Abstract
The results of the advanced computer analysis of the influence of time and gas atmosphere of the chemical activation process on the microporous structure formation of activated carbons prepared from oil palm shell via microwave irradiation and activation, using potassium hydroxide as an activation agent, are presented in this paper. The quenched solid density functional theory (QSDFT) and the new numerical clustering-based adsorption analysis (LBET) methods were used especially in the analysis of the microporous structure of the activated carbons, taking into account the surface heterogeneity, and the results obtained were confronted with the simple results achieved earlier using Brunauer–Emmett–Teller (BET) and T-plot methods. On the basis of the computer analysis carried out and taking into account the results obtained, it has been shown that the material with the best adsorption properties and suitable for practical industrial applications is activated carbon obtained in a gaseous nitrogen atmosphere at an activation time of 30 min. Moreover, the value of the heterogeneity parameter indicates that the surface area of this activated carbon is homogeneous, which is of particular importance in the practical application. The paper emphasizes that an erroneous approach to the interpretation of analytical results based on gas adsorption isotherms, which consists in basing conclusions only on the values of a single parameter such as specific surface area or micropore volume, should be avoided. Therefore, it is recommended to use in the analysis of measurement data, several methods of porous structure analysis, including methods considering the heterogeneity of the surface, and when interpreting the results one should also take into account the adsorption process for which the analyzed materials are dedicated.
1. Introduction
Activated carbons are amorphous carbonaceous materials, characterized, among other things, by very good adsorption properties, including a developed large specific surface area. Due to their unique properties, these materials are widely used in many industrial technologies [1,2,3]. The activated carbons are usually obtained by physical activation preceded by carbonization, or by one-step chemical activation. Physical activation consists in partly gasifying a char with steam or carbon dioxide [4]. The extent to which the porous structure develops during physical activation is mainly determined by temperature and duration of the process [5]. The resultant porous structure strongly depends on the corresponding burn-off but, in general, the use of carbon dioxide as an activator produces mostly micro- and ultra-microporous structures, whereas steam leads to a broader distribution of pores and to a higher mesoporosity [6]. The degree to which the porous structure is developed during physical activation is determined to a large extent by process temperature and time; the time depends on the reactivity of the carbonaceous material and the activity of the gasifying agent [4,5,6].
The main advantage of physical activation is the ability to maintain the shape and texture of the precursor, provided that the raw material is selected for this purpose. This physical activation feature enables the production of low abrasiveness activated carbon from very hard raw materials such as nut shells or fruit stones. On the other hand, the physical activation of fibrous materials allows the production of non-wovens and acticated carbon fabrics and mats with large surfaces and very good adsorption properties. Moreover, the method is also theoretically ecological due to the absence of the need to use toxic chemicals, but it is quite time and energy consuming [4,5,6].
Alternative processes for producing activated carbons are chemical activation methods [7]. Recently, the use of potassium hydroxide as an activator has attracted more and more interest, which allows to obtain microporous materials with a high degree of pore homogeneity, high adsorption capacity and specific surface area [7,8,9]. The factors that influence the formation of a microporous structure in the chemical activation are: type of raw material, process temperature, atmosphere, and the mass ratio of the activator to the carbonized raw material [10,11].
Chemical activation processes have many advantages over physical activation processes. Among the advantages is the fact that the chemical activation process reduces the formation of tar during pyrolysis, which allows for a more efficient production process. In addition, unlike physical activation, the chemical activation process can be performed at lower temperatures and in shorter periods of time, obtaining a more microporous structure, thus reducing the energy consumption of the production process and, consequently, its costs. After the activation process, however, the activators must be removed by washing, which increases the cost of production of activated carbons [7,8,9,10,11].
Apart from the raw material and activator, the method of heating the raw material also has an important influence on the formation of the microporous structure of activated carbons. One of the most commonly used technologies for obtaining activated carbons is conventional heating, in which the heat source is located outside the raw material deposit. However, this method is characterized by long manufacturing process time and high energy consumption. Therefore, the use of microwave radiation in the chemical activation process is gaining more and more interest, which provides not only uniform heating throughout the charge volume but also lower energy consumption [12,13,14,15,16,17,18,19,20]. The porous structure and the adsorption properties of activated carbons depend, however, first of all significantly on the structure of the original raw material. Hence, the choice of an appropriate precursor is not less important than the selection of the production technology and the determination of the optimum process conditions. Therefore, the search for new raw materials that would be useful in the production of activated carbons has been carried out, and particular attention has been paid in this regard to biomass waste [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
2. Materials and Methods
Hesas et al. [39] presented the results of a study in which oil palm shell were used as raw material to produce activated carbons via microwave irradiation and KOH chemical activation. During the activation process of the hydroxide-impregnated raw material, nitrogen or carbon dioxide was passed through a gas reactor at a flow rate of 300 cm3/min. The effects of activation time and gaseous atmosphere in the reactor on the porous structure formation of the derived activated carbons were analyzed. In the aforementioned studies, among other activation times ranging from 5 to 45 min were analyzed, and nitrogen and carbon dioxide were tested as gaseous atmospheres. The resulting activated carbons were characterized by nitrogen adsorption isotherms determined at −196 °C by volumetric gas adsorption Micromeritics ASAP 2020 Series (Micromeritics Instrument Corporation, Norcross, GA, USA).
The BET [40] and T-plot [41] methods were used in the analysis of adsorption isotherms. These methods have many well-known limitations, including not taking into account, among other things, surface heterogeneity, which could significantly affect the reliability of the obtained results.
Therefore, the concept of carrying out a new original series of analyses using more advanced and reliable methods of porous structure analysis, i.e., QSDFT [42,43,44,45] and the LBET [46,47,48,49,50] methods, taking into account, among other things, surface heterogeneity.
In the present research, the following parameters and indicators were determined: the specific surface area SQSDFT [m2g−1], the volume of pores VQSDFT [cm3g−1], and the micropores size distribution PSD obtained via the QSDFT method [42,43,44,45], the value of parameters of the porous structure. i.e., the volume of the first adsorbed layer VhA [cm3g−1], the dimensionless energy parameter for the first adsorbed layer QA/RT, the dimensionless energy parameter for the higher adsorbed layers, BC, the geometrical parameter of the porous structure determining the height of the adsorbate molecule clusters α, the geometrical parameter of the porous structure determining the width of the adsorbate molecule clusters β calculated via the LBET method [46,47,48,49,50]. Additionally, via the fast numerical multivariate identification procedure of adsorption systems, the following information were determined: the value of the surface heterogeneity parameter h, the value of the dispersion of fitting errors σe, the value of the indices of identification reliability wid and the adsorption energy distributions AED on the first adsorbed layer, obtained for all analyzed activated carbons.
The values of the parameters determined via QSDFT and LBET method, based on nitrogen isotherms, are summarized in Table 1 and Table 2 for activated carbons obtained under a flow of carbon dioxide and nitrogen, respectively, at different activation times, where they are confronted with the previously obtained values of the specific surface areas SBET determined for particular samples by BET method as well as micropore volume Vmic and total pore volume VT obtained via the T-plot method. The analyzed adsorption isotherms of nitrogen, and the corresponding results of fast multivariate identification of adsorption systems and adsorption energy distributions AED on the first layer obtained via LBET method and pore size distributions PSD determined by QSDFT method are presented in Figure 1 and Figure 2 for activated carbons obtained under a flow of carbon dioxide CO2 and nitrogen N2, respectively, at different activation times.

Table 1.
The results of the analysis of a microporous structure of activated carbons derived from oil palm shell via microwave irradiation and chemical activation, using potassium hydroxide, activated under a flow of carbon dioxide CO2 at different activation times, based on nitrogen adsorption isotherms, using the QSDFT, LBET, BET, and T-plot methods; the micropore specific surface area, SQSDFT [m2g−1] and the volume of micropores, VQSDFT [cm3g−1], calculated via QSDFT method; the volume of the first adsorbed layer, VhA [cm3g−1]; the dimensionless energy parameter for the first adsorbed layer, QA/RT; the dimensionless energy parameter for the higher adsorbed layers, BC; the geometrical parameter of the porous structure determining the height of the adsorbate molecule clusters, α, and the geometrical parameter of the porous structure determining the width of the adsorbate molecule clusters, β; the surface heterogeneity parameter, h; the dispersion of fitting errors σe; the identifiability index wid; the specific surface area calculated by the BET method, SBET [m2g−1], and the micropore volume Vmic [cm3g−1], and the total pore volume VT [cm3g−1] calculated by the T-plot method.

Table 2.
The results of the analysis of a porous structure of activated carbons derived from oil palm shell via microwave irradiation and chemical activation, using potassium hydroxide, activated under a flow of nitrogen N2 at different activation times, based on nitrogen adsorption isotherms, using the QSDFT, LBET, BET, and T-plot methods.
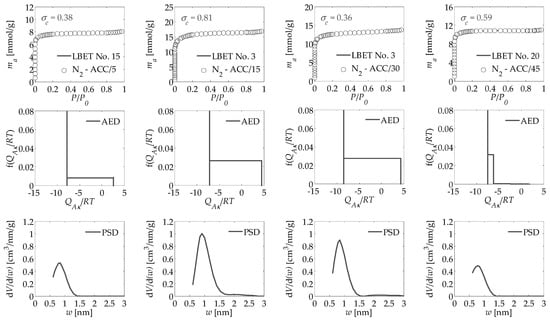
Figure 1.
Nitrogen adsorption isotherms for the activated carbons derived from oil palm shell via microwave irradiation and chemical activation, using potassium hydroxide, activated under a flow of carbon dioxide CO2 at different activation times and the fast multivariate identification procedure results obtained using the LBET method, as well as the adsorption energy distribution AED in the first adsorption layer and the pore size distribution PSD obtained for all analyzed samples of activated carbons via the QSDFT method.
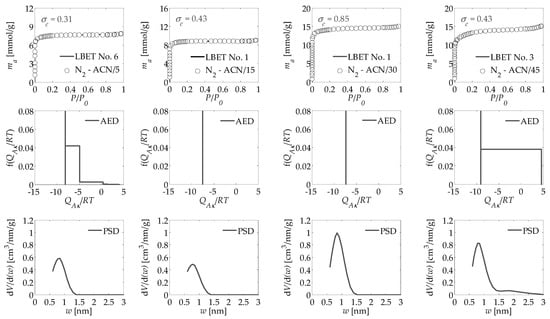
Figure 2.
Nitrogen adsorption isotherms for the activated carbons derived from oil palm shell via microwave irradiation and chemical activation, using potassium hydroxide, activated under a flow of nitrogen N2 at different activation times and the fast multivariate identification procedure results obtained using the LBET method, as well as the adsorption energy distribution AED in the first adsorption layer and the pore size distribution PSD obtained for all analyzed samples of activated carbons via the QSDFT method.
3. Discussion of the Obtained Results
On the basis of the results gathered in Table 1, it can be concluded that activated carbon obtained via microwave irradiation and KOH chemical activation in carbon dioxide atmosphere at 5 min activation time is characterized by a moderately expanded pore structure, including micropores, indicated by the values of the structure parameters, i.e., SQSDFT, VQSDFT, VhA, SBET, Vmic, and VT. The number of best-fited LBET model No. 18 shows the limitations of adsorbate cluster expansion related to the high adsorption energy in layers n > 1, which is also confirmed by the value of energy parameter BC for adsorbed layers higher than 1. The value of the surface heterogeneity parameter h (h = 2) point to that on the surface of the tested material, there is a dominant narrow range of sites that are homogeneous in terms of energy, and a range of sites with high energy heterogeneity of the surface. The values of geometrical parameters, i.e., α and β point to the formation of monolayer branching clusters of adsorbate molecules in the pores of the studied material. On the other hand, the shape of adsorption energy distribution on the first adsorbed layer (AED) determined by LBET method indicates that there are adsorption sites with the same or very similar adsorption energy, i.e., mainly small micropores where only single molecules can adsorb and sites with wide energy spectrum, i.e., bigger micropores and small mesopores.
The activated carbon sample marked as ACC/15, i.e., obtained with an activation time of 15 min, is characterized by significantly higher values of structural parameters: SQSDFT, VQSDFT, VhA, SBET, Vmic, and VT, which point to the expansion of the microporous structure of the material under study with increasing temperature of the activation process. In the case of activated carbon sample ACC/15, an increase in the value of parameter BC (BC = 7.07), i.e., the energy parameter for layers higher than the first one, with a simultaneous decrease in the value of the energy parameter of the first adsorbed layer QA/RT (QA/RT = −7.14) compared to the ACC/5 sample, and thus, the appearance of preferential conditions for multilayer adsorption.
This conclusion is also confirmed by the values of geometrical parameters calculated for the sample of activated carbon ACC/15 i.e., α and β indicating the formation of medium-sized, non-branching clusters of adsorbate particles in the pores of the analyzed material (α = 0.31 and β = 1.00). The number of the best fitted LBET model, i.e., No. 3, indicates that there are limitations in the growth of clusters of adsorbate molecules in the active carbon pores of ACC/15 due to the competitive expansion of neighboring clusters.
Very interesting results were obtained for the sample ACC/30, i.e., activated carbon prepared with 30 min activation time for parameters SQSDFT, VQSDFT, VhA, Vmic, and VT, which, taking into account the simultaneous increase in the value of the parameter SBET parameter indicates the wall scorching between the part of micropores. For the sample obtained at an activation time of 30 min, there was also a significant change in the values of the energy parameters (QA/RT = −8.49, and BC = 5.72) compared to the ACC/15 sample obtained at 15 min, indicating a decreased preference for multilayer adsorption and an increase in adsorption energy for the first adsorbed layer. Values of geometric parameters obtained for Sample ACC/30 as well as α and β values obtained for ACC/30 are practically identical to those for ACC/15 obtained at the activation time of 15 min and indicate, also taking into account the values of the structure parameters, that with an increase in the activation time to 30 min, only the thinnest walls between micropores and micropores located in the areas with the highest temperature effects, such as the outer surface of grains or their edges, were burnt.
The next analyzed activated carbon sample obtained from oil palm shell via microwave irradiation and KOH chemical activation was ACC/45 sample obtained with an activation time of 45 min. In the case of the aforementioned activated carbon, a significant decrease in the values of the structure parameters can be observed in SQSDFT, VQSDFT, VhA, Vmic, and VT, with a simultaneous significant increase in the value of the parameter SQSDFT, in comparison with the activated carbon sample ACC/30 obtained with an activation time of 30 min. The values of both energy parameters were also changed in QA/RT and BC as well as geometrical parameters indicating, respectively, preferential energetic conditions for monolayer adsorption and geometric conditions enabling formation of monolayer and branching clusters of adsorbate molecules in the micropores of analyzed ACC/45 material. Note also that the surface of sample ACC/45 is heterogeneous, as indicated by the value of the heterogeneity parameter h = 3.
Analysis of the pore size distribution PSD, determined by QSDFT method for activated carbons obtained in carbon dioxide gas atmosphere, shows that the material studied is dominated by micropores in the range of 0.5 to 1.5 nm, which confirms the results obtained using the LBET method. It is worth noticing the very similar shapes of PSD for all the analyzed activated carbons, which indicates the significant influence of the structure of the primary material on the formation of the porous structure of the obtained activated carbons. In turn, the value of the dispersion of the fitting error σe values indicate a very good fit of the model to the experimental data, and the values of the identification indicators indicate a very good identifiability of the adsorption systems, which indicates that the parameters determined by the LBET method are reliable and credible.
In the course of the study, samples of activated carbons obtained from oil palm shell were successively analyzed by chemical activation with potassium hydroxide KOH during which nitrogen was passed through a gas reactor at a flow rate of 300 cm3/min. Based on the calculation results presented in Table 2, it can be observed that the structural parameters obtained for sample ACN/5 are very close to those obtained for sample ACC/5, which allows us to conclude that, at short activation time, the effect of gaseous atmosphere is negligible. Some differences can already be observed in the case of geometrical parameter values, namely in the case of a sample obtained in nitrogen atmosphere, i.e., ACN/5 low and non-branching clusters of adsorbate molecules were formed in contrast to monolayer branching clusters formed in the pores of activated carbon ACC/5 obtained in the case of chemical activation with KOH in carbon dioxide atmosphere.
In the case of the energy parameters determined for activated carbon ACN/5, however, a significant effect of the gaseous atmosphere can already be observed, namely a higher value of the energy parameter for the first adsorbed layer QA/RT, as well as a significantly higher value of the energy parameter for adsorbed layers higher than the first one BC, which indicates preferential energetic conditions for the development of multilayer adsorption process, however, blocked by geometrical constraints. Moreover, the surface of the analyzed ACN/5 activated carbon is heterogeneous, as indicated by the value of the heterogeneity parameter h = 3.
Another sample obtained via microwave irradiation and KOH chemical activation in a nitrogen atmosphere was activated carbon ACN/15 prepared with an activation time of 15 min. A significant increase in the values of the structure parameters was observed for this activated carbon, compared to the ACN/5 sample obtained with an activation time of 5 min, however, these values are significantly lower compared to the sample obtained with the same activation process time but in a carbon dioxide atmosphere. This observation highlights the greater influence of the carbon dioxide atmosphere on the formation of the microporous structure of activated carbon. However, the low value of the parameter BC, indicates unfavorable energetic conditions for multilayer adsorption process. Values of geometrical parameters α = 0.03 and β = 1.00 obtained for activated carbon ACN/15 indicate that single adsorbate molecules adsorb in the micropores of the investigated sample, and the value of the surface inhomogeneity parameter h = 0 indicates that the surface of the tested material is homogeneous.
On the basis of the adsorption energy distribution determined for the sample ACN/15 by LBET method, it can be concluded that in the material studied, there are only adsorption sites of equal or very close adsorption energy, i.e., sites able to adsorb a single adsorbate molecule, i.e., micropores of size comparable to adsorbate molecules.
The next activated carbon sample analyzed was ACN/30, i.e., one obtained at an activation time of 30 min. The analysis showed that in comparison with sample ACN/15, the values of structure parameters increased significantly SQSDFT, VQSDFT, VhA, SBET, Vmic, and VT, as well as to a small extent, the value of the energy parameter for higher layers, BC. Furthermore, activated carbon ACN/30 shows practically the same values of geometrical parameters, the same degree of surface uniformity, and the same shape of adsorption energy distribution on the first layer as ACN/15 sample obtained with shorter activation time.
In the case of another activated carbon marked as ACN/45 and obtained at the longest activation time, i.e., 45 min, burning out of the walls of some micropores was observed, which was indicated by a decrease in the values of structural parameters, SQSDFT, VhA, SBET, and Vmic, with a simultaneous increase or unchanged value of the parameters, VQSDFT, VhA and VT, in relation to activated carbon ACN/30. Activated carbon ACN/45 was also characterized by high values of energy parameters QA/RT and BC (i.e., −9.02 and 7.50, respectively), indicating preferential energy conditions for the multilayer adsorption process. The values of the geometric parameters (α = 0.51 and β = 1.00) indicate the formation of medium-height non-branching clusters of adsorbate molecules in the pores of the ACN/45 activated carbon sample.
The value of surface heterogeneity parameter as well as the shape of adsorption energy distribution on the surface of ACN/45 activated carbon indicate the occurrence of both sites with equal adsorption energy, i.e., micropores capable of holding only a single nitrogen molecule, and adsorption sites with a wide energy spectrum, i.e., larger pores capable of adsorbing more nitrogen molecules. The shape of the pore volume distribution PSD indicates a dominant contribution of pores in the range of 0.5 to 1.5 nm to the total pore volume as in the other samples analyzed in the activated carbons study, and some contribution of pores in the range of 1.5 to 2.5 nm, which confirms the results obtained with the LBET method.
On the basis of the performed research, the results of which are presented in this paper, it has been shown, taking into account all the determined structural, energetic and geometrical parameters, that the material showing generally the best adsorption properties, which can find practical industrial application, is activated carbon obtained in a gaseous nitrogen atmosphere at the activation time equal to 30 min.
The mentioned sample of activated carbon is characterized by high values of specific surface area and micropore volume determined by QSDFT method, i.e., SQSDFT = 1107 m2/g and VQSDFT = 0.475 cm3/g, as well as the volume of the first adsorbed layer determined by the LBET method (VhA = 0.459 cm3/g). The values of energetic parameters determined by LBET method indicate preferential energetic conditions for monolayer adsorption, and geometric parameters for adsorption of single nitrogen molecules in the micropores of the studied material. The value of the specific surface area determined by the BET method (SBET = 1069 m2/g), as well as the volume of micropores determined by the T-plot method (Vmic = 0.42 cm3/g), or total pore volume VT = 0.52 cm3/g confirm the above conclusion.
4. Conclusions
The analyses conducted in the present study provided interesting and valuable results from the point of view of both scientific research and activated carbon production technology. As shown in the present study, basing only on the results of the analysis using the BET method, i.e., the values of the specific surface area determined by this method, and forgetting about the assumptions and limitations of this method, it can be erroneously concluded that the material with the best adsorption properties is the sample of activated carbon ACC/45 obtained in a gaseous atmosphere of carbon dioxide and with an activation time of 45 min, because the value of the specific surface area determined for this sample is the highest among all the analyzed activated carbons. If other structure, energy, and geometrical parameters are taken into account, then the earlier conclusion is not so obvious anymore. The matter becomes even more complicated if the degree of surface heterogeneity is taken into account. Moreover, as is well known, the homogeneous surface of adsorption materials is a highly desirable feature in most technologies and practical applications of activated carbons. Therefore, as shown in the paper, it is a mistake to rely only on a single parameter such as specific surface area or volume of micropores, as it may turn out that these parameters in a dedicated industrial technology for the production of activated carbons are no less important than the parameters that are not taken into account. Therefore, based on the results of the studies presented in this work, it is recommended to perform the analysis of the porous structure and adsorption properties using several analysis methods, and LBET method deserves special attention.
Funding
This research was funded by the Research Subvention from the Polish Ministry of Science and Higher Education for the AGH University of Science and Technology in Krakow, grant number 16.16.210.476. The APC was funded by the AGH University of Science and Technology in Krakow.
Data Availability Statement
The data presented in this work can be made available on request.
Acknowledgments
I sincerely thank Roozbeh Hoseinzadeh Hesas from Department of Chemical Engineering, Faculty of Engineering, University of Malaya, Kuala Lumpur, Malaysia, for providing the data, i.e., adsorption isotherms, and for permission for their use, to perform the original analyses presented in this article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Conde-Rivera, L.R.; Suarez-Escobar, A.F.; Marin-Perez, J.J.; Junco-Rodriguez, M.J.; Lopez-Suarez, F.E. TiO2 supported on activated carbon from tire waste for ibuprofen removal. Mater. Lett. 2021, 291, 129590. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ahmed, M.M.M.; Du, W.T.; Kuo, S.W. Meso/microporous carbons from conjugated hyper-crosslinked polymers based on tetraphenylethene for high-performance CO2 capture and supercapacitor. Molecules 2021, 26, 738. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.C.W.; Hou, C.H. A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ubaid, A.M. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—A review. J. Clean. Prod. 2019, 242, 118409. [Google Scholar] [CrossRef]
- Miliotti, E.; Rosi, L.; Bettucci, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Characterization of chemically and physically activated carbons from lignocellulosic ethanol lignin-rich stream via hydrothermal carbonization and slow pyrolysis pretreatment. Energies 2020, 13, 4101. [Google Scholar] [CrossRef]
- Chen, W.; He, F.; Zhang, S.; Xv, H.; Xv, Z. Development of porosity and surface chemistry of textile waste jute-based activated carbon by physical activation. Environ. Sci. Pollut. Res. Int. 2018, 25, 9840–9848. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E. An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Coll. Surf. A 2017, 529, 443–453. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, G.B.; Hong, B.U.; Hwang, S.Y. Regeneration of activated carbons spent by waste water treatment using KOH chemical activation. Appl. Sci. 2019, 9, 5132. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Ma, R.; Hu, X.; Wang, L.; Wang, X.; Radosz, M.; Fan, M. CO2 adsorption on hazelnut-shell-derived nitrogen-doped porous carbons synthesized by single-step sodium amide activation. Ind. Eng. Chem. Res. 2020, 59, 7046–7053. [Google Scholar] [CrossRef]
- Perdana, Y.A.; Joni, R.; Aziz, E.H. Effect of KOH activator on the performance of activated carbon from oil palm kernel shell as supercapacitor electrode material. J. ACEH Phys. Soc. 2020, 9, 13–19. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. C 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Sun, Y.; Wang, W.; Yue, Q.; Yang, T. Comparative study on characterization of activated carbons prepared by microwave and conventional heating methods and application in removal of oxytetracycline (OTC). Chem. Eng. J. 2011, 171, 1446–1453. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Porous structure and adsorptive properties of pineapple peel based activated carbons prepared via microwave assisted KOH and K2CO3 activation. Microporous Mesoporous Mater. 2012, 148, 191–195. [Google Scholar] [CrossRef]
- Hoseinzadeh Hesas, R.; Arami-Niya, A.; Wan Daud, W.M.; Sahu, J.N. Comparison of oil palm shell-based activated carbons produced by microwave and conventional heating methods using zinc chloride activation. J. Anal. Appl. Pyrol. 2013, 104, 176–184. [Google Scholar] [CrossRef]
- Hoseinzadeh Hesas, R.; Arami-Niya, A.; Wan Daud, W.M.A.; Sahu, J.N. Preparation of granular activated carbon from oil palm shell by microwave-induced chemical activation: Optimisation using surface response methodology. Chem. Eng. Res. Des. 2013, 91, 2447–2456. [Google Scholar] [CrossRef]
- Kundu, A.; Gupta, B.S.; Hashim, M.A.; Sahu, J.N.; Mujawar, M.; Redzwan, G. Optimisation of the process variables in production of activated carbon by microwave heating. RSC Adv. 2015, 5, 35899–35908. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A. Preparation and characterization of raw and carbon from banana peel by microwave activation: Application in citric acid adsorption. J. Environ. Chem. Eng. 2015, 3, 2435–2447. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave pyrolysis of lignocellulosic biomass: Heating performance and reaction kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Wahi, R.; Zuhaidi, N.F.Q.; Yusof, Y.; Jamel, J.; Kanakaraju, D.; Ngaini, Z. Chemically treated microwave-derived biochar: An overview. Biomass Bioenergy 2017, 107, 411–421. [Google Scholar] [CrossRef]
- Liu, C.; Chen, W.; Hong, S.; Pan, M.; Jiang, M.; Wu, Q.; Mei, C. Fast microwave synthesis of hierarchical porous carbons from waste palm boosted by activated carbons for supercapacitors. Nanomaterials 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Kalderis, D.; Diamadopoulos, E. Numerical analysis of the influence of the impregnation ratio on the microporous structure formation of activated carbons, prepared by chemical activation of waste biomass with phosphoric acid. J. Phys. Chem. Solids 2017, 105, 81–85. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A.; Mohd Zobir, S.A. Electrochemical energy storage potentials of waste biomass: Oil palm leaf- and palm kernel shell-derived activated carbons. Energies 2018, 11, 3410. [Google Scholar] [CrossRef] [Green Version]
- Peredo-Mancilla, D.; Ghouma, I.; Hort, C.; Ghimbeu, C.M.; Jeguirim, M.; Bessieres, D. CO2 and CH4 adsorption behavior of biomass-based activated carbons. Energies 2018, 11, 3136. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Barreto, D.; Rodriguez Estupiñan, P.; Moreno-Piraján, J.; Ramírez, R.; Giraldo, L. Adsorption and photocatalytic study of phenol using composites of activated carbon prepared from onion leaves (allium fistulosum) and metallic oxides (ZnO and TiO2). Catalysts 2020, 10, 574. [Google Scholar] [CrossRef]
- Yang, P.; Rao, L.; Zhu, W.; Wang, L.; Ma, R.; Chen, F.; Lin, G.; Hu, X. Porous carbons derived from sustainable biomass via a facile one-step synthesis strategy as efficient CO2 adsorbents. Ind. Eng. Chem. Res. 2020, 59, 6194–6201. [Google Scholar] [CrossRef]
- Lorero, I.; Vizcaíno, A.J.; Alguacil, F.J.; López, F.A. Activated carbon from winemaking waste: Thermoeconomic analysis for large-scale production. Energies 2020, 13, 6462. [Google Scholar] [CrossRef]
- Melouki, R.; Ouadah, A.; Llewellyn, P.L. The CO2 adsorption behavior study on activated carbon synthesized from olive waste. J. CO2 Util. 2020, 42, 101292. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.W. Directly synthesized nitrogen-and-oxygen–doped microporous carbons derived from a bio-derived polybenzoxazine exhibiting high-performance supercapacitance and CO2 uptake. Euro. Polym. J. 2020, 138, 109954. [Google Scholar] [CrossRef]
- Borhan, A.; Yusuf, S. Activation of rubber-seed shell waste by malic acid as potential CO2 removal: Isotherm and kinetics studies. Materials 2020, 13, 4970. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Hu, X. Analysis of the effect of conditions of preparation of nitrogen-doped activated carbons derived from lotus leaves by activation with sodium amide on the formation of their porous structure. Materials 2021, 14, 1540. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhang, H.; Shao, L.M.; Lü, F.; He, P.J. Preparation and application of hierarchical porous carbon materials from waste and biomass: A review. Waste Biomass Valor. 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Goel, C.; Mohan, S.; Dinesha, P. CO2 capture by adsorption on biomass-derived activated char: A review. Sci. Total Environ. 2021, 798, 149296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Dissanayake, P.D.; Gao, B.; Liu, W.J.; Lee, K.B.; Ok, Y.S. Review on upgrading organic waste to value-added carbon materials for energy and environmental applications. J. Environ. Manag. 2021, 296, 113128. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- El-Nahas, S.; Salman, H.M.; Saber, A.M. Production of low-price carbon for removal of aluminium ions in potable water. J. Environ. Eng. Sci. 2021, 16, 145–164. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.V.N.; Gnana Prakash, D.; Antonysamy, A.J.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yu, Q.; Li, M.; Zhao, H.; Jin, S.; Huang, Y.; Fan, J.; Chen, J. Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review. J. Environ. Chem. Engin. 2021, 9, 105905. [Google Scholar] [CrossRef]
- Hoseinzadeh Hesas, R.; Arami-Niya, A.; Wan Daud, W.M.A.; Sahu, J.N. Microwave-assisted production of activated carbons from oil palm shell in the presence of CO2 or N2 for CO2 adsorption. J. Ind. Eng. Chem. 2015, 24, 196–205. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Zhao, L.; Wu, Y.W.; Han, J.; Wang, H.X.; Liu, D.J.; Lu, Q.; Yang, Y.P. Density functional theory study on mechanism of mercury removal by CeO2 modified activated carbon. Energies 2018, 11, 2872. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.; Yang, S.; Jiang, Y.; Zhao, F.; Li, Z.; Zaman, B.; Fayaz, M.; Li, X.; Chen, Y. Artefact peaks of pore size distributions caused by unclosed sorption isotherm and tensile strength effect. Adsorption 2020, 26, 633–644. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micromesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Fierro, V.; Celzard, A. Numerical studies of the effects of process conditions on the development of the porous structure of adsorbents prepared by chemical activation of lignin with alkali hydroxides. J. Colloid Interface Sci. 2017, 486, 277–286. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Fierro, V.; Celzard, A. Confrontation of various adsorption models for assessing the porous structure of activated carbons. Adsorption 2019, 25, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Delgadillo, D.P.V. Computer analysis of the effect of the type of activating agent on the formation of the porous structure of activated carbon monoliths. J. Mater. Res. Technol. 2019, 8, 4457–4463. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Serafin, J.; Booth, A.M.; Michalkiewicz, B. Computer analysis of the effect of activation temperature on the microporous structure development of activated carbon derived from common polypody. Materials 2021, 14, 2951. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E.; Fierro, V.; Celzard, A. An Evaluation of the impact of the amount of potassium hydroxide on the porous structure development of activated carbons. Materials 2021, 14, 2045. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).