Abstract
Sludge pre-treatments are emerging as part of the disposal process of solid by-products of wastewater purification. One of their benefits is the increase in methane production rate and/or yield, along with higher loading capacities of existing digesters. In this study, we report the performance of a pilot-scale compartmentalized digester (volume of 18.6 m3) that utilized a mild thermal pre-treatment at 70 °C coupled with hydrogen peroxide dosing. Compared with a reference conventional anaerobic digester, this technique allowed an increased organic loading rate from 1.4 to 4.2 kg volatile solids (VS)/(m3d) and an increment in the solids degradation from 40 to 44%. To some extent, these improvements were promoted by the solubilization of the tightly-bound fraction of the extracellular polymeric substances to looser and more accessible fractions without the formation of refractory compounds. In sum, our results suggest that this pre-treatment method could increase the treatment capacity of existing digesters without significant retrofitting.
1. Introduction
Population growth and increase in the coverage of sanitation are factors that increase the volume of waste activated sludge (WAS) to be treated. Anaerobic digestion is a common approach to reduce the organic sludge mass; however, the low hydrolysis rate (khyd) of WAS results in the need for long retention times and large reactor volumes. This is particularly problematic when an existing plant, without the possibility for expansion, is faced with an increased volume of WAS to be treated. Consequently, WAS pre-treatments have emerged with advantages such as increased chemical oxygen demand (COD) solubilization and biodegradation, among others [1,2]. The application of pre-treatments with low consumption of energy as well as the avoidance of sophisticated and complex processes is desirable in order to increase the energy recovery from sludge processing, while additional expenses on investment and maintenance remain low. In this regard, mild-temperature pre-treatment coupled with hydrogen peroxide dosing in combination with compartmentalized digestion seems to be a low-cost approach with potentially positive outcomes [3,4,5,6].
Thermal pre-treatment at temperatures below 100 °C utilizes less energy compared with pre-treatment at higher temperatures and pressures [7]. The required energy can be supplied in the form of waste heat and does not require pressurized vessels. Moreover, low-temperature pre-treatment can be improved via the addition of hydrogen peroxide; Jung and co-workers documented that pre-treatment at 60 °C together with 362 mg H2O2/g total solids (TSs) achieved a 10% increase in biogas production compared with standalone thermal pre-treatment [3]. Similarly, Cacho Rivero and Suidan observed that a pre-treatment at 90 °C, applying between 0 and 412 mg H2O2/g total suspended solids (TSSs), increased volatile suspended solids’ (VSSs) degradation from 46 to 71.6% [4]. Finally, Özön and Erdinçler reported an absolute increase in biodegradation of 9% at 75 °C and 1000 mg H2O2/g TS compared with the control sample [8]. These studies were performed at laboratory scale.
Compartmentalized digestion, i.e., staging of anaerobic digestion using the same temperature, but subjected to different pH values in separated compartments, has also been applied with positive results. For instance, Maspolim and colleagues studied two-staged mesophilic digestion with pH values of 5.5 and 7.0 for the first and second compartment, respectively [5]. Their system was able to maintain a stable methane production and volatile solids’ (VSs) degradation after an increase in the organic loading rate (OLR) from 2.2 to 3.5 kg COD/m3d, equivalent to 1.3–2.1 kg VS/m3d. In contrast, under the same settings, the single-staged reactor delivered a diminished performance in both biogas and degradation of solids. Similarly, Bhattacharya et al. used a two-stage configuration to digest a mixture of primary and secondary sludge with a hydraulic retention time (HRT) of 2.7 d for the first reactor and 10 d for the second one [6]. Their reactor achieved a 6% absolute increase in VS degradation compared with one-stage digestion at the same HRT. The reasons for the observed improvements in phased digestion have been associated with the optimization of environmental conditions for hydrolytic/fermentative bacteria in terms of pH, retention time, and loading rate [5,6,9].
It is well-known that hydrolysis is the rate-limiting step in particulate substrate degradation such as excess sewage sludge. In this study, we examined to what extent mild thermal pre-treatment provoked an increase in the WAS hydrolysis rate, which in turn allowed a substantial increase in applicable OLR in a compartmentalized digester, combining the advantages of the two treatment approaches. In addition, the impact of H2O2 dose during mild temperature pre-treatment was evaluated at lab-scale. Finally, pros and cons of this process amendment are discussed as well as possible mechanisms underlying the proposed pre-treatment technique.
2. Materials and Methods
2.1. Substrate, Inoculum, and Pre-Treatment
Secondary sludge with a TS concentration of 5–6% from wastewater treatment plant (WWTP) Nieuwgraaf (Arnhem, The Netherlands) was used for lab-scale experiments. Digestate from the anaerobic digesters, operated at an HRT of 21 days and 37 °C, was incubated for about one week, after which it was filtered with a 1 mm sieve and used as inoculum. Temperatures of 60, 70, and 80 °C were applied to the secondary sludge, as well as exposure times of 20, 30, or 240 min. Afterwards, 0–250 mg H2O2/g TS of a 30% solution (Carl Roth GmbH, Karlsruhe, Germany) was applied to the warm sludge and the sample was mixed until temperature decreased to 35 °C. The pilot-scale reactors were fed with a blend of primary and secondary sludge in a mass ratio of 25:75. Only the secondary sludge was subjected to thermal pre-treatment, and the temperature, peroxide dose, and exposure time were 70 °C, 15 mg H2O2/g TS, and 30 min, respectively.
2.2. Anaerobic Digestion
During all lab-scale experiments, the cumulative methane production (CMP) assays were performed in triplicate, using an AMPTS-2 (BPC Instruments AB, Lund, Sweden). Bottles with a capacity of 500 mL were filled with 400 mL of a mixture of inoculum and substrate in a ratio of 2 on VS basis. Hereafter, phosphate buffer and nutrient solutions were added, as described by [10], and the bottles were sealed and flushed with a blend of 80% nitrogen and 20% CO2 by volume, until the free space was replaced at least five times to displace oxygen in the headspace. Afterwards, a mixing motor was coupled to each bottle, which were incubated at 35 °C. The produced biogas was directed to a 3 M NaOH scrubber solution that adsorbs CO2 and the flow of methane was then quantified via a calibrated cell, which produces an electrical signal every ~10 N mL of gas.
The pilot-scale set-up consisted of two treatment lines (Figure 1): a reference digester consisting of a single stage reactor (volume of 13.7 m3) and a compartmentalized digester composed of two staged reactors of 9.3 m3 each. During the pilot-scale experiment, biogas was measured via a gas-flow meter Model Proline Prosonic Flow B 200 (Endress and Hauser AG, Reinach, Switzerland). This device is also able to quantify the methane content of biogas. Each compartment was equipped with one meter. The working volume of the pilot reactors fluctuated following the applied OLR. The compartmentalized digester had a minor recirculation from the second to the first compartment, which was adjusted based on the pH in the first digester.
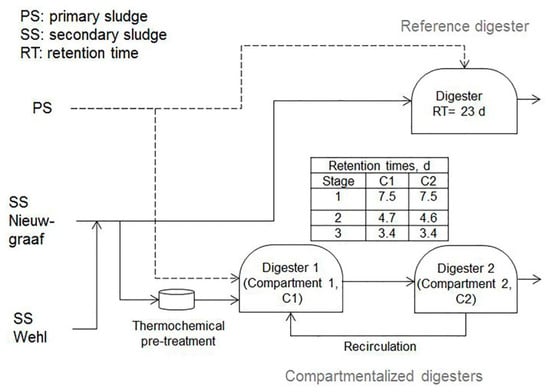
Figure 1.
Scheme of the pilot-scale test.
2.3. Analytical Methods
Total solids(TS) and VS were quantified according to standard methods 2540B and 2540E [11]. For the determination of chemical oxygen demand (COD), the open reflux method (5220B) was used. The soluble fraction (sCOD) was obtained by centrifuging the sample at 15,000× g for 10 min and filtering the supernatant through a polytetrafluoroethylene (PTFE) filter (Macherey-Nagel GmbH & Co, Düren, Germany) with a nominal pore-size of 0.45 μm.
E. coli determination was performed via the pour plate method 9215B [11], by means of Compact Dry EC (Nissui Pharmaceutical Co., Japan). Dilutions were in the range of 101 to 103 for pre-treated samples and up to 105 for raw sludge. The water for dilution was sterile saline solution of 0.9% NaCl.
Volatile fatty acids (VFAs) were measured using the procedure of [12]. Samples of digestate were centrifuged at 14,500 rpm for 5 min and the supernatant was filtered with the PTFE filter mentioned above and diluted by a factor of 2 using a 320 mg/L solution of 1-pentanol as solvent and 10 µm of 98% formic acid. The working volume of the vial was 1.5 mL.
Extracellular polymeric substances (EPSs) were extracted based on the method proposed by [13]. Ca2+, Mg2+, Na+, and K+ were determined using an ion-chromatograph 883 cation system (Metrohm AG, Herisau, Switzerland) equipped with a metrosep C4-150/4 column (Metrohm AG, Herisau, Switzerland). The eluent used was 3 mM HNO3 at a flow rate of 0.9 mL/min.
Microbial composition analyses were performed using DNA extractions, taking duplicate samples of 0.5 g from the liquors of both the reference and compartmentalized digesters. DNA was extracted with a FastDNA™ SPIN Kit for Soil (MP Biomedicals LLC, Santa Ana, CA, USA), while its quality and quantity were evaluated by a Qubit3.0 DNA detection kit (Qubit® dsDNA HS Assay Kit, Life Technologies, Carlsbad, CA, USA). High throughput sequencing was performed by the HiSeq Illumina platform with universal primer 515F/806R for bacterial and archaeal 16S rRNA genes (Novogene Limited, Cambridge, UK). Finally, sequences were further analyzed by the quantitative insights into microbial ecology (QIIME) pipelines (version 1.7.0) [14]) to pair forward and reverse sequences, and chimeras were removed by the UCHIME algorithm [15]. Sequences with ≥97% similarity were clustered into one operational taxonomic unit (OTU) by UCLUST algorithm [16]. Singletons were removed and OTUs with an occurrence less than three times in at least one sample were excluded. Taxonomic assignation was performed in Mothur software (University of Michigan, Ann Harbor, MI, USA) against the SILVA Database. The variation in relative abundance of bacteria at the phylum level and that of archaea at the genus level was plotted with MS Excel.
Dewaterability was assessed by measuring the capillary suction time (CST) according to method 2710G [11] using a CST type 304M (Triton Electronics Ltd., Essex, UK) and chromatography paper manufactured by the same company. Press-filter assays were performed with a Mareco Minipress MMP3 (Afmitech, Friesland, The Netherlands). The used conditioning agent (polymer) was Superfloc C-82090 (Kemira Oyj, Helsinki, Finland), the same as the one used in the full-scale process. Different doses were assayed until optima were found for each system. Firstly, the amount of polymer corresponding to the specified dose was applied to the digestate in a flocculator (Velp Scientifica Srl, Usmate Velate, Italy) at two mixing regimes: 15 s at 250 rpm and 1 min at 22 rpm, until a lump of sludge was formed. Secondly, 250 mL of the resulting suspension was added to the working cell of the press-filter. The pneumatic pressure was 6 bar with a disc-pressing speed of ~5 mm/min. Finally, a pilot-scale centrifuge model UCD 205-00-32 (GEA Group AG, Düsseldorf, Germany) with a set rotational speed of 3600 rpm and a maximum flow of 5 m3/h was used for comparison.
2.4. Biodegradation and Methane Production Rate
Sludge biodegradation (β) was calculated according to Equation (1). β0 corresponded to the amount of methane produced during the digestion divided by the theoretical production of methane from one gram of substrate COD. For simplicity, the COD required for cell maintenance and synthesis was not considered. The methane production rate (kCH4) was calculated based on methane production under the assumption of following first-order kinetics [17], using a two-substrate kinetic model as given in Equation (2). The division between kCH4-rapid and kCH4-slow was set based on the visual examination of the slopes of the methane production curve, which occurred between day 3 and 4.
2.5. Design of Experiments and Statistical Analyses
The lab-scale phase followed a full-factorial design of experiments. Either one- or two-way analysis of variance (ANOVA) was used to assess the effect of pre-treatment temperature and exposure time by means of Origin Pro 9 (Origin Lab Corp., Northampton, MA, USA). Standard deviation was used as a measurement of experimental error.
For the pilot-scale experiment, various statistical tools and techniques were applied to identify differences between the two reactors. Firstly, histograms were plotted to examine the data distribution and to define proper statistical hypotheses and models to be applied. A Shapiro–Wilks test was used to assess the normality of the data sets. Secondly, a Wilcoxon signed rank test (the non-parametric equivalent of the Student’s t-test) was applied. Finally, the statistics associated with the Wilcoxon test (W) and its p-value were calculated to assess the statistical significance between the studied digesters. The analysis of data was performed with the statistical software R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria). Linear adjustments were obtained with a linear regression model in MS Excel. Statistical analyses for both the lab and pilot scale considered a 95% confidence level (α = 0.05).
3. Results and Discussion
3.1. Lab-Scale Experiments
The effects of pre-treatment temperature, exposure time, and peroxide dose on biodegradation, kCH4, sCOD, E. coli, CST, and VS degradation were studied in a series of three batch experiments. In experiment #1, we investigated the effect of temperature, i.e., 60, 70, and 80 °C, and holding times, i.e., 20, 30, and 240 min, with a peroxide dose of 10 mg/gTS for all samples. The combination that resulted in the highest methane production extent was implemented in experiment #2, at peroxide doses of 0, 5, 10, and 15 mg H2O2/gTS. Finally, the same temperature and exposure time combination was applied in experiment #3, using peroxide doses of 15, 30, 50, 100, and 250 mg H2O2/gTS. Overall, the experimental design allowed a comparison of the effects of temperature, exposure time, and H2O2 dose (Table 1). A discussion of the relevant findings is presented below.

Table 1.
Effects of the studied variables related to applied temperature, H2O2 dose, and exposure time. CST, capillary suction time; sCOD, soluble fraction chemical oxygen demand; VS, volatile solid; TS, total solid.
3.1.1. Effects of Temperature and Time
Despite a notorious COD solubilization (Figure S1), pre-treatment at 60 °C had a similar methane production compared with the non-heated sample (named as reference in Figure 2a). Pre-treatment conditions at 70 °C during 30 min revealed the highest yield in terms of cumulative methane production, while COD solubilization was found to be highest at 80 °C during 240 min. However, pre-treatment at 80 °C did not show any considerable difference in methane production compared with 60 °C pre-treatment and the non-heated sample. Moreover, the residual sCOD after 30 days of digestion was highest for 80 °C pre-treatment, with an increasing trend at increased heating time (Figure 2b and Table 1). No VFA analyses were conducted during digestion in this stage of the study. However, the absence of a sigmoidal shape in any of the cumulative methane production curves (Figure S2) suggests that methanogenesis was not the rate limiting step. Moreover, the overall VS degradation for the 80 °C pre-treatment was lower than for the lower temperatures (Table 1). Thus, it was assumed that about one-third of the solubilized COD during pre-treatment at 80 °C was recalcitrant, in contrast to the other temperatures that degraded almost all of the solubilized COD.
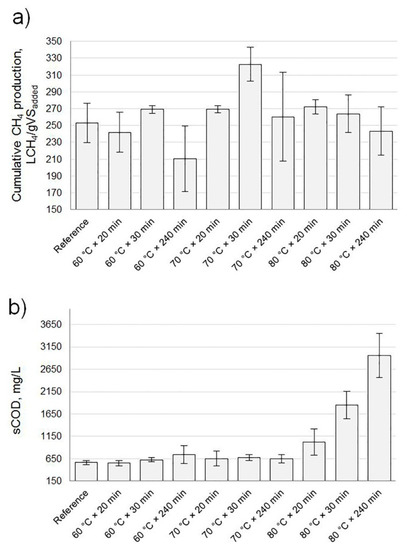
Figure 2.
(a) Cumulative methane production for the assayed conditions; (b) soluble fraction chemical oxygen demand (sCOD) of the digestates after 30 days of digestion. Note: all conditions (including reference) included 10 mg H2O2/g total solids (TSs). VS, volatile solid.
Regarding the duration of pre-treatment, an exposure time of 240 min produced less methane compared with shorter times at any temperature. This finding agrees with previous studies showing that temperature and exposure time have a combined effect on biogas production under similar conditions to those in this research [18,19,20].
Apparently, during pre-treatment, only the digestible fraction of the COD was solubilized, which may explain the observed rise in the hydrolysis rate found by [21] at 60 °C. Methane production rates reflecting solids hydrolysis were determined only for the reference and WAS pre-treated at a temperature of 70 °C (Figure 3) and (Table S1). The combination of 70 °C and 5–15 mg H2O2/gTS resulted in an increase in kCH4-rapid, while kCH4-slow was unaffected by pre-treatment (Figure 3). In contrast, the application of hydrogen peroxide to non-thermally treated WAS resulted in a decrease in kCH4-rapid, probably because of the oxidative stress caused by peroxide reaching anaerobic digestion. This finding suggests that the exposure to a specific temperature is pivotal for applying H2O2 during the pre-treatment and to prevent H2O2 from entering the anaerobic digesters.
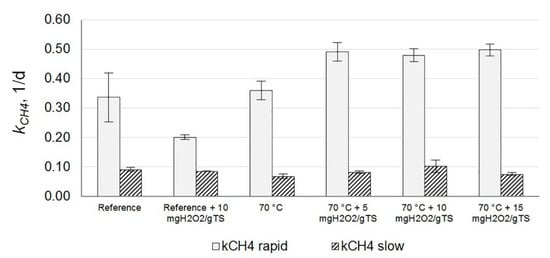
Figure 3.
Methane production rates following pre-treatments during experiment #2, at an exposure time of 30 min. n = 3 except for reference + 10 mg H2O2/gTS, which had n = 2.
The observed increase in kCH4-rapid could have originated from the possible production of VFAs during pre-treatment. To verify this, a sample of activated sludge was pre-treated at 70 °C with 15 mg H2O2/gTS. The results, shown in Figure S3, demonstrated that the concentration of VFAs during pre-treatment was below 30 mg/L. On the other hand, the VFA concentrations during digestion of a sludge sample that was similarly pre-treated accumulated to values between 100 and 500 mg/L. The disparity between the VFA concentrations during pre-treatment and digestion clearly shows that the solubilized COD produced during pre-treatment was only very limitedly composed of VFAs.
CST was measured as a proxy for dewaterability of the digestate. Compared with the reference, a pre-treatment temperature of 80 °C produced digestates with lower CSTs, indicating an improvement in dewaterability (Figure S4). On the other hand, temperatures of 60 °C and 70 °C resulted in CST times similar to the control sample. These results partially agree with those of [22], who did not find any changes in CST after exposing WAS to 60 and 80 °C, compared with the untreated sample. On the other hand, Laurent and colleagues observed a reduction in average particle size from 118 μm to 82 μm for a pre-treatment temperature of 75 °C and 120 min of exposure time [23]. It is inferred that small particles increase the surface area for water bonding, and thus will negatively affect the dewaterability [24]. In our present study, the particle size distribution was not measured.
The concentration of E. coli was measured to assess hygienization of the pre-treated sludge. Figure S5 shows that E. coli declined proportionally to temperature and treatment time. At the harshest pre-treatment combination (80 °C and 240 min), their content fell below the detection level in the treated sample. After digestion, E. coli were detected even in higher concentrations compared with the untreated sample (Figure S6). Because the batch digesters were inoculated with digestate, residual E. coli could have derived from either the substrate or the inoculum. Apparently, digester conditions, that is, abundant presence of nutrients and trace elements that are released during pre-treatment or digestion, are suitable for further manifestation or even possible growth of E. coli [25,26].
3.1.2. Effects of Peroxide
The addition of H2O2 at concentrations ≤15 mg/g TS did not increase either methane production or sCOD, nor did it affect the concentration of E. coli. The results showed that a positive effect of the addition of peroxide was evident only when it was accompanied by thermal pre-treatment at 70 °C, as it increased the methane production rate from 0.34 to 0.49 d−1 (Table 1). The assessed kCH4 reflected the solids’ hydrolysis rate, which is related to the particle size of the substrate [27,28]. Therefore, an increase in kCH4 with the addition of H2O2 likely can be ascribed to a reduction in particle size. A previous study demonstrated that the addition of 20 mg H2O2/gTS at 90 °C triggered a decrease in the particle size of treated sludge compared with the sole application of thermal pre-treatment [29]. Alternatively, a Fenton mechanism may have played a role in the increased degradation rates; Fenton chemistry is related to the utilization of peroxide for the formation of reactive oxidative species (particularly hydroxyl radicals) under the presence of ferrous iron as catalyst [8,29]. Although not very likely in our current experimental set-up, a Fenton reaction might have occurred in the batch assays for two reasons. Firstly, catalase—the enzyme that decomposes H2O2 into water; and molecular oxygen—denaturizes at temperatures higher than 60 °C [30,31], preventing peroxide from being scavenged in the WAS matrix after pre-treatment. Secondly, the remaining H2O2 molecules in WAS might have reacted with iron species or other transition metal catalysts during heating, which could have produced hydroxyl radicals. However, for an efficient Fenton reaction, iron should remain in solution, for which a pH < 4 is optimal [32,33]. However, during the pre-treatment, the pH of sludge fluctuated between 6.0 and 6.5 (Figure S3).
3.1.3. Reproducibility
The assays of cumulative methane production were performed with WAS and anaerobic digestate samples taken at different moments in a time span of three months. The results revealed limited reproducibility of the specific methane productions of the untreated samples (see Figure S7, flask R1, R2, and R3). In addition, the WAS responded substantially different to pre-treatment (Figure S2, flask E and M; and N and Q), with absolute variations in biodegradation (β0) ranging from 11% to 29% at the same pre-treatment conditions. The differences in specific methane production of the control samples could be a consequence of the fluctuating seasonal composition of the WAS [34], which could also have influenced the pre-treatment results [35,36]. Therefore, a detailed characterization of the substrate is relevant for a meaningful comparison between studies.
3.2. Pilot-Scale Experiments
The best settings of the pre-treatment method were applied in the pilot-scale experiment with the objective to markedly increase the OLR, VS degradation, and biogas production. To fulfil this goal, a combination of mild thermal pre-treatment methods with peroxide dosing and the more proven compartmentalized anaerobic digestion was chosen. The applied pre-treatment settings were adopted from the batch test: a temperature of 70 °C, an exposure time of 30 min, and the addition of 15 mg H2O2/gTS. The OLR was the independent variable, whereas biogas production and the degradation of organics were the response variables. The test consisted of three stages: for stage #1, the single stage reference reactor had an OLR of 1.4 kg VS/m3d (2.1 kg COD/ m3d) corresponding to a retention time of 23 days, a typical value for anaerobic digestion of WAS. The OLR and retention time of the compartmentalized reactor were 4.2 kg VS/m3d (5.8 kg COD/ m3d) and 15 days. The collection of data started after at least three retention times for attaining a stabilized condition after the previous feeding regime. In addition, during days 30 to 49, the reactor was being adapted for the conditions of the next stage (transition period), thus those data were excluded for statistical analyses. During stages #2 and #3, the compartmentalized reactor was tested to examine its ability to withstand peak loads of 6.5 and 10 kg VS/m3d, while the reference reactor was stopped. The duration of these peak load experiments was 23 and 7 days, respectively. The outcomes of these pilot tests are discussed below.
3.2.1. Stage #1
Figure 4 shows the sets of data of biogas production and VS degradation for both reactors, whereas Table 2 presents the average values obtained along the test. The spreading of data complicates differentiation between the two reactors, as is observed from the histograms in Figure S8. The Shapiro–Wilks normality test showed that the assumption of normality was not accomplished for the reference reactor (p-value = 0.0191). This is also shown in the slight bias to the left side of the histogram in Figure S8. On the other hand, the dataset of the compartmentalized reactor can be considered extracted from a normal distribution (p-value = 0.1540), despite the absence of symmetry around the mean. As at least one of the reactors did not comply with the assumption of normality, and the non-parametric Wilcoxon signed rank test was applied to assess the differences between the two processes. The application of such a test requires the fulfilment of four assumptions [37], which are evaluated in Table S2. The Wilcoxon signed rank test indicated that there was a significant difference in biogas production between the two reactors (W = 321; p-value = 0.0011). In other words, the compartmentalized reactor produced around 15% more biogas compared with the reference (Table 2).
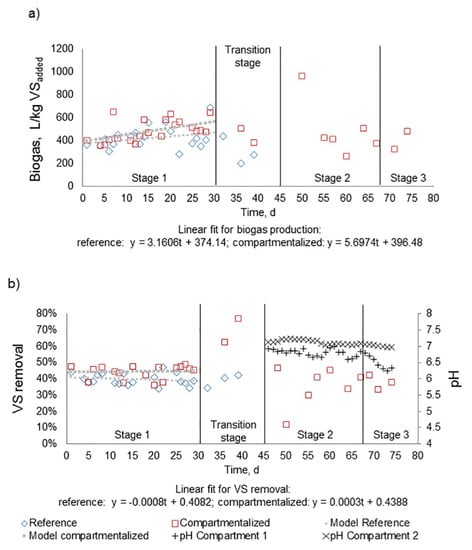
Figure 4.
(a) Specific biogas production; (b) VS degradation and pH of both reactors.

Table 2.
Reactor performance. OLR, organic loading rate; VFA, volatile fatty acid.
The resulting linear regression curves obtained from biogas production data (Figure 4a) show a positive slope, suggesting that both reactors were not fully stable during the experiment and were likely still adapting to the substrate. During stage #1, the compartmentalized reactor generated around 85% of the biogas in the first compartment (i.e., during the first 7 days of digestion) (Figure S9), coinciding with the lab-scale observation on the increase in kCH4 (Figure 3 and Table S1).
In line with the results of biogas production, the histograms for VS degradation are shown in Figure S10. The Shapiro–Wilks normality test results showed that the compartmentalized reactor did not have a normal data distribution (p-value = 0.0058). The Wilcoxon test was applied and resulted in a significant difference between the reference and the compartmentalized reactor for VS degradation (W = 281; p-value = 0.0227), which represents an increase of 5% in absolute VS degradation (Table 2).
Finally, during stage #1, VFA analyses were performed three times, showing that their concentration was below 10 mg/L for both reactors (Table 2), suggesting the absence of substantial VFA accumulation and hydrolysis being the rate limiting step in stage #1.
Previous research has shown that improved hydrolysis caused by thermal pre-treatment benefits the stability of the digestion process at reduced retention times [38,39,40]. Our results support these findings and clearly show that a higher OLR can be attained with a mild thermal pre-treatment in combination with compartmentalized digestion. In addition, the latter reactor showed an increase in biogas production and VS degradation. However, a previous study showed opposite results and reported a decline in VS degradation at similar pre-treatment conditions [38]. They applied a pre-treatment at 70 °C for two hours to a mixture of slaughterhouse products (digestive tract content, drum-sieved waste, dissolved air flotation sludge, and grease) and secondary sludge in a ratio 1 to 7 by volume. The OLR in the anaerobic digester was raised from 1.8 to 3.7 kg VS /m3d, thus reducing the retention time from 25 to 14 days. They observed a drop in absolute VS degradation from 40% (reference) to 33% (pre-treated sample). It should be noticed that the addition of a highly reduced substrate, such as slaughterhouse products, could have contributed to the observed unstable digestion performance [38].
3.2.2. Stages #2 and #3
The objective of executing the final experimental stages was to test the resilience of the compartmentalized reactor when imposing short-term operational changes. During Stage #2, a 23-day peak load was tested of 6.5 kg VS/m3d with a primary to secondary sludge ratio of 25:75 by weight. The solids retention time (SRT) was 4.7 and 4.6 days in compartment 1 and 2, respectively. In Stage #3, the peak load was further augmented to 10 kg VS/m3d during 7 days, by increasing the more rapidly bio-degradable primary sludge to a feed ratio of 40:60 (primary to secondary sludge). The SRT in each compartment was 3.4 days during this stage. Unfortunately, there was no reference reactor available for comparing the response of a conventional digester to these kinds of peak loadings. In Stages #2 and #3, VFA and pH were monitored, which are indicators of process perturbation of anaerobic digestion.
During Stage #2, operation at an OLR of 6.5 kg VS/m3d, the concentration of VFA reached 180 mg VFA/L in the first compartment and was below the detection limit in the second reactor (Table 2). Nonetheless, a further increase in OLR to 10 kg VS/m3d in Stage #3 resulted in 1900 and 330 mg VFA/L for the first and second compartment, respectively. The accumulation of VFAs was also reflected in the decline in pH from 7.0 to 6.2 in the first compartment (Figure 4b). During the highest loading condition, the ratio of acetate to propionate was 1:0.86, while the concentration of butyrate and valerate was in excess of 50 mg/L each. Such VFAs’ concentrations have been associated with imbalances in full-scale processes and suggest that, in this stage, methanogenesis became the rate limiting step. The observed VFAs’ values in Stage #3 agree with previously reported values using thermal-phased anaerobic digestion, in which a concentration of 4400 mg VFA/L was reached at a retention time of 7 days [39]. Similarly, an OLR of 8 kg VS/m3d (retention time of 4.2 days) caused acidification and interruption of biogas production [41]. In the latter studies, the loss of performance at such loading rates was ascribed to the washout of methanogens. Nonetheless, our results suggest that the compartmentalized reactor was able to handle peak loadings for at least one retention time (between 7 and 9 days).
3.2.3. Effects of Mild Thermal Pre-Treatment Coupled with Hydrogen Peroxide
Because of the configuration of the experiment, it was not possible to allocate the observed performance to either thermal pre-treatment or compartmentalized digestion. Nonetheless, based on existing literature, the potential impact of either or both approaches is further discussed. In the present study, mild-temperature pre-treatment coupled with hydrogen peroxide pre-treatment solubilized 13.2% ± 5.5 (n = 23) of the total COD of the sludge along the complete test duration. The concentration of residual sCOD in the effluent after digestion of both the reference and compartmentalized digester was comparable. It is hypothesized that the increment in sCOD could be a response of the variation in the structural arrangement of extracellular polymeric substances (EPSs). EPSs form a mechanically stable network of proteins, polysaccharides, and lipids that immobilize cells [42] and comprise a major constituent of WAS. The debilitation of this network is expected to diminish the resistance of sludge to biodegradation [43]. During stage #1, mild-temperature pre-treatment coupled with hydrogen peroxide pre-treatment triggered the transfer of the tightly-bound fraction to soluble fractions of EPS (Figure 5a). This effect can be assigned solely to the temperature effect during pre-treatment, as, according to Cai and Liu, the addition of 0–80 mg H2O2 /gTS caused a negligible increase in the solubilization of organic matter [44].
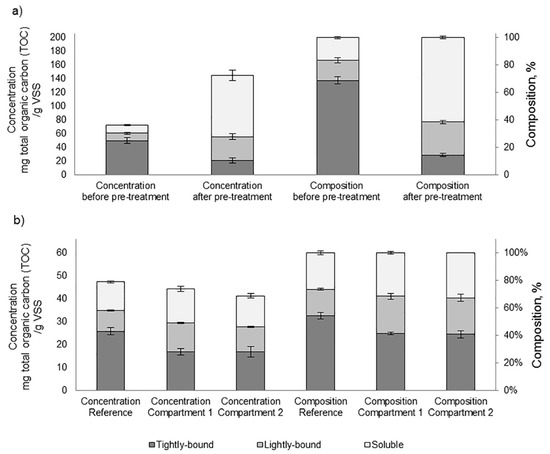
Figure 5.
Extracellular polymeric substance (EPS) concentration and fractionation of EPS (a) before and after pre-treatment, n = 6; and (b) in the anaerobic liquors, n = 2. VSS, volatile suspended solid.
Likewise, sCOD and EPS were analyzed in the liquors of the first and second compartments, as well as in the reference reactor. During Stage #1, the digestates of both compartments showed similar a sCOD concentration, demonstrating that sCOD was mostly converted in the first compartment (Table 2). On the other hand, the digestate of the second compartment had a slightly higher amount of sCOD compared with the reference. This observation might be due to (a) the formation of recalcitrant compounds during mild-temperature pre-treatment coupled with H2O2 pre-treatment; and/or (b) the presence of residual biodegradable sCOD as a result of the shorter retention time of the compartmentalized reactor. The lab-scale experiments showed that, after 30 days of digestion, around 95% of the solubilized COD at 70 °C was consumed. Therefore, we infer that the pre-treatment produced mostly biodegradable sCOD rather than recalcitrant compounds.
Figure 5b shows the fractionation of EPS of the anaerobic liquors. As these samples included both primary sludge and the anaerobic biomass, a direct comparison with the profiles of pre-treated WAS is not possible. Nonetheless, it is observed that the concentration and composition of EPS of sludge from the first and second compartment of the compartmentalized digester were similar. In contrast, the reference reactor had a higher amount of tightly-bound EPS and a slightly lower concentration of soluble EPS. This shift evidences that pre-treatment debilitated the EPS network through the breakdown of its most recalcitrant part and transformed it into a more accessible fraction.
3.2.4. Effects of Compartmentalized-Digestion and Recirculation
The results of the present research suggest that compartmentalization of digestion indeed provoked a separation of the phases of anaerobic digestion; the first compartment produced the majority of the VFA and biogas, while the second one acted as a polishing and buffering step. This was also reflected in the pH values. During Stage #2, the pH value of the first compartment was lower compared with the second one, i.e., 6.8 ± 0.1 and 7.1 ± 0.1, respectively. Similarly, recirculation has been reported to improve the hydraulics of reactors and the fluxes of nutrients and metabolites [45]. Indeed, recirculation from the second to the first compartment was devised to control pH and alkalinity. The literature has demonstrated that a recirculation of 60% caused an increase in methane yield from 290 to 330 L CH4/kg VSadded [45]. Remarkably, in our study, the applied recirculation rate appeared to be irrelevant to affect performance. We hypothesize that the improved performance in the present research was fostered by (a) the supply of hydrolyzed sludge via mild-temperature pre-treatment coupled with hydrogen peroxide pre-treatment; and (b) an improved contact between the substrate and the anaerobic biomass resulting from compartmentalization.
3.2.5. Microbial Composition and Structure
Samples for microbial community analysis were taken on days 19 (during Stage #1) and 67 (at the end of Stage #2). In our study, the main limitation for properly interpreting the results was the short-term operation, i.e., about three retention times, or about six retention times for the individual compartments, a short time for full adaptation of the microbial community. Thus, care should be taken with correlating the observed performance to changes in microbial populations. The dominant four bacteria phyla, i.e., proteobacteria, firmicutes, bacteroidetes, and actinobacteria, accounted for at least 65% of abundance of the community (Figure S11), matching previous works [46,47]. The pre-treatment step reduced the abundance of Proteobacteria and Bacteroidetes, whereas it increased the relative share of Firmicutes and Actinobacteria in the secondary sludge. This implies that Firmicutes and Actinobacteria were likely more resistant to the combined pre-treatment. Similarly, compared with the reference, Firmicutes had a higher abundance in both digester compartments. It has been documented that higher OLRs increase the number of Firmicutes [48], a phylum that has been related to better performance during phased digestion [49].
Regarding the archaeal domain, Methanosaeta outweighed Methanosarcina for both the reference and compartmentalized reactors (Figure S12). The striking dominance of Methanosaeta, which is characterized by a low growth rate, might have resulted in increased acetate concentrations during the subsequent stages at high OLR.
3.2.6. Dewaterability
The dewaterability of the digestate was determined by assessing the CST and by the performance of dewatering tests using a lab-scale filter press and a pilot-scale centrifuge. The dewaterability of the digestate of the compartmentalized digester deteriorated, as indicated by the higher CST, polymer doses, and water content in the cake compared with the reference (Table 3). The observed decline in dewaterability contrasts with the CST results obtained during the lab-scale experiments, which, however, differed in substrate composition. Nonetheless, we assume that more trustworthy results were obtained during the continuous pilot-scale experiments, because more methods were applied and more samples were analyzed.

Table 3.
Results of dewaterability assays of pilot-scale experiment.
Nonetheless, the decrease in dewaterability may be explained by a decrease in particle size and the solubilization of EPS. As postulated earlier, the mild-temperature pre-treatment coupled with hydrogen peroxide pre-treatment might have dropped the mean particle size of the sludge, resulting in a concomitant increase in surface area for water bounding. The possible decrease in the average particle size might be ascribed to the thermal pre-treatment, as the sole application of hydrogen peroxide had no effect on particle size or specific resistance to filtration in the range of 2.5–30 mg H2O2/g TSS [50]. Similarly, the observed variation in the composition of EPS (Figure 5) could have caused a change in the water fractions of the sludge. According to [51], the presence of soluble and loosely-bound EPS fractions is detrimental for the aggregation of aerobic flocs. In addition, the destruction of the tightly-bound fraction triggers the liberation of proteins to the soluble fraction, which could further deteriorate the dewaterability of both aerobic and anaerobic sludge [52,53,54]. The latter consideration agrees with the observed poorer dewatering of the digestate of the compartmentalized reactor.
3.3. Energy Balance and Cost Implications
A hypothetical WWTP with a capacity of 250,000 population equivalent (p.e.) was considered for the elaboration of an energy balance of the reference and the compartmentalized digester (Table 4). The assumptions for the calculation are presented in the Supplementary Data. Considering the values obtained during Stage #1 of the pilot-scale phase, both the reference and the compartmentalized digester have favorable energy balances, i.e., are net producers of heat and electricity from the WAS-enclosed biochemical energy. Whereas the conventional digester produced more excess heat, the compartmentalized digester had a potentially higher electricity production. It must be noted that 60% heat recovery after the thermal pre-treatment is required in order to obtain a favorable balance. However, the applied peroxide dose represents a cost of $2.24 USD per kg TS of sludge, while the 25% higher polymer dose for dewatering would result in a proportional cost increase. Accepting this, further research should be concentrated on (a) optimization of the required H2O2 dosage and (b) optimization of polymer dosage for sludge dewatering. It should be noted that the availability of an increased digester capacity prevents expenditures on digester construction costs. This benefit may off-set the additional cost of chemicals.

Table 4.
Energy balance 1.
4. Conclusions
- Pre-treatment resulted in increased methane production rates of waste activated sludge, which were attributed to increased solids hydrolysis, partly linked to a shift from the tightly-bound to the loosely-bound and soluble EPS fractions.
- Pre-treatment and compartmentalized digestion accommodated an increase in applicable OLR from 1.4 to 4.1 kg VS/m3d, resulting in a slight increase in both biogas production and volatile solids degradation. However, digestate dewaterability deteriorated compared with the reference conventional digester. The increase in loading rate can be translated to a reduction in retention time from 23 to 15 days.
- No detrimental process imbalance was observed at a peak OLR of 10 kg VS/m3d during at least one retention time (7 days).
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/13/22/6059/s1, Figure S1: COD solubilization of samples under different pre-treatment temperatures and application times; Figure S2: Cumulative methane production from experiment #1. The reference is shown in a black solid line, with standard deviation (n = 3); Figure S3: Temperature, VFA production, and pH profile of activated sludge treated at 70 °C; Figure S4: Capillary suction times (corrected by solids concentration) of digestates after methane production assays; Figure S5: E. Coli counts of WAS after pre-treatment at different conditions, (n = 3); Figure S6: E. Coli counts of the sludge mixture (seed + treated sludge) after methane production assays, (n = 3); Figure S7: Cumulative methane production for different pre-treatment settings; Figure S8: Histograms for biogas production for (a) single stage digester and (b) compartmentalized digester; Figure S9: Proportional generation of methane per compartment;; Figure S10: Histograms for biogas production for (a) the reference and (b) compartmentalized reactor; Figure S11: Bacterial phyla and their relative abundances; Figure S12: Archaeal genera and their relative abundances. Table S1: Methane production rates following pre-treatment during Experiment #2, at an application time of 30 min. n = 3 except for reference + 10 mg H2O2/gTS, which had n = 2; Table S2: Assumptions of the Wilcoxon ranked sign test for biogas production and VS degradation and assumptions for energy balance.
Author Contributions
Conceptualization, C.P. and A.H.; formal analysis, A.G., H.G., and O.O.-I.; investigation, A.G. and H.G.; Methodology, M.d.K.; project administration, A.H.; resources, J.B.v.L. and M.d.K.; supervision, M.d.K.; visualization, A.G., C.P., and A.H.; writing—original draft, A.G.; writing—review and editing, J.B.v.L. and M.d.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Economic Affairs, The Netherlands, with the Topsector Energy funding.
Acknowledgments
To the Mexican National Council of Science and Technology (CONACyT) for the scholarship No. 410688 granted to the first author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.; Delgenès, J.; Steyer, J.-P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Pesante, S.; Venegas, M.; Vidal, G. Developments in pre-treatment methods to improve anaerobic digestion of sewage sludge. Rev. Environ. Sci. Biotechnol. 2016, 15, 173–211. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.; Lee, S.; Lee, C. Effect of mild-temperature H2O2 oxidation on solubilization and anaerobic digestion of waste activated sludge. Environ. Technol. 2014, 35, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.A.C.; Suidan, M.T. Effect of H2O2 dose on the thermo-oxidative co-treatment with anaerobic digestion of excess municipal sludge. Water Sci. Technol. 2006, 54, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Maspolim, Y.; Zhou, Y.; Guo, C.; Xiao, K.; Ng, W. Comparison of single-stage and two-phase anaerobic sludge digestion systems—Performance and microbial community dynamics. Chemosphere 2015, 140, 54–62. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Madura, R.L.; Walling, D.A.; Farrell, J.B. Volatile solids reduction in two-phase and conventional anaerobic sludge digestion. Water Res. 1996, 30, 1041–1048. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hendriks, A.; Van Lier, J.; De Kreuk, M. Pre-treatments to enhance the biodegradability of waste activated sludge: Elucidating the rate limiting step. Biotechnol. Adv. 2018, 36, 1434–1469. [Google Scholar] [CrossRef]
- Özön, E.; Erdinçler, A. Effects of microwave, H2O2/MW and H2O2/heat pre-treatments on the methane production from wastewater sludges: Experimental and modeling approach. Environ. Sci. Pollut. Res. 2019, 26, 35411–35421. [Google Scholar] [CrossRef]
- Lv, W.; Schanbacher, F.L.; Yu, Z. Putting microbes to work in sequence: Recent advances in temperature-phased anaerobic digestion processes. Bioresour. Technol. 2010, 101, 9409–9414. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Spanjers, H.; Van Lier, J.B. Performance of inorganic coagulants in treatment of backwash waters from a brackish aquaculture recirculation system and digestibility of salty sludge. Aquac. Eng. 2014, 61, 9–16. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Ghasimi, D.S.M.; De Kreuk, M.; Maeng, S.K.; Zandvoort, M.H.; Van Lier, J.B. High-rate thermophilic bio-methanation of the fine sieved fraction from Dutch municipal raw sewage: Cost-effective potentials for on-site energy recovery. Appl. Energy 2016, 165, 569–582. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.; Wang, D.; Li, X.; Zhong, Y.; Li, X.; Deng, Y.; Wang, L.; Yi, K.; Zeng, G. Enhanced dewaterability of waste activated sludge by Fe(II)-activated peroxymonosulfate oxidation. Bioresour. Technol. 2016, 206, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, L.; Jiang, G.; Jensen, P.D.; Batstone, D.J.; Yuan, Z. A novel free nitrous acid (FNA)-based technology for enhancing methane production from waste activated sludge. Environ. Sci. Technol. 2013, 47, 11897–11904. [Google Scholar] [CrossRef]
- Appels, L.; Degreve, J.; Van Der Bruggen, B.; Van Impe, J.F.; Dewil, R. Influence of low temperature thermal pre-treatment on sludge solubilisation, heavy metal release and anaerobic digestion. Bioresour. Technol. 2010, 101, 5743–5748. [Google Scholar] [CrossRef]
- Hiraoka, M.; Takeda, N.; Sakai, S.; Yasuda, A. Highly Efficient Anaerobic Digestion with Thermal Pretreatment. Water Sci. Technol. 1985, 17, 529–539. [Google Scholar] [CrossRef]
- Koupaie, E.H.; Johnson, T.; Eskicioglu, C. Advanced anaerobic digestion of municipal sludge using a novel and energy-efficient radio frequency pretreatment system. Water Res. 2017, 118, 70–81. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Zhang, Y.; Liu, C.; Chen, Q. Accelerated high-solids anaerobic digestion of sewage sludge using low-temperature thermal pretreatment. Int. Biodeterior. Biodegrad. 2016, 106, 141–149. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Prorot, A.; Marin, J.; Droste, R.L.; Kennedy, K.J. Synergetic pretreatment of sewage sludge by microwave irradiation in presence of H2O2 for enhanced anaerobic digestion. Water Res. 2008, 42, 4674–4682. [Google Scholar] [CrossRef]
- Laurent, J.; Pierra, M.; Casellas, M.; Dagot, C.C. Fate of cadmium in activated sludge after changing its physico-chemical properties by thermal treatment. Chemosphere 2009, 77, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Mowla, D.; Tran, H.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378. [Google Scholar] [CrossRef]
- Higgins, M.J.; Chen, Y.-C.; Murthy, S.N.; Hendrickson, D.; Farrel, J.; Schafer, P. Reactivation and growth of non-culturable indicator bacteria in anaerobically digested biosolids after centrifuge dewatering. Water Res. 2007, 41, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Jiang, Q.; Liu, H.; Liu, H. Occurrence and reactivation of viable but non-culturable E. coli in sewage sludge after mesophilic and thermophilic anaerobic digestion. Biotechnol. Lett. 2013, 36, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.; Geerink, M.; Zeeman, G.; Lettinga, G. Anaerobic hydrolysis kinetics of particulate substrates. Water Sci. Technol. 2000, 41, 17–24. [Google Scholar] [CrossRef]
- Vavilin, V.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef]
- Lo, K.V.; Ning, R.; De Oliveira, C.K.Y.; De Zetter, M.; Srinivasan, A.; Liao, P.H. Application of Microwave Oxidation Process for Sewage Sludge Treatment in a Continuous-Flow System. J. Environ. Eng. 2017, 143, 04017050. [Google Scholar] [CrossRef]
- Guwy, A.; Martin, S.; Hawkes, F.; Hawkes, D. Catalase activity measurements in suspended aerobic biomass and soil samples. Enzym. Microb. Technol. 1999, 25, 669–676. [Google Scholar] [CrossRef]
- Nadler, V.; Goldberg, I.; Hochman, A. Comparative study of bacterial catalases. Biochim. Biophys. Acta (BBA) Gen. Subj. 1986, 882, 234–241. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Theng, B.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, B.; Cerutti, A.; Campo, G.; Scibilia, G.; Lorenzi, E.; Zanetti, M. Improvement of energy recovery from the digestion of waste activated sludge (WAS) through intermediate treatments: The effect of the hydraulic retention time (HRT) of the first-stage digestion. Appl. Energy 2019, 240, 191–204. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Dai, X.; Dong, B. The influence of organic-binding metals on the biogas conversion of sewage sludge. Water Res. 2017, 126, 329–341. [Google Scholar] [CrossRef]
- Dai, X.; Xu, Y.; Dong, B. Effect of the micron-sized silica particles (MSSP) on biogas conversion of sewage sludge. Water Res. 2017, 115, 220–228. [Google Scholar] [CrossRef]
- Meek, G.E.; Ozgur, C.; Dunning, K. Comparison of the t vs. Wilcoxon Signed-Rank Test for Likert Scale Data and Small Samples. J. Mod. Appl. Stat. Methods 2007, 6, 91–106. [Google Scholar] [CrossRef]
- Luste, S.; Luostarinen, S. Anaerobic co-digestion of meat-processing by-products and sewage sludge—Effect of hygienization and organic loading rate. Bioresour. Technol. 2010, 101, 2657–2664. [Google Scholar] [CrossRef]
- Akgul, D.; Cella, M.A.; Eskicioglu, C. Influences of low-energy input microwave and ultrasonic pretreatments on single-stage and temperature-phased anaerobic digestion (TPAD) of municipal wastewater sludge. Energy 2017, 123, 271–282. [Google Scholar] [CrossRef]
- Mehdizadeh, S.N.; Eskicioglu, C.; Bobowski, J.; Johnson, T. Conductive heating and microwave hydrolysis under identical heating profiles for advanced anaerobic digestion of municipal sludge. Water Res. 2013, 47, 5040–5051. [Google Scholar] [CrossRef]
- Gou, C.; Yang, Z.; Huang, J.; Wang, H.; Xu, H.; Wang, L. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 2014, 105, 146–151. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Genet. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y.; Dai, X.; Liu, M.; Dai, L.; Dong, B. Spatial Configuration of Extracellular Organic Substances Responsible for the Biogas Conversion of Sewage Sludge. ACS Sustain. Chem. Eng. 2018, 6, 8308–8316. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Y. Comparative study of dissolved organic matter generated from activated sludge during exposure to hypochlorite, hydrogen peroxide, acid and alkaline: Implications for on-line chemical cleaning of MBR. Chemosphere 2018, 193, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Performance of two-stage vegetable waste anaerobic digestion depending on varying recirculation rates. Bioresour. Technol. 2014, 162, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, Y.; Ni, B.-J.; Han, X.; Fan, L.; Yuan, Z. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb. Cell Fact. 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, R.H.; McIlroy, S.J.; Kristensen, J.M.; Nierychlo, M.; Karst, S.M.; Dueholm, M.S.; Albertsen, M.; Nielsen, P.H. The impact of immigration on microbial community composition in full-scale anaerobic digesters. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Regueiro, L.; Lema, J.M. Microbial management of anaerobic digestion: Exploiting the microbiome-functionality nexus. Curr. Opin. Biotechnol. 2015, 33, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production–A review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Tang, Y.; Shoeib, T.; Li, A.; Yang, H. Evaluating the effects of the preoxidation of H2O2, NaClO, and KMnO4 and reflocculation on the dewaterability of sewage sludge. Chemosphere 2019, 234, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Liu, J. Composition analysis of fractions of extracellular polymeric substances from an activated sludge culture and identification of dominant forces affecting microbial aggregation. Sci. Rep. 2016, 6, 28391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, P.; Yang, X.; Chen, Z.; Wang, D. Insights into the respective role of acidification and oxidation for enhancing anaerobic digested sludge dewatering performance with Fenton process. Bioresour. Technol. 2015, 181, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-H.; He, P.-J.; Shao, L.-M.; He, P.-P. Stratification Structure of Sludge Flocs with Implications to Dewaterability. Environ. Sci. Technol. 2008, 42, 7944–7949. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Novak, J.T. Factors Affecting Floc Properties During Aerobic Digestion: Implications for Dewatering. Water Environ. Res. 1999, 71, 197–202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).