Abstract
The comprehensive and quantitative assessment of the contribution of minerals with different occurrence forms to particulate matter with an aerodynamic diameter of less than 10 μm (PM10) emitted from the combustion of Zhundong coal is of great significance for its clean utilization and for the development of particulate matter formation mechanisms. Samples with simplified occurrence forms of inorganic species were prepared by water-, salt-, and acid-washing of Zhundong coal. The samples were combusted in a drop-tube furnace under 20 vol % oxygen at 1250 °C, and the emitted PM10 was collected. The effects of the minerals in different forms on the PM10 emissions were analyzed by comparing the mass concentration distributions, yields, and elemental compositions of PM10. The results showed that water-soluble, ion-exchangeable, acid-soluble, and acid-insoluble minerals contributed 8.3%, 37.8%, 29.7%, and 24.2% of the PM10 emissions, respectively. The distributions of the Na, Mg, Ca, and Fe contents in PM10 were bimodal, as follows: 63.6% of Na and 54.5% of Fe were deported to the ultrafine mode PM, while 63.6% of Mg and 86.6% of Ca were deported to the coarse mode PM. The distributions of the Si and Al contents were unimodal, namely: 92.9% of Si and 90.5% of Al were deported to the coarse mode PM. Water-soluble Na; ion-exchanged Mg, Ca, and Fe; and acid-insoluble Si and Al played decisive roles in the distribution of minerals in PM10.
1. Introduction
Particulate matter (PM) with an aerodynamic diameter of less than 10 μm (PM10) is a vital pollutant produced in the process of coal utilization. For meeting the increasingly stringent emission standards, the formation mechanism and control method of PM10 during coal combustion have always been the focus of research [1,2,3,4]. In the target of the ultra-low emission of pollutants from coal-fired boilers, the emission limit of PM10 is less than 5 mg/m3 [4]. Zhundong coal, found in Xinjiang, China, has vast reserves, and is expected to be widely used in the future, not only in China [5,6]. In order to realize the clean utilization of this important energy source, it is necessary to make a specialized and detailed study on the PM10 emission from its combustion.
PM10 can be divided into ultrafine-mode, intermediate-mode, and coarse-mode PM, according to its mass concentration distribution with particle size [7,8,9,10]. Each mode has a different mechanism of formation, namely: ultrafine mode PM is formed by the vaporization and condensation of minerals [11,12], coarse mode PM is the result of mineral coalescence of char [12,13], and the central mode PM is affected by multiple mechanisms [14]. The release of volatiles forms a large number of pores in the char [15,16], and the fragmentation of char caused by these pores during combustion is an important factor affecting the central mode PM.
PM10 was transformed during coal combustion from minerals that mainly contain K, Na, Ca, Mg, Fe, Si, and Al. The occurrence forms of minerals can be divided into water-soluble, ion-exchange, acid-soluble, and acid-insoluble, according to the way of their chemical extraction [17,18]. The transformation process and results of minerals in different occurrence forms to different mode PM showed significant differences [19,20,21,22]. Water-soluble Na and K, such as their chlorides, are easy to vaporize, and are therefore enriched in ultrafine-mode PM [23,24], while acid-insoluble Na and K, such as Na and K in composite aluminosilicate, can exist stably at high temperatures and mainly become part of coarse-mode particles [25]. Ion-exchangeable Mg, Ca, and Fe are bound to a carbon matrix by chemical bonds, and are easily decomposed and released during combustion [26], thereby significantly contributing to all three modes of PM [21]. Acid-soluble Mg, Ca, and Fe, such as their carbonates, are usually transformed into coarse-mode PM by melt and coalescence after coal burnout [27]. The most common minerals of Si and Al are acid-insoluble, such as aluminosilicate and silica [28,29]. They are gasified in the form of their suboxides, with a small amount under high temperature and reductive conditions [30,31], and most of them are converted into coarse-mode PM [32,33]. It has also been reported that significant amounts of ion-exchangeable Si and Al exist in some low-rank coals, which tend to be transformed into ultrafine-mode PM [21].
Given the vital influence of mineral forms on PM10 emission, a comprehensive and quantitative assessment of this effect is helpful to the development of the formation mechanisms. The effect of different forms of minerals on PM10 emissions has been reported for Victorian lignite coal [21].
In the studies of PM10 emissions from Zhundong coal, the yield and composition of PM, the conversion process of one or more minerals to PM, and the influence of combustion parameters have been reported [34,35,36,37]; however, there is still a lack of study from the perspective of the mineral occurrence forms.
Alkali metals and alkaline earth metals (these elements play a decisive role in the formation of ultrafine-mode PM) have a high proportion in the minerals of Zhundong coal, and their occurrence forms are various [38]. These characteristics make Zhundong coal a suitable medium for studying the influence of the mineral occurrence forms on the PM10 emissions.
At the same time, the pre-removal of minerals from coal before combustion is the most direct and effective way to reduce PM emissions. The evaluation of the emission reduction effect by different pre-removal methods has an essential application value.
Based on the similarities between the pre-removal methods of minerals and the separation methods of minerals with different occurrence forms, we combined the two kinds of study on Zhundong coal. Water-, salt-, and acid-washing coal were prepared by washing Zhundong raw coal with deionized water, ammonium acetate solution, and dilute nitric acid solution, respectively. The water-washed coal contained no water-soluble minerals, the salt-washed coal contained neither water-soluble nor ion-exchangeable minerals, and the acid-washed coal contained only acid-insoluble minerals. The four coal samples (including raw coal) were combusted in a drop-tube furnace under 20 vol % O2 at 1250 °C, and the PM10 was collected and analyzed to reveal the transformation rules of the minerals with different occurrence forms into PM10, and to provide the theoretical basis for controlling PM10 emission.
2. Materials and Methods
2.1. Sample Preparation
Zhundong coal, crushed and then sieved to a narrow size range of 63–90 μm, was termed raw coal. Its proximate and ultimate analyses are shown in Table 1.

Table 1.
Proximate and ultimate analyses of raw coal.
The raw coal contained four form minerals, namely: water-soluble, ion-exchangeable, acid-soluble, and acid-insoluble minerals [17,18]. Water-washed coal was prepared by mixing raw coal (50 g) and deionized water (1 L); stirring for 2 h at 60 °C; then rinsing, filtering, and drying the sample. The same preparation process was used for the salt- and acid-washed coals, using 0.1 mol/L ammonium acetate and 0.1 mol/L nitric acid solutions, respectively, instead of deionized water. By following these procedures, the water-washed coal contained ion-exchangeable, acid-soluble, and acid-insoluble minerals; the salt-washed coal contained acid-soluble and acid-insoluble minerals; and there were only acid-insoluble minerals remaining in the acid-washed coal. The mineral compositions of the four samples were different so that they could be used for comparative experiments.
2.2. Experimental System
All of the experiments were carried out in a drop-tube furnace (Figure 1). The system was divided into five sections, namely, combustion, feeding, gas distribution, cooling, and sampling.
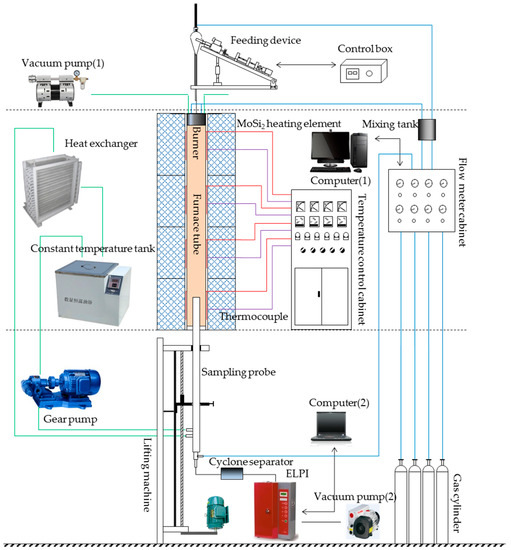
Figure 1.
Schematic diagram of the experimental system. ELPI—electrical low-pressure impactor.
The gas distribution section contained gas cylinders, a flow-meter cabinet, a mixing tank, and a computer (1). The feeding section contained a self-made feeding device and control box. The feed rate of the samples was 0.1 g/min, with a carrier gas flowrate of 0.5 L/min and a composition of 20 vol % oxygen (O2) and 80 vol % nitrogen (N2). The combustion section contained a burner, furnace tube, MoSi2 heating element, thermocouple, and temperature-control cabinet. The inner diameter of the furnace tube was 80 mm, and the length of the constant-temperature section was 2 m. The temperature in the furnace was held at 1250 °C during the experiments. The burner was made of Al2O3, with a central feed pipe and two intake pipes. The samples entered the furnace tube from the central feed pipe, and the reaction gas (9.5 L/min, 20 vol % O2, 80 vol % N2) entered from the intake pipe. The reaction gas mixed with the samples in the furnace tube, which then burned out. The cooling section contained a vacuum pump (1), heat exchanger, constant-temperature tank, and gear pump. The vacuum pump (1) was used to cool down the burner. The other devices were used to cool the sampling probe. The cooling medium was conductive heating oil.
The sampling section contained a sampling probe, cyclone separator, electrical low-pressure impactor (ELPI; ELPI, Dekati, Finland), vacuum pump (2), computer (2), and self-made lifting machine. The sampling probe was used to collect the PM from the furnace tube. Nitrogen was used to quench and dilute the gas containing the PM, and the conductive heating oil further cooled the PM. The cyclone separated the PM with an aerodynamic diameter exceeding 10 μm, while the ELPI could divide PM10 into twelve grades of particle size ranges [39,40], as shown in Table 2.

Table 2.
Particle size ranges of twelve grades of particulate matter (PM) collected by ELPI (μm).
2.3. Sample Analysis
The coal samples were solubilized by microwave digestion (Ethos one, Milestone, Italy), and then analyzed by inductively coupled plasma atomic emission spectrometry (ICP–AES; iCAP 7000, Thermo Fisher Scientific, USA). The contents of the main mineral elements (Na, K, Mg, Ca, Fe, Si, and Al) are shown in Table 3.

Table 3.
Contents of main mineral elements in the coal samples (mg/g_coal).
Ca was the dominant mineral element (8.207 mg/g_coal) in the raw coal, followed by Si, Na, Mg, Fe, Al, and K, in decreasing order. The total proportion of alkali (Na and K) and alkaline-earth (Mg and Ca) elements in the raw coal was 67.9%, which differed from a high-rank coal that contains more stable elements (Si and Al) [27,29]. The K content was very small (0.148 mg/g_coal), which was less than 1% of the total mineral element content. It had little effect on the PM emission, and was not considered in the subsequent analysis.
The mass fraction of the mineral elements in PM10 was analyzed by field-emission scanning electron microscopy (FE-SEM; Apreo C, Thermo Fisher Scientific, USA).
2.4. Analytical Method
The minerals in four occurrence forms were separated step by step, and their quantities were calculated by major mineral elements (Na, Mg, Ca, Fe, Si, and Al). The contributions of the minerals in different occurrence forms to the PM10 emission are described intuitively, and are quantitatively based on the differences of the PM10 yield from the combustion of raw coal, water-washed coal, salt-washed coal, and acid-washed coal. The contents of the minerals in different forms and their contribution to the PM10 were compared so as to analyze their transformation process. Equations (1)–(5) were used to determine (i) the contents of these four form minerals (Figure 2), (ii) the contributions of these four forms of minerals to the PM10 emissions, and (iii) their contributions to the elements present in the PM10.
Wraw coal = Wwater-soluble + Wion-exchangeable + Wacid-soluble + Wacid-insoluble,
Wwater-soluble = ∑(wi, raw coal − wi, water-washed coal),
Wion-exchangeable = ∑(wi, water-washed coal − wi, salt-washed coal),
Wacid-soluble = ∑(wi, salt-washed coal − wi, acid-washed coal),
Wacid-insoluble = ∑wi, acid-washed coal.
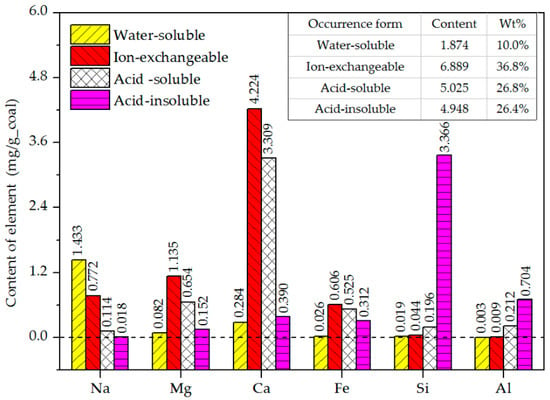
Figure 2.
Contents and proportions of four of the form mineral elements in the coal samples.
In the calculation of (i), W (mg/g_coal) was the total content of all of the mineral elements, wi (mg/g_coal) was the content of a certain mineral element (given in Table 3), and i was a certain mineral element (Na, Mg, Ca, Fe, Si, or Al); in the calculation of (ii), W (mg/g_coal) was the yield of PM, wi (mg/g_coal) was the mass concentration of PM in a certain size range, and i was the size range of PM (given in Table 2); in the calculation of (iii), W (μg/g_coal) was the total content of the mineral elements in PM, wi (μg/g_coal) was the mass concentration of the mineral elements in PM with a certain size range (equal to 10 times the product of the mass concentration of PM and the mass fraction of mineral elements in PM), and i was the size range of the PM (given in Table 2).
3. Results and Discussions
3.1. Contents and Forms of Minerals in the Coal Samples
Figure 2 shows the contents and relative proportions of the different forms of mineral elements. Na mainly existed in water-soluble and ion-exchangeable forms, Mg and Ca were mainly present in ion-exchangeable and acid-soluble forms, Fe was not present in the water-soluble components, and Si and Al mainly existed in acid-insoluble minerals. The total content of the ion-exchangeable minerals was the highest (36.8%), followed by the acid-soluble (26.8%), acid-insoluble (26.4%), and water-soluble (10.0%) minerals.
Table 4 shows the relative proportions of the major mineral elements in the different forms (results from Figure 2). Combining the data of Figure 2 and Table 4, the following was found:

Table 4.
Relative proportions of the main elements in the four form minerals (wt %).
- The total content of the water-soluble mineral elements was 1.874 mg/g_coal, of which the Na content was the highest (1.433 mg/g_coal), accounting for 76.5% of this category. This water-soluble Na mainly existed as chloride [38].
- The total content of the ion-exchangeable mineral elements was 6.889 mg/g_coal, and mainly included Ca, Mg, Na, and Fe. These elements occurred as cations, which combined with carboxyl and hydroxyl groups and were dispersed in the coal. The Ca content was the highest at 4.224 mg/g_coal, accounting for 61.3% of the total ion-exchangeable mineral elements.
- The total content of the acid-soluble mineral elements was 5.025 mg/g_coal, and comprised mainly Ca, Mg, and Fe. These minerals were mainly carbonates, silicates, and aluminates, which were present as dispersed mineral particles [38]. Ca was the most abundant, accounting for 65.9% of this group. The Ca mainly existed as carbonate.
- The total content of the acid-insoluble mineral elements was 4.948 mg/g_coal, and mainly included Si, Al, Ca, and Fe. The Si content was 3.366 mg/g_coal, accounting for 68.0% of the total. Acid-insoluble Si mainly existed as SiO2 and silicate-aluminates, while acid-insoluble Al and Ca mainly existed as silicate-aluminates. These were all present as dispersed mineral particles that had stable physical and chemical properties [21,38].
There were obvious differences in the contents and dominant elements of the four forms of minerals. Owing to these differences, the effects of the four forms of minerals on the yields and compositions of PM10 were significantly different, as described in Section 3.2 and Section 3.3, respectively.
3.2. Effect of Different Form Minerals on Mass Concentration Distribution and Yield of PM10
Figure 3 shows the mass concentration distributions and yields of PM from the combustion of the raw, water-washed, salt-washed, and acid-washed coals. The trends of four curves are similar, with two peaks (at 0.137 and 5.176 μm) and an obvious inflection point (at 0.81 μm); however, the curves generally moved down with the decrease of mineral content. The three regions between the four curves reflected the contributions of water-soluble, ion-exchangeable, and acid-soluble minerals to PM10. The effect of the water-soluble minerals was more obvious for a particle size less than 0.137 μm. The ion-exchangeable minerals had a strong effect at all of the particle sizes. The effect of acid-soluble minerals was mainly on particles of 0.137–10 μm.
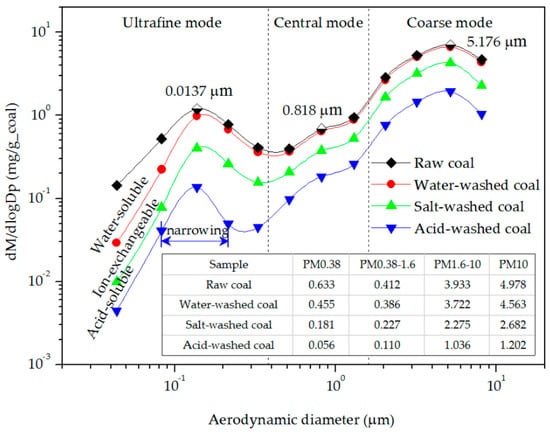
Figure 3.
Mass concentration distributions and yields of PM from the combustion of the coal samples.
The mass concentrations of PM10 emitted from the combustion of the four coal samples displayed a trimodal distribution, comprising ultrafine, central, and coarse modes [7,8,9]. According to the characteristics of these modes, and the positions of the peaks and inflection points of the distribution curves [9,41], the size ranges of the three modes of PM were defined as follows: 1.6–10 μm for the coarse mode PM (PM1.6–10), 0.38–1.60 μm for the central mode PM (PM0.38–1.6), and less than 0.38 μm for the ultrafine mode PM (PM0.38). The PM10 emissions from the raw coal combustion contained contributions from all four form minerals. The total content of PM10 was 4.798 mg/g_coal, comprised of 0.633 mg/g_coal of PM0.38, 0.412 mg/g_coal of PM0.38–1.6, and 3.933 mg/g_coal of PM1.6–10.
Figure 4 shows the yields and proportional contributions of the PM emitted from the four form minerals, as follows:
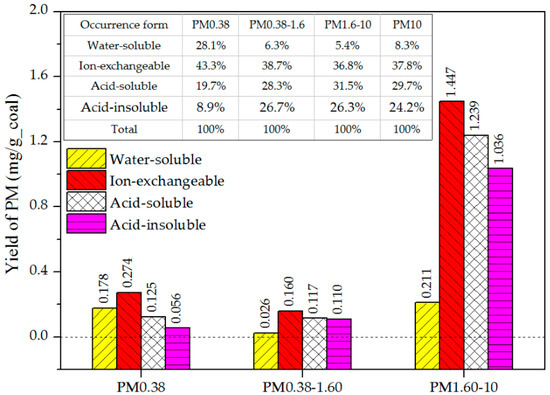
Figure 4.
Yields and proportional contributions of the PM emitted from the four form minerals.
- The water-soluble, ion-exchangeable, acid-soluble, and acid-insoluble minerals contributed 8.3%, 37.8%, 29.7%, and 24.2% of the PM10 yield, respectively. The ion-exchangeable minerals had a high content and were dispersed in the coal, and so were easily transformed into PM during combustion, resulting in the highest contribution to PM10 [21].
- Of the three modes of PM, the contribution of water-soluble minerals to the ultrafine mode was the highest (28.1%). Water-soluble minerals are active, and are easily converted into ultrafine mode PM by vaporization and condensation [24]; it acted through condensation on the PM surface, and had little effect on the emissions of the central and coarse mode PM [10].
- The acid-soluble minerals contributed to the coarse mode PM more than to the other mode PM, accounting for 31.5%. The acid-soluble minerals or their decomposition products are relatively stable and difficult to gasify—most gradually melted and coalesced into coarse mode PM at a high temperature during coal combustion.
- The contributions of the acid-insoluble minerals to all three modes of PM were less than that of the acid-soluble minerals. This result was due to the low melting point of the Si-containing matter (such as SiO2) in the acid-insoluble minerals, which was prone to coalesce and be converted into larger particles (PM10+), thus reducing the formation of PM10. The subsequent analysis of PM10+ produced by the raw coal showed that its Si mass fraction exceeded 20%, which was four times that in the coarse mode PM (PM1.6–10).
- The ultrafine mode PM resulting from the acid-insoluble minerals originated from the gasification of high-boiling-point minerals (such as quartz and aluminosilicate). These minerals are gasified to atoms or suboxides at a high temperature and under a local reductive atmosphere [30,31]. As a result of the low extent of the gasification of acid-insoluble minerals, the yield of PM0.38 was small, and the peak area of ultrafine mode PM resulting from acid-washed coal was narrowed (Figure 3).
In summary, owing to the differences in their contents and compositions, the contributions of ion-exchangeable, acid-soluble, and acid-insoluble minerals to all three modes of PM decreased in this order; the contributions of water-soluble minerals to central and coarse mode PM were the lowest, and the contribution to ultrafine mode PM was less than that of ion-exchangeable minerals and more than that of acid-soluble minerals. In the above comparison, the interactions between the minerals with different occurrence forms were not considered. The study on the interaction can ensure a more accurate assessment of their contribution, and is also of great significance for further understanding the formation mechanism of PM10.
3.3. Effect of Different from Minerals on Element Composition and Content of PM10
The effects of the mineral elements in different occurrence forms were analyzed by comparing the differences of the element composition in PM10 emitted from the four coal samples. The transformation amount and process of mineral elements (Na, Mg, Ca, Fe, Si, and Al) in different forms to PM were described in detail by combining the mass fraction and content of elements in PM.
Table 5 shows the contents and proportions of the mineral elements in PM, from the combustion of raw coal, including the contributions of all four form minerals. The distributions of the contents and mass fractions of Na, Mg, Ca, Fe, Si, and Al in PM, emitted from the combustion of the four samples, are shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10, respectively. The differences of the mineral content in PM formed during the combustion of the four samples indicated their relative transformation to PM from the different form minerals.

Table 5.
Contents and proportions of mineral elements in PM from the combustion of raw coal (μg/g_coal).
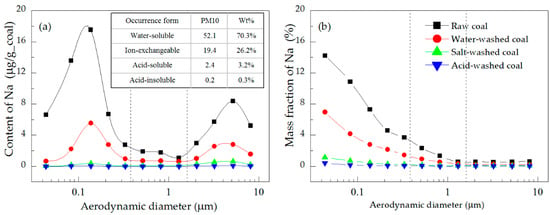
Figure 5.
Distributions of the (a) content and (b) mass fraction of Na in the PM.
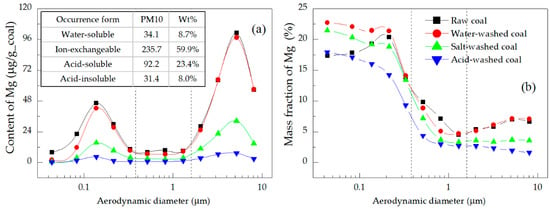
Figure 6.
Distributions of the (a) content and (b) mass fraction of Mg in the PM.
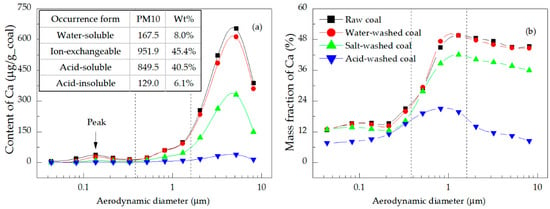
Figure 7.
Distributions of the (a) content and (b) mass fraction of Ca in the PM.
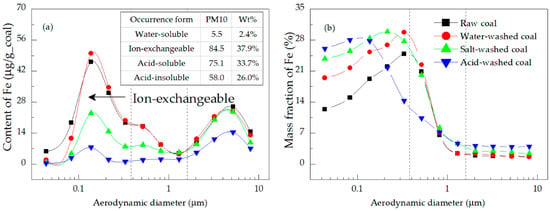
Figure 8.
Distributions of the (a) content and (b) mass fraction of Fe in the PM.
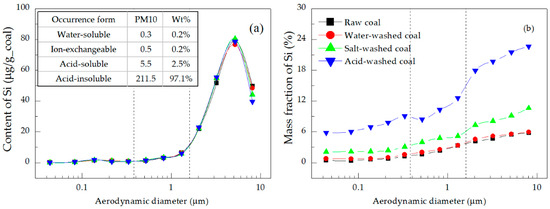
Figure 9.
Distributions of the (a) content and (b) mass fraction of Si in the PM.
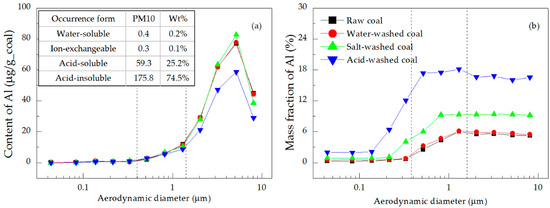
Figure 10.
Distributions of the (a) content and (b) mass fraction of Al in the PM.
Figure 5a shows that the amounts of water-soluble, ion-exchangeable, acid-soluble, and acid-insoluble Na transferred into PM10 were 52.1, 19.4, 2.4, and 0.2 μg/g_coal, respectively. This was due to the combined effect of two factors, namely: firstly, the content of Na in the four forms of minerals correspondingly decreased (Figure 2); secondly, the gasification ability of water-soluble Na (NaCl) was stronger than that of ion-exchangeable Na [26], and the gasification ability of acid-soluble and acid-insoluble Na was inhibited by aluminosilicate and SiO2 [25].
Figure 5 shows that the distribution of the Na content in PM10 had two peaks, which corresponded to those of the mass concentration distribution (Figure 3). The mass fraction of Na decreased with the increase of particle size, indicating that the mass concentration distribution of PM played a crucial role in the distribution of the Na content. Of the two peaks, the one in the ultrafine mode region was higher than the one in the coarse mode region, reflecting the enrichment of Na in small-size PM [24]. Specifically, the content of Na in PM0.38 emitted from raw coal combustion accounted for 63.6% of the total PM10 (Table 5). This result was because the enrichment of Na in small-size PM depended on its transformation. Na is a highly active mineral element, and is easily gasified into Na vapor during combustion. Some Na vapor directly formed nano-sized PM with a high mass fraction of Na by vaporization and condensation, and then grew into ultrafine mode PM during the processes of collision and coalescence [27]. Na vapor was also adsorbed onto the existing PM by surface condensation. The smaller the particle size and the larger the specific surface area, the more easily the Na vapor was absorbed and the higher its mass fraction. This transformation mechanism led to a decrease of the mass fraction of Na with an increase of particle size, manifesting as the enrichment of Na in the ultrafine mode PM.
Mg and Ca showed a similar performance in the formation of PM when they occurred in the same form [21,42]. Figure 6a and Figure 7a illustrate two points, namely (1) Ion-exchangeable Mg and Ca contributed the most to Mg and Ca in PM10 (59.9% for Mg and 45.4% for Ca), followed by acid-soluble, water-soluble, and acid-insoluble Mg and Ca in decreasing order. This result was determined by the content and gasification ability of the different forms of these elements, which were higher for ion-exchangeable Mg and Ca than for the acid-soluble elements, although the contents of the acid-soluble Mg and Ca were far higher than those of the water-soluble and acid-insoluble elements. (2) The contents of Mg and Ca in PM10 displayed two peaks in their distributions, with the higher appearing in the coarse mode region, reflecting that Mg and Ca tended to be more readily transformed into the coarse mode PM. Specifically, the contents of Mg and Ca in PM1.6-10 emitted from raw coal combustion accounted for 63.6% and 86.6% of the total Mg and Ca in PM10, respectively (Table 5). The peak value of Mg in the coarse mode region was 1.7–2.3 times that in the ultrafine mode region, while that of Ca was 15.5–32.2 times larger, which indicated that Mg was more easily transformed into ultrafine mode PM. Figure 6b and Figure 7b show that with an increase of particle size, the mass fraction of Mg was first high and then dropped, and the mass fraction of Mg in ultrafine mode PM was higher; the trend for Ca was just the opposite. The mass fraction of Mg in ultrafine mode PM was higher than that of Ca, which confirmed the higher conversion of Mg to PM0.38.
Figure 8a shows that the contributions of the water-soluble, ion-exchangeable, acid-soluble, and acid-insoluble Fe to PM10 were 2.4%, 37.9%, 33.7%, and 26.0%, respectively. The distribution of the Fe content had peaks located in the ultrafine and coarse mode regions [21]. The peak value of the ultrafine mode area was higher for the PM emitted from the combustion of raw and water-washed coal; for the salt-washed coal, the two peaks were similar in size; and for the acid-washed coal, the peak value of the coarse mode area was higher. According to the difference in the contents and forms of the four coal samples (Table 3 and Figure 2), it was found that the ion-exchangeable Fe made an important contribution to the ultrafine mode PM (area indicated by the arrow in Figure 8a) [21]; the acid-insoluble Fe tended to be a component of the coarse mode PM. The sum of the Fe in the ultrafine mode PM converted from four form minerals was more than that in the coarse mode PM. Specifically, the Fe content in the PM0.38 emitted from the raw coal combustion accounted for 54.5% of the total Fe in PM10 (Table 5). As shown in Figure 8b, its mass fraction distribution had only one peak, located in the ultrafine mode region. For the ultrafine mode PM emitted from the combustion of all four coal samples, all of the mass fractions of Fe were higher, which indicated that Fe was the main component [36]. At a high temperature and under a local reductive atmosphere during the coal combustion, Fe gasified considerably from all of the mineral forms.
Figure 9a and Figure 10a show that acid-insoluble Si and Al contributed 97.1% and 74.5% of total Si and Al in PM10, respectively [32,33]. Their content distributions had only one peak, located in the coarse mode region, indicating that these elements mainly existed in the coarse mode PM. Specifically, the contents of Si and Al in the PM1.6–10 emitted from the raw coal combustion accounted for 92.9% and 90.5% of the total PM10 (Table 5), respectively. These elements were mainly present as acid-soluble and acid-insoluble minerals, and were hard to gasify—they mostly melted and coalesced into coarse mode PM during coal combustion [31,32]. The mass fractions of Si and Al were very low in the ultrafine mode PM, but high in the coarse mode PM, as shown in Figure 9b and Figure 10b, respectively. The difference between the mass fraction distribution of Si (increasing with the increase of particle size) and that of Al (step-type increase) was due to the stronger gasification ability of Si [9,43].
In summary, the distributions of the Na, Mg, Ca, and Fe contents in PM10 showed two peaks, while those of Si and Al showed a single peak. Na and Fe were mainly distributed in the ultrafine mode PM, and Mg and Ca were mainly distributed in the coarse mode PM, while and Si and Al were almost all distributed in the coarse mode PM. Water-soluble Na; ion-exchangeable Mg, Ca, and Fe; and acid-insoluble Si and Al yielded important contributions to PM10 emissions.
4. Conclusions
This study reported the effects of different forms of minerals on PM10 emissions during the combustion of Zhundong coal. The following conclusions were drawn:
- The contribution of water-soluble mineral elements, mainly Na and Ca, to PM10 was only 8.3%, but their contribution to ultrafine mode PM was relatively larger, accounting for 28.1% of the total PM0.38. The ion-exchangeable mineral elements, mainly Mg, Ca, Na, and Fe, contributed 37.8% of PM10. The acid-soluble mineral elements, mainly Ca, Mg, and Fe, contributed 29.7% of PM10. The acid-insoluble mineral elements, mainly Si, Al, Ca, and Fe, contributed 24.2% to PM10, and mainly transformed into coarse PM.
- The distributions of the Na, Mg, Ca, and Fe contents in PM10 showed two peaks, as follows: 63.6% of Na and 54.5% of Fe deported to the ultrafine mode PM, while 63.6% of Mg and 86.6% of Ca were in the coarse mode PM. The Si and Al contents showed a single peak distribution, with 92.9% of Si and 90.5% of Al reporting to the coarse mode PM.
- Water-soluble Na; ion-exchangeable Mg, Ca, and Fe; and acid-insoluble Si and Al contributed considerably to PM10, as follows: water-soluble Na contributed 70.4% of the total Na; ion-exchangeable Mg, Ca, and Fe contributed 59.9%, 45.4%, and 37.9% of the total Mg, Ca, and Fe, respectively; and acid-insoluble Si and Al contributed 97.1% and 74.5% of these elements, respectively.
Author Contributions
L.Z. performed the experiments, analyzed the data, and wrote the paper; Q.D., J.G., and S.W. supervised this project and revised the paper.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2017YFF0209805.
Acknowledgments
The authors are grateful to the sample testing support received from the Analytical and Testing Center at Harbin Institute of Technology, and the Material Analytical and Testing Center at Harbin University of Science and Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taniguchi, M.; Kamikawa, Y.; Shibata, T.; Yamamoto, K.; Kobayashi, H. Application of the NOx reaction model for development of low-NOx combustion technology for pulverized coals by using the gas phase stoichiometric ratio index. Energies 2011, 4, 545–562. [Google Scholar] [CrossRef]
- Ghauri, M.; Shahzad, K.; Inayat, A.; Ali, Z.; Cliffe, K.R. High pressure oxydesulphurisation of coal using KMnO4—Effect of coal slurry concentration, pH and alkali. Energies 2016, 9, 289. [Google Scholar] [CrossRef]
- Chen, G.B.; Chatelier, S.; Lin, H.; Wu, F.; Lin, T. A study of sewage sludge co-combustion with Australian black coal and shiitake substrate. Energies 2018, 11, 3436. [Google Scholar] [CrossRef]
- Sui, Z.F.; Zhang, Y.S.; Peng, Y.; Norris, P.; Cao, Y.; Pan, W.P. Fine particulate matter emission and size distribution characteristics in an ultra-low emission power plant. Fuel 2016, 185, 863–871. [Google Scholar] [CrossRef]
- Wang, X.; Qin, D.; Zhang, D.; Guan, W.; Xu, M.; Wang, X.; Zhang, G. Evolution characteristics of overburden strata structure for ultra-thick coal seam multi-layer mining in Xinjiang East Junggar Basin. Energies 2019, 12, 332. [Google Scholar] [CrossRef]
- Zhou, J.; Zhuang, X.; Alastuey, A.; Querol, X.; Li, J. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China. Int. J. Coal Geol. 2010, 82, 51–67. [Google Scholar] [CrossRef]
- Linak, W.P.; Miller, C.A.; Seames, W.S.; Wendt, J.O.L.; Ishinomori, T.; Endo, Y.; Miyamae, S. On trimodal particle size distributions in fly ash from pulverized-coal combustion. Proc. Combust. Inst. 2002, 29, 441–447. [Google Scholar] [CrossRef]
- Seames, W.S. An initial study of the fine fragmentation fly ash particle mode generated during pulverized coal combustion. Fuel Process. Technol. 2003, 81, 109–125. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Yao, H.; Liu, X.; Zhou, K. A new method for identifying the modes of particulate matter from pulverized coal combustion. Powder Technol. 2008, 183, 105–114. [Google Scholar] [CrossRef]
- Xu, M.; Yu, D.; Yao, H.; Liu, X.; Qiao, Y. Coal combustion-generated aerosols: Formation and properties. Proc. Combust. Inst. 2011, 33, 1681–1697. [Google Scholar] [CrossRef]
- Neville, M.; Quann, R.J.; Haynes, B.S.; Sarofim, A.F. Vaporization and condensation of mineral matter during pulverized coal combustion. Symp. (Int.) Combust. 1981, 18, 1267–1274. [Google Scholar] [CrossRef]
- Flagan, R.C. Submicron particles from coal combustion. Symp. (Int.) Combust. 1979, 17, 97–104. [Google Scholar] [CrossRef]
- Yan, L.; Gupta, R.P.; Wall, T.F. The implication of mineral coalescence behavior on ash formation and ash deposition during pulverized coal combustion. Fuel 2001, 80, 1333–1340. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Yao, H.; Liu, X.; Zhou, K.; Li, L.; Wen, C. Mechanisms of the central mode particle formation during pulverized coal combustion. Proc. Combust. Inst. 2009, 32, 2075–2082. [Google Scholar] [CrossRef]
- Smolinski, A.; Howaniec, N. Analysis of porous structure parameters of biomass chars versus bituminous coal and lignite carbonized at high pressure and temperature—A chemometric study. Energies 2017, 10, 1457. [Google Scholar] [CrossRef]
- Kim, G.M.; Kim, J.P.; Lisandy, K.Y.; Jeon, C.H. Experimental model development of oxygen-enriched combustion kinetics on porous coal char and non-porous graphite. Energies 2017, 10, 1436. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.; Yan, Z.; Liu, K.; Xu, Y.; Sheng, C. The varying characterization of alkali metals (Na, K) from coal during the initial stage of coal combustion. Energy Fuels 2001, 15, 786–793. [Google Scholar] [CrossRef]
- Wei, X.; Huang, J.; Liu, T.; Fang, Y.; Wang, Y. Transformation of alkali metals during pyrolysis and gasification of a lignite. Energy Fuels 2008, 22, 1840–1844. [Google Scholar] [CrossRef]
- Quann, R.J.; Neville, M.; Sarofim, A.F. A laboratory study of the effect of coal selection on the amount and composition of combustion generated submicron particles. Combust. Sci. Technol. 1990, 74, 245–265. [Google Scholar] [CrossRef]
- Tian, C.; Lu, Q.; Liu, Y.; Zeng, H.; Zhao, Y.; Zhang, J.; Gupta, R. Understanding of physicochemical properties and formation mechanisms of fine particular patter generated from Canadian coal combustion. Fuel 2016, 165, 224–234. [Google Scholar] [CrossRef]
- Gao, X.; Rahim, M.U.; Chen, X.; Wu, H. Significant contribution of organically-bound Mg, Ca, and Fe to inorganic PM10 emission during the combustion of pulverized Victorian brown coal. Fuel 2014, 117, 825–832. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, C. Correlation of the sub-micrometer ash yield from pulverized coal combustion with coal ash composition. Energy Fuels 2018, 32, 9961–9970. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, C.A.; Yan, Y.; Jin, X.; Liu, Y.H.; Che, D.F. Release and transformation of sodium during combustion of Zhundong coals. J. Energy Inst. 2016, 89, 48–56. [Google Scholar] [CrossRef]
- Huang, Q.; Li, S.; Li, G.; Yao, Q. Mechanisms on the size partitioning of sodium in particulate matter from pulverized coal combustion. Combust. Flame 2017, 182, 313–323. [Google Scholar] [CrossRef]
- Ruan, R.; Xiao, J.; Du, Y.; Tan, H.; Wang, X.; Yang, F. Effect of interaction between sodium and oxides of silicon and aluminum on the formation of fine particulates during synthetic char combustion. Energy Fuels 2018, 32, 6756–6762. [Google Scholar] [CrossRef]
- Quyn, D.M.; Wu, H.; Bhattacharya, S.P.; Li, C. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part II. Effects of chemical form and valence. Fuel 2002, 81, 151–158. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; Yao, H.; Yu, D.; Gao, X.; Cao, Q.; Cai, Y. Effect of combustion parameters on the emission and chemical composition of particulate matter during coal combustion. Energy Fuels 2007, 21, 157–162. [Google Scholar] [CrossRef]
- Tian, S.D.; Zhuo, Y.Q.; Zhan, Z.H.; Shu, X.Q.; Kang, Z.Z. Distribution of clay minerals in light coal fractions and the thermal reaction products of these clay minerals during combustion in a drop tube furnace. Energies 2016, 9, 428. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, K.; Meng, K.; Song, L.; Lin, Q. Effects of SiO2/Al2O3 ratios on sintering characteristics of synthetic coal ash. Energies 2017, 10, 242. [Google Scholar] [CrossRef]
- Schick, H.L. A thermodynamic analysis of the high temperature vaporization properties of silica. Chem. Rev. 1960, 60, 331–362. [Google Scholar] [CrossRef]
- Brewer, L. The thermodynamic properties of the oxides and their vaporization processes. Chem. Rev. 1953, 52, 1–75. [Google Scholar] [CrossRef]
- Teramae, T.; Takarada, T. Fine ash formation during pulverized coal combustion. Energy Fuels 2009, 23, 2018–2024. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Sato, A.; Ninomiya, Y.; Yamashita, T. Effects of coal blending on the reduction of PM10 during high-temperature combustion 1. Mineral transformations. Fuel 2008, 87, 2997–3005. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Li, B.; Sun, S.; Zhang, S. Combustion characteristics of char from pyrolysis of Zhundong sub-bituminous coal under O2/steam atmosphere: Effects of mineral matter. Int. J. Greenh. Gas Control 2019, 80, 54–60. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Huang, Q.; Yao, Q. Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace. Fuel 2015, 143, 430–437. [Google Scholar] [CrossRef]
- Fan, B.; Wen, C.; Zeng, X.; Wu, J.; Yu, X. Emission behaviors of inorganic ultrafine particles during Zhundong coal oxy-fuel combustion with characterized oxygen input fractions comparable to air combustion. Appl. Sci. 2018, 8, 1486. [Google Scholar] [CrossRef]
- Gao, Q.; Li, S.; Yuan, Y.; Zhang, Y.; Yao, Q. Ultrafine particulate matter formation in the early stage of pulverized coal combustion of high-sodium lignite. Fuel 2015, 158, 224–231. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Yang, H.; Lu, J. Improved sequential extraction method for determination of alkali and alkaline earth metals in Zhundong coals. Fuel 2016, 181, 951–957. [Google Scholar] [CrossRef]
- Keskinen, J.; Pietarinen, K.; Lehtimäki, M. Electrical low pressure impactor. J. Aerosol Sci. 1992, 23, 353–360. [Google Scholar] [CrossRef]
- Marjamäki, M.; Keskinen, J.; Chen, D.; Pui, D.Y.H. Performance evaluation of the electrical low-pressure impactor (ELPI). J. Aerosol Sci. 2000, 31, 249–261. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Hinkley, J.T.; Gupta, R.P.; Wall, T.F.; Nelson, P.F. Submicron ash formation from coal combustion. Fuel 2005, 84, 1206–1214. [Google Scholar] [CrossRef]
- Miller, S.F.; Schobert, H.H. Effect of the occurrence and composition of silicate and aluminosilicate compounds on ash formation in pilot-scale combustion of pulverized coal and coal-water slurry fuels. Energy Fuels 1994, 8, 1197–1207. [Google Scholar] [CrossRef]
- Quann, R.J.; Sarofim, A.F. Vaporization of refractory oxides during pulverized coal combustion. Symp. (Int.) Combust. 1982, 19, 1429–1440. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).