Abstract
Alkaloids, typical nitrogen compounds, were found to be abundant in tobacco waste. The recovery of alkaloids from tobacco waste for biological pesticides could reduce the use of traditional chemical pesticides and avoid the pollution of farmland by the leaching of alkaloids from tobacco waste. Considering the fact that alkaloids can easily volatilize, thermal treatment is expected to be a potential technology to achieve the release and recovery of alkaloids from tobacco waste. For better understanding of conversion behavior of nitrogen-containing compounds in tobacco waste during thermal treatment, purge/trap-GC/MS (gas chromatography mass spectrometry), PY-GC/MS (pyrolysis-gas chromatography mass spectrometry), and fixed-bed/ATD-GC/MS (auto-thermal desorption gas chromatography mass spectrometry) were adopted to detect the ingredients and concentration of nitrogen-containing compounds in tobacco waste and/or volatiles. The results of purge/trap-GC/MS showed that nitrogen-containing compounds in tobacco waste could be effectively evaporated at 180 °C in the forms of N-benzyl-N-ethyl-P-isopropyl benzamide, 2-Amino-4-methylphenol, or N-butyl-tert-butylamine. Specifically, N-benzyl-N-ethyl-P-isopropyl benzamide was the main nitrogenous compound in the volatiles of tobacco wastes accordingly. (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was dominant in N-compounds in pyrolysis condition according to the results of Py-GC/MS. In air atmosphere, with the heating temperature increasing, the concentration of main (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was firstly increased and then decreased. Besides, the interactions between the released volatiles could be accelerated at a high temperature. Accordingly, these findings suggested that pyrolysis under proper conditions could effectively promote the extraction of alkaloids from tobacco waste.
1. Introduction
Tobacco waste is a type of special biomass waste, which contains hazardous alkaloids [1,2]. Unlike ordinary biomass waste, the disordered accumulation of large amount of tobacco waste might lead to the leaching of high concentration of alkaloids into the surrounding soil and water, causing serious damage to the microbial environment [3,4]. On one hand, production of tobacco absolute was considered as a potential high value application of tobacco waste [5]. On the other hand, if collected and used at a controlled low concentration, alkaloids could act as a safe and environmental biological pesticide for the control of disposal of diseases and pests [6,7]. However, one of the key issues involved in the procedure of alkaloids collection is to find a proper treatment of tobacco waste for the extraction of alkaloids. Generally, alkaloids are nitrogenous compounds with poor thermal stability and can be evaporated after being heated at certain temperatures [8,9]. More importantly, compared to the wet leaching method, thermal treatment could effectively reduce the volume of remained tobacco waste, and simultaneously transfer it into land-useful biochar to supplement the loss of soil organic matter and inorganic mineral elements in the process of tobacco planting [10,11,12,13,14].
Generally, there are many kinds of nitrogen-containing compounds in tobacco waste. Schmeltz et al. systematically studied the composition of nitrogen compounds in tobacco and showed more than 400 nitrogen compounds were detected, such as pyridine bases, N-heterocyclic compounds, and aromatic amines [15]. Naturally, thermal volatilization and decomposition characteristics of these nitrogen compounds were different due to their various thermal stability [16]. Wen et al. found that protein in tobacco was of poor thermal stability and could be released/decomposed at 50 °C [17]. In contrast, aromatic amines could maintain thermal stable at 150 °C [18]. Meanwhile, Cui’s research indicated that the increase in temperature could promote the cracking of nitrogen compounds with poor stability, resulting in the ammonia emission and the formation of small molecule tar [19]. In addition, heating rate would significantly affect the thermal conversion pathway of tobacco waste [20]. McGrath et al. found that low heating rate could preserve the binding forms of nitrogen compounds well. Moreover, a low heating process could increase the residence time of volatiles, enhancing the secondary reactions among these compounds. In contrast, a fast heating rate was supposed to attenuate secondary reaction, with some nitrogen compounds decomposed [21,22]. Consequently, selecting suitable heat treatment conditions was of high significance for the effective extraction of nitrogen compounds from tobacco waste.
On the other hand, the detection of nitrogen compounds in the volatiles was the basis of revealing the transport and transformation mechanism of nitrogen components in thermal treatment. Nie et al. adopted microwave-assisted deep eutectic solvent extraction coupled with a headspace solid-phase microextraction technique to extract and analyze the components of volatiles in tobacco. The results demonstrated that the detection results would be more accurate by improving the extraction method to reduce the reaction between volatiles [23]. Furthermore, the purge/trap–gas chromatography mass spectrometry (GC/MS) technique combined extraction and detection could detect more types of tobacco flavor components. In addition, auto-thermal desorption gas chromatography mass spectrometry (ATD-GC/MS) was widely used in the detection of components in tobacco smoke to attenuate secondary reaction [2]. The interactions between various nitrogen compounds and other volatiles would reduce the yields of the target products [24,25,26]. Exploring the appropriate extraction conditions was conducive to improving the extraction efficiency and to clarify conversion mechanism of nitrogen compounds.
Thermal treatment was considered as a potential technology to release and collect nitrogen-containing substances from tobacco waste. The migration of nitrogen compounds in tobacco waste during thermal treatment were investigated in the present study in order to determine the appropriate thermal treatment conditions and effectively extract nitrogen compounds. Purge/trap-GC/MS was adopted to detect components and concentrations of nitrogen-containing compounds in tobacco waste. The influence of heating temperature on migration of nitrogen compounds was discussed via the experiment conducted in fixed-bed/ATD-GC/MS. Meanwhile, the effect of heating rate on the extraction of nitrogen-containing components was proposed through the results of pyrolysis-gas chromatography mass spectrometry (PY-GC/MS) and fixed-bed/ATD-GC/MS.
2. Materials and Methods
2.1. Materials
Four tobacco wastes were selected in our experiments and were labeled as TW-1, TW-2, TW-3, and TW-4, respectively. For sample preparation, the selected wastes were crushed with a grinder (TIANQI, CS-2000) and particles with the size of 0.1 to 0.2 mm were sieved (YINGCHAO, 140 meshes and 75 meshes) for the subsequent tests.
2.2. Experimental and Analysis Methods
For further understanding of volatiles emission behavior from the tobacco wastes, a thermogravimetric analyzer (Netzsch Sta 449 F3) was employed to heat the samples at a low heating (10 °C/min) from room temperature to the terminal temperature of 1000 °C. Pure nitrogen as well as simulated air (21% O2 + 78% N2) atmosphere was used to study the thermal procedures of the volatiles during pyrolysis or combustion, which helps choose proper treatment temperature and atmosphere for thermal extraction of N-volatiles from tobacco wastes.
In order to obtain the overall distribution characteristics of volatiles from the tobacco wastes, purge/trap-GC/MS was adopted to further explore to detect the trace N-containing substances. Agilent 7890A/5975C gas chromatography-mass spectrometer was used for purge/trap experiment, and 2 g tobacco waste were placed in a sample vessel. The volatiles were released during the heating volatilization of the sample and were then condensed and collected in a trap tube. Subsequently, the trap tube was heated to evaporate volatiles for GC/MS analysis. Meanwhile, the probe temperature was controlled at 40 °C and purged for 60 min with high purity helium, including dry blowing for 1 min and desorption at 180 °C for 30 s. In addition, the oven temperature of GC was maintained at 280 °C. GC/MS determination was performed with an HP-5 column (30 m-0.25 mm-0.25 μm, with a split ratio of 10:1) as follows: Firstly, remain at 40 °C for 4 min. Then, heat to 200 °C at a heating rate of 5 °C/min and keep for 10 min. Finally heat to 240 °C at 10 °C/min and maintain for 2 min. The MS was performed as an EI (electron ionization) method and 70 eV ionization energy. NIST (National Institute of Standards and Technology)14.L mass spectral library was applied for spectrum retrieval, and the scan quality scanning range was 16~300 amu.
PY-GC/MS (CDS Pyroprobe 6150 pyrolysis sampler and an Agilent 7890A/5975C GC/MS) was used for the fast pyrolysis of tobacco waste. In detail, 1.00 mg samples were tested for each detection and the pyrolysis temperature was set at 280 °C with He as the purge gas (at a flow rate of 30 mL/min). These conditions are determined based on torrefaction, specific kind of pyrolysis. HP-5 column (30 m-0.25 mm-0.25μm, with a split ratio of 100:1) was used for GC/MS. The oven temperature was heated from 40 °C to 280 °C at a heating rate of 15 °C/min. The MS was performed as EI ionization methods and 70 eV ionization energy. NIST 14.L mass spectral library was applied for spectrum retrieval and the scan quality scanning range was 16~300 amu.
As shown in Figure 1, thermal extraction of the N-containing volatiles from tobacco waste samples were eventuated in a self-designed reaction platform. In each experiment, 1.5 g samples were placed in a quartz tube and were heated up to the test temperature (setting at 280 °C, 295 °C, 310 °C, 325 °C and 340 °C, respectively). The simulated air (79% N2 and 21% O2) was used as the carrier gas, and its flow rate was precisely controlled by an electronic mass flowmeter (FLOWMETHOD, FLC-500D). An absorption tube filled organic polymers and molecular sieves (tenax TA, Cartograph 1TD and Carboxen 1000) were used to absorb and to collect the volatiles produced from the tobacco wastes. In addition, ATD-GC/MS was used to desorb and analyze the flue gas components captured by the absorption tube [27]. The desorption temperature of the sampling tube was set at 320 °C and maintain 30 min with He as the desorption gas. By using splitless injection, the chromatographic column was maintained at 40 °C for 4 min and then was heated to 250 °C at a heating rate of 5 °C/min. The MS was performed as EI ionization methods and 70 eV ionization energy. NIST 14.L mass spectral library was applied for spectrum retrieval and the scan quality scanning range was 16~300 amu.
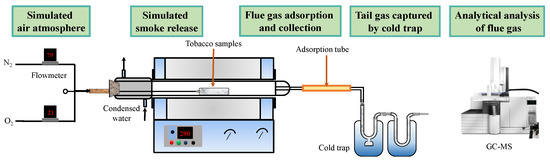
Figure 1.
Schematic diagram of thermal treatment of tobacco in a tube furnace.
3. Results and Discussion
3.1. Components and Distribution Characteristics of Tobacco Waste
The results regarding proximate and ultimate analysis of various tobacco wastes are shown in Table 1. It was found that the volatiles were dominant in dry basis (accounting from 54.43% to 63.29%) of tobacco wastes. In addition, according to the results of ultimate analysis, it could be seen that C and O were the main elements. The content of N in different tobacco waste varied from 3.19% to 5.32%, and TW-1 contained the highest N content among the four tobacco wastes.

Table 1.
Characteristics of the selected tobacco wastes.
Thermogravimetry analysis (TGA) experiments in air atmosphere were firstly carried out in air atmosphere to investigate the combustion characteristics of the selected tobacco wastes, and the results are shown in Figure 2. According to TG (thermogravimetric analysis) curves and DTG (derivative thermogravimetric analysis) curves, the decomposition process could be divided into three stages through the mass-loss process. In stage I, a mild mass loss ranging from 120 to 225 °C was observed, which was attributed to the release of volatile components with lower point. Secondly, a continuous mass loss was found in stage II at temperatures ranging from 225 to 380 °C, probably due to the release of volatile components with a higher boiling point. Thirdly, the combustion reactions among the released fixed carbon components resulted in a distinct mass loss process in the temperature range of 380 to 550 °C (stage III). Although ignition temperature of the four tobacco wastes (316 °C, 326 °C, 300 °C, and 320 °C, respectively) varied from 300 to 330 °C, there were few differences in volatilization and release of small molecules the first two steps. That is to say, the variation in composition properties of various tobacco wastes might cause different ignition and combustion phenomena, the thermal stability of small molecule compounds (including nitrogen-containing compounds) remained similar, which provided a possibility for the extraction of nitrogen compounds from tobacco wastes through thermal treatment.
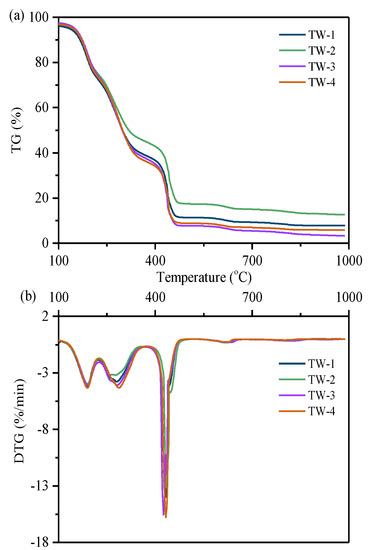
Figure 2.
Combustion of tobacco wastes under 10 °C/min, (a) thermogravimetric analysis (TG) curves and (b) derivative thermogravimetric analysis (DTG) curves.
To further understand the cracking behavior of typical tobacco wastes, thermogravimetry was carried out in nitrogen atmosphere to explore the evaporation of small molecules. Figure 3 shows the TG-DTG curves of tobacco wastes at the heating rate of 10 K/min under nitrogen atmosphere. Similar to the DTG curves in air atmosphere, three mass-loss peaks were obviously observed, corresponding to the temperature range at 180–220 °C, 220–280 °C, and 280–340 °C, respectively. In Table 2 and Table 3, the mass changes related to the first two peaks in TG curves are compared under combustion and pyrolysis condition [28]. The results indicated that the mass-loss of each curve did not change dramatically with the properties of different tobacco wastes. Interestingly, the mass-loss of the second peak under nitrogen atmosphere was much lower than that under air atmosphere. It was suggested that existence of air accelerated the release of small molecule volatiles by oxidation. The properties of nitrogen-containing compounds extracted from tobacco wastes could maintain stable in an oxygen-lean environment.
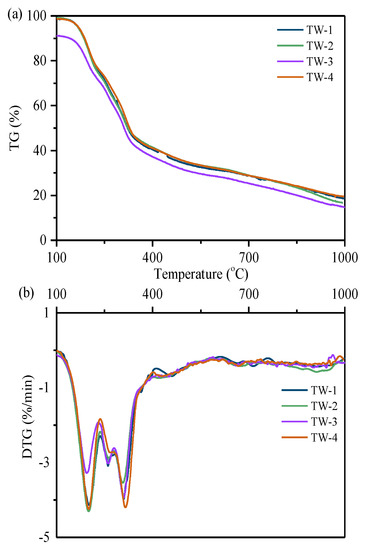
Figure 3.
Pyrolysis of tobacco wastes under 10 °C/min, (a) TG curves and (b) DTG curves.

Table 2.
Characteristics of the TGA experiments of tobacco waste under combustion condition.

Table 3.
Characteristics of the TGA experiments of tobacco waste under pyrolysis condition.
Typically, tobacco waste was one kind of biomass mainly composed of cellulose, hemicellulose, and lignin [29,30,31,32]. For the cracking of hemicellulose, the mass-loss temperature range was 210–310 °C, which matched the first mass-loss peak of tobacco wastes. However, the first mass-loss peak of tobacco wastes (180–220 °C) was ahead that of hemicellulose due to the release of nitrogenous compounds in tobacco wastes. Moreover, the mass-loss temperature range of cellulose and lignin were 310–400 °C and 160–900 °C, probably resulting in the second (220–280 °C) and third (280–340 °C) mass-loss peak of tobacco wastes. The mass-loss process of tobacco wastes was very fast before 400 °C because of the evaporated volatiles (especially for some small molecular weight compounds) in tobacco decomposition. After 400 °C, the process was extremely slow, which was mainly related to the decomposition and carbonization process of lignin and macromolecules in tobacco wastes. As mentioned above, the first two curves were caused by the release of small molecule compounds (including nitrogen-containing compounds). In addition, the nitrogenous compounds in tobacco wastes were supposed to bring forward the release of a volatile at relatively low temperatures.
The results of thermogravimetry showed the general features of tobacco waste during heating up. However, the conversion pathways of specific components in tobacco waste were unclear. Therefore, the purge/trap-GC/MS was applied to detect the composition of volatile from the thermal treatment of tobacco wastes to clearly the conversion pathway of N-compounds. The ingredients and concentration from tobacco wastes during purge/trap-GC/MS is shown in Figure 4. The distribution percentage in Figure 4 shows the relative percentage of the overall detected and identified volatiles during purge/trap-GC/MS.
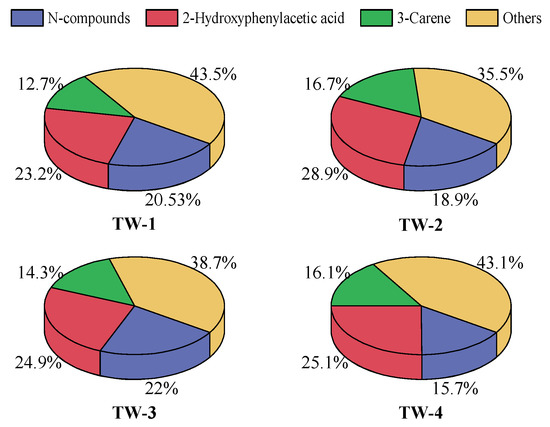
Figure 4.
The main products of tobacco wastes during purge/trap-GC/MS.
The products of tobacco wastes during purge/trap-GC/MS were divided into N-compounds, 2-Hydroxyphenylacetic acid, 3-Carene, and others in Figure 4. Aromatics, organic acids, aliphatic hydrocarbons, etc., which were not related to the conversion of nitrogen-containing compounds, were classified in others. Nitrogen-containing substances in tobacco waste could be effectively extracted at 180 °C in the forms of N-benzyl-N-ethyl-P-isopropyl benzamide and 2-Amino-4-methylphenol. N-benzyl-N-ethyl-P-isopropyl benzamide was the main yield N-compounds in the volatiles of tobacco wastes, with the maximum concentration up to 20.5% in TW-3. For other tobacco waste samples, the concentrations of N-benzyl-N-ethyl-P-isopropyl benzamide were both over 10%. Given the fact that TW-4 contained the lowest N content among the four tobacco wastes according to Table 1, the concentration of N-benzyl-N-ethyl-P-isopropyl benzamide in TW-4 was the lowest. Moreover, 3-Carene, as a typical flavor substance, was found in the volatiles of tobacco wastes during purge/trap-GC/MS.
3.2. Conversion Behaviors of Nitrogen Compounds during Thermal Treatment
In order to detect the volatile components of tobacco waste in thermal treatment, pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) was applied to achieve fast pyrolysis of tobacco wastes and on-line analysis of the pyrolysis vapors. The pyrolysis temperature was set at 280 °C, which was close to the second peak temperature for the release of volatiles in pyrolysis condition as discussed in Section 3.1. Figure 5 shows the chromatograms of the four selected tobacco wastes. As can be seen, (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was the main nitrogenous compound in tobacco wastes. Besides, glycerol and squalene were accounted for a large proportion in the products. Except for major products, there were also a small amount of allose and ketones.
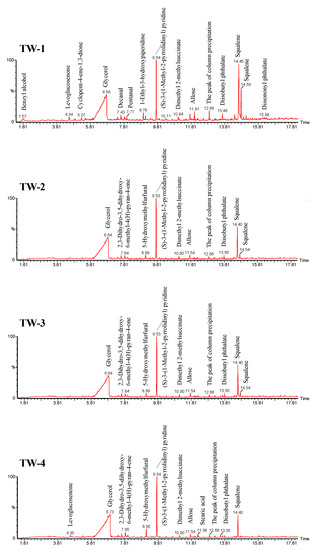
Figure 5.
Py-GC/MS chromatograms of tobacco waste.
According to the chromatograms, the products were divided into (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine, glycerol, squalene, other N-compounds, and other compounds. The yield of products are compared in Figure 6. Generally, N-containing compounds in tobacco waste could release easily, as a result of which (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine and other N-compounds were detected in Py-GC/MS at 280 °C. (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was dominated in N-compounds, and the yields of it from tobacco waste pyrolysis were 9.6% (TW-1), 12.9% (TW-2), 10.4% (TW-3), and 10.0% (TW-4), respectively. Glycerin, as the main component of tobacco smoke, was detected in large quantities. In detail, the yield of glycerin of each tobacco waste was more than 60%. Furthermore, squalene was detected in each sample and was accounted for about 4–20%. The antioxidant effect of squalene made it possible for value-added utilization of tobacco wastes. Generally, the results of Py-GC/MS showed that the nitrogenous components in tobacco wastes would decompose when heated, resulting in the difference of components in the products.
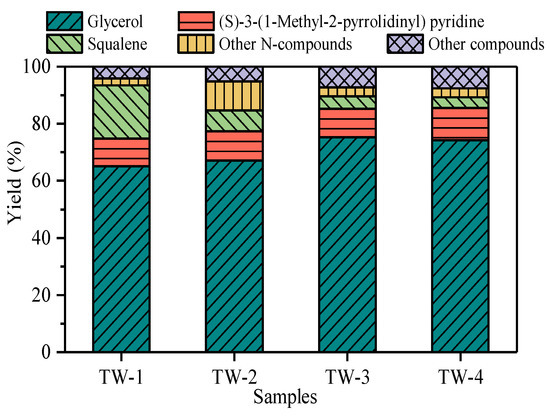
Figure 6.
The main products of tobacco waste during Py-GC/MS.
In order to investigate the effect of temperature on the emission and conversion of volatiles, especially for nitrogenous compounds, thermal treatment of tobacco wastes at five temperatures, 280 °C, 295 °C, 310 °C, 325 °C, and 340 °C was conducted. TW-1, containing the most nitrogenous components as can be found in Table 1, was adopted as the material for investigating the effect of temperature on the composition of volatile. Absorption tube was applied to collect the volatile, and then ATD-GC/MS was applied to detect the composition of the volatile. The yields of total N-compounds and (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine at different temperatures are shown in Figure 7. The detail distributions of main organic species from tobacco wastes at different temperatures are listed in Table S2.
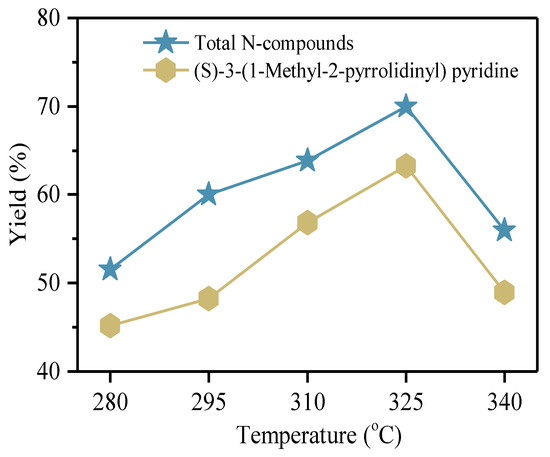
Figure 7.
The yields of total N-compounds and (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine at different temperatures.
The yield of total N-compounds was over 50% at each temperature. For (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine, a typical nitrogenous compound, the concentration was increased with temperature rising up to 325 °C, reaching the highest value 63.26%, indicating that among detected products, the (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine made up the largest percentage distribution. Then, the concentration of (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine showed a downward trend with temperature increasing from 325 °C to 340 °C. The tendency showed that the releasing of (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was firstly promoted and then inhibited with temperature increase. The effects of temperature on the release of volatiles from tobacco wastes showed two aspects. On one hand, the increase in temperature would promote the decomposition of macromolecular substances in tobacco waste to release volatiles. Therefore, the increase in temperature could promote the decomposition of N-containing components, making more nitrogenous substances release into the volatiles. In addition, the decreasing of N-containing components ((S)-3-(1-Methyl-2-pyrrolidinyl) pyridine) is due to its decomposition at temperatures higher than 320 °C [33,34,35,36].
4. Conclusions
The transformation behavior of nitrogen compounds in the pyrolysis/combustion process of four tobacco wastes were discussed in present study. Nitrogenous compounds in tobacco wastes brought forward release of volatile at relatively low temperatures as evident by the results of thermogravimetry experiments. N-benzyl-N-ethyl-P-isopropyl benzamide was the main nitrogenous compound in the volatiles of tobacco wastes, according to the results of purge/trap-GC/MS. (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was dominant in N-compounds in pyrolysis condition according to Py-GC/MS. In air atmosphere, with the heating temperature increasing, the concentration of the main (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine was firstly increased and then decreased. The effects of temperature on the release of volatiles from tobacco wastes were presented in two aspects. The increase in temperature would promote the releasing of (S)-3-(1-Methyl-2-pyrrolidinyl) pyridine before 325 °C. On the other hand, the decomposition of N-compounds could take place at high temperatures. Accordingly, pyrolysis under proper conditions could effectively promote the extraction of alkaloids from tobacco waste.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/13/18/4619/s1. Table S1: The main products of tobacco wastes during purge/trap-GC/MS, Table S2: Distributions of main organic species from tobacco wastes at different temperatures.
Author Contributions
Conceptualization, M.W., M.Y., H.H.; methodology, X.S.; formal analysis, R.L.; investigation, X.P., Y.H.; resources, M.W., H.H.; data curation, F.Y., Q.G.; writing—original draft preparation, M.W., F.Y.; writing—review and editing, M.W., F.Y., M.Y., H.H.; funding acquisition, M.Y., H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (51906081) and Major Technical Innovation Projects of Hubei Province, China (2018ACA151).
Acknowledgments
The authors are grateful to the Analytical and Testing Center of Huazhong University of Science and Technology for the experimental measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, Y.Q.; Chu, G.H. Rapid determination of the volatile components in tobacco by ultrasound-microwave synergistic extraction coupled to headspace solid-phase microextraction with gas chromatography-mass spectrometry. J. Sep. Sci. 2016, 39, 1173–1181. [Google Scholar] [CrossRef]
- Savareear, B.; Brokl, M. Thermal desorption comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry for vapour phase mainstream tobacco smoke analysis. J. Chromatogr. A 2017, 1525, 126–137. [Google Scholar] [CrossRef]
- D’Asaro, J.A.; Telepchak, M.J. Determination of tobacco alkaloids using solid-phase extraction. Am. Lab. 2004, 36, 13–17. [Google Scholar]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase is inhibited by tobacco smoke: β-carboline alkaloids act as potent and reversible inhibitors. Biochem. Biophys. Res. Commun. 2005, 326, 378–386. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.Z. Extraction technology of tobacco absolute oil by compound Solvent. Acad. Period. Farm Prod. Process. 2012, 274, 55–59. [Google Scholar]
- An, T.Y.; Huang, R.Q. Alkaloids from Cynanchum komarovii with inhibitory activity against the tobacco mosaic virus. Phytochemistry 2001, 58, 1267–1269. [Google Scholar] [CrossRef]
- Jones, N.M.; Gabriela, B.G. Comparison of methods for extraction of tobacco alkaloids. J. AOAC Int. 2001, 84, 309–316. [Google Scholar] [CrossRef]
- Strezov, V.; Popovic, E. Assessment of the thermal processing behavior of tobacco waste. Energy Fuels 2012, 26, 5930–5935. [Google Scholar] [CrossRef]
- Chen, M.; Liu, S. Catalytic effects of several additives on co-pyrolysis of tobacco stalk and Waste Rubber Tire Powder. In Proceedings of the International Conference on Materials for Renewable Energy & Environment, Shanghai, China, 20–22 May 2011; pp. 322–326. [Google Scholar]
- Seredych, M.; Bandosz, T.J. Tobacco waste/industrial sludge based desulfurization adsorbents: Effect of phase interactions during pyrolysis on surface activity. Environ. Sci. Technol. 2007, 41, 3715–3721. [Google Scholar] [CrossRef] [PubMed]
- Onorevoli, B.; Pereira, D.S.M.G. Characterization of feedstock and biochar from energetic tobacco seed waste pyrolysis and potential application of biochar as an adsorbent. J. Environ. Chem. Eng. 2018, 6, 1279–1287. [Google Scholar] [CrossRef]
- Kim, D.; Yoshikawa, K. Characteristics of biochar obtained by hydrothermal carbonization of cellulose for renewable energy. Energies 2015, 8, 14040–14048. [Google Scholar] [CrossRef]
- Liang, J.Y.; Yu, Z.S. Microwave pretreatment power and duration time effects on the catalytic pyrolysis behaviors and kinetics of water hyacinth. Bioresour. Technol 2019, 286, 121369. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.B.; Ashfaque, A.C. Pyrolysis of municipal green waste: A modelling, simulation and experimental analysis. Energies 2015, 8, 7522–7541. [Google Scholar]
- Schmeltz, I.; Hoffmann, D. Nitrogen-containing compounds in tobacco and tobacco smoke. Chem. Rev. 1977, 77, 295–311. [Google Scholar] [CrossRef]
- Hameed, S.; Yu, Y. A review on biomass pyrolysis models: Kinetic, network and mechanistic models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Zhang, W.D.; Li, Y.P. Effects of Curing Condition on contents of three nitrogen compounds of flue-cured tobacco leaves. J. Anhui Agric. Sci. 2009, 37, 16325–16327. [Google Scholar]
- McGrath, T.E.; Brown, A.P. Phenolic compound formation from the low temperature pyrolysis of tobacco. J. Anal. Appl. Pyrolysis 2009, 84, 170–178. [Google Scholar] [CrossRef]
- Cui, Q.W.; Tian, L.L. Study on correlation between main nitrogen compounds and flue gas ammonia emission of flue-cured tobacco in baking process. Acta Agric. Jiangxi 2012, 24, 95–97. [Google Scholar]
- Yang, J.; Yang, S. Investigation of thermogravimetry and pyrolysis behavior of tobacco material in two heat-not-burn cigarette brands. Acta Table Sin. 2015, 21, 7–13. [Google Scholar]
- Mcgrath, T.E.; Wooten, J.B. Formation of polycyclic aromatic hydrocarbons from tobacco: The link between low temperature residual solid (char) and PAH formation. Food Chem. Toxicol. 2007, 45, 1039–1050. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J. Influence of Operating Conditions on the Thermal Behavior and Kinetics of Pine Wood Particles Using Thermogravimetric Analysis. Energies 2020, 13, 2756. [Google Scholar] [CrossRef]
- Nie, J.; Yu, G. Microwave-assisted deep eutectic solvent extraction coupled with headspace solid-phase microextraction followed by GC-MS for the analysis of volatile compounds from tobacco. Anal. Methods 2017, 9, 856–863. [Google Scholar] [CrossRef]
- Zheng, S.J.; Gu, W.B. Influence of cigarette smoking parameters on burning temperature and delivery levels of smoke constituents. Acta Table Sin. 2007, 13, 6–11. [Google Scholar]
- Torikai, K.; Yoshida, S. Effects of temperature, atmosphere and pH on the generation of smoke compounds during tobacco pyrolysis. Food Chem. Toxicol. 2004, 42, 1409–1417. [Google Scholar] [CrossRef]
- Liu, S.; Tang, P.P. Smoke release characteristics of tobacco leaves under heating. Tob. Sci. Technol. 2015, 48, 27–31. [Google Scholar]
- Nsaful, F.; Collard, F.X. Lignocellulose pyrolysis with condensable volatiles quantification by thermogravimetric analysis—Thermal desorption/gas chromatography–mass spectrometry method. J. Anal. Appl. Pyrolysis 2015, 116, 86–95. [Google Scholar] [CrossRef]
- Gunasee, S.; Carrier, M. Pyrolysis and combustion of municipal solid wastes: Evaluation of synergistic effects using TGA-MS. J. Anal. Appl. Pyrolysis 2016, 121, 50–61. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, D. Study on the interaction effect of seaweed bio-coke and rice husk volatiles during co-pyrolysis. J. Anal. Appl. Pyrolysis 2018, 132, 111–122. [Google Scholar] [CrossRef]
- Taylor, M.J.; Michopoulos, A.K. Probing Synergies between Lignin-Rich and Cellulose Compounds for Gasification. Energies 2020, 13, 2590. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Matsakas, L. Aromatics from Beechwood Organosolv Lignin through Thermal and Catalytic Pyrolysis. Energies 2019, 12, 1606. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltran, M.I. Nicotine/mesoporous solids interactions at increasing temperatures under inert and air environments. J. Anal. Appl. Pyrolysis 2016, 116, 162–172. [Google Scholar] [CrossRef]
- Baker, R.R. A review of pyrolysis studies to unravel reaction steps in burning tobacco. J. Anal. Appl. Pyrolysis 1987, 11, 555–573. [Google Scholar] [CrossRef]
- Liu, Z.C.; Lu, S.M. Investigation of the Pyrolysis Products of Nicotine by PY/GC-MS. Fine Chem. 2009, 26, 480–484. [Google Scholar]
- Liu, B.Z.; Yao, W. Racemization of S-(−)-nicotine during smoking and its relationship with pyrolysis process. J. Anal. Appl. Pyrolusis 2008, 81, 157–161. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).