1. Introduction
In the future, a major hurdle of realizing carbon constraining world is storage and transportation of energy. While there are many potential green sources of electrical energy generation (e.g., solar and wind), both the intermittency and remoteness of these sources will severely limit their practical application at large scale [
1]. Storage of the electric energy produced by intermittent sources would allow a leveling of the supply that should enable the “green-grid”. There are many possible ways to store the energy, but chemical bonds represent perhaps the cheapest option. Hydrogen generated from electrolysis suffers from several drawbacks: it is a gas and therefore requires either large volumes or high pressures to store [
2]. Similar issues arise in the application of hydrogen as a fuel in automotive applications. Although the commercial hydrogen fuel cell vehicle can store 70 MPa of hydrogen with about 5.5 mass% including tank, improvement of hydrogen storage technology with higher hydrogen density (U.S. DOE ultimate target: 6.5 mass% of system) is required [
2]. The promising way to store hydrogen with high density is hydrogen storage material; therefore, many different hydrogen storage media have been considered to improve their gravimetric and volumetric density of usable hydrogen [
3]. One of the recent high profile candidates is ammonia borane (AB; NH
3BH
3) because of its 19.6 mass% of hydrogen capacity and moderate initial dehydrogenation temperature (~110 °C) [
4]. AB desorbs hydrogen over a wide range of temperatures in three idealized steps, as follows [
5]:
In reality, the decomposition pathway of AB is more complicated and the “NH
2BH
2” and “NHBH” species are better represented as polymers. While, on the face of it, AB looks like an excellent material for hydrogen storage, there are several issues to be overcome before practical use could be a reality [
6]. Issues with the kinetics of hydrogen release have been well-examined by many research groups using a wide range of approaches such as catalysts [
7], Lewis and Brønsted acids [
8], metal hydrides [
9], proton sponge activation [
10], ionic liquids [
11], confinements [
12], and metal-substitution as metal amidoboranes [
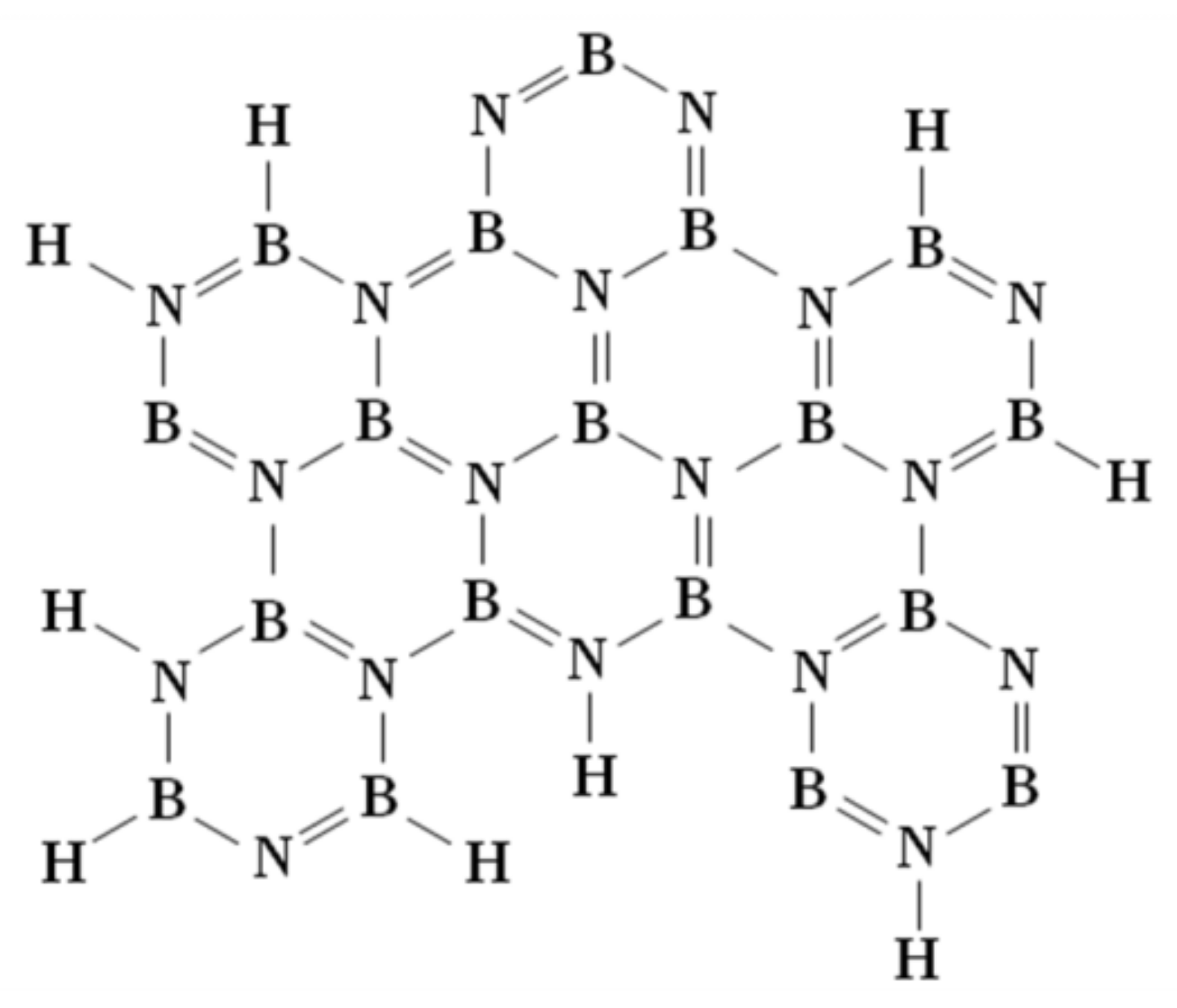
13]. However, the major limitation of AB has always been the exothermic nature of hydrogen release although this nature has advantages from a technological point of view, in that no extra energy is required to obtain hydrogen from AB. This nature prohibits the direct rehydrogenation of the dehydrogenated material form back to AB by simple pressurizing hydrogen gas. Only chemical recycle route (regeneration) is possible to recharge hydrogen into dehydrogenated AB. The dehydrogenated material, or “spent fuel” as it is often called, is a complex mixture of polymeric {BNH
x}
n species (polyborazylene: PB), generally containing hydrogen. PB has linear or B
2N
3 ring structures with many degrees of polymerization, which depends on amount and condition of released hydrogen. The full release of hydrogen from AB, which is the ceramic boron nitride (h-BN), is also spent fuel, but h-BN was considered too robust to be ever converted back to AB due to quite strong bonds in B
3N
3 ring structure. Therefore, less than 2.5 equivalents of hydrogen released from AB would be favorable for regeneration, which means hydrogen in the {BNH
x}
n species would facilitate the chemical reformation of AB. The proto-typical spent fuel, which is ring-structured PB made from borazine (B
3N
3H
6) [
14], has been successfully converted back to AB by using benzenethiol [
15] as a digesting agent followed by tin hydrides [
16] for hydrogen transfer. Moreover, we eventually established a quite simple one-pot AB regeneration technique from PB using hydrazine (Hz) in liquid ammonia (liq. NH
3) as the hydrogen transfer reagent [
17]. This “one-pot regeneration” rapidly proceeds within 24 h via two reaction routes: hydrazine reacts with PB to form hydrazine borane (N
2H
4BH
3) and then AB forms by the ligand exchange from N
2H
4 to NH
3 [
17]; HB(NH
2)
2 forms by the reaction between PB and ammonia in the liquid ammonia and then AB and B(NH
2)
3 form by the disproportionation of HB(NH
2)
2 [
18]. The theoretical approach indicates that only B-H bond can react with NH
3 and N
2H
4 [
17,
18], which means that B-H bonding is mandatory for regeneration.
While some reports of AB regeneration [
19,
20,
21] and lithium amidoborane (LiNH
2BH
3) [
22] have been published, there is still a great challenge remaining which is full-regeneration of AB from hexagonal boron nitride (h-BN). Based upon simple thermodynamic argument, h-BN is too far downhill to be considered as a precursor for AB (endothermic reaction, Δ
H = 215.4 kJ/mol) due to full-dehydrogenated state of AB [
23,
24]. Another fact, that h-BN has no B-H bonding, is also a big issue for regeneration. Therefore, direct hydrogenation of bulk h-BN to AB might be difficult to proceed. Graphite, which has similar structure to h-BN, is also difficult to hydrogenate directly at room temperature but ball-milling under hydrogen pressure, a strong hydrogenation tool, realizes hydrogenation of carbon at room temperature (RT) [
25]. Even ball-milling hydrogenation method has been employed, no AB formed but only surface on h-BN was hydrogenated taking the form of “BNH
x”, which still has many B
3N
3 rings (
hklm = 0002) supported by X-ray diffraction (XRD) [
26]. The hydrogenation of h-BN during the ball milling process relies upon the formation of defects at the surface of h-BN, which is an apparently thermodynamic favorable process. Although BNH
x is smaller hydrogen coordination (x < 0.5) than PB (x = 1.0) and has a similar structure to that of PB (
Figure 1), the infrared (IR) spectrum of BNH
x showed both B-H and N-H stretching peaks with a structure similar to polyaminoborane (PAB; {BH
2NH
2}
n). Therefore, BNH
x is different from PAB in terms of BH
2 species and number of B
3N
3 ring structure. Now activated BNH
x is no longer as thermodynamically stable as h-BN and direct chemical conversion to AB using N
2H
4/NH
3 is possible. Hence, the purpose in this study is to transform back to AB upon N
2H
4/NH
3 treatment from B-H bonds in milling hydrogenated h-BN. We also investigated the reaction mechanism of this transformation including the reaction route.
2. Materials and Methods
The starting material, h-BN nanopowder (<150 nm, 99%, Aldrich Co. Ltd.) was used as received or after dried at 200 °C for 8 h under vacuum condition. BNH
x and BN-NH
x (N-H bonding coated h-BN) were synthesized by using the ball milling method under 1 MPa of H
2 or 0.8 MPa of NH
3 at RT for 80 h using vibration milling (SEIWA GIKEN Co. Ltd., RM-10) as the previous report [
26]. In a typical reaction, 100 mg of each sample (neat h-BN, BNH
x, and BN-NH
x) was placed into stainless steel vessel (about 50 mL) and cooled down to −78 °C using a dry ice/ethanol mixture. Then NH
3 was condensed into the container. After the addition of about 30 mL of liquid NH
3, 200 µL of anhydrous hydrazine (98%, Aldrich Co. Ltd.) was added. The reaction vessel was then sealed and heated at 40 °C for 1 week. These vessels were opened under atmospheric pressure containing condensed NH
3 by cooling in liquid nitrogen before opening to prevent blowing up sample in the container. After warming up and the evaporation of NH
3 under atmospheric pressure for 5 h, residual NH
3 and N
2H
4 were completely removed under dynamic vacuum maintained for 5 h. All procedures were carried out without exposing air.
11B and 1H solution nuclear magnetic resonance (NMR: Bruker, AVANCE 300, 96.29 MHz for 11B and 300.13 MHz for 1H) of BNHx and N2H4/NH3 treated samples were performed at room temperature using d8-THF as the solvent. Samples for NMR were prepared by filtering by THF (about 50 mL) then dynamic evacuation of filtered solution. External calibration of 11B NMR was performed using BF3-etherate standard (0.0 ppm). We also measured 11B solution NMR (Bruker, AVANCE 400, 128.38 MHz) of BNHx after soaking in each solvent (NH3 and N2H4) at RT for 24 h in order to confirm intermediates.
Thermogravimetric analysis (TGA) of BNHx after the N2H4/NH3 treatment (whole sample including soluble and insoluble species) was performed by LABSYS evo (SETARAM). At the same time, gas analyses were performed by residual gas analyzer (RGA: INFICON, Transpector® CPM) and infrared (IR: Thermo Nicolet, AVATAR 360 FT-IR) TGA-RGA measurement of BNHx before the treatment and residual (after removal of AB) treated BNHx was simultaneously performed by TG 8120 (Rigaku) coupled with M-QA200TS (Canon Anelva Corporation). Both measurements were performed with a scanning rate of 10 °C/min. Powder X-ray diffraction (XRD) measurements were operated by RINT-2100 (Rigaku, Cu Kα radiation) for BNHx and D8 Discover (Bruker, Cu Kα radiation) for N2H4/NH3 treated BNHx. All procedures have been performed without exposing the samples to air by handling in the Ar-filled glovebox except the TGA-RGA-IR measurement of BNHx after the treatment, where the exposure time was minimized (<10 sec).
3. Results and Discussion
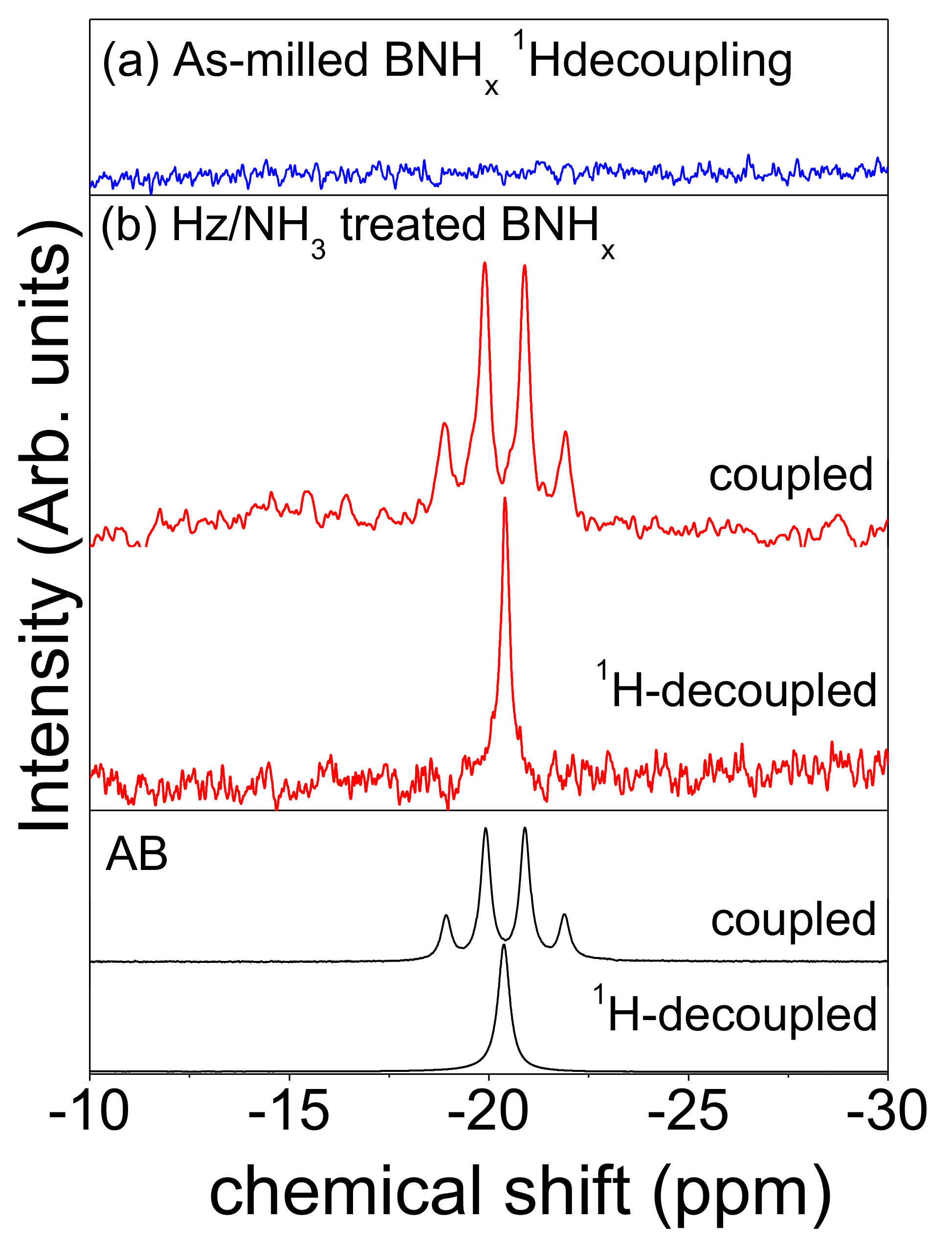
The regeneration of AB is most simply followed by
11B solution NMR. The reaction of neat h-BN with N
2H
4/NH
3 showed no evidence of the formation of AB or any other molecular species even after prolonged time (
Figure S1a in supplementary materials). Extraction of BNH
x, hydrogenated h-BN by ball milling, with THF yielded no observable B-H species in solution (
Figure 2a). In contrast, when BNH
x was treated with N
2H
4/NH
3 for 1 week, a soluble BH
3 containing species (−20.4 ppm,
JB,H = 94 Hz) is presented (
Figure 2b). This resonance is identified as AB by comparison with commercial AB. In addition, mixture of this soluble species and the commercial AB showed single peak in
11B NMR spectrum (
Figure S1b). Although hydrazine borane (HzB) can be produced as an intermediate compound or by the reaction between AB and N
2H
4, in liquid NH
3 all of HzB can be converted to AB by metathesis (
Figure 2b). The
1H NMR spectrum of this product also shows the presence of resonances identified as triplet NH
3 species in AB at 4ppm (
Figure S2).
Unfortunately, the yield of AB is not significant overall being less than 5% based upon the mass of treated BNH
x. It is important to reveal the mechanism of this reaction in order to improve AB yield using this method. It is likely that only the surface of h-BN is hydrogenated during the ball milling process and only this surface is susceptible to be attacked by NH
3 and N
2H
4. We have reported that BNH
x, hydrogenated h-BN by ball milling, is still dominated by the hexagonal N
3B
3 ring structure of h-BN, even after 80 h milling [
26]. Indeed, our X-ray diffraction analysis (XRD) of BNH
x and the product after N
2H
4/NH
3 treatment have diffraction peaks corresponding to h-BN ring structure (
hklm = 0002,
Figure S3). BN-NH
x, which is milled h-BN under ammonia pressure for 80 h [
22], has not been shown in our hands to generate AB by the Hz/NH
3 treatment (
Figure S1c), which is the same as the failure of neat h-BN to show any reaction with N
2H
4/NH
3. This is part of the evidence that presence of the B-H bonds is critical to this reaction as confirmed. Moreover, the calculation results in the previous report support that B-H bonds are only reactive with NH
3 and N
2H
4 but not N-H bonds [
17,
18]. TG-RGA-IR analysis could support this speculation. RGA profile of BNH
x shows wide temperature range of hydrogen (and small amount of N
2 and NH
3) release (
Figure 3a). On the other hand, residue (N
2H
4/NH
3 treated BNH
x after removal of AB) desorbed mainly NH
3 (and small amount of N
2 and H
2) below 500 °C (
Figure 3b), which is similar to that of BN-NH
x denoted in
Figure 3c. This phenomenon indicates that weak B-H bond in BNH
x was substituted from NH
3 and N
2H
4 during treatment. Increase of BNH
x mass after the treatment (100 to 170 mg) can be this evidence. Additional evidence has been found in IR results because the residue has weaker B-H (about 2500 cm
−1) and stronger N-H peaks (about 3500 cm
−1) than these BNH
x peaks (
Figure S4). Overall untreated BNH
x loses about 2.5 mass% up to 900 °C whereas the residue loses 11.5 mass% mostly due to NH
3 desorption. This is also evidence of substitution from B-H to N-H bonds.
The reaction path is also an important issue to be clarified for improvement of this method. As mentioned, the intermediate of N
2H
4 route is N
2H
4BH
3, while the intermediates of NH
3 route are HB(NH
2)
2 and B(NH
2)
3 (decomposition product of HB(NH
2)
2). The simple way to confirm their presence is NMR measurement after soaking BNH
x in liquid NH
3 or N
2H
4.
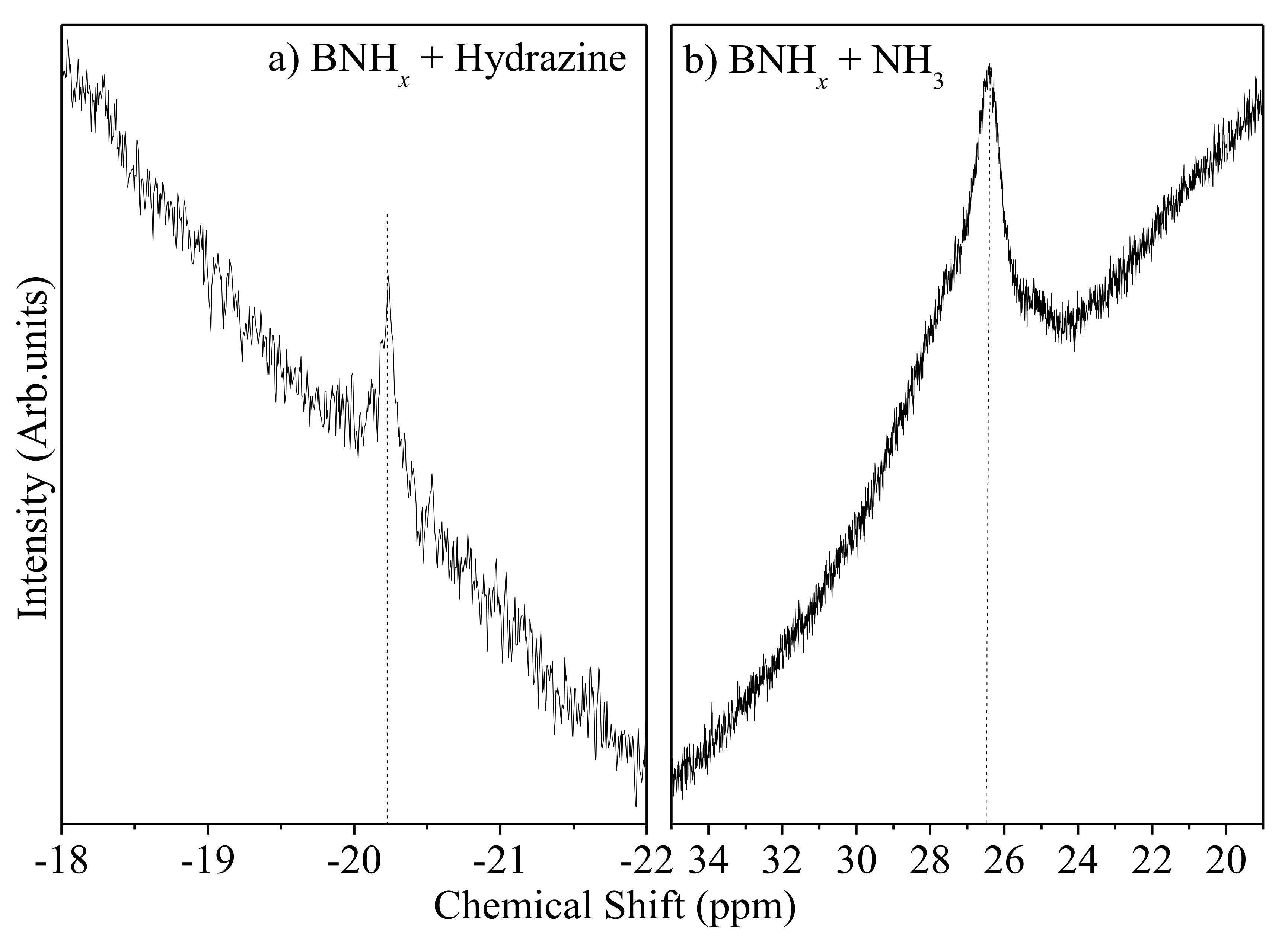
1H-decoupled
11B solution NMR spectra (solvent: THF) after soaking in both N
2H
4 and liquid NH
3 had a weak peak at −20.2 ppm (
Figure 4a) and a strong peak at 26.5 ppm with a small shoulder (
Figure 4b), respectively. Although the signals in the coupled spectrum of N
2H
4-soaked sample were too weak to identify, this peak position is close to N
2H
4BH
3. The peak in the NMR spectrum in liquid NH
3-soaked sample locates almost the same as B(NH
2)
3. Other peak was not observed in both spectra. Thus, regeneration route of BNH
x could be the same as PB regeneration route as we reported.
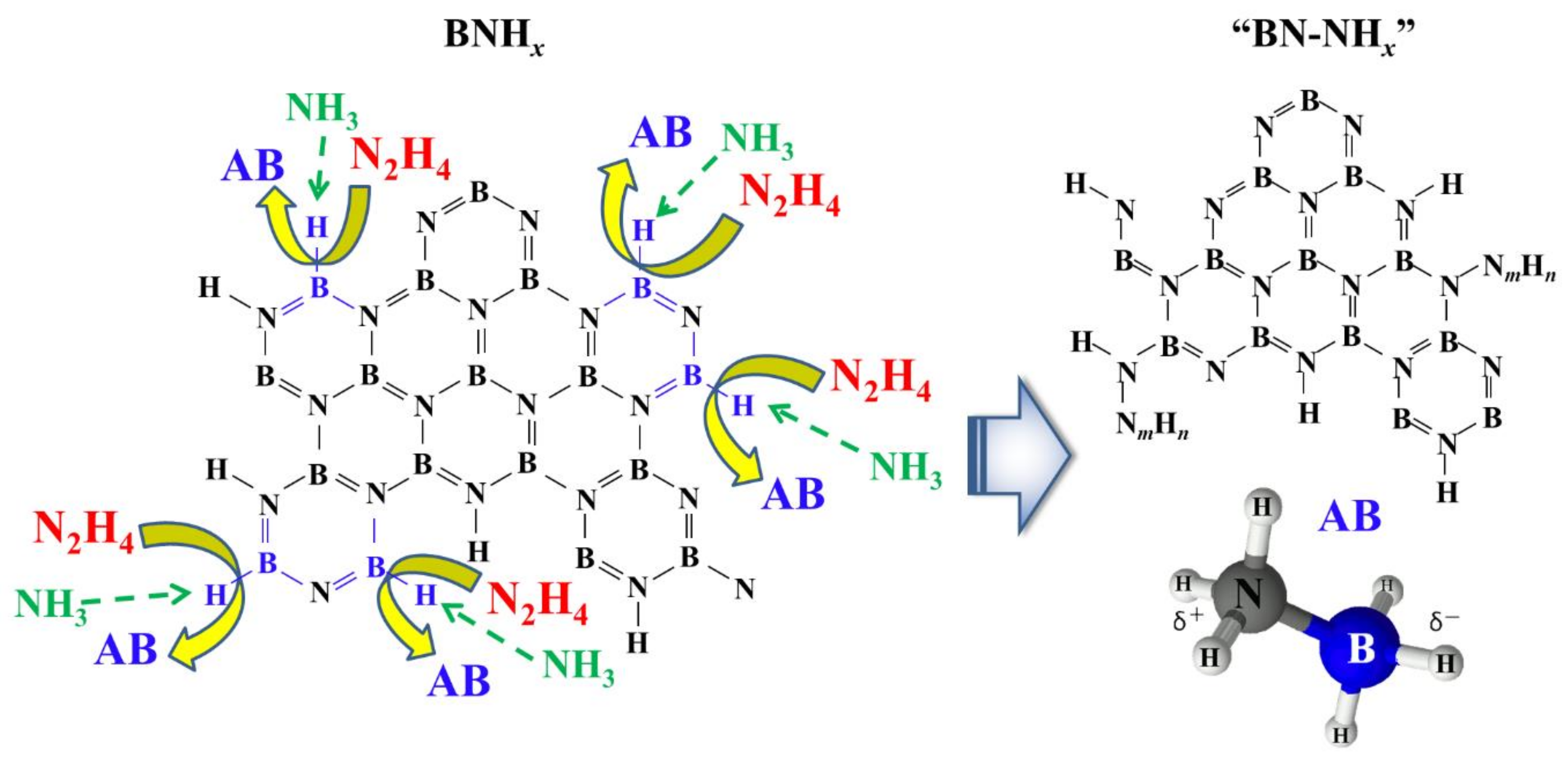
When we consider these observations, a probable reaction mechanism for the formation of the AB from h-BN can be envisioned. Firstly, all of the reactions, from the ball milling through to the reactions with N
2H
4/NH
3, only occur at the BN surface. Previous calculations indicate that only the B-H bonds in (BNH
x)
n have an ability to form AB on reaction with N
2H
4 and NH
3, while the B
3N
3 ring structure is unaffected by them. Once B-H bonds created by milling are attacked by N
2H
4 and NH
3, N
mH
n bonds are formed resulting in release of AB and an N
mH
n coated surface. The sample mass may increase due to this substitution reaction. The coated N
mH
n surface could be similar to BN-NH
x. The B-H bonds reacted with NH
3 and N
2H
4 could be weak bond energy with N atom. Considering the main B-H bond in BNH
x is PAB ({BNH
2}
n), this weak B-H bond could be BH
2. A schematic depiction of this model is displayed in
Figure 5 but the real reaction might be more complicated because the edge of BNH
x would have many kinds of structure. We need to identify “active” edge by the theoretical approach to clarify deeper reaction mechanism of this regeneration method. Further experimental work is also necessary to minimize or break the volume of N
3B
3 ring structure and therefore increase the amount of B-H bonds in the BNH
x by ball-milling (or other method).