Abstract
In view of the difficulty of producing heavy oil from carbonate reservoirs, the surfactant SDY-1 was synthesized by homogeneous solution polymerization with a homogeneous solution polymerization technique using aliphatic amine polyoxyethylene ether (PAEn) H(OCH2CH2)nNR(CH2CH2O)nH as the raw material, epichlorohydrin as the reaction intermediate, tetrabutylammonium bromide and pentamethyldivinyltriamine as the promoters, and alkylphenol as the catalyst. Based on the analysis of reservoir fluid and rock properties, the performance of the surfactant SDY-1 was evaluated in terms of its heat resistance, its salinity tolerance, its ability to change the heavy oil–water interfacial tension and rock wettability and its oil washing efficiency. The results show that when the salinity of the formation water is 2.23 × 105 mg/L, the addition of surfactant SDY-1 can lower the super-heavy oil–water interfacial tension with an asphaltene concentration of 30.19 wt.%, which is aged at a temperature of 140 °C for 3 days, from 22.41 to 0.366 mN/m. In addition, the surfactant SDY-1 can change the contact angle of super-heavy oil–water–rock from 129.7 to 67.4° and reduce the adhesion of crude oil to the rock surface by 99.26%. The oil displacement experiment indicates that the oil washing efficiency of the surfactant SDY-1 can reach 78.7% after ageing at a temperature of 140 °C for 3 days. Compared with petroleum sulfonate flooding, the addition of SDY-1 can improve the displacement efficiency by 33.6%, and the adsorption loss is only 0.651 mg/g oil sand. It has broad application prospects for heavy oil reservoirs with high temperatures, high pressures and high asphaltene contents.
1. Introduction
Heavy oil is an important part of global crude oil resources. With the decrease in conventional light oil recoverable reserves, the efficient production of heavy oil has become increasingly important [1]. Heavy oil is characterized by a high asphaltene concentration, high viscosity and poor flow ability. It is usually harder for heavy oil to flow in fractured reservoirs than it is for light oil due to the higher capillary force and contact angle on the surface of the rock arising from the higher concentration of asphaltene, which is defined as the heavy, polar and surface active component of crude oil, especially in carbonate reservoirs, which are more oil-wet than other types of reservoir and have a high degree of heterogeneity [2,3]. Tahe oilfield is a typical carbonate reservoir which has the characteristics of ultra-deep, high temperature and high salinity. The depth of the reservoir is 5300~7000 m, the formation temperature is 120~150 °C and the total salinity of formation water is (20–25) × 104 mg/L. This makes the efficient production of heavy oil the most challenging problem in China, even in the world.
A large amount of research has been performed on surfactants to address this problem, surfactants have the advantage of lower energy consumption compared with thermal recovery technologies. Among them, surfactants with tolerance to high-temperature and high-salinity environments have attracted increasing attention due to their wider application potential under harsh reservoir conditions. There are two main approaches to develop high-salinity and high-temperature tolerant surfactants. The first approach is mixing anionic and nonionic surfactants to obtain a synergistic effect. It has been demonstrated that a surfactant molecule with both anionic and nonionic surface active groups, especially sulfonate groups, not only can avoid chromatographic separation but also has a high stability under high-salinity and high-temperature conditions. Gale et al. [4] developed a kind of sulfonate polyoxyethylene ether polyoxypropylene ether anionic/nonionic surfactant with C6–24 alkyl chains and 1–10 oxyethylene and oxypropylene groups, which has a salinity tolerance up to 10.4 × 104 mg/L. Adkins et al. [5,6,7] developed a series of alkylalkoxyl carboxylate and alkylalkoxyl sulfate surfactants with high-temperature and high-salinity tolerance by alkoxylation and sulfonation, and their mixtures with C15–18 internal olefin sulfonate (IOS) or C19–23 IOS can be used at temperatures of 120 °C and salinity concentrations of (4–10) × 104 mg/L. Yang et al. [8] synthesized polyoxyethylene polyoxypropylene ether sulfate surfactant; after mixing it with C15–18 IOS, ultralow interfacial tension between crude oil and brine (21 × 104 mg/L salinity) was obtained at a temperature of 100 °C.
The second approach is modifying existing surfactants by introducing functional groups into the main molecular structure. Berger et al. [9] prepared new aryl alkyl sulfonates by introducing sulfonic acid groups on alkyl chains, which showed good performance in decreasing crude oil-brine interfacial tension. Another kind of typical modified surfactant that has good temperature and salinity tolerance and that has been extensively reported on is aryl-substituted alpha-olefin sulfonate (AOS) and internal olefin sulfonate (IOS) [10,11]. The results for several AOSs showed that the optimum carbon chain length in the molecular structure to yield ultralow interfacial tensions (IFTs) is C11~C20 according to the equivalent alkane carbon number (EACN) of crude oil. With respect to IOS, long hydrophobic chains with high carbon numbers, such as C20–28, showed better performance in reducing interfacial tension than short hydrophobic chains, and their solubility in salinityy solution increased with increasing temperature [12,13]. Paul et al. [14] synthesized a new aryl-substituted alkyl sulfonate by reacting alkyl benzene with AOS. They showed that oil–water systems with this sulfonate can yield ultralow IFTs in the absence of alkali under 120 °C and 13 × 104 mg/L salinity concentrations. Zhao et al. [15] synthesized a new kind of surfactant, aliphatic amine polyoxyethylene ether sulfonate (ACS), by allylation and sulfonation with aliphatic amine polyoxyethylene ethers as raw materials, which can reduce oil/water interfacial tension (IFT) to ultralow magnitudes under Tarim reservoir conditions of a temperature of 110 °C, salinity of 11.5 × 104 mg/L and Ca2+ and Mg2+ of 7654 mg/L.
In recent years, researchers have devoted themselves to the development of surfactants in high temperature and high salinity reservoirs. Kamal et al. [16] synthesized a zwitterionic surfactant. The hydrophilic head of the surfactant is both positively and negatively charged, and the hydrophobic tail length is 14 carbon. It was found that the zwitterionic surfactant has salt resistance up to 20% at 80 °C and can be used in a high salt reservoir. Generally, the reservoir temperature can be as high as 80~120 °C; thus, the synthesized surfactant which is used for the EOR (Enhance Oil Recovery) process should have a high temperature tolerance. Hussain et al. [17] synthesized a series of quaternary ammonium Gemini surfactants (C8, C10 and C12) with different spacer lengths. They studied the critical micelle concentration and surface tension of these surfactants in reservoirs at 90 °C. At the same temperature, Li Gang et al. [18] studied the enhanced heavy oil recovery potential of alkyl polyglycoside (APG) under high temperature and high salinity conditions. It was found that under the condition of 90 °C and 30 g/L salinity, the EOR of APG can reach 10.1%, which is much higher than 5% of the commonly used surfactant in tertiary oil recovery. Ilous et al. [19] found that increasing the number of hydrophilic ethoxyls can make surfactants have stronger salt resistance, while slightly hydrophobic propoxy groups can reduce the salt resistance. For more severe reservoir environment, Cai et al. [20] studied a new type of gabetalkoxybetaine(GAB) surfactant, which was prepared by introducing the ethylene oxide (EO) functional group into a betaine molecule and selecting Gerber alcohol as a hydrophobic group. Under the condition of salinity of 2.75 × 105 mg/L and temperature of 120 °C, ultra-low interfacial tension can be obtained by using 0.03%~0.20% gab dilution solution. Although a considerable amount of progress has been made to develop effective surfactants for enhancing oil recovery in high-salinity and high-temperature carbonate reservoirs, there are few studies on heavy oil with asphaltene concentrations higher than 10 wt.% and viscosities higher than 500 mPa·s under reservoir conditions, which leave numerous questions unresolved due to the presence of asphaltenes limiting the absorption of surfactants at the oil–water interface.
In this paper, we studied a new surfactant system, SDY-1, which is suitable for carbonate reservoirs to improve heavy oil recovery and can achieve a heat resistance of 140 °C and salinity tolerance of 2.33 × 105 mg/L.
2. Experiments
2.1. Materials
A heavy crude oil sample with a viscosity of 52.5 mPa·s at 135 °C under reservoir conditions was obtained from the Tahe oilfield in Xinjiang Province, China. The saturate, aromatic, resin and asphaltene (SARA) contents of the crude oil sample measured by Iatroscan MK-6s thin layer chromatography (LSI Medience Corp., Tokyo, Japan) were 16.84, 29.39, 23.58 and 30.19 wt.%, respectively, indicating that the oil sample had a high concentration of asphaltene, which tends to aggregate and adsorb on the rock surface. This will significantly hinder the absorption of surfactant molecules at the oil–water interface and enhance the oil wettability of the rock surface. Therefore, asphaltene dispersion is an important problem to be solved during surfactant flooding in heavy oil reservoirs. The chemical composition of a formation water sample from the Tahe oilfield in Xinjiang Province, China, was determined by ion chromatography, as shown in Table 1 [21]. Table 1 shows that the main anions included in the formation water sample were Cl−, HCO3−, CO32− and SO42− and that the main cations were K+, Na+, Ca2+ and Mg2+, with a total salinity of 2.23 × 105 mg/L and Ca2+ and Mg2+ concentrations of 1.01 × 104 mg/L, reflecting a high hardness and salinity.

Table 1.
Composition and concentration of ions in formation water from the Tahe oilfield.
Aliphatic amine polyoxyethylene ether (PAEn), epichlorohydrin, tetrabutyl ammonium bromide, pentamethyldiethylene triamine, alkylphenol, 2-sodium chloroethyl sulfonate and aromatics (methylbenzene and ethylbenzene), K2CO3 and AlCl3 were purchased from Boen Chemical China, Ltd., Shanghai, China.
2.2. Synthesis of the Surfactant SDY-1
The surfactant SDY-1 was synthesized via a homogeneous solution polymerization technique using aliphatic amine polyoxyethylene ether (PAEn) H(OCH2CH2)nNR(CH2CH2O)nH as the surfactant raw material; epichlorohydrin as the reaction intermediate; tetrabutyl ammonium bromide and pentamethyldiethylene triamine as the promotors; and alkylphenol, 2-sodium chloroethyl sulfonate and aromatics (methylbenzene and ethylbenzene) as functionalized molecule providers. The detailed synthesis process can be found in Appendix A. It should be noted that the length of the alkyl chain in PAEAn was determined according to the equivalent alkane carbon number (EACN) of crude oil to reduce the oil–water interfacial tension as much as possible [22]. In this work, C12 aliphatic amine polyoxyethylene ether was used.
2.3. Morphology and Droplet Size Distribution Measurements
Two dimethylbenzen solutions containing 1 wt.% asphaltene were prepared; 0.1 wt.% SDY-1 was added to one of them. Both samples were sealed and ultrasonicated for 15 min after standing for 6 h. Then, we used a dynamic light scattering instrument (DelsaTM Nano C particle analyzer and ZetaCAD, Beckman Coulter Co., Brea, CA, USA) equipped with a 100 nm lens to measure the particle size distributions of the two asphaltene dispersion systems at 30 °C. Each measurement was repeated 3 times.
An optical microscope was used to observe the morphology of the asphaltene particles in the solutions. Two droplets of the two freshly prepared asphaltene solutions were placed on two glass slides and gently covered with a coverslip. The images were processed using a Labophot-2 microscope (Olympus Co., Tokyo, Japan).
2.4. Interfacial Tension (IFT) Measurements
At 30 °C, a spinning drop interface tensiometer was used to measure the interfacial tension between the 0.1 wt.% SDY-1 aqueous solution and crude oil samples and between the petroleum sulfonate aqueous solution and crude oil samples. The water used in this test was the formation water.
2.5. Thermal Stability Performance
The oil–water interfacial tension and displacement efficiency were tested to investigate the abilities of temperature-resistant surfactants. Each test was repeated three times, and then the average result was obtained. For this experiment, the displacement efficiency represented the ability to break the crude oil away from the surface of the rock.
The measurement of the changes in oil–water interfacial tension: 0.1 wt.% SDY-1 and petroleum sulfonate aqueous solution were put into the high-pressure vessel and aged for 3 days at a temperature of 140 °C. The interfacial tensions between crude oil and surfactant solutions were measured before and after ageing.
Displacement efficiency evaluation experiment: Tahe lithoclasts and crude oil were mixed to prepare oil sand with a mass ratio of lithoclast:crude oil = 17:3, which was sealed at a constant temperature of 140 °C for 30 days. Then, we put the mixture of oil sand and 0.1 wt.% surfactant aqueous solution that had been ageing under a high temperature at a mass ratio of 1:1 into a cylinder with a stopper, and then put the cylinder into an oven under a temperature of 140 °C for 1 h. Finally, we recorded the volume of crude oil separated from the oil sand and calculated the displacement efficiency by the following formula.
where ω is the displacement efficiency, ρ is the crude oil density, V is the volume of the crude oil separated from the oil sand and m is the mass of the oil sand.
2.6. Contact Angle Measurements
An E5637-C interfacial tension tester (Sanchez Technologies, Frépillon, France) was used to measure the contact angle of the heavy crude oil on the surface of the carbonate rock from the Tahe oilfield with or without surfactant according to the China Petroleum Industry standard SY/T5153-2007 (reservoir rock wettability determination method) [23]. The aqueous solutions were all prepared by using formation water from the Tahe oilfield.
2.7. Visual Oil Displacement Experiments
Because fractures and caves in the Tahe carbonate reservoir are well developed, the reservoirs are mainly composed of tectoclase caused by tectonic deformation and pores, holes and seams formed by karstification, shown in Figure 1. The large caverns provide most of the reservoir space [24]. Tectoclases provide not only reservoir spaces but also communication channels. The carbonate matrix has a low matrix permeability with various pore shapes of very different sizes and an uneven distribution, creating anisotropy. Therefore, in this experiment, frosted glass with a large pore structure was used as a model to simulate the Tahe subsurface deposit in landscape orientation. The dimension of the glass was 30 × 30 × 1 cm, the distance between the two glasses was 1 mm and their surfaces have a number of pores and holes with a size of less than 5 mm, as shown in Figure 2. The frosted glass experiment was carried out to visually compare the crude oil displacement performance of SDY-1 with current commercial surfactants in a qualitatively simulated reservoir with high capillary force and heterogeneity.
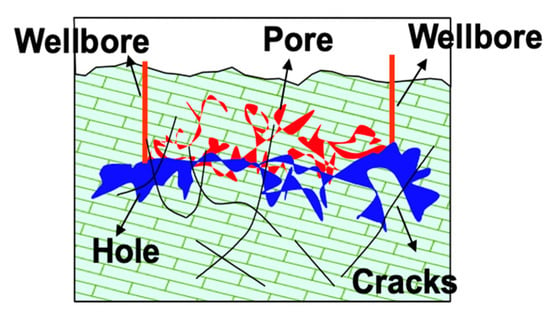
Figure 1.
Spatial structure model of Tahe reservoir.

Figure 2.
Images of frosted glass.
The processes of this experiment are shown as follows:
According to the methods shown in Figure 3, we installed the experimental facilities and saturated this model by injection of formation water for 48 h at 30 °C. Then, simulated crude oil was injected into the model for 48 h at 140 °C. Meanwhile, we calculated the saturated oil volume by the volume of formation water displaced with oil. At a speed of 2 mL/min, the petroleum sulfonate aqueous solution was injected into the model, and we recorded the real-time volumes of crude oil and water in the measuring cylinder. When the water content reached 98%, we stopped the injection and determined the displacement efficiency of petroleum sulfonates. Based on the oil recovery of petroleum sulfonates, at a speed of 2 mL/min, we injected the 0.1 wt.% SDY-1 aqueous solution into the model. In the same way, we calculated the displacement efficiency of SDY-1.
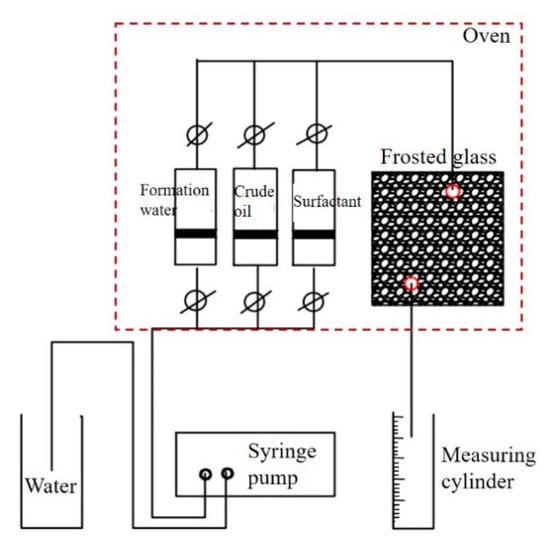
Figure 3.
Schematic diagram of the visual oil displacement experiment device.
The oil displacement efficiency η was calculated as:
where is the volume of oil displaced by the surfactant solutions.
2.8. Adsorption Measurements
In the process of oil washing and oil displacement, the loss of surfactant will affect the oil displacement efficiency due to the adsorption of oil onto the sand. The adsorption loss of surfactant was studied by observing the change in the concentration of surfactant in the solution before and after equilibrium with the rock for 12 h at 140 °C. Equation (3) quantifies the relationship between the different variables and amount of adsorption per unit weight of adsorbent.
where Γ is the adsorption capacity, %; C0 is the initial mass fraction of the adsorbate solution, dimensionless within the range 0–1; C is the instantaneous mass fraction of adsorbate solution, dimensionless within the range 0–1; M is the mass of the adsorbate solution, g; and m is the mass of the oil sand, g. The mass fraction of the adsorbed surfactant was measured at 20 °C by a UV-2100 ultraviolet spectrophotometer (Unico (Shanghai) Instrument Co., Ltd., Shanghai, China).
3. Results and Discussion
3.1. Characterization of SDY-1
The IR experiment was performed on the synthesized surfactant SDY-1, and the spectra results are shown in Figure 4. The stretching vibration peak of C−H, due to the methyl and methylene groups in the alkyl chain, appears at wavenumbers 2950–2850 cm−1, and the bending vibration peak of C−H in the alkyl chain appears at 1350.1 and 1459.9 cm−1. The bending vibration peak of C−H in the methylene appears at 1298.8 cm−1. The peak at a wavenumber of 1189.0 represents the C−O−C group. The stretch vibration peak of fatty tertiary amine
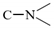 appears at 1050.7 cm−1, the peak of ester C=O appears at 1609.6 cm−1, the peak of sulfonate S−O appears at 1000–750 cm−1, and the peak of C=C in the phenyl group appears at 1400–1600 cm−1. The peaks at wavenumbers 3358.8 and 3472.9 cm−1 represent O−H arising from the free water. It can be concluded from the IR spectra results that both temperature-tolerant groups and aromatic groups are included in the synthesized surfactant SDY-1.
appears at 1050.7 cm−1, the peak of ester C=O appears at 1609.6 cm−1, the peak of sulfonate S−O appears at 1000–750 cm−1, and the peak of C=C in the phenyl group appears at 1400–1600 cm−1. The peaks at wavenumbers 3358.8 and 3472.9 cm−1 represent O−H arising from the free water. It can be concluded from the IR spectra results that both temperature-tolerant groups and aromatic groups are included in the synthesized surfactant SDY-1.
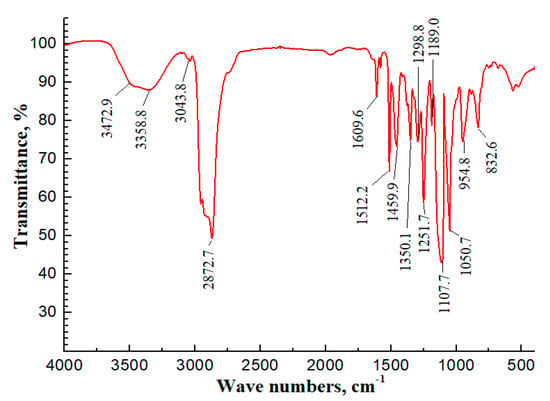
Figure 4.
IR spectrum of surfactant SDY-1 in KBr.
Figure 5 presents the n-alkane carbon number distribution of SDY-1 and heavy crude oil measured by using a Finnigan SSQ710 GC-MS with a 50–100 eV electron impact ion source. The n-alkane carbon number distribution of SDY-1 is mainly C7–C25, and the highest mass fraction appears at approximately C12, which shows good agreement with the heavy crude oil sample. With a closer carbon number between the surfactant molecule and oil molecule, a lower oil–water interfacial tension and better wettability alteration ability can be obtained according to EACN theory; thus, SDY-1 is the surfactant that we require for surfactant flooding to recover Tahe crude oil with a high concentration of asphaltene.
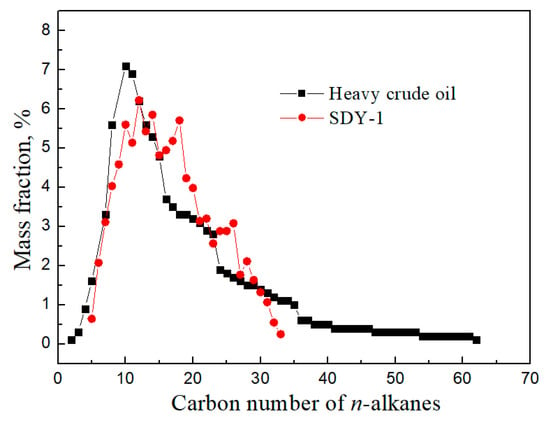
Figure 5.
The n-alkane carbon number distribution of the SDY-1 and heavy crude oil samples.
3.2. Asphaltene Dispersion Effect
The optical microscopy technique was used to examine the influence of SDY-1 at a concentration of 0.1 wt.% on the morphology of the asphaltene in the dimethylbenzene solution. The images presented in Figure 6 show that the number of asphaltene aggregates was lower and the dispersion was greater with SDY-1 compared with those without SDY-1. The particle size distributions shown in Figure 7 reveal that an average particle size of 5.25 μm was obtained for the pure 1.0 wt.% asphaltene dimethylbenzene system, while a reduced average particle size of 1.75 μm was obtained in the presence of 0.1 wt.% SDY-1, indicating that SDY-1 can be used to effectively disperse asphaltene aggregates.
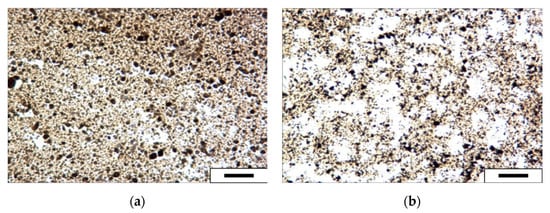
Figure 6.
Optical microscopy images of a 1 wt.% asphaltene xylene system without SDY-1 (a) and with 0.1 wt.% SDY-1 (b). The scale bar is 5 μm.
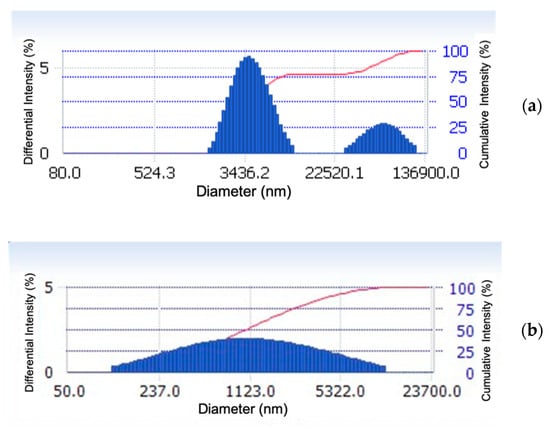
Figure 7.
Asphaltene particle size distribution of the 1 wt.% asphaltene xylene system. (a) Without SDY-1 and (b) with 0.1 wt.% SDY-1.
By the function of relatively strong intermolecular forces and steric hindrance, surfactant molecules consisting of aromatic groups and long-chain alkanes can permeate and distribute into flake asphaltene molecules, partly breaking up the planes [25]. Asphaltene molecules will overlap and pile up into aggregates that have a loose structure, low order and small spatial elongation and that form new bonds. This can promote the distribution and dissolution of asphaltenes in the continuous phase, which can reduce the amount and size of asphaltene aggregates.
More dispersed asphaltenes contribute to a lowered oil/water IFT due to a higher absorption of the surfactant molecules at the oil–water interface.
3.3. IFT between Heavy Oil and Formation Water
As Figure 8 shows, in this work, SDY-1 can lower the interfacial tension between heavy oil and formation water from 22.41 to 0.36 mN/m. Moreover, at present, petroleum sulfonate solution, which has been widely applied in light oil fields, can only lower the oil–water interfacial tension to 10.35 mN/m. This showed that SDY-1 still had superior interfacial activity under conditions of higher salinity (total salinity 2.23 × 105 mg/L) and higher hardness. Therefore, SDY-1 with high salinity tolerance can be applied suitably well in high-salinity reservoirs, which is attributed to the large hydrophobic branched chain, sulfonate groups and more E-O groups in the surfactant molecules. This reduction in oil–water IFT has an important effect on enhanced heavy oil recovery. For tertiary oil recovery, washing oil breaks crude oil away from the rock surface. The work of the adhesion of crude oil to a rock surface is as follows.
where is the oil–water interfacial tension and θ is the contact angle between the crude oil and the rock surface. According to Equation (4), the higher the surfactant’s interfacial activity, the lower the oil-water interfacial tension, the smaller the adhesion work, the easier it is to detach the crude oil from the rock surface and the higher the oil washing efficiency.
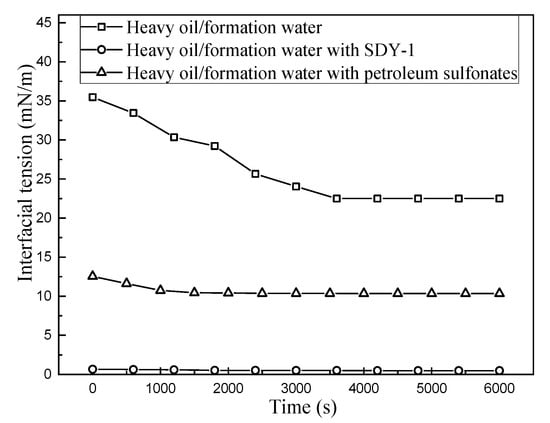
Figure 8.
The variations in interfacial tension with different surfactants.
3.4. Thermal Stability of SDY-1
To further study the oil–water IFT and washing oil efficiency, we investigated the heat resistance of the surfactant SDY-1. As shown in Figure 9, the oil-water IFT of the petroleum sulfonate increased greatly after high-temperature ageing. Before and after high-temperature ageing, the IFT of the SDY-1 was 0.366 and 0.883 mN/m, respectively; it changed only slightly. This finding demonstrated that the SDY-1 had a higher interfacial activity after high-temperature ageing and had an excellent performance in heat resistance. This is because sulfonic groups were introduced into the structure of the SDY-1 surfactant molecules, and their main-chain and branch-chain functional groups were dominated by structures -C-C- and -C-N-.
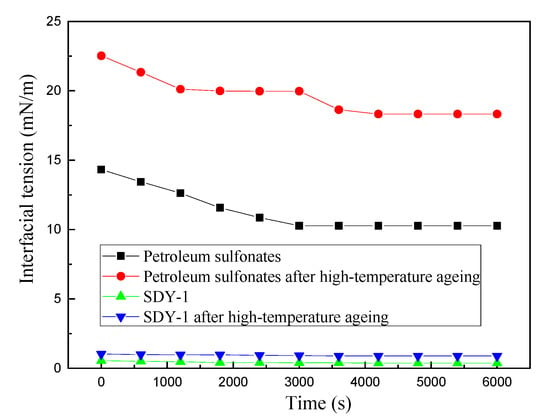
Figure 9.
The variations in interfacial tension of the surfactants before and after high-temperature ageing.
Figure 10 and Table 2 indicate the changes in washing oil efficiency of different surfactants before and after 3 days of high-temperature ageing. As Table 2 shows, after ageing, the washing oil efficiency of petroleum sulfonate decreased substantially, which was directly caused by the poor heat resistance and vulnerability of the molecular structure under a high temperature. The washing oil efficiency of SDY-1 changed slightly, and SDY-1 maintained an efficiency of approximately 80%, reflecting outstanding heat resistance. Therefore, SDY-1, with superior heat resistance, can provide a higher interfacial activity and excellent washing oil efficiency. As a result, SDY-1 is perfectly suitable for high-temperature reservoirs.

Figure 10.
Efficiency of washing heavy crude oil before and after ageing for 3 d at 140 °C. (1) Petroleum sulfonates before ageing, (2) petroleum sulfonates after ageing, (3) SDY-1 before ageing and (4) SDY-1 after ageing.

Table 2.
Efficiency of washing oil before and after ageing for 3 d at 140 °C.
3.5. Wettability Alteration
Key factors of enhanced washing oil efficiency are to effectively lower the IFT and change the wettability of the rock surface [26,27]. For carbonate reservoirs, the main mechanism of enhanced oil recovery is to make water infiltrate into reservoir rocks to displace the crude oil. On the one hand, for hydrophobic reservoirs, capillary forces can hinder water infiltration, and most of the injected water moves along larger cracks, not through the matrix. On the other hand, for hydrophilic reservoirs, capillary force is the main driving force of infiltration and contributes greatly to the water displacing the oil. Therefore, one of the best ways to enhance the oil recovery of hydrophobic carbonate reservoirs is to change the wettability of the rock surface from lipophilic to hydrophilic. In this way, a decrease in interfacial tension can also reduce the capillary resistance. Finally, the transformation of wettability and reduction in the IFT can lower the adhesion work of the crude oil to the rock surfaces, which makes oil easier to separate from the rocks.
As Figure 11 shows, after carbonate rock slices were soaked in the SDY-1 aqueous solution, the average oil–water–rock contact angle was reduced from 129.7 (lipophilic) to 67.4° (hydrophilic). The SDY-1 successfully transforms the wettability and is useful for improving the displacement of crude oil by the permeation of water into rocks.
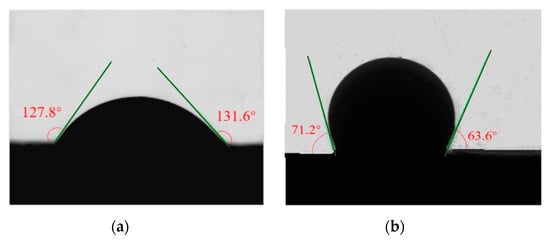
Figure 11.
Contact angles of heavy oil on the surface of Tahe carbonate rock. (a) Without SDY-1. (b) With SDY-1.
Based on Equation (4), after adding the SDY-1, the adhesion work was reduced sharply from 43.436 and 0.300 J/m2. Once the wettability changed, oil could not easily reattach to the rock surface.
3.6. Visual Oil Displacement Efficiency
According to Section 2.7, further experiments were carried out. We evaluated the displacement efficiency of SDY-1 and verified its mechanism. The oil remaining in the model during the displacement process is shown in Figure 12. After using the petroleum sulfonate solution, the displacement efficiency was 53.2%, and nearly half of the oil remained in the model. This is related to the poor adaptability of petroleum sulfonates to high-asphaltene crude oil. This is consistent with the production status of Tahe oil fields [28]. The remaining oil content is high when water flooding extraction is developed to a high water content. We continued injecting 0.1 wt.% SDY-1 solution into the reservoir and improved the displacement efficiency by 33.6%. Comparing the experiential results of the petroleum sulfonate and SDY-1, we found that SDY-1 can preferably spread block-shaped crude oil out and can permeate some areas not affected by petroleum sulfonate.
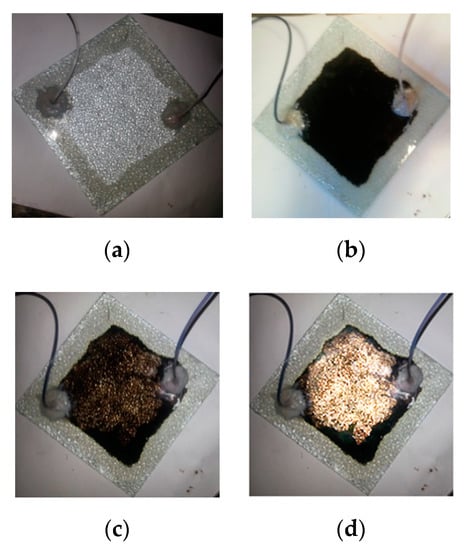
Figure 12.
Oil displacement experiments by surfactants: (a) after saturating with formation water, (b) after saturating with heavy crude oil, (c) after petroleum sulfonate flooding, (d) after SDY-1 flooding.
On the basis of the experimental findings, we propose SDY-1’s functional mechanisms. Due to the aromatic groups and long-chain alkanes of the molecules, stronger hydrogen bonds, π-π conjugation and steric hindrance form of the SDY-1, which can effectively spread asphaltene aggregates out and reduce their size, asphaltene will be distributed well into the oil phase so that more surfactant molecules can be distributed in the oil–water interface and rock surface. Furthermore, at high concentrations, SDY-1 molecules, including quaternary ammonium groups with weak positive electricity, desorb organic carboxylate from the rock surface. At the same time, the wettability is transferred from lipophilic to hydrophilic. Following this transformation, the adhesion work is lower, which means that it will be easier for oil to separate from the rock surface. In addition, under the condition of oil and water shearing, the oil will be further distributed, an O/W emulsion will form and the mobility ratio will and sweep efficiency will improve. The more oil droplets are broken off, the greater the possibility of collision and the larger the oil zone. The remaining oil is pushed close to the outlet of the model. Thus, oil recovery is enhanced.
3.7. Adsorption Performance
In rock, surfactant has a large tendency to be absorbed, which leads to the loss of surfactant and increases the expense of production. Therefore, it is necessary and meaningful to evaluate the absorbability of surfactant. Based on Figure 13, in the low-concentration range, the adsorption capacity of SDY-1 on carbonate increased with increasing surfactant concentration. Initially, electrostatic interactions and intermolecular forces caused by surfactant molecules and rock surfaces formed adsorption. When the surfactant molecules completely occupied the high-energy surface of the rock solid, the surfactant molecules aggregated to form a double layer structure and reached adsorption equilibrium through the intertail hydrophobic effect. The saturation adsorption capacity was 0.651 mg, lower than 1.0 mg/g [29] which is the standard value for surfactant flooding. It also can be seen from Figure 13 that the adsorption capacity of SDY-1 was much lower than anionic surfactant petroleum sulfonate which has been widely used in crude oil production, indicating that SDY-1 meets the requirement of adsorption loss.
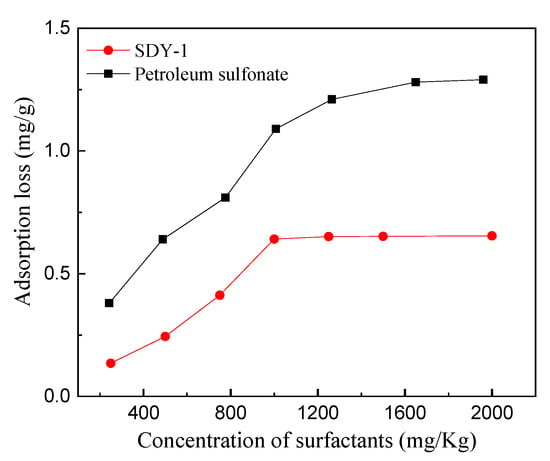
Figure 13.
The adsorption isotherm curve of SDY-1 on the surface of rock.
4. Conclusions
Referring to surfactant modification methods, we introduced temperature and salinity resistance and aromatic and other functional groups. Aiming at enhancing super-heavy oil recovery, SDY-1, which increases the asphaltene distribution, emulsification, heat and salinity resistance and transforms the wettability, was developed. This kind of surfactant has the following properties:
- (1).
- For super-heavy oil including 30.19 wt.% asphaltene, SDY-1 can lower the IFT from 22.41 to 0.366 mN/m. At 140 °C, after ageing for 3 days, the IFT is mostly unchanged. The sodium salinity resistance and salinity resistance of calcium and magnesium ions can reach 2.23 × 105 and 1.01 × 104 mg/L, respectively. Thus, SDY-1 has excellent performance in terms of its tolerance to high-temperature and high-salinity conditions. SDY-1 is perfectly suitable for carbonate reservoirs in these conditions.
- (2).
- SDY-1 can transform the wettability of the carbonate rock surface from 129.7 (lipophilic) to 67.4° (hydrophilic) and can lower the adhesion work of the rock surface by 99.26%. Thus, SDY-1 can effectively enhance the recovery of super-heavy oil.
- (3).
- After ageing at 140 °C for 3 days, the oil washing efficiency of the SDY-1 surfactant solution can reach 78.7%. Compared with petroleum sulfonate flooding, the displacement efficiency with SDY-1 can be increased by 33.6%, and the adsorption loss is only 0.651 mg/g oil sand. SDY-1 has broad application prospects for high-temperature, high-salinity and high-asphaltene heavy oil reservoirs.
Author Contributions
Resources, Y.-Q.Y., and J.-X.G.; investigation, L.L., and S.-L.Z.; writing—original draft, Y.-Q.Y.; visualization, X.W.; format analysis, Y.-Q.F.; supervision, X.-Q.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovative Environment Construction Plan (Innovative talents) Project of Karamay, grant number 2020CXRC0031; the the Research Start-up Fund of China University of Petroleum-Beijing at Karamay, grant number XQZX20200013; and the National Natural Science Foundation of China, grant number 51274210.
Conflicts of Interest
All authors have read, and declare no conflict of interest.
Nomenclature
| IOS | Internal olefin sulfonate |
| AOS | Alpha-olefin sulfonate |
| EACN | Equivalent alkane carbon number |
| IFT | Interfacial tension |
| PAEn | Aliphatic amine polyoxyethylene ether |
| TBAB | Tetrabutyl ammonium bromide |
| SARA | Saturate, aromatic, resin, and asphaltene |
| APG | Alkyl polyglycoside |
| GAB | Gabetalkoxybetaine |
| EO | Ethylene oxide |
| EOR | Enhance oil recovery |
Appendix A
Detailed synthesis process of the Surfactant SDY-1
First, a mixture of PAEn (2 ≤ n ≤ 10) tetrabutyl ammonium bromide (TBAB) and anhydrous potassium carbonate with a mass ratio of PAEn:TBAB:K2CO3 = 40:1:13 was placed in a reactor with n-hexane solvent. After stirring at 70−80 °C, protected by a N2 stream, epichlorohydrin with a mass ratio of PAEn:C3H5ClO = 1.5:1 was slowly added into the mixture in 1 h, and intermediate product A was obtained after the reflux reaction took place for 5−9 h, followed by filtering of the solid residue. Then, intermediate product A, alkylphenol and 2-sodium chloroethyl sulfonate with a molar ratio of 2:1:1 and pentamethyldiethylene triamine as a promoter were homogenized under magnetic stirring in a thermostated water bath at 90 °C for 12 h until a clear pale yellow liquid was obtained, which is aliphatic amine alkyl phenol sodium sulfonate RC6H5OH-(CH2)3-(OCH2CH2)nNR(CH2CH2O)n-(CH2)3-SO3Na. Lastly, we prepared an aromatic mixture of methylbenzene and ethylbenzene with a mass ratio of 1:1. After stirring, this mixture, together with AlCl3, was slowly mixed into the pale yellow liquid followed by reaction for 48 h in a thermostated oil bath at 200 °C. During mixing, we used a molar ratio of 1:1:0.5 for the RC6H5OH-(CH2)3-(OCH2CH2)nNR(CH2CH2O)n-(CH2)3-SO3Na, aromatic mixture, and AlCl3. After cooling the resulting solution to room temperature, we eventually obtained a dark yellow mixture, i.e., the surfactant SDY-1, where sulfonic group, alkyl phenol and alkyl phenyl groups were introduced so that it can possess both a high-temperature tolerance and asphaltene dispersion ability. In addition, the surfactant SDY-1 is electrically neutral in water solution and can chelate metal ions, which makes it have good salinity tolerance.
References
- Gao, C.H. Advances of Polymer Flood in Heavy Oil Recovery. In Proceedings of the Society of Petroleum Engineers SPE Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 12–14 December 2011. [Google Scholar]
- Wei, B.; Lu, L.; Pu, W.-F.; Jiang, F.; Li, K.; Sun, L.; Jin, F. From Phase Behavior to Understand the Dominant Mechanism of Alkali-Surfactant-Polymer Flooding in Enhancing Heavy Oil Recovery. J. Surfactants Deterg. 2016, 20, 355–366. [Google Scholar] [CrossRef]
- Kumar, M.; Hoang, V.T.; Satik, C.; Rojas, D.H. High-Mobility-Ratio Waterflood Performance Prediction: Challenges and New Insights. SPE Reserv. Eval. Eng. 2008, 11, 186–196. [Google Scholar] [CrossRef]
- Gale, W.W.; Puerto, M.C.; Ashcraft, T.L.; Saunders, R.K.; Reed, R.L. Propoxylated Ethoxylated Surfactants and Method of Recovering Oil Therewith. Patent CA1107750A, 25 August 1981. [Google Scholar]
- Adkins, S.; Liyanage, P.; Arachchilage, G.W.P.P.; Mudiyanselage, T.; Pope, G. In A New Process for Manufacturing and Stabilizing High-Performance EOR Surfactants at Low Cost for High-Temperature, High-Salinity Oil Reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Lu, J.; Britton, C.; Solairaj, S.; Liyanage, P.J.; Kim, D.H.; Adkins, S.; Arachchilage, G.W.P.P.; Weerasooriya, U.; Pope, G.A. Novel Large-Hydrophobe Alkoxy Carboxylate Surfactants for Enhanced Oil Recovery. SPE J. 2014, 19, 1024–1034. [Google Scholar] [CrossRef]
- Adkins, S.; Arachchilage, G.W.P.; Solairaj, S.; Lu, J.; Weerasooriya, U.; Pope, G. Development of Thermally and Chemically Stable Large-Hydrophobe Alkoxy Carboxylate Surfactants. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar] [CrossRef]
- Yang, H.T.; Britton, C.; Liyanage, P.J.; Solairaj, S.; Kim, D.H.; Nguyen, Q.P.; Weerasooriya, U.; Pope, G.A. In Low-cost, high-performance chemicals for enhanced oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Berger, P.D.; Lee, C.H. New anionic alkylaryl surfactants based on olefin sulfonic acids. J. Surfactants Deterg. 2002, 5, 39–43. [Google Scholar] [CrossRef]
- Baviére, M.; Bazin, B.; Noik, C. Surfactants for EOR: Olefin Sulfonate Behavior at High Temperature and Hardness. SPE Reserv. Eng. 1988, 3, 597–603. [Google Scholar] [CrossRef]
- Levitt, D.; Jackson, A.; Heinson, C.; Britton, L.N.; Malik, T.; Dwarakanath, V.; Pope, G.A. Identification and Evaluation of High-Performance EOR Surfactants. SPE Reserv. Eval. Eng. 2009, 12, 243–253. [Google Scholar] [CrossRef]
- Zhao, P.; Jackson, A.; Britton, C.; Kim, H.; Britton, L.N.; Levitt, D.; Pope, G.A. Development of High-Performance Surfactants for Difficult Oils. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar] [CrossRef]
- Puerto, M.; Hirasaki, G.J.; Miller, C.A.; Barnes, J.R. Surfactant Systems for EOR in High-Temperature, High-Salinity Environments. SPE J. 2012, 17, 11–19. [Google Scholar] [CrossRef]
- Berger, P.D.; Berger, C.H.; Hsu, I.K. Anionic Surfactants Based on Alkene Sulfonic Acid. Patent EP1056693A1, 6 December 2000. [Google Scholar]
- Zhao, J.; Dai, C.; Ding, Q.; Du, M.; Feng, H.; Wei, Z.; Chen, A.; Zhao, M. The structure effect on the surface and interfacial properties of zwitterionic sulfobetaine surfactants for enhanced oil recovery. RSC Adv. 2015, 5, 13993–14001. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussain, S.M.S.; Fogang, L.T. A Zwitterionic Surfactant Bearing Unsaturated Tail for Enhanced Oil Recovery in High-Temperature High-Salinity Reservoirs. J. Surfactants Deterg. 2018, 21, 165–174. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Kamal, M.S.; Murtaza, M. Synthesis of Novel Ethoxylated Quaternary Ammonium Gemini Surfactants for Enhanced Oil Recovery Application. Energies 2019, 12, 1731. [Google Scholar] [CrossRef]
- Li, G.; Chen, L.; Ruan, Y.; Guo, Q.; Liao, X.; Zhang, B. Alkyl polyglycoside: A green and efficient surfactant for enhancing heavy oil recovery at high-temperature and high-salinity condition. J. Pet. Explor. Prod. Technol. 2019, 9, 2671–2680. [Google Scholar] [CrossRef]
- Illous, E.; Ontiveros, J.F.; Lemahieu, G.; Lebeuf, R.; Aubry, J.-M. Amphiphilicity and salt-tolerance of ethoxylated and propoxylated anionic surfactants. Colloids Surfaces A 2020, 601, 124786. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Q.; Luo, W.; Wang, H.; Zhou, X.; Li, J.; Zheng, Y. Guerbet Alkoxy Betaine Surfactant for Surfactant-Polymer Flooding in High Temperature, High Salinity Reservoirs. In Proceedings of the SPE International Conference on Oilfield Chemistry, Society of Petroleum Engineers (SPE), Galveston, TX, USA, 8–9 April 2019. [Google Scholar]
- Gao, S.; Zhang, J.; Zhang, T.; Cui, Y.; Wang, Z.; Sun, X.; Li, J.; Zhang, L. Study on Influence Factors and Governance Countermeasures of Movable Gel Prepared with Backfilling Waste Water. IOP Conf. Series: Earth Environ. Sci. 2018, 153, 062074. [Google Scholar] [CrossRef]
- Hayes, M.; El-Emary, M.; Schechter, R.; Wade, W. The relation between the EACNmin concept and surfactant HLB. J. Colloid Interface Sci. 1979, 68, 591–592. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Jin, Y.; Lu, Y.; Liang, C.; Li, W.; Wang, Y.; Tan, P.; Zou, D. Experimental study and artificial neural network simulation of the wettability of tight gas sandstone formation. J. Nat. Gas Sci. Eng. 2016, 34, 387–400. [Google Scholar] [CrossRef]
- Li, Y.-B.; Pu, W.; Wei, B.; Chen, Y.-F.; Bai, B. The feasibility of CO2 and N2 injection for the Tahe fracture-cavity carbonate extra-heavy oil reservoir: An experimental study. Fuel 2018, 226, 598–606. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Cheng, Z.; Wu, W.; Zhang, J.; Zhang, J.; Yang, Z.; Zhang, D. New Composite Viscosity Reducer with Both Asphaltene Dispersion and Emulsifying Capability for Heavy and Ultraheavy Crude Oils. Energy Fuels 2017, 31, 1159–1173. [Google Scholar] [CrossRef]
- Lu, J.; Goudarzi, A.; Chen, P.; Kim, H.; Delshad, M.; Mohanty, K.K.; Sepehrnoori, K.; Weerasooriya, U.P.; Pope, G. Enhanced oil recovery from high-temperature, high-salinity naturally fractured carbonate reservoirs by surfactant flood. J. Pet. Sci. Eng. 2014, 124, 122–131. [Google Scholar] [CrossRef]
- Sarafzadeh, P.; Niazi, A.; Oboodi, V.; Ravanbakhsh, M.; Hezave, A.Z.; Ayatollahi, S.; Raeissi, S. Investigating the efficiency of MEOR processes using Enterobacter cloacae and Bacillus stearothermophilus SUCPM#14 (biosurfactant-producing strains) in carbonated reservoirs. J. Pet. Sci. Eng. 2014, 113, 46–53. [Google Scholar] [CrossRef]
- Cao, G.; Li, Z.; Li, Q.; Bai, Y.; Zhang, X.; Liu, Y. Research of Decreasing Injection Pressure and Increasing Injection Rate by Petroleum Sulfonate-Based Surfactant in Xingnan Oilfield. J. Petrochem. Univ. 2017, 30, 27–30. [Google Scholar]
- Dang, C.T.Q.; Chen, Z.; Nguyen, N.T.B.; Bae, W.; Phung, T.H. Development of Isotherm Polymer/Surfactant Adsorption Models in Chemical Flooding. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition; Society of Petroleum Engineers (SPE), Jakarta, Indonesia, 20–22 September 2011. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).