Multicomponent Shale Oil Flow in Real Kerogen Structures via Molecular Dynamic Simulation
Abstract
1. Introduction
2. Methodology
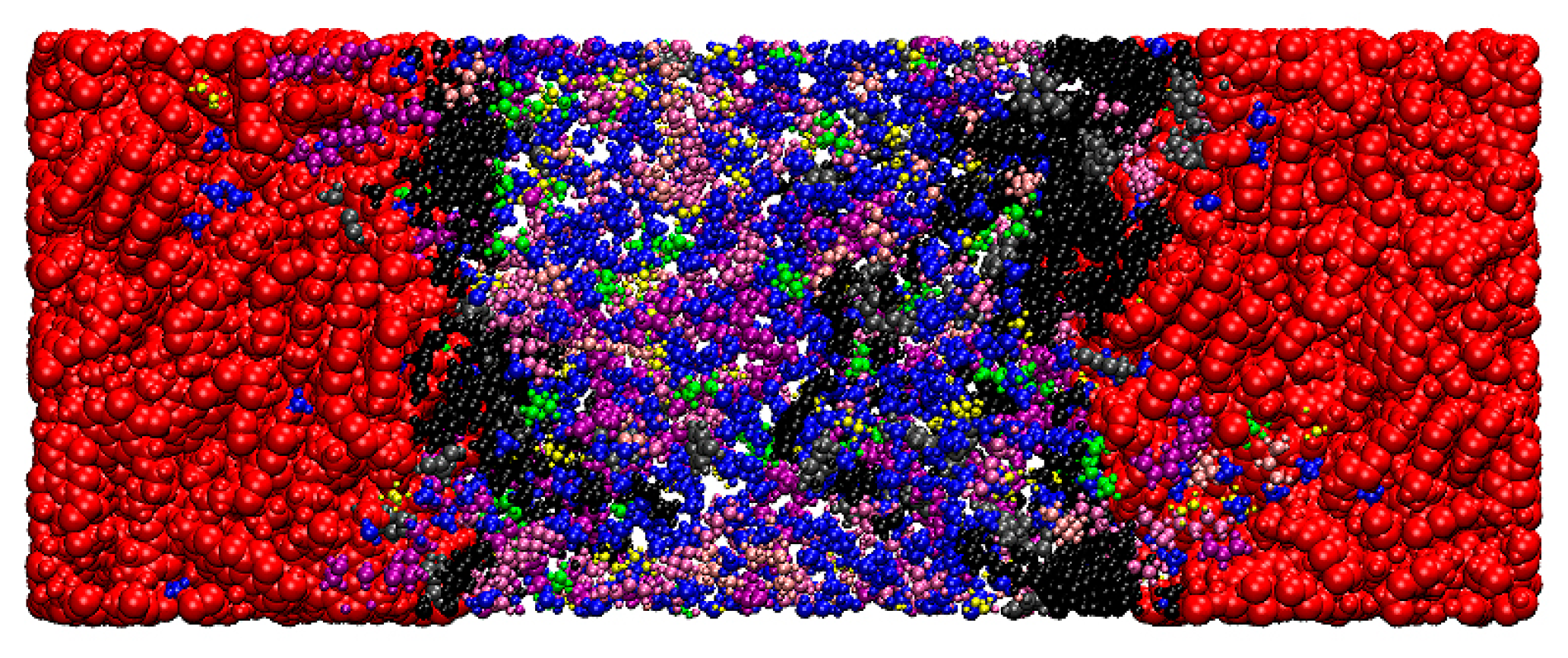
2.1. Molecular Models
2.2. Simulation Methods
3. Results and Discussions
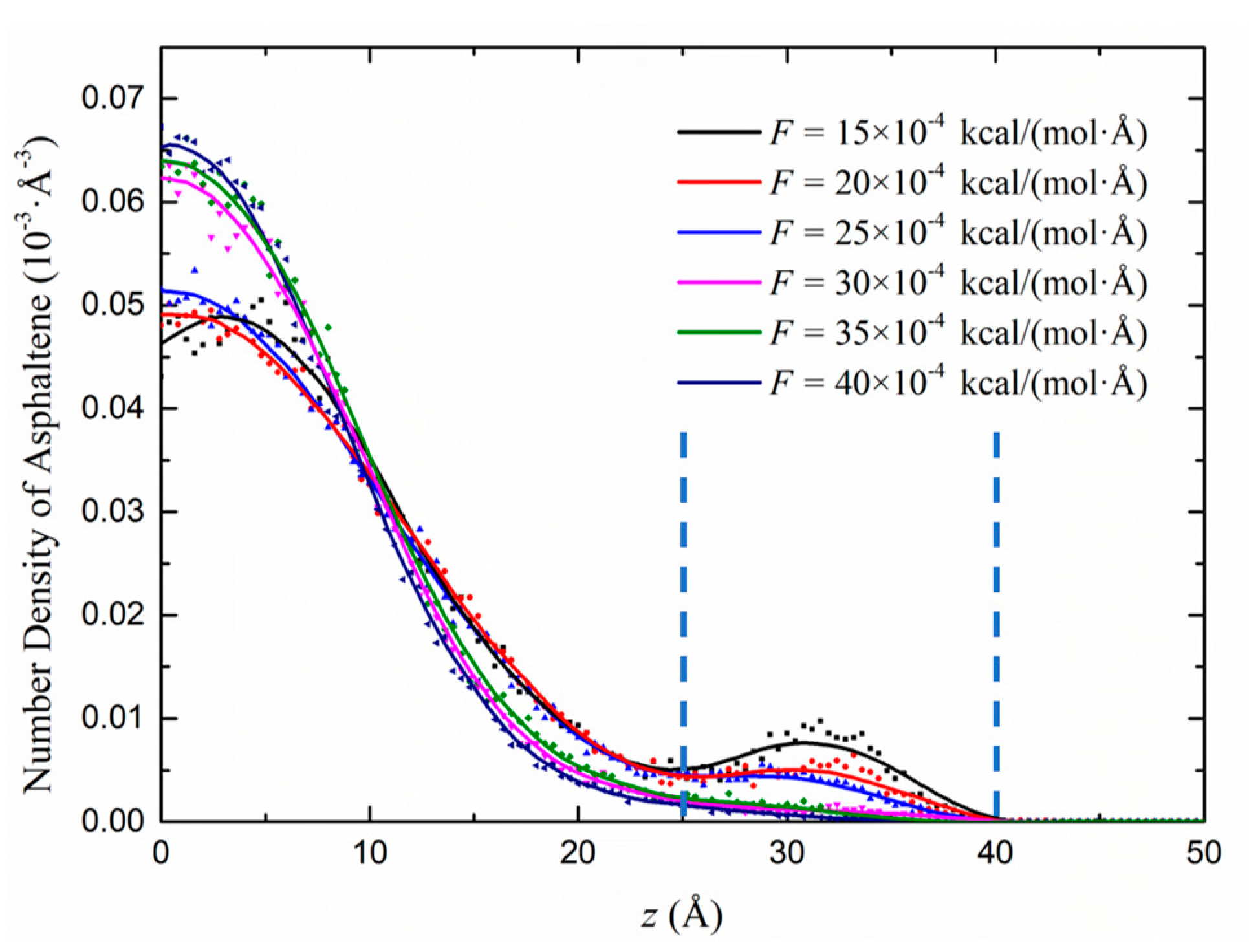
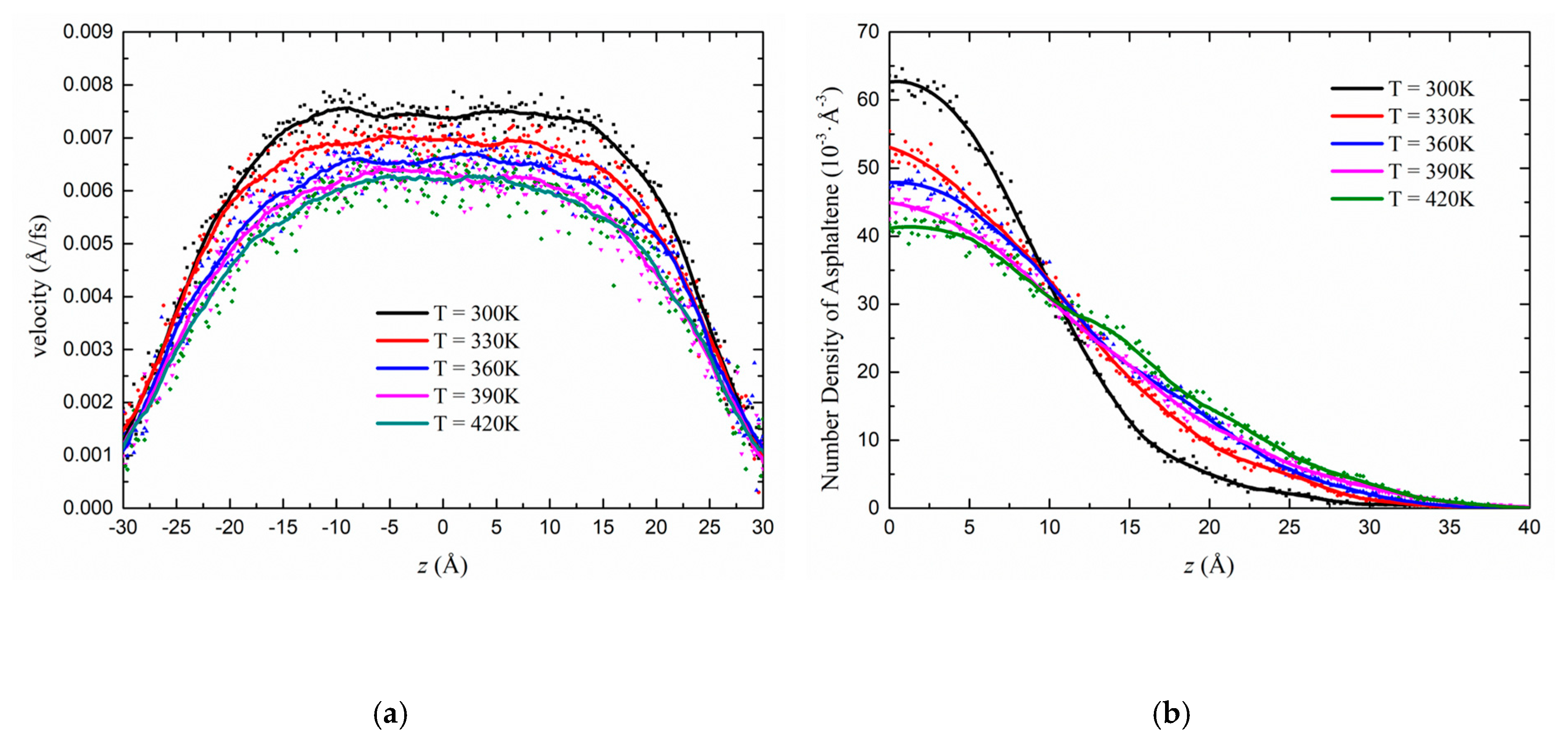
3.1. Heterogeneous Density Distribution Analysis
3.2. Sensitivity Analysis
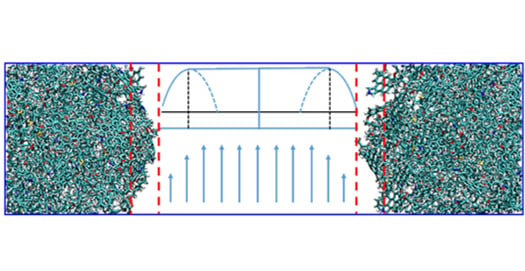
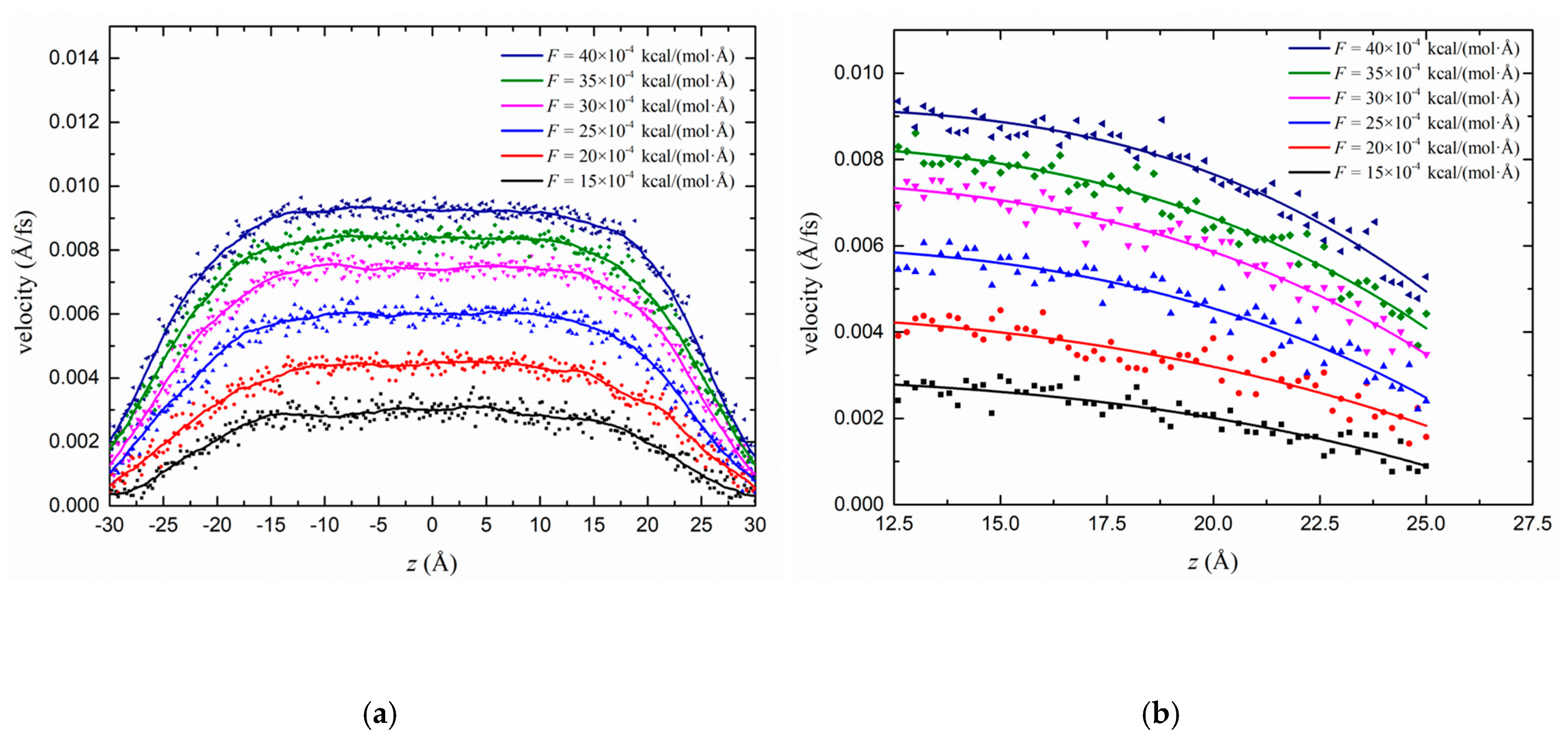
3.2.1. Effect of the Driving Force on the Flow Behaviour in the Kerogen Slit
3.2.2. Effect of the Temperature on the Flow Behaviour in the Kerogen Slit
3.3. Slip Analysis and Fitting
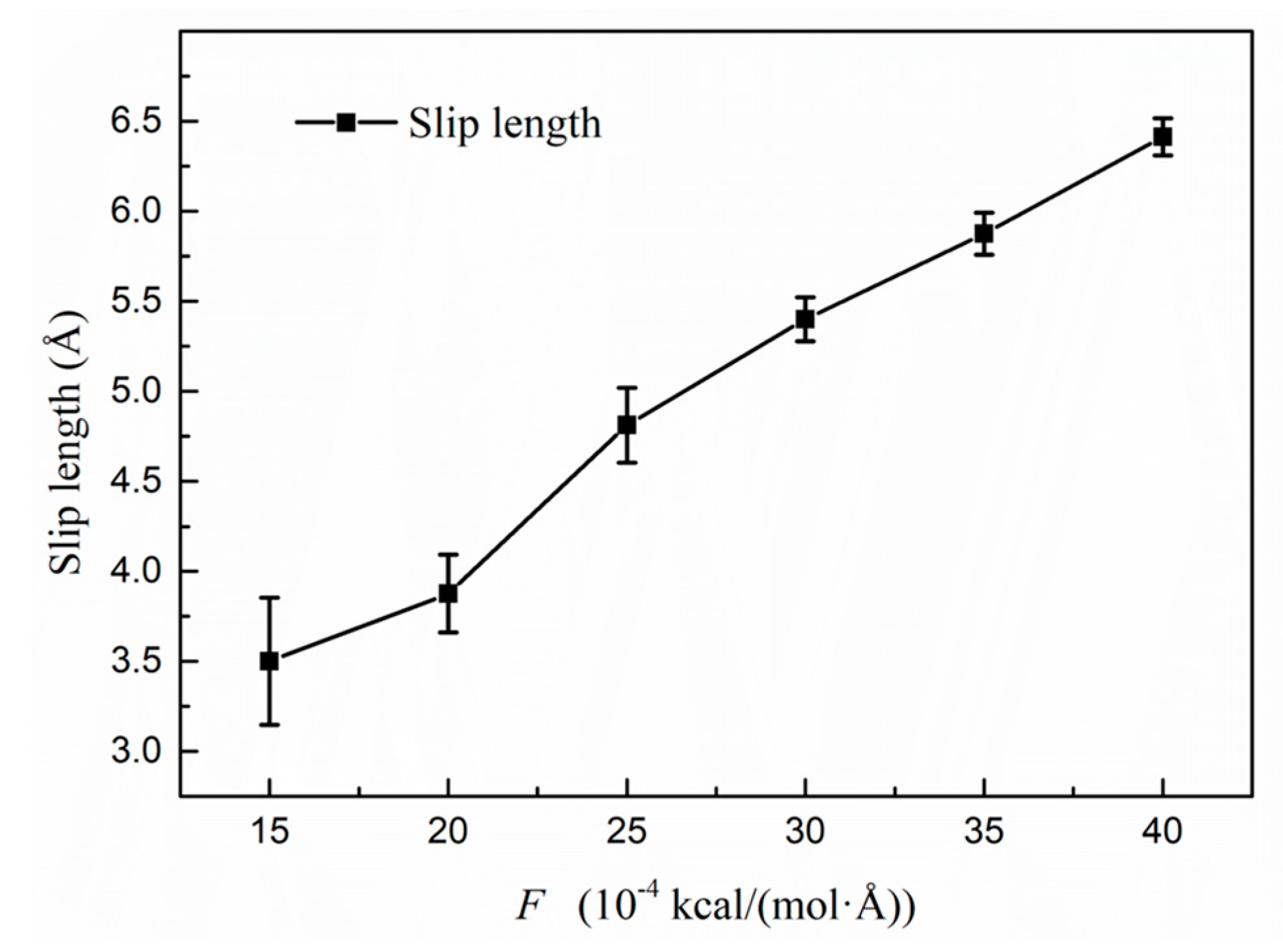
3.3.1. Slip Length Analysis of the Transport Condition
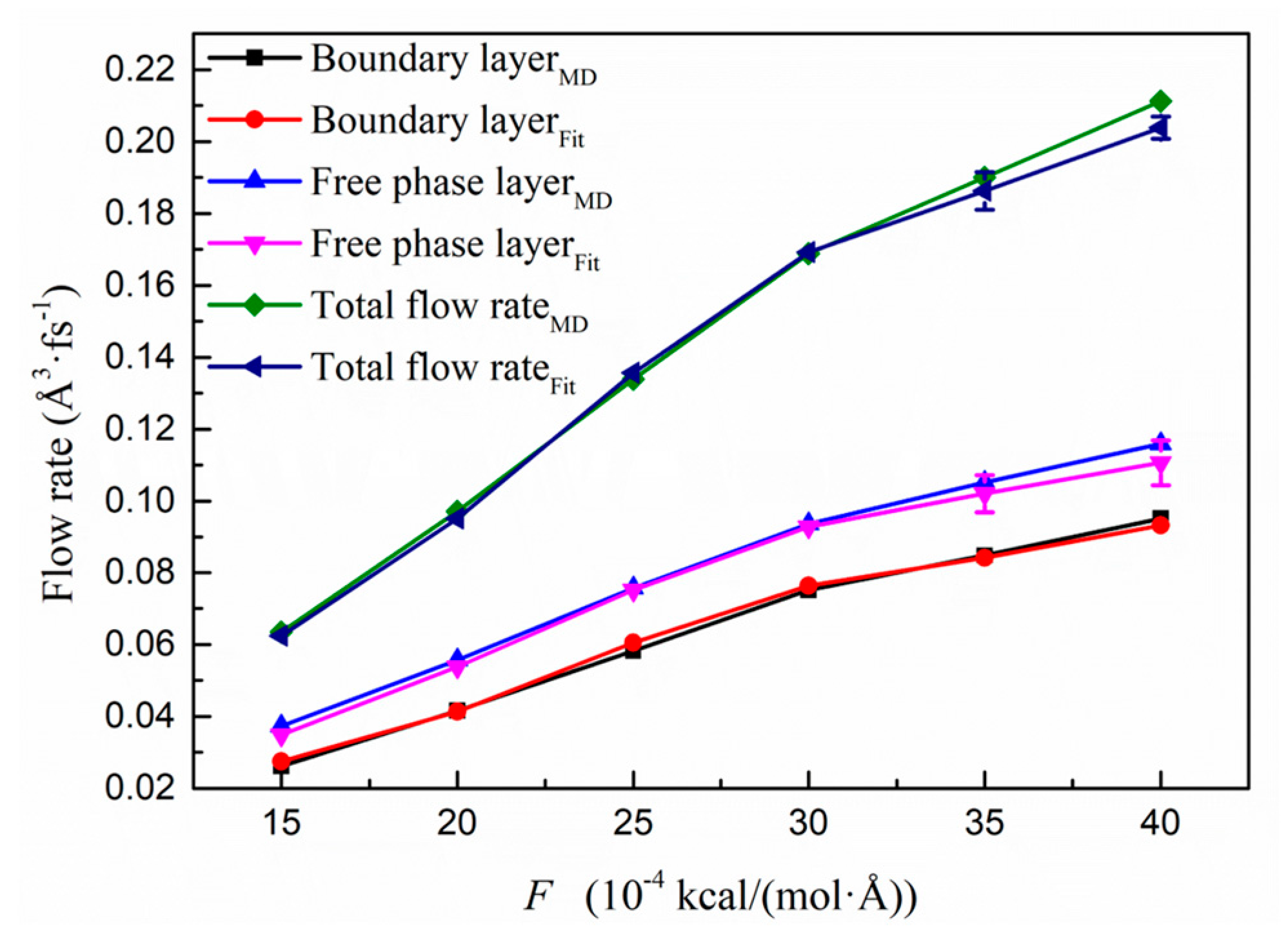
3.3.2. Segment Fitting on the Flow Behaviour in the Kerogen Slit
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, J.D. Energy: A reality check on the shale revolution. Nature 2013, 494, 307–308. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Rycroft, C.H.; Grigory Isaakovich, B. A mathematical model of fluid and gas flow in nanoporous media. Proc. Natl. Acad. Sci. USA 2012, 109, 20309–20313. [Google Scholar] [CrossRef]
- Smith, J.F. New Study: “The Shale Oil Boom: A U.S. Phenomenon”; Harvard Kennedy School, Belfer Center for Science and International Affairs: Cambridge, MA, USA, 2013. [Google Scholar]
- Kerr, R.A. Natural Gas From Shale Bursts Onto the Scene. Science 2010, 328, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Nanogeochemistry: Nanostructures, emergent properties and their control on geochemical reactions and mass transfers. Chem. Geol. 2014, 378–379, 1–23. [Google Scholar] [CrossRef]
- Javadpour, F. Nanopores and Apparent Permeability of Gas Flow in Mudrocks (Shales and Siltstone). J. Can. Pet. Technol. 2009, 48, 16–21. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q. Molecular dynamics simulations of oil transport through inorganic nanopores in shale. Fuel 2016, 171, 74–86. [Google Scholar] [CrossRef]
- Perez, F.; Devegowda, D. Spatial distribution of reservoir fluids in mature kerogen using molecular simulations. Fuel 2019, 235, 448–459. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, K.; Zhang, L.; Sun, H.; Zhang, K.; Ma, J.S. Pore-scale simulation of shale oil flow based on pore network model. Fuel 2019, 251, 683–692. [Google Scholar] [CrossRef]
- Wang, X.; Yin, H.; Zhao, X.; Li, B.; Yang, Y. Microscopic remaining oil distribution and quantitative analysis of polymer flooding based on CT scanning. Adv. Geo-Energy Res. 2019, 3, 448–456. [Google Scholar] [CrossRef]
- Zhou, S.; Ning, Y.; Wang, H.; Liu, H.; Xue, H. Investigation of methane adsorption mechanism on Longmaxi shale by combining the micropore filling and monolayer coverage theories. Adv. Geo-Energy Res. 2018, 2, 269–281. [Google Scholar] [CrossRef]
- Cai, J.; Hu, X. Petrophysical Characterization and Fluids Transport in Unconventional Reservoirs; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, Z.; Yao, J.; Ren, Z.; Sun, H.; Zhang, L.; Yang, Y.; Fan, J.; Kou, J. Accumulation behaviors of methane in the aqueous environment with organic matters. Fuel 2019, 236, 836–842. [Google Scholar] [CrossRef]
- Song, W.H.; Yao, J.; Li, Y.; Sun, H.; Zhang, L.; Yang, Y.F.; Zhao, J.L.; Sui, H.G. Apparent gas permeability in an organic-rich shale reservoir. Fuel 2016, 181, 973–984. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Q.; Farzam, J.; Zha, M.; Cui, R. Multiscale Modeling of Gas Transport in Shale Matrix: An Integrated Study of Molecular Dynamics and Rigid-Pore-Network Model. SPE J. 2020, 25, 1416–1442. [Google Scholar] [CrossRef]
- Yang, Y.F.; Yao, J.; Wang, C.C.; Gao, Y.; Zhang, Q.; An, S.Y.; Song, W.H. New pore space characterization method of shale matrix formation by considering organic and inorganic pores. J. Nat. Gas Sci. Eng. 2015, 27, 496–503. [Google Scholar] [CrossRef]
- JM, H.; GW, J. Oil and organic matter in source rocks of petroleum. AAPG Bull. 1956, 40, 477–488. [Google Scholar]
- Bousige, C.; Ghimbeu, C.M.; Vix-Guterl, C.; Pomerantz, A.E.; Suleimenova, A.; Vaughan, G.; Garbarino, G.; Feygenson, M.; Wildgruber, C.; Ulm, F.J. Realistic molecular model of kerogen’s nanostructure. Nat. Mater. 2016, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, P.; Collell, J.; Yiannourakou, M. Molecular Modeling of the Volumetric and Thermodynamic Properties of Kerogen: In fluence of Organic Type and Maturity. Energy Fuels 2015, 29, 91–105. [Google Scholar] [CrossRef]
- Obliger, A.; Pellenq, R.; Ulm, F.J.; Coasne, B. Free Volume Theory of Hydrocarbon Mixture Transport in Nanoporous Materials. J. Phys. Chem. Lett. 2017, 7, 3712–3717. [Google Scholar] [CrossRef]
- B, Z.; R, X.; P, J. Novel molecular simulation process design of adsorption in realistic shale kerogen spherical pores. Fuel 2016, 180, 718–726. [Google Scholar]
- Collell, J.; Galliero, G.; Gouth, F.; Montel, F.; Pujol, M.; Ungerer, P.; Yiannourakou, M. Molecular simulation and modelisation of methane/ethane mixtures adsorption onto a microporous molecular model of kerogen under typical reservoir conditions. Microporous Mesoporous Mater. 2014, 197, 194–203. [Google Scholar] [CrossRef]
- Kou, J.; Yao, J.; Lu, H.; Zhang, B.; Li, A.; Sun, Z.; Zhang, J.; Fang, Y.; Wu, F.; Fan, J. Electromanipulating Water Flow in Nanochannels. Nanochannels 2014, 54, 2351–2355. [Google Scholar]
- Kerstin, F.; Felix, S.; Laurent, J.; Netz, R.R.; Lydéric, B. Ultralow liquid/solid friction in carbon nanotubes: Comprehensive theory for alcohols, alkanes, OMCTS, and water. Langmuir ACS J. Surf. Colloids 2012, 28, 14261. [Google Scholar]
- Li, Y.; Xu, J.; Li, D. Molecular dynamics simulation of nanoscale liquid flows. Microfluid. Nanofluidics 2010, 9, 1011–1031. [Google Scholar] [CrossRef]
- Falk, K.; Coasne, B.; Pellenq, R.; Ulm, F.J.; Bocquet, L. Subcontinuum mass transport of condensed hydrocarbons in nanoporous media. Nat. Commun. 2014, 6, 6949. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Roxin, A.; Snurr, R.Q.; Wang, Q.; Lichter, S. Molecular mechanisms of liquid slip. J. Fluid Mech. 2008, 600, 257–269. [Google Scholar] [CrossRef]
- Qiao, R. Effects of molecular level surface roughness on electroosmotic flow. Microfluid. Nanofluidics 2007, 3, 33–38. [Google Scholar] [CrossRef]
- Stefano, B.; Todd, B.D.; Searles, D.J. Thermostating highly confined fluids. J. Chem. Phys. 2010, 132, 1016. [Google Scholar]
- Arya, G.; Chang, H.C.; Maginn, E.J. Molecular Simulations of Knudsen Wall-slip: Effect of Wall Morphology. Mol. Simul. 2003, 29, 697–709. [Google Scholar] [CrossRef]
- Sun, H.; Hui, Z.; Na, Q.; Ying, L. Molecular Insights into the Enhanced Shale Gas Recovery by Carbon Dioxide in Kerogen Slit-Nanopores. J. Phys. Chem. C 2017, 121, 10233–10241. [Google Scholar] [CrossRef]
- Tesson, S.; Firoozabadi, A. Methane Adsorption and Self-Diffusion in Shale Kerogen and Slit Nanopores by Molecular Simulations. J. Phys. Chem. C 2018, 122, 23528–23542. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Yao, J.; Kou, J.; Li, Z.; Wu, T.; Zhang, K.; Zhang, L.; Sun, H. Adsorption Behaviors of Shale Oil in Kerogen Slit by Molecular Simulation. Chem. Eng. J. 2020, 387, 124054. [Google Scholar] [CrossRef]
- Collell, J.; Ungerer, P.; Galliero, G.; Yiannourakou, M.; Montel, F.; Pujol, M. Molecular Simulation of Bulk Organic Matter in Type II Shales in the Middle of the Oil Formation Window. Energy Fuels 2015, 28, 7457–7466. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q. Fast mass transport of oil and supercritical carbon dioxide through organic nanopores in shale. Fuel 2016, 181, 741–758. [Google Scholar] [CrossRef]
- Waldman, M.; Hagler, A.T. New combining rules for rare gas van der Waals parameters. J. Comput. Chem. 1993, 14, 1077–1084. [Google Scholar] [CrossRef]
- York, D.M.; Darden, T.A.; Pedersen, L.G. The effect of long-range electrostatic interactions in simulations of macromolecular crystals: A comparison of the Ewald and truncated list methods. J. Chem. Phys. 1993, 99, 8345–8348. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; Ashok, I.; Jônsson, H.; Kalonji, G.; Zahorjan, J. Parallel short-range molecular dynamics using the Ādhāra runtime system. Comput. Phys. Commun. 1997, 102, 28–43. [Google Scholar] [CrossRef]
- Travis, K.P.; Todd, B.D.; Evans, D.J. Poiseuille flow of molecular fluids. Phys. A-Stat. Mech. Appl. 1997, 240, 315–327. [Google Scholar] [CrossRef]
- Sundaram, D.S.; Puri, P.; Yang, V. Thermochemical Behavior of Nickel-Coated Nanoaluminum Particles. J. Phys. Chem. C 2013, 117, 7858–7869. [Google Scholar] [CrossRef]
- Nose, S.; Klein, M.L. A study of solid and liquid carbon tetrafluoride using the constant pressure molecular dynamics technique. J.Chem. Phys. 1983, 78, 6928–6939. [Google Scholar] [CrossRef]
- Sokhan, V.P.; Nicholson, D.; Quirke, N. Fluid flow in nanopores: An examination of hydrodynamic boundary conditions. J.Chem. Phys. 2001, 115, 3878–3887. [Google Scholar] [CrossRef]
- Nagayama, G.; Ping, C. Effects of interface wettability on microscale flow by molecular dynamics simulation. Int. J. Heat Mass Transf. 2004, 47, 501–513. [Google Scholar] [CrossRef]
- Peter, D.; Billy, T. Challenges in Nanofluidics—Beyond Navier–Stokes at the Molecular Scale. Processes 2018, 6, 144. [Google Scholar]
- Sankar, D.S.; Hemalatha, K. A non-Newtonian fluid flow model for blood flow through a catheterized artery—Steady flow. Appl. Math. Model. 2007, 31, 1847–1864. [Google Scholar] [CrossRef]
- Hughes, C.; Lerman, C.; Schwartz, M.; Peshkin, B.N.; Wenzel, L.; Narod, S.; Corio, C.; Tercyak, K.P.; Hanna, D.; Isaacs, C. Effects of couple-stresses on the dispersion of a soluble matter in a pipe flow of blood. Rheol. Acta 1980, 19, 710–715. [Google Scholar]
- Zheng, Y.; Li, Q.; Rui, H.; Gray, M.R.; Chou, K.C. Competitive Adsorption of Toluene and n-Alkanes at Binary Solution/Silica Interfaces. J. Phys. Chem. C 2009, 113, 20355–20359. [Google Scholar]
- Christenson, H.K.; Gruen, D.W.R.; Horn, R.G.; Israelachvili, J.N. Structuring in liquid alkanes between solid surfaces: Force measurements and mean-field theory. Cheminform 1987, 87, 1834–1841. [Google Scholar]
- Chaturani, P.; Upadhya, V.S. A two-fluid model for blood flow through small diameter tubes. Biorheology 1979, 16, 109–118. [Google Scholar] [CrossRef]
- Niavarani, A.; Priezjev, N.V. Rheological study of polymer flow past rough surfaces with slip boundary conditions. J. Chem. Phys. 2008, 129, 425. [Google Scholar] [CrossRef]
- Niavarani, A.; Priezjev, N.V. Slip boundary conditions for shear flow of polymer melts past atomically flat surfaces. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2008, 77, 041606. [Google Scholar] [CrossRef]
- Holt, J.K.; Hyung Gyu, P.; Yinmin, W.; Michael, S.; Artyukhin, A.B.; Grigoropoulos, C.P.; Aleksandr, N.; Olgica, B. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- BoţAn, A.; Rotenberg, B.; Marry, V.; Turq, P.; Noetinger, B.T. Hydrodynamics in Clay Nanopores. J. Phys. Chem. C 2011, 115, 16109–16115. [Google Scholar] [CrossRef]
- Wua, K.; Chena, Z.; Lib, J.; Lib, X.; Xua, J.; Dong, X. Wettability effect on nanoconfined water flow. PNAS 2017, 114, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Předota, M.; Cummings, P.T.; Wesolowski, D.J. Electric Double Layer at the Rutile (110) Surface. 3. Inhomogeneous Viscosity and Diffusivity Measurement by Computer Simulations. J. Phys. Chem. C 2007, 111, 3071–3079. [Google Scholar] [CrossRef]
- Bitsanis, I.; Vanderlick, T.K.; Tirrell, M.; Davis, H.T. A Tractable Molecular Theory of Flow in Strongly Inhomogeneous Fluids. J. Chem. Phys. 1988, 89, 3152–3162. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, Y.; Yang, Y.; Mei, Q.; Yang, S.; Wang, C. Multicomponent Shale Oil Flow in Real Kerogen Structures via Molecular Dynamic Simulation. Energies 2020, 13, 3815. https://doi.org/10.3390/en13153815
Liu J, Zhao Y, Yang Y, Mei Q, Yang S, Wang C. Multicomponent Shale Oil Flow in Real Kerogen Structures via Molecular Dynamic Simulation. Energies. 2020; 13(15):3815. https://doi.org/10.3390/en13153815
Chicago/Turabian StyleLiu, Jie, Yi Zhao, Yongfei Yang, Qingyan Mei, Shan Yang, and Chenchen Wang. 2020. "Multicomponent Shale Oil Flow in Real Kerogen Structures via Molecular Dynamic Simulation" Energies 13, no. 15: 3815. https://doi.org/10.3390/en13153815
APA StyleLiu, J., Zhao, Y., Yang, Y., Mei, Q., Yang, S., & Wang, C. (2020). Multicomponent Shale Oil Flow in Real Kerogen Structures via Molecular Dynamic Simulation. Energies, 13(15), 3815. https://doi.org/10.3390/en13153815