flames and pointed out that high laminar burning velocities correspond to high radical concentrations in the reaction zone.
In the constant-volume method, the next power law is used to show the dependence of the laminar burning velocity on the initial pressure [75]:
In Equation (3), Su,0 is the laminar burning velocity at reference conditions; p0,ref is the reference pressure (in this study p0,ref was chosen to be 1 bar); β is the baric coefficient obtained from the linear regressions ln(Su) against ln(p/p0). The results of these regressions are presented in Table 4. One can observe that the values of β range between −0.349 and −0.212. Despite these differences, the values fall within the reported values characteristics to methane–air mixtures (β = −0.500 [36] and β = −0.170 [1]).

Table 4.
The baric coefficients of laminar burning velocities (β) and overall reaction orders (n).
The influence of the pressure on the laminar burning velocity of stoichimetric methane–air mixtures was studied by many authors and the reported results of baric coefficients are quite scattered. For example, [6] reported a baric coefficient of −0.215 from experiments at initial pressures between 0.4 and 1.5 bar performed in a cylindrical vessel. Another research [38] reported β = −0.370 after measurements in a cylindrical vessel at initial pressures which range between 1 and 5 atm. This value is similar with that reported in [41] (β = −0.374) from data measured in a spherical vessel at initial pressures between 0.1 and 1.0 MPa. Reyes et al. [39] reported a baric coefficient of β = −0.352 using measurements in a spherical vessel at various initial pressures (0.1–0.7 MPa) and temperatures (320–480 K). A smaller value of −0.435 was reported by Elia et al. [42] using experiments in a spherical vessel and initial pressures ranging between 0.75 and 70 atm. On the other hand, Stone et al. [46] reported β = −0.297.
Some researchers have studied the dependence of a baric coefficient on initial pressure. Their studies showed that this parameter decreases with initial pressure increase. For example, Hill and Hung [76] recommended a baric coefficient of −0.299 for initial pressures between 0.2 and 1 bar. Accordingly to Rallis and Garforth [77], it is suitable to use a baric coefficient of −0.145 for initial pressures of up to 0.6 atm; a baric coefficient of −0.265 for initial pressures between 0.6 and 3 atm; and a baric coefficient of −0.510 for initial pressures between 3 and 10 atm. Taking into account these suggestions, we can consider that the baric coefficients obtained in the present study using the laminar burning velocity at initial pressures between 0.4 and 1.4 bar agree well with the values recommended in [76,77].
The empirical power law of the laminar burning velocity dependence on pressure (Equation (3)) is used not only to predict the laminar burning velocities for certain initial pressures for which experimental measurements are not possible, but it is also used in applied engineering models for design of engines or gas turbines [29].
The baric coefficient β was related with the overall reaction order (n) which represents the sensitivity of the reaction rate to pressure and temperature variation. Moreover, the consequence of pressure on the chain mechanism can be detected and quantified through this parameter.
At constant initial temperature, the overall reaction order can be evaluated using the equation proposed by Potter and Berlad [78]:
This equation shows that the dependence of the laminar burning velocity on pressure includes on one hand, the effect of density variation (−1), and on the other hand, the effect of a chemical reaction (n/2) [79]. The overall reaction order is not just an important parameter which characterizes, from a kinetic point of view, the dependency of the laminar burning velocity on pressure of a given fuel–air mixture, but it is also one of the indispensable parameters used to modelling the deflagration process carried out in vented or closed vessels. As pointed out by Konnov et al. [10], for most hydrocarbon–air mixtures, the overall reaction order was found to be less than 2 for Su < 50 cm/s, indicating that the laminar burning velocity decreases when an increase in initial pressure appears. The authors also mentioned that this is the reason for a negative value of the baric coefficient.
Table 4 depicts the overall reaction orders obtained in this study. The present values of the overall reaction orders obtained from experimental laminar burning velocities range between 1.42 (from pressures-time curves achieved in the spherical vessel and processed using the burnt mass fraction) and 1.50 (from pressures-time curves achieved in the cylindrical vessel C2 and processed using both cubic law and burnt mass fraction). The values of overall reaction orders obtained in the present work are in the reference range of data (0.84–1.50) specific to methane–air mixtures examined at ambient initial temperature as reported in [30,37,69].
6. Conclusions
In this work, an experimental and numerical study on the laminar burning velocities of stoichiometric methane–air mixture at various initial pressures (0.4–1.4 bar) and ambient initial temperature was conducted by using the volume-constant method and a numerical modelling. The pressure-time histories achieved in three vessels with distinct volume and geometrical aspects were processed using two different correlations in order to obtain the laminar burning velocity.
The experimental burning velocities ranging from 35.3 cm/s (data from spherical vessel S, obtained using the burnt mass fraction) to 37.5 cm/s (data from cylindrical vessel C1, obtained using the cubic law) are inside the interval of confidence reported by other researchers thus confirming that the volume-constant method produce reliable and comparable data of laminar burning velocities.
Through numerical modelling it was observed that data obtained from runs with the INSFLA package exceed the experimental ones regardless of the vessel or correlation used.
The effect of the initial pressure on the laminar burning velocity was examined using an empirical power law, and from the linear regressions ln(Su) against ln(p/p0) the baric coefficients were obtained. The baric coefficients were further used to obtain the overall reaction order.
The baric coefficients obtained in this study ranging between −0.349 and −0.212 fall within the reported values (−0.500 …−0.170) characteristic to methane–air mixtures. The overall reaction order ranging between 1.42 and 1.5 was also found to be in the reference range of data specific to methane–air mixtures examined at ambient initial temperature (0.84 … 1.50).
In our future work, we will extend the comparative studies to other fuel–oxidizer and fuel–oxidizer–additive mixtures (oxidizers: O2 or N2O). Hence, the primary results should be useful to improve the kinetic studies regarding the combustion reaction and to deliver useful data for safety recommendations.
Author Contributions
Conceptualization, V.G.; software, V.G. and M.M.; validation, V.G., M.M. and C.M.; investigation, V.G., M.M. and C.M.; data curation, M.M.; formal analysis, V.G.; writing—original draft preparation, V.G.; visualization, V.G., M.M. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully thank U. Maas (Heidelberg University, Germany) and D. Markus (Physikalisch-Technische Bundesanstalt, Braunschweig, Germany) for the permission to run the program INSFLA and for the provided assistance. The present study was partially supported by the Romanian Academy under research project “Dynamics of fast oxidation and decomposition reactions in homogeneous systems” of Ilie Murgulescu Institute of Physical Chemistry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cammarota, F.; Di Benedetto, A.; Di Sarli, V.; Salzano, E.; Russo, P. Combined effects of initial pressure and turbulence on explosions of hydrogen-enriched methane/air mixtures. J. Loss Prevent. Proc. Ind. 2019, 22, 607–613. [Google Scholar] [CrossRef]
- Hinton, N.; Stone, R. Laminar burning velocity measurements of methane and carbon dioxide mixtures (biogas) over wide ranging temperatures and pressures. Fuel 2014, 116, 743–750. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, M.; Wen, X. Comparative study of the propagation of methane/air and hydrogen/air flames in a duct using large eddy simulation. Proc. Saf. Environ. Prot. 2018, 120, 45–56. [Google Scholar] [CrossRef]
- Konnov, A.A. The Temperature and Pressure Dependences of the Laminar Burning Velocity: Experiments and Modelling. In Proceedings of the European Combustion Meeting (ECM 2015), Budapest, Hungary, 30 March–2 April 2015. [Google Scholar]
- Mitu, M.; Prodan, M.; Giurcan, V.; Razus, D.; Oancea, D. Influence of inert gas addition on propagation indices of methane–air deflagrations. Process Saf. Environ. Prot. 2016, 102, 513–522. [Google Scholar] [CrossRef]
- Prodan, M.; Mitu, M.; Razus, D.; Oancea, D. Spark ignition and propagation properties of methane-air mixtures from early stages of pressure history. Rev. Roum. Chim. 2016, 61, 299–307. [Google Scholar]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Prodan, M.; Oancea, D. Propagation indices of methane-air explosions in closed vessels. J. Loss Prev. Process. Ind. 2017, 47, 110–119. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on propagation velocity of methane–air laminar flames. Rev. Chim. 2018, 69, 196–200. [Google Scholar] [CrossRef]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.I.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel + air mixtures. Progr. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Fan, W.P.; Gao, Y.; Zhang, Y.M.; Chow, C.L.; Chow, W.K. Experimental studies and modeling on flame velocity in turbulent deflagration in an open tube. Proc. Saf. Environ. Prot. 2019, 129, 291–307. [Google Scholar] [CrossRef]
- Sulaiman, S.Z.; Khan, N.A.M.H.; Izhab, I.; Shaarani, S.M.; Mudalip, S.K.A.; Man, R.C.; Arshad, Z.I.; Kasmani, R.M.; Sulaiman, S. Explosion characteristics assessment of premixed biogas/air mixture in a 20-L spherical vessel. Chem. Eng. Commun. 2021, 208, 583–591. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zheng, L.; Yu, M.; Pan, R.; Shan, W. Study on the propagation characteristics of hydrogen/methane/air premixed flames in variable cross-section ducts. Proc. Saf. Environ. Prot. 2020, 135, 135–143. [Google Scholar] [CrossRef]
- Luo, Z.; Liang, H.; Wang, T.; Cheng, F.; Su, B.; Liu, L.; Liu, B. Evaluating the effect of multiple flammable gases on the flammability limit of CH4: Experimental study and theoretical calculation. Proc. Saf. Environ. Prot. 2021, 146, 369–376. [Google Scholar] [CrossRef]
- Coward, H.F. Explosions in Gaseous Mixtures, Encyclopedia of Chemical Technology; Kirk, R., Othmer, D., Eds.; The Interscience Encyclopedia Inc.: New York, NY, USA, 1950; Volume 5. [Google Scholar]
- Chaudron, G.; Trombe, F. Les Hautes Températures et Leurs Utilizations en Physique et en Chimie; Masson et Cie: Paris, France, 1973; Volume 1. [Google Scholar]
- Lewis, B.; von Elbe, G. Combustion, Flames and Explosion of Gases, 3rd ed.; Academic Press: New York, NY, USA; London, UK, 1987. [Google Scholar]
- Hattwing, M.; Steen, H. Handbook of Explosion Prevention and Protection; Chapter 3; WILEY-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Egolfopoulos, F.; Hansen, N.; Ju, Y.; Kohse-Höinghaus, K.; Law, C.K.; Qi, F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Progr. Energy Combust. Sci. 2014, 43, 36–67. [Google Scholar] [CrossRef]
- Diederichsen, J.; Wolfhard, H.G. The burning velocity of methane flames at high pressure. Trans. Faraday Soc. 1956, 52, 1102–1109. [Google Scholar] [CrossRef]
- Mazas, A.; Fiorina, B.; Lacoste, D.; Schuller, T. Effects of water vapor addition on the laminar burning velocity of oxygen-enriched methane flames. Combust. Flame 2011, 158, 2428–2440. [Google Scholar] [CrossRef]
- Wu, Y.; Modica, V.; Rossow, B.; Grisch, F. Effects of pressure and preheating temperature on the laminar flame speed of methane/air and acetone/air mixtures. Fuel 2016, 185, 577–588. [Google Scholar] [CrossRef]
- Chan, Y.; Zhu, M.; Zhang, Z.; Liu, P.; Zhang, D. The effect of CO2 dilution on the laminar burning velocity of premixed methane/air flames. Energy Procedia 2015, 75, 3048–3053. [Google Scholar] [CrossRef] [Green Version]
- Dyakov, I.V.; Konnov, A.A.; De Ruyck, J.; Bosschaart, K.J.; Brook, E.C.M.; De Goey, L.P.H. Measurement of adiabatic burning velocity in methane-oxygen-nitrogen mixtures. Combust. Sci. Technol. 2001, 172, 81–96. [Google Scholar] [CrossRef]
- Bosschaart, K.J.; De Goey, L.P.H. The laminar burning velocity of mixtures of hydrocarbon and air as measured with the heat-flux method. Combust. Flame 2004, 136, 261–269. [Google Scholar] [CrossRef]
- Nonaka, H.O.B.; Pereira, F.M. Experimental and numerical study of CO2 content effects on the laminar burning velocity of biogas. Fuel 2016, 182, 382–390. [Google Scholar] [CrossRef]
- Yan, B.; Wu, Y.; Liu, C.; Yu, J.F.; Li, B.; Li, Z.S.; Chen, G.; Bai, X.S.; Alden, M.; Konnov, A.A. Experimental and modeling study of laminar burning velocity of biomass derived gases/air mixtures. Int. J. Hydrogen Energy 2011, 36, 3769–3777. [Google Scholar] [CrossRef]
- Zahedi, P.; Yousefi, K. Effects of pressure and carbon dioxide, hydrogen and nitrogen concentration on laminar burning velocities and NO formation of methane-air mixtures. J. Mech. Sci. Technol. 2014, 28, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wang, Z.; Wang, S.; Whiddon, R.; He, Y.; Lv, Y.; Konnov, A.A. Parametrization of the temperature dependence of laminar burning velocity for methane and ethane flames. Fuel 2019, 239, 1028–1037. [Google Scholar] [CrossRef]
- Zhu, D.; Egolfopoulos, F.; Law, C.K. Experimental and Numerical Determination of Laminar Flame Speeds of Methane (Ar, N2, CO2)-Air Mixtures as Function of Stoichiometry, Pressure and Flame Temperature. Proc. Combust. Inst. 1989, 22, 1537–1545. [Google Scholar] [CrossRef]
- Law, C.K. A Compilation of Experimental Data on Laminar Burning Velocities, in Reduced Kinetic Mechanisms for Applications in Combustion Systems; Lecture Notes in Physics Monographs; Springer: Heidelberg/Berlin, Germany, 1993; Volume 15. [Google Scholar]
- Vagelopoulos, C.M.; Egolfopoulos, F.N. Direct experimental determination of laminar flame speeds. Proc. Combust. Inst. 1998, 27, 513–519. [Google Scholar] [CrossRef]
- Rahim, F.; Elia, M.; Ulinski, M.; Metghalchi, M. Burning velocity measurements of methane-oxygen-argon mixtures and an application to extend methane-air burning velocity measurements. Int. J. Engine Res. 2002, 3, 81–92. [Google Scholar] [CrossRef]
- Halter, F.; Tahtouh, T.; Rousselle, C.M. Nonlinear effects of stretch on the flame front propagation. Combust. Flame 2010, 157, 1825–1832. [Google Scholar] [CrossRef]
- Pizzuti, L.; Martins, C.A.; dos Santos, L.R.; Guerra, D.R. Laminar burning velocity of methane/air mixtures and flame propagation speed close to the chamber wall. Energy Procedia 2017, 120, 126–133. [Google Scholar] [CrossRef]
- Bradley, D.; Hundy, G.F. Burning Velocities of Methane-Air Mixtures using Hot-Wire Anemometers in Closed-Vessel Explosions. Proc. Combust. Inst. 1989, 13, 575–583. [Google Scholar] [CrossRef]
- Rozenchan, G.; Zhu, D.L.; Law, C.K.; Tse, S.D. Outward propagation, burning velocities, and chemical effects of methane flames up to 60 atm. Proc. Combust. Inst. 2002, 29, 1461–1470. [Google Scholar] [CrossRef]
- Han, P.; Checkel, M.D.; Fleck, B.A.; Nowicki, N.L. Burning velocity of methane/diluent mixture with reformer gas addition. Fuel 2007, 86, 585–596. [Google Scholar] [CrossRef]
- Reyes, M.; Sastre, R.; Giménez, B.; Sesma, C. Experimental, kinetic modeling and morphologic study of the premixed combustion of hydrogen/methane mixtures. Energies 2022, 15, 3722. [Google Scholar] [CrossRef]
- Reyes, M.; Tinaut, F.V.; Horrillo, A.; Lafuente, A. Experimental characterization of burning velocities of premixed methane-air and hydrogen-air mixtures in a constant volume combustion bomb at moderate pressure and temperature. Appl. Therm. Eng. 2018, 130, 684–697. [Google Scholar] [CrossRef]
- Gu, X.J.; Haq, M.Z.; Lawes, M.; Woolley, R. Laminar burning velocity and Markstein lengths of methane–air mixtures. Combust. Flame 2000, 121, 41–58. [Google Scholar] [CrossRef]
- Elia, M.; Ulinski, M.; Metghalchi, M. Laminar Burning Velocity of Methane–Air–Diluent Mixtures. J. Eng. Gas Turbines Power 2001, 123, 190–196. [Google Scholar] [CrossRef]
- Iijima, T.; Takeno, T. Effects of temperature and pressure on burning velocity. Combust. Flame 1986, 65, 35–43. [Google Scholar] [CrossRef]
- Sharma, S.P.; Agrawal, D.D.; Gupta, C.P. The Pressure and Temperature Dependence of Burning Velocity in a Spherical Combustion Bomb. Proc. Combust. Inst. 1989, 18, 493–501. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Luong, V.V.; Pham, Q.T.; Phung, M.T.; Do, P.N. Effect of ignition energy and hydrogen addition on laminar flame speed, ignition delay time, and flame rising time of lean methane/air mixtures. Energies 2022, 15, 1940. [Google Scholar] [CrossRef]
- Stone, R.; Clarke, A.; Beckwith, P. Correlations for the laminar-burning velocity of methane/diluent/air mixtures obtained in free-fall experiments. Combust. Flame 1998, 114, 546–555. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, W.; Hu, E. Experimental study of the effect of nitrogen addition on gas explosion. J. Loss Prev. Proc. Ind. 2013, 26, 1–9. [Google Scholar] [CrossRef]
- Liao, S.Y.; Jiang, D.M.; Cheng, Q. Determination of laminar burning velocities for natural gas. Fuel 2004, 83, 1247–1250. [Google Scholar] [CrossRef]
- Law, C.K. Dynamics of stretched flames. Proc. Combust. Inst. 1989, 22, 1381–1402. [Google Scholar] [CrossRef]
- Faghih, M.; Chen, Z. The constant-volume propagating spherical flame method for laminar flame speed measurement. Sci. Bull. 2016, 61, 1296–1310. [Google Scholar] [CrossRef]
- Giurcan, V.; Movileanu, C.; Musuc, A.M.; Mitu, M. Laminar Burning Velocity of Biogas-Containing Mixtures. A Literature Review. Processes 2021, 9, 996. [Google Scholar] [CrossRef]
- Razus, D.; Oancea, D.; Movileanu, C. Burning velocity evaluation from pressure evolution during the early stage of closed-vessel explosions. J. Loss Prev. Process. Ind. 2006, 19, 334–342. [Google Scholar] [CrossRef]
- Xiouris, C.; Ye, T.; Jayachandran, J.; Egolfopoulos, F.N. Laminar flame speeds under engine-relevant conditions: Uncertainty quantification and minimization in spherically expanding flame experiments. Combust. Flame 2016, 163, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Saxena, P.; Kumar, S. Laminar burning velocity of methane-air mixtures at elevated temperatures. Energy Fuels 2013, 27, 3460–3466. [Google Scholar] [CrossRef]
- Movileanu, C.; Mitu, M.; Munteanu, V.; Razus, D.; Oancea, D. CO2 effect on the flame velocity in gaseous flammable mixtures. Preliminary tests on stoichiometric ethylene-air mixture. An. Univ. Buc. Chim. 2004, 1–2, 249–254. [Google Scholar]
- Mitu, M.; Movileanu, C.; Giurcan, V. Deflagration characteristics of N2-diluted CH4-N2O mixtures in the course of the incipient stage of flame propagation. Energies 2021, 14, 5918. [Google Scholar] [CrossRef]
- Warnatz, J.; Maas, U.; Dibble, R. Combustion, 4th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006. [Google Scholar]
- COSILAB., Version 3.0.3; Rotexo-Softpredict-Cosilab GmbH & Co KG: Bad Zwischenhahn, Germany, 2013.
- Brinzea, V.; Mitu, M.; Movileanu, C.; Musuc, A.; Razus, D.; Oancea, D. Propagation velocities of propane-air deflagrations at normal and elevated pressures and temperatures. Rev. Chim. 2012, 63, 289–292. [Google Scholar]
- Razus, D.; Brinzea, V.; Mitu, M.; Movileanu, C.; Oancea, D. Burning velocity of propane–air mixtures from pressure–time records during explosions in a closed spherical vessel. Energy Fuels 2012, 26, 901–909. [Google Scholar] [CrossRef]
- Manton, J.; Lewis, B.; von Elbe, G. Burning-velocity measurements in a spherical vessel with central ignition. Proc. Symp. Combust. 1953, 4, 358–363. [Google Scholar] [CrossRef]
- Oancea, D.; Razus, D.; Ionescu, N.I. Burning velocity determination by spherical bomb technique. I. A new model for burnt mass fraction. Rev. Roum. Chim. 1994, 39, 1187–1196. [Google Scholar]
- Brinzea, V.; Mitu, M.; Movileanu, C.; Razus, D.; Oancea, D. Deflagration parameters of stoichiometric propane-air mixture during the initial stage of gaseous explosions in closed vessels. Rev. Chim. 2011, 62, 201–205. [Google Scholar]
- Garforth, A.M.; Rallis, C.J. Laminar burning velocity of stoichiometric methane-air: Pressure and temperature dependence. Combust. Flame 1978, 31, 53–68. [Google Scholar] [CrossRef]
- Prodan, M.; Ghicioi, E.; Laszlo, R.; Nalboc, I.; Suvar, S.; Nicola, A. Experimental and numerical study of ignition and flame propagation for methane–air mixtures in small vessels. Processes 2021, 9, 998. [Google Scholar] [CrossRef]
- Burke, M.P.; Ju, Y.; Dryer, F.L. Effect of cylindrical confinement on the evolution of outwardly propagating flames. In Proceedings of the Meeting of the Eastern States Section of the Combustion Institute, University of Virginia, Charlottesville, VA, USA, 21–25 October 2007. [Google Scholar]
- Wang, D.; Ji, C.; Wang, S.; Yang, J.; Meng, H. Numerical study of geometric effects on burning velocity measurement by constant volume method. Combust. Sci. Technol. 2020, 192, 1475–1492. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Goldenberg, B.E.M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C.; Lissianski, V.V.; et al. GRI-Mech 3.0. 1999. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 19 February 2021).
- Hu, E.; Li, X.; Meng, X.; Chen, Y.; Cheng, Y.; Xie, Y.; Huang, Z. Laminar flame speeds and ignition delay times of methane–air mixtures at elevated temperatures and pressures. Fuel 2015, 158, 1–10. [Google Scholar] [CrossRef]
- Yuan, Z.; Xie, L.; Sun, X.; Wang, R.; Li, H.; Liu, J.; Duan, D. Effects of water vapor on auto-ignition characteristics and laminar flame speed of methane/air mixture under engine-relevant conditions. Fuel 2022, 315, 123169. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Cho, P.; Law, C.K. Laminar flame speeds of methane-air mixtures under reduced and elevated pressures. Combust. Flame 1989, 76, 375–391. [Google Scholar] [CrossRef]
- Coffee, T.P. Kinetic mechanisms for premixed, laminar, steady state methane/air flames. Combust. Flame 1984, 55, 161–170. [Google Scholar] [CrossRef]
- Warnatz, J. The structure of laminar alkane-, alkene-, and acetylene flames. Symp. Combust. 1981, 18, 369–384. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Miao, H. Experimental and numerical study on laminar burning velocities and flame instabilities of hydrogen–air mixtures at elevated pressures and temperatures. Int. J. Hydrogen Energy 2009, 34, 8741–8755. [Google Scholar] [CrossRef]
- Metghalchi, H.; Keck, J.C. Laminar burning velocity of propane–air mixtures at high temperature and pressure. Combust. Flame 1980, 38, 143–154. [Google Scholar] [CrossRef]
- Hill, P.G.; Hung, J. Laminar burning velocities of stoichiometric mixtures of methane with propane and ethane additives. Combust. Sci. Technol. 1988, 60, 7–30. [Google Scholar] [CrossRef]
- Rallis, C.J.; Garforth, A.M. The determination of laminar burning velocity. Prog. Energy Combust. Sci. 1980, 6, 303–329. [Google Scholar] [CrossRef]
- Potter, A.E., Jr.; Berlad, A.L. The effect of fuel type and pressure on flame quenching. Symp. Combust. 1957, 6, 27–36. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Law, C.K. Chain mechanisms in the overall reaction orders in laminar flame propagation. Combust. Flame 1990, 80, 7–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).