DNS Study of Spherically Expanding Premixed Turbulent Ammonia-Hydrogen Flame Kernels, Effect of Equivalence Ratio and Hydrogen Content
Abstract
:1. Introduction
2. Numerical Set-Up and Conditions
3. Results and Discussion
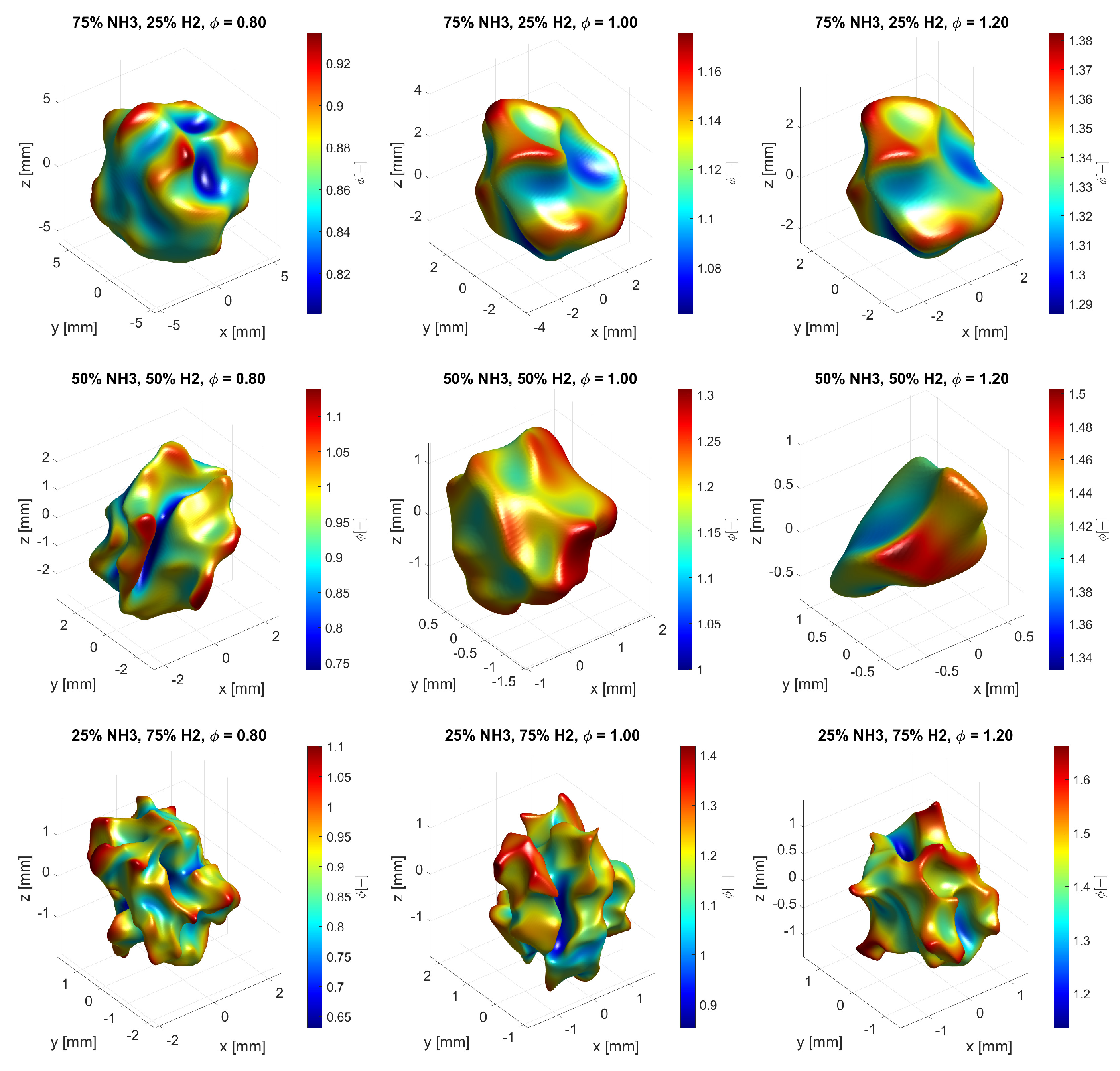
3.1. Flame Structure
3.2. Curvature Effects on Flame Chemistry
3.3. Time Evolution of Temperature and Heat Release
4. Conclusions
- Due to variations in local equivalence ratio, the flame surface is distorted for all cases;
- As the equivalence ratio is increased for each amount of hydrogen, the flame surface smoothens out, forming strongly curved leading edges with positive curvature;
- As the amount of hydrogen is increased, the flame structure becomes more wrinkled due to the increase in spread of the local equivalence ratios as the preferential diffusion effects of hydrogen becomes stronger.
- Due to the increase in equivalence ratio to near stoichiometry for all lean cases, the reactivity of all reactions are increased compared to the unstrained flat flame cases;
- For all rich cases, the opposite is observed again as the equivalence ratio is pushed to high levels, decreasing reactivity;
- The effect of the sign of the curvature influences the flame significantly. The role of the H radical is quite important in this perspective;
- The existence of a critical amount of hydrogen in the fuel mixture is observed, after which the trend of some reactions with respect to the sign of the curvature is flipped speculated to be due to competing reactive and diffusive timescales of hydrogen radicals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramage, J. Energy: A Guidebook; Oxford University Press: Oxford, UK, 1998; Volume 35, pp. 35–3899. [Google Scholar] [CrossRef]
- Felderhoff, M.; Weidenthaler, C.; Von Helmolt, R.; Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Phys. Chem. Chem. Phys. 2007, 9, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Hussein, N.A.; Valera-Medina, A.; Alsaegh, A.S. Ammonia-Hydrogen Combustion in a Swirl Burner with Reduction of NOx Emissions. Energy Procedia 2019, 158, 2305–2310. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Tang, G.; Jin, P.; Bao, Y.; Chai, W.S.; Zhou, L. Experimental investigation of premixed combustion limits of hydrogen and methane additives in ammonia. Int. J. Hydrogen Energy 2021, 46, 20765–20776. [Google Scholar] [CrossRef]
- Wiseman, S.; Rieth, M.; Gruber, A.; Dawson, J.R.; Chen, J.H. A comparison of the blow-out behavior of turbulent premixed ammonia/hydrogen/nitrogen-air and methane–air flames. Proc. Combust. Inst. 2021, 38, 2869–2876. [Google Scholar] [CrossRef]
- Mashruk, S.; Xiao, H.; Valera-Medina, A. Rich-Quench-Lean model comparison for the clean use of humidified ammonia/hydrogen combustion systems. Int. J. Hydrogen Energy 2021, 46, 4472–4484. [Google Scholar] [CrossRef]
- Bioche, K.; Bricteux, L.; Bertolino, A.; Parente, A.; Blondeau, J. Large Eddy Simulation of rich ammonia/hydrogen/air combustion in a gas turbine burner. Int. J. Hydrogen Energy 2021, 46, 39548–39562. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Hatakeyama, S.; Hayakawa, A.; Kobayashi, H. Numerical study of a low emission gas turbine like combustor for turbulent ammonia/air premixed swirl flames with a secondary air injection at high pressure. Int. J. Hydrogen Energy 2017, 42, 27388–27399. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical kinetic modeling of ammonia oxidation with improved reaction mechanism for ammonia/air and ammonia/hydrogen/air combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Netzer, C.; Ahmed, A.; Gruber, A.; Løvås, T. Curvature effects on NO formation in wrinkled laminar ammonia/hydrogen/nitrogen-air premixed flames. Combust. Flame 2021, 232, 111520. [Google Scholar] [CrossRef]
- Rieth, M.; Gruber, A.; Williams, F.A.; Chen, J.H. Enhanced burning rates in hydrogen-enriched turbulent premixed flames by diffusion of molecular and atomic hydrogen. Combust. Flame 2021, 239, 111740. [Google Scholar] [CrossRef]
- Jiang, Y.; Gruber, A.; Seshadri, K.; Williams, F. An updated short chemical-kinetic nitrogen mechanism for carbon-free combustion applications. Int. J. Energy Res. 2020, 44, 795–810. [Google Scholar] [CrossRef]
- Lele, S.K. Compact finite difference schemes with spectral-like resolution. J. Comput. Phys. 1992, 103, 16–42. [Google Scholar] [CrossRef]
- de Lange, H.C. Inviscid flow modelling using asymmetric implicit finite difference schemes. Int. J. Numer. Methods Fluids 2005, 49, 1033–1051. [Google Scholar] [CrossRef]
- Kennedy, C.A.; Carpenter, M.H.; Lewis, R.M. Low-storage, explicit Runge–Kutta schemes for the compressible Navier–Stokes equations. Appl. Numer. Math. 2000, 35, 177–219. [Google Scholar] [CrossRef] [Green Version]
- Poinsot, T.J.; Lele, S.K. Boundary conditions for direct simulations of compressible viscous flows. J. Comput. Phys. 1992, 101, 104–129. [Google Scholar] [CrossRef]
- Yu, K.; Colonius, T.; Pullin, D.I.; Winckelmans, G. Dynamics and decay of a spherical region of turbulence in free space. J. Fluid Mech. 2021, 907, A13. [Google Scholar] [CrossRef]
- de Swart, J.A.M. Modeling and Analysis of Flame Stretch and Preferential Diffusion in Premixed Flames; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2009. [Google Scholar]
- Somers, L. The Simulation of flat Flames with Detailed and Reduced Chemical models. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Peters, N. Turbulent Combustion; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Ern, A.; Giovangigli, V. EGLIB: A General-Purpose Fortran Library for Multicomponent Transport Property Evaluation, CERMICS Internal Report; Technical Report 96-51; 1996; Available online: https://www.cmap.polytechnique.fr/www.eglib/manual.ps (accessed on 21 June 2022).
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia chemistry in oxy-fuel combustion of methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/syngas/air premixed flames. Combust. Flame 2020, 213, 1–13. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An experimental and modeling study of ammonia with enriched oxygen content and ammonia/hydrogen laminar flame speed at elevated pressure and temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]













| Case | % NH3 | [K] | [m/s] | [m] | Re | [m/s] | [m] | [s] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 75 | 0.8 | 1763 | 0.09 | 1.51 × 10 | 4.64 | 1 | 4.64 | 0.425 | 4.78 × 10 | 3.55 × 10 |
| B | 75 | 1.0 | 1847 | 0.14 | 1.13 × 10 | 4.64 | 1 | 4.64 | 0.673 | 3.58 × 10 | 1.68 × 10 |
| C | 75 | 1.2 | 1685 | 0.17 | 1.02 × 10 | 4.64 | 1 | 4.64 | 0.777 | 3.22 × 10 | 1.31 × 10 |
| D | 50 | 0.8 | 1644 | 0.27 | 6.08 × 10 | 4.64 | 1 | 4.64 | 1.258 | 1.92 × 10 | 4.83 × 10 |
| E | 50 | 1.0 | 1740 | 0.39 | 5.14 × 10 | 4.64 | 1 | 4.64 | 1.818 | 1.63 × 10 | 2.83 × 10 |
| F | 50 | 1.2 | 1749 | 0.43 | 4.94 × 10 | 4.64 | 1 | 4.64 | 1.978 | 1.56 × 10 | 2.50 × 10 |
| G | 25 | 0.8 | 1442 | 0.68 | 3.69 × 10 | 4.64 | 1 | 4.64 | 3.140 | 1.17 × 10 | 1.17 × 10 |
| H | 25 | 1.0 | 1493 | 0.97 | 3.38 × 10 | 4.64 | 1 | 4.64 | 4.492 | 1.07 × 10 | 7.53 × 10 |
| I | 25 | 1.2 | 1534 | 1.13 | 3.12 × 10 | 4.64 | 1 | 4.64 | 5.249 | 9.85 × 10 | 5.93 × 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukundakumar, N.; Bastiaans, R. DNS Study of Spherically Expanding Premixed Turbulent Ammonia-Hydrogen Flame Kernels, Effect of Equivalence Ratio and Hydrogen Content. Energies 2022, 15, 4749. https://doi.org/10.3390/en15134749
Mukundakumar N, Bastiaans R. DNS Study of Spherically Expanding Premixed Turbulent Ammonia-Hydrogen Flame Kernels, Effect of Equivalence Ratio and Hydrogen Content. Energies. 2022; 15(13):4749. https://doi.org/10.3390/en15134749
Chicago/Turabian StyleMukundakumar, Nithin, and Rob Bastiaans. 2022. "DNS Study of Spherically Expanding Premixed Turbulent Ammonia-Hydrogen Flame Kernels, Effect of Equivalence Ratio and Hydrogen Content" Energies 15, no. 13: 4749. https://doi.org/10.3390/en15134749
APA StyleMukundakumar, N., & Bastiaans, R. (2022). DNS Study of Spherically Expanding Premixed Turbulent Ammonia-Hydrogen Flame Kernels, Effect of Equivalence Ratio and Hydrogen Content. Energies, 15(13), 4749. https://doi.org/10.3390/en15134749







