Abstract
The reduction of high acid value (AV) of inedible jatropha oil (JO) by esterification with isopropanol (IPA), which is a common alcohol solvent waste in Taiwan’s high-tech industry, was studied. The decrease of AV is beneficial for the subsequent transesterification to produce JO biodiesel (i.e., biodiesel of fatty acid isopropyl ester (FAIE)). Acid catalyst (H2SO4) and a novel mixing/emulsion technique using ultrasound irradiation (UI) were applied to promote and facilitate the esterification process. The results showed that increased IPA/oil molar ratio (MIOE) can significantly reduce the AV, kinematic viscosity (KV), density (ρLO), and water content (MW) of esterified JO, while also providing the benefit of enhancing the yield (YF) of biodiesel of FAIE. For example, with MIOE = 5 at esterification temperature (TE) = 394.2 K (393.8–394.7 K), a reduction of AV of 99.25% with YF of 67.15% can be achieved. Free fatty acid (FFA) was reduced from 18.06 wt.% to 0.14 wt.%, indicating 17.92 wt.% out of 18.06 wt.% of FFA was esterified to FAIE. As a result, among the YF of 67.15%, 49.23% (= 67.15 wt.% deducting 17.92 wt.%) was contributed by the transesterification of triglycerides. By esterification of high FFA-containing raw JO with acid catalyst, one can not only avoid saponification, but also reduce the loading of the subsequent alkali-catalyzed transesterification. Moreover, increasing TE from 394.2 to 454.4 K further reduced AV (from 0.27 to 0.084 mg KOH/g) and MW (from 0.27 to 0.043 wt.%), but, on the other hand, it increased KV (from 14.62 to 25.2 mm2/s) and ρLO (from 901.6 to 913.3 kg/m3), while it decreased YF (from 67.15 to 25.84%). In sum, IPA was successfully used as a replacement for methanol in the esterification of JO while UI provided mixing/emulsion along with heating resulting from cavitation for the system.
1. Introduction
Biomass energy has been widely applied around the world [1,2,3,4,5]. Among the different options, the main liquid biofuels are biodiesel and bioethanol. In particular, biodiesel can be mixed with fossil diesel and used as a commercial fuel without any infrastructure change issues. The use of biodiesel not only reduces energy consumption, but also decreases the reliance on fossil fuels. Furthermore, partial self-supplied energy can be expected by developing the techniques of manufacturing biodiesel. In the beginning, edible oil, with advantages of low acid value and a simplified manufacturing process, was used as raw material [6,7,8,9]. However, with the passage of time, the techniques for manufacturing biodiesel have progressed greatly. These methods include transesterification, pyrolysis, and micro-emulsification [10,11]. Compared with fossil diesel, biodiesel has better performance characteristics, such as high flash point, lubricity, cetane number, and biodegradability, while it emits less pollutants during combustion [12].
To avoid the use of edible oils with relatively high prices and the potential problem of food scarcity, inedible oils have become major raw materials for manufacturing biodiesel nowadays [13,14,15]. For example, jatropha oil (JO) has received much attention in this respect [16,17,18,19]. Jatropha curcas is an inedible plant, that can grow in poor environments. It has many uses, for example, the husk [20] and seeds [21] may be employed to manufacture activated carbon. By crushing its seeds, which have high oil content (50–60%), the obtained bio-oil can be processed to produce biodiesel [22]. In comparison with other biodiesels, jatropha-oil biodiesel has a relatively high flash point (163 °C) and cetane number (57.1), and a low cloud point (4 °C) [9]. The application of JO for biodiesel has been studied for decades [23,24,25,26,27].
Most of the biodiesel manufacturing processes use the transesterification technique with methanol to convert the triglycerides of a raw bio-oil into fatty acid methyl ester (FAME) biodiesel, more commonly referred to as biodiesel. However, when this technique is applied using raw materials like JO with a high content of free fatty acids (FFAs), esterification with liquid acidic catalysts must be applied as a first step pretreatment. This is to reduce FFAs to avoid soap-forming saponification in the alkali-catalyzed transesterification which has much better reaction efficiency than acid-catalyzed transesterification [28,29,30]. It is known that esterification can effectively reduce FFA levels in raw bio-oil to below 1 wt.% [29,30,31,32]. Moreover, the esterification of oil with high acid value (AV) by acid catalysts has another advantage by reducing the loading of the alkali-catalyzed transesterification for manufacturing biodiesel [33].
According to the Taiwan Environmental Protection Administration, more than 140,000 tons of waste isopropyl alcohol (IPA) are produced every year as IPA is an effective industrial solvent commonly used in Taiwan’s high-tech industry [34]. Therefore this study used IPA as an alternative alcohol reactant in order to tackle the waste problem and reduce the cost. Previous studies have shown that fatty acid isopropyl esters (FAIE) biodiesel manufactured by the two-step process of esterification and transesterification emitted less air pollutants such as CO, hydrocarbons (HCs), and smoke after combustion than fossil diesel, while has better cold-flow properties than methyl esters because of its branched chain [35,36]. As a result, IPA would be a suitable alcohol reactant for biodiesel production, particularly in Taiwan.
Esterification and transesterification involve mixing of oil, alcohol, and catalyst, therefore, the selection of reaction system is crucial to achieve satisfactory yields. High frequency ultrasonic irradiation (UI) offers advantages of uniform mixing and quick heating [25,26]. The temperature is increased and retained by the oscillations of the UI. All these effects enhance the efficiency of esterification and transesterification [37]. With UI, the reaction rate, conversion efficiency of FFA, and yield of biodiesel can be increased with low energy demand [22,38].
This study examined the esterification of JO with IPA using H2SO4 as acid-catalyst in semi-batch and batch UI processes. The properties of the esterified products were measured under different experimental conditions. Then, the proper conditions for esterification were elucidated. The obtained information should be useful for engineering and the economic assessment of recycling IPA for biodiesel production.
2. Results and Discussion
2.1. The Properties of Jatropha Oil
The JO sampled possessed acid value (AV), iodine value (IV), kinematic viscosity (KV), density (ρLO), and water content (MW) of about 36.12 mg KOH/g, 105.37 g I2/100 g, 33.38 mm2/s, 918.35 kg/m3, and 0.13 wt.%, respectively. These values are close to those of Andrade-Tacca et al. [25,26]. The low IV meets the requirements of our experiments. However, the high AV of JO of about 36.12 mg KOH/g infers that the content of FFA (MFFA) is as high as about 18.06 wt.%. Note that the AV is defined as the number of milligrams of KOH required to neutralize the FFA present in one gram of fat. Therefore, the content of FFAs such as oleic acid could be estimated by AV/1.99, which has been shown by Hakoda et al. [39] and Augustine et al. [40] to provide acceptable precision for vegetable oils. The high MFFA of JO may be attributed to its inherent characteristics as well as the long storage time causing the observed rancidity. Thus a pretreatment of raw JO such as esterification reducing its high AV and KV would be beneficial for the subsequent production of biodiesel satisfying the Taiwan Biodiesel Standard (CNS 15072) from esterified JO [41]. Note that the esterification can also lower the ρLO. The reduction of MW can be easily achieved by simple proper processing steps such as evaporation, fractionation or dewatering with a drying adsorbent.
The classification of the complicated components of JO can be simplified and presented in terms of percent distillate vs. boiling point defined as the simulated distillation characteristic (SDC) of the sample. The sample was analyzed by gas chromatography. The retention times of corresponding components were identified and compared with those of standards containing n-hexane to n-tetratetracontane (C6–C44). Therefore, the boiling points in the SDC plot corresponded to those of the components for standards but not for sample such as raw JO containing free fatty acids which are not volatile. Thus, instead of presenting the sample contents as a cumulative percent of components vs. retention time, SDC expresses the results as percent distillate vs. boiling point which has been commonly used for the specification of refinery feed-stocks and products. Figure 1 shows the SDC of JO along with those of some other fuel oils like diesel, heavy oil, and boat oil deduced from gas chromatogram results for comparison. In the range of 20–80% distillate, the major components of diesel, heavy oil, boat oil, and JO are C11–C15, C12–C17, C12–C27, and C20–C42, respectively. It indicates that characteristics of JO are not suitable for the direct use as fuel oil. Thus, treatments, such as two-step esterification and transesterification, and catalytic or non-catalytic thermal cracking with or without hydrogen, are needed to upgrade its fuel properties before use.
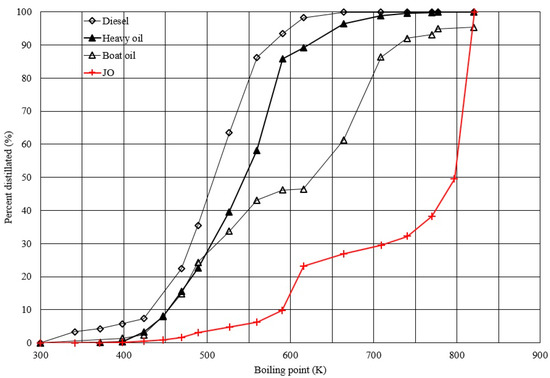
Figure 1.
Simulated distillation characteristics of jatropha oil (JO), diesel, heavy oil and boat oil.
2.2. Effects of Molar Ratio of IPA to Oil on the Performance of Semi-Batch Esterification
The effects of the molar ratio of IPA to oil (MIOE) on the performance of semi-batch esterification were examined with MIOE of 0.5, 1, 3, 4, 5, 6, and 11 at an esterification temperature (TE) of about 394.2 K (393.8–394.7 K) (121.2 °C) as shown in Table 1 and Figure 2. Although TE of 394.2 K is above the boiling point (BP) of IPA (356 K (83 °C)), however, a semi-batch process with continuous addition of IPA/H2SO4 (IPA/H) mixture at room temperature into the hot reactant can reduce the evaporation of IPA. Also, the temperature (394.2 K) can be properly control for continuous feeding of the IPA/H mixture. The TE of 394.2 K higher than the BP of water (373 K) assists in the removal of water from the reaction system, thus enhancing the forward esterification reaction.

Table 1.
Effects of molar ratio of IPA to oil (MIOE) on the performance of esterification of JO by continuous addition of IPA/H2SO4 (IPA/H) mixture under conditions of mass of initial oil (mO) = 200 g, esterification temperature (TE) = 394.2 K (393.8–394.7 K), weight percent of sulfuric acid to sum of sulfuric acid and oil (MH/HO) = 0.94 wt.% and flow rate of IPA/H mixture (QIPA/H) = 1.5 mL/min. Power of ultrasonic irradiation (PUI) = 420 W. Other notations: tE, AV, KV, ρLO, MW, YF = esterification time, acid value, kinematic viscosity, density of liquid or oil, water content, yield of biodiesel of fatty acid isopropyl esters (FAIE).
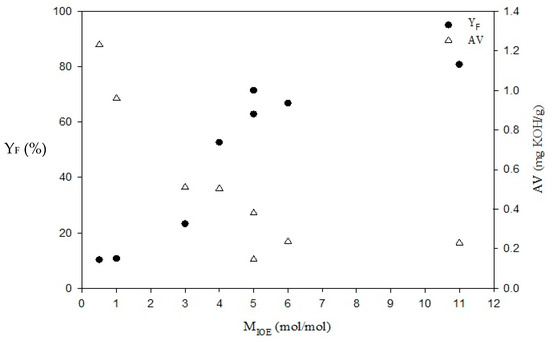
Figure 2.
Effects of MIOE on YF and AV for esterification of JO. mO = 200 g, TE = 394.2 K, MH/HO = 0.94 wt.%, QIPA/H = 1.5 mL/min. PUI = 420 W.
In the study, the proper MIOE was determined by examining its effects on the AV of esterified JO and yield of FAIE biodiesel (YF). We note that both the esterification of FFA and the transesterification of triglycerides may contribute to YF. The content of FFA (MFFA) in wt.% is calculated by AV/1.99. The measurements of AV of JO before (AV0) and after (AV) esterification can give the conversion efficiency of FFA via esterification (ηA (%) = [(AV0 − AV)/AV0] × 100%). The esterification time (tE) increases with increasing MIOE because more IPA/H mixture was needed when added at a fixed flow rate. More energy was consumed with longer tE. Other esterification variables are the weight percent of acid to the sum of acid and oil (MH/HO) = 0.94 wt.% and flow rate of the IPA/H mixture (QIPA/H) = 1.5 mL/min.
According to Table 1 and Figure 2, increased MIOE can significantly reduce the AV, KV, and ρLO of the esterified JO while increasing YF. The highest YF with the lowest values of AV, KV, and ρLO occured at MIOE = 11. When MIOE increases higher than 5, the effect of MIOE in assisting the esterification with UI on these values becomes gradually less significant. In comparison with the Taiwan Biodiesel Standard, the KV and water content of esterified JO for the cases examined cannot meet the requirements (KV: 3.5–5.0 mm2/s; water content: <500 mg/kg), and only ρLO (899.23 kg/m3) of esterified JO at MIOE = 11 is below the maximum limit (900 kg/m3).
As esterification is regarded as pretreatment of alkali-catalyzed transesterification to reduce FFA content, AV is the main parameter considered. Generally, AV has to be reduced to below 2 mg KOH/g (or MFFA < 1%) for transesterification [32,42,43]. In this study, AV of FAIE is expected to meet the Taiwan Biodiesel Standard, which is below 0.5 mg KOH/g. According to Figure 2, shen MIOE is 4, the AV of the esterified JO was decreased from 36.12 to 0.50 mg KOH/g which is about the limit value. When MIOE was increased to 5, the AV of esterified JO can be further reduced to about 0.27 mg KOH/g (average of two measurements of 0.15 and 0.38 as shown in Figure 2) thus satisfying the Taiwan Biodiesel Standard [41].
The other important factor YF is also shown in Figure 2. From the AV properties of JO, the maximum yield of biodiesel from direct esterification can be calculated as 18.06% because JO only contains 18.06 wt.% FFA. If YF is larger than 18.06%, the extra contribution can indicate that transesterification also occurs in this acid-catalyzed esterification process. When MIOE exceeds 3, esterification accompanying with transesterification is obvious because YF is much greater than 18.06% which represents an advantage of reducing the loading of the subsequent alkali-catalyzed transesterification.
As mentioned above, with increasing MIOE to 5, YF is increased considerably, while AV is decreased. However, the significance of the effect of increasing YF while decreasing AV is reduced after MIOE is increased to over 5. Thus, in order to avoid wasting more IPA and energy by exceeding MIOE, a proper condition of MIOE = 5 is chosen. This condition is effective for esterification, giving ηA and YF as high as 99.25 and 67.15 wt.%, respectively. Notice that FFA is reduced from 18.06 to 0.14 wt.%, which means 17.92 wt.% of free fatty acid is esterified to FAIE (a biodiesel). Thus, among 67.15 wt.% of YF, 49.23% (=67.15 wt.% deducting 17.92 wt.%) is contributed to by the transesterification of triglycerides.
We note that the esterification of JO mainly serves as a pretreatment to obtain esterified JO with better qualities, such as lower AV, KV, and ρLO, especially the lower AV, which are beneficial for the follwing transesterification to produce JO biodiesel. The formation of some FAIE biodiesel is an additional benefit of esterification, reducing the IPA loading for the subsequent transesterification. This can be reflected by the YF. Thus, the properties measured as presented in this study did not include the FAIE compositions, which of course would be helpful for further understanding the quality of the FAIE produced. Although the esterification generally would not be used to produce good-quality biofuel ready for combustion application, however, a further study on the comparison of the combustion characteristics of esterified JO with those of raw JO would illustrate the feasibility of the application of esterification.
2.3. Effects of Esterification Temperature on Performance of Semi-Batch Esterification
The effects of esterification temperatures TE below (337 and 354 K) and above (375, 394, 415, 434, and 454 K) the BP of IPA of 356 K on system performance are shown in Table 2 and Figure 3. Note that the limitation of BP of alcohol usually makes performing esterification at higher temperatures difficult. As TE increases, YF is enhanced from 16.4% to 20.92% for TE below IPA’s BP, while it decreases from 75.47% to 25.84% for TE values above IPA’s BP. The highest YF of 75.47% occurs at TE = 375 K (102 °C) above IPA’s BP. The values of ηA at all TE examined were higher than 99.25% with AV below 0.27 mg KOH/g, indicating that for the overall YF achieved about 17.92–18.06 wt.% is contributed by esterification while the balance comes from transesterification. The slightly lower YF of 16.4 wt.% at TE = 337 K than 18.06 wt.% may be due to experimental error. It is obvious that temperature is a significant factor for both esterification and transesterification. Temperatures higher than the alcohol’s BP may have a positive effect on both reactions if a proper operation method such as a semi-batch process is adopted.

Table 2.
Effects of esterification temperature TE on the performance of esterification of JO by continuous addition of IPA/H under conditions of mO = 200 g, MIOE = 5, MH/HO = 0.94 wt.%, QIPA/H = 1.5 mL/min, and tE = 58.5 min. PUI = 420 W.
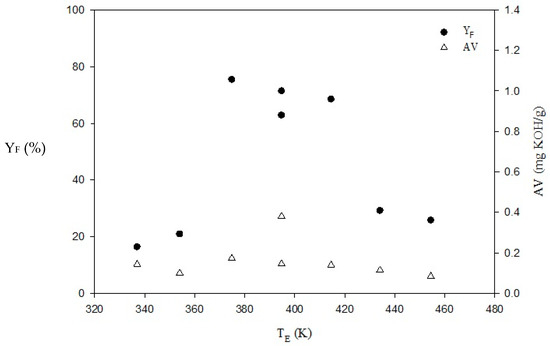
Figure 3.
Effects of TE on YF and AV for esterification of JO. MIOE = 5, MH/HO = 0.94 wt.%, QIPA/H = 1.5 mL/min, tE = 58.5 min. PUI = 420 W.
In this study, a semi-batch process can preheat JO at temperatures higher than IPA’s BP, while IPA/H at room temperature was subsequently added into reactor with a fixed flow rate. The continuous inflow of liquid-phase IPA/H can lead to homogeneous esterification accompanied by transesterification as soon as it enters the reactor contacting JO. However, when the temperature difference between IPA/H and JO is too broad, IPA tends to evaporate quickly resulting in poor reaction performance. Consequently, YF drops drastically as the esterification temperature is increased greatly above IPA’s BP, say 434 and 454 K.
In order to trap the evaporated water and recover the gasified IPA during esterification, an exhaust outlet attached to a condenser is placed after the reactor. With exception of TE = 337.0 and 354.0 K below water’s BP in Table 2, the other cases have evidently low water content below 0.27 wt.%. Water content is generally decreased with increasing esterification temperature, as expected. When the esterification temperature is higher than 434 K, the water contents of esterified JO of 0.043 wt.% meet the Biodiesel Standard (500 mg/kg). Consequently, this semi-batch process design provides advantages of high-temperature homogeneous esterification and the possibility of alcohol recovery. On the other hand, after esterification, KV and ρLO in Table 2 did not meet the requirements of the Biodiesel Standard CNS 15072 [41]. Only ρLO of 895 kg/m3 of esterification at 374.8 K can meet the standard (860–900 kg/m3 at 15 °C). The deficit, however, can be resolved afterwards in the subsequent transesterification.
Referring the above assessments on the parameters examined and considering the energy savings, TE is chosen at 394 K for the semi-batch process to engage in the next alkali-catalyzed transesterification stage. Esterification under this condition (394.2 K) can produce a moderately high biodiesel yield (67.15%, accompanied by transesterification), along with low AV (0.27 mg KOH/g below 0.5 mg KOH/g), water content (0.27 wt.%), and density (901.57 kg/m3) and a proper energy consumption. In comparison with biodiesel standards, KV (14.62 mm2/s) is much higher than that of those standards (3.5–5.0 mm2/s at 40 °C), while the AV of esterified JO satisfies the CNS 15072 (<0.5 mg KOH/g), EN 14214 (<0.5 mg KOH/g) and ASTM D6751 (<0.80 mg KOH/g) standards [41,44,45].
In Table 1 and Table 2 and Figure 2 and Figure 3, the yield of biodiesel of FAIE YF was determined from the results of the nuclear magnetic resonance (NMR) spectrum of esterified JO. A detailed description of the NMR analysis and computation methods is presented in Section 3.4. A typical NMR spectrum result of esterified JO for the case at TE = 374.8 K with mO = 200 g, MIOE = 5, MH/HO = 0.94 wt.%, QIPA/H = 1.5 mL/min, and tE = 58.5 min in Table 2 is shown in Figure 4 for illustration. Positions 2.3 and 4.9 ppm were where the signals of hydrogen atoms (H’ and H’’) attached to specific carbons of FFA and FAIE (e.g., RCH’2COOCH”(CH3)2) were exhibited, respectively. The R is the carbon chain of the fatty acid. The integrals of proton signals in positions 2.3 and 4.9 ppm are 1.725 and 0.651, respectively. Thus, the corresponding YF is calculated as YF = (2 × Integral of proton signal at 4.9 ppm/Integral of proton signal at 2.3 ppm) × 100% = 75.48%.
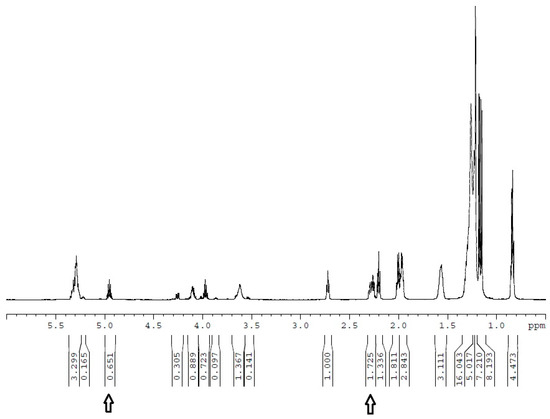
Figure 4.
NMR spectrum of esterified JO for the case at TE = 374.8 K with mO = 200 g, MIOE = 5, MH/HO = 0.94 wt.%, QIPA/H = 1.5 mL/min, and tE = 58.5 min. PUI = 420 W.
2.4. Comparisons of Esterified JO by Different Types of UI Processes
In batch process without temperature control, the esterification temperature TE increased with time. The results from different conditions of esterification times and time-average TE are listed in Table 3. In comparison with the semi-batch process at pre-set TE of 337.0–354.0 K (64.0–81.0 °C) close to the batch process in the time-averaged TE range of 340.3–354.9 K, the AV of esterified JO in the batch process can only be decreased to 1.16–3.5 mg KOH/g, i.e., inferior to those seen in the semi-batch process of 0.10–0.14 mg KOH/g. This result indicates that semi-batch process is better than batch process for the esterification of JO in reducing AV before conducting the followed stage of alkali-catalyzed transesterification. Notice that, for the case of batch process with the time-average TE of 354.9 K (81.9 °C), the maximum temperature already reaches 365.6 K (92.6 °C). This higher temperature leads to YF of 24.88%, slightly higher than the YF of 20.92% of esterification by the semi-batch process at pre-set TE of 354.0 K (81.0 °C), due to the enhancing effect of higher temperature. The KV, ρLO, and water content MW of the two processes with pre-set and average TE lower than 373 K (100 °C) are close. However, the MW of esterified JO via the semi-batch process at pre-set TE higher than 373 K (say, 394.2 K) with MIOE = 5 is greatly reduced and lower than that via the batch process because of the continuous removal of water.

Table 3.
Comparisons of properties of esterified JOs from semi-batch and batch UI processes using IPA/H of this study with those from sequential UI [26] using methanol/H2SO4 (M/H) and biodiesel standards.
Comparisons of the properties of the esterified JOs from semi-batch and batch processes with some biodiesel oil standards are shown in Table 3. Note that biodiesel oil standards are not applicable to oil or esterified oil. However, as for reference, the comparisons indicate that semi-batch esterification is very beneficial for reducing AV, meeting with the need of low AV of biodiesel.
Table 3 also presents the properties of esterified JO using methanol and H2SO4 (M/H) by sequential M/H dose-UI process [26], for which the experimental conditions were: initial weight of JO = 188.32 g, TE = 382.3 K, molar ratio of methanol to JO = 11, weight percent of H2SO4 to JO = 0.92 wt.%, total volume of mixture of methanol and H2SO4 = 100 mL, and power of UI = 270 W. The comparison indicates that the listed properties of AV, ρLO, and MW of esterified JO of semi-batch processes using IPA/H are as good as those of sequential UI applying methanol/H2SO4. The KV for the process employing IPA/H is higher than that adopting methanol/H2SO4. However, it can be further reduced by the subsequent transesterification. The results of this study showed that IPA could be employed instead of methanol offering the opportunity to reutilize the abundant waste IPA. The obtained esterified JO nevertheless contains some FAIE biodiesel as indicated by the yield YF, however, the esterified JO commonly needs further transesterification in order to meet the biodiesel standards. Therefore, only the characteristics of esterified JO but not FAIE were shown and compared.
Three cases of applying UI and thermal heating reported by Andrade-Tacca et al. [25] are also shown in Table 3. Case UNVT-E30S10 applied UI with temperature-rise effect without temperature control, where the temperature varied from initial ambient temperature; TE = 338.4 K at tE = 30 min, with average temperature = 329.7 K; settling time = 10 min after tE. Case MHFT-E30S10 adopted mechanical mixing with temperature controlled by employing external thermal heating using a 338 K water bath; TE = 320.1 K at tE = 30 min, with average temperature = 320.6 K. Case MNAT-E30S10 used mechanical mixing without temperature control with the initial temperature set at ambient temperature; TE = 294.7 K at tE = 30 min, with average temperature = 296.3 K. It indicates that UI with temperature-rise effect without temperature control gives AV of 6.13 mg KOH/g lower than mechanical mixing with temperature controlled by employing external thermal heating with AV of 15.70 mg KOH/g. Among these three cases, mechanical mixing without external thermal heating giving AV of 21.03 mg KOH/g is the least effective in reducing AV via esterification. Thus, the heat induced due to cavitation by UI is very beneficial for the esterification. The UI also assists the mixing/emulsion for the liquid-liquid heterogeneous reaction system of esterification of JO. Because no radical conserving agent was present, therefore, the enhancement effect of UI on the esterification is by nature controlled by the intrinsic kinetics and mass transfer along with heat transfer. The sonochemical effect was not involved [46]. Regarding the heat energy required for esterification, it can come from the heat induced due to cavitation for the UI, however an external heating is needed for the mechanical mixing with heating.
3. Experimental Methods
3.1. Materials
JO sample was imported from Indonesia by Ozone Environmental Technology Co. (Yi-Lan County, Taiwan). Other chemicals including isopropyl alcohol (IPA, 99.5%, C3H7OH) and sulfuric acid (H, 96.3%, H2SO4) were from J.T. Baker Inc. (Phillipsburg, NJ, USA).
3.2. Equipment
The ultrasonic irradiation sonicator (Ultrasonic 600) employed for esterification of JO was purchased from Ho-Yu Technology Co. (New Taipei City, Taiwan). Its frequency and maximal power (PUI) are 25 kHz and 600 W, respectively. The ultrasonic system which was used in both semi-batch and batch operations is shown in Figure 5. As for semi- batch process, IPA and H2SO4 mixture was continuously fed into UI reactor by peristaltic pump (SINDOS 10, KNF Co., Balterswil, Switzerland). The system is also equipped with a thermal probe in the reactor to monitor the reaction temperature.
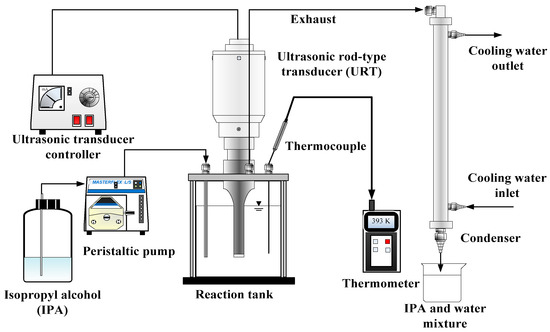
Figure 5.
Schematic diagram of ultrasonic irradiation (UI) system.
3.3. Experiments
3.3.1. Semi-Batch System
JO (200 g) was preheated to reach the preset TE by UI operated at power PUI of 420 W (70% of maximum power of 600 W). IPA and H2SO4 were pre-mixed in flask and denoted as IPA/H. The volume of H2SO4 was 1.02 mL. IPA content refers to the alcohol/oil molar ratio for esterification (MIOE). The flow rate of mixture of IPA/H (QIPA/H) was 1.5 mL/min while TE was controlled at the setting value. The tE can then be computed with the given MIOE and QIPA/H. The vaporized IPA and water can be recovered from the condenser.
After esterification, the reaction mixture was poured into a 1000 mL beaker for obtaining esterified JO, which contains some transesterified biodiesel, by washing with saturated brine. The esterified JO was subjected for measuring the yield of biodiesel of FAIE (YF), water content (MW), and other properties.
Notice that the use of PUI at 420 W was to ensure it provided sufficient power to reduce the AV via esterification. This can be referred to the work of Manh et al. [47] that studied biodiesel production from tung oil and other blended oils applying alkali-catalyzed transesterification via UI at PUI of 120–270 W. Their results showed that YF increases gradually to 20% at PUI = 120 W, while rapidly to 80% at 150 W. It reaches 90% at 270 W. PUI at 420 W may not be the optimal power, however, is sufficient and thus employed in this study.
3.3.2. Batch System
JO (200 g) was charged into the UI reactor. It then followed a batch addition of mixture of IPA (amount referred to MIOE) and H2SO4 (1.02 mL) and was immediately subjected to UI. Parameters examined included composition, TE, and tE. The followed treatments and analyses of esterified JO from batch esterification were the same as those from semi-batch esterification.
3.4. Analyses
Nuclear magnetic resonance (NMR) spectrometer (Bruker AVIII HD-600, Bruker Co., Manning Park, Billerica, MA, USA) was used to measure the proton spectrum (1H-NMR) of samples [48,49]. The solvent used was CDCl3. The signals of hydrogen atoms (H’ and H’’) attached to specific carbons of FFA and FAIE (e.g., RCH’2COOCH”(CH3)2) were in positions of 2.3 and 4.9 ppm in spectrum, respectively, where R denotes the fatty acid carbon chain. The integrals of proton signals at 2.3 and 4.9 ppm in the NMR spectrum were then obtained, respectively. Consequently, YF (yield of biodiesel of FAIE, %) can be calculated by integrals of proton signals of these two positions:
YF = (2 × Integral of proton signal at 4.9 ppm/Integral of proton signal at 2.3 ppm) × 100%
The AV (mg KOH/g) was analyzed by automatic potentiometric titrator (KEN/AT-510, Kyoto Electronics Manufacturing Co., Shinjuku, Tokyo, Japan) with titration of solutions of KOH and IPA (0.1 M). The conversion efficiency of FFA via esterification (ηA, %) is then computed as:
where AV0 and AV represent the AV of JO before and after esterification.
ηA (%) = [(AV0 − AV)/AV0] × 100%
Kinematic viscosity (KV, mm2/s) was measured by viscometry (SimpleVIS size 100 (U851) and 200 (T453), CANNON Instrument Co., State College, PA, USA). After sample flowed through the viscometer, the process time was multiplied by coefficient of the viscometer (with values = 0.01398 for size 100 of U851 and 0.09196 for size 200 of T453) to obtain KV. Different sizes of viscometers were suited for oils with different ranges of viscosity. Size 100 (for 3–15 mm2/s) and size 200 (for 20–100 mm2/s) were used for detecting the KV of esterified JO.
Density of liquid or oil (ρLO) was determined by portable density meter (DMA35, Anton Paar Benelux, Everdenberg, Oosterhout, The Netherlands). According to Chinese National Standard (CNS 14474 [49]), when sample was drawn into U-type oscillating tube, sample with different density induces different oscillating frequency. The density of sample was detected by comparing with the reading of meter.
Water content MW was determined with the method of Karl Fischer by using the moisture titration apparatus (model 851 Titrando Karl Fischer Titrator, Metrohm, Riverview, FL, ISA).
Gas chromatography with a flame ionization detector (Agilent, 6890, Chemstation, Hewlett Packard Inc., Wilmington, DE, USA) and a fused-silica capillary column (UA1(HT)—15W—0.25F, 15 m × 0.53 mm × 0.25 μm, max. Temp: 693 K, Frontier Laboratories Ltd., Fukushima, Japan) was employed to elucidate the components of sample with corresponding retention times. The initial settings of GC-conditions were: Carrier gas: H2, 40 mL min−1; air, 180 mL min−1; auxiliary gas: H2, 4.5 mL min−1; oven temperature: 313 K; injector temperature: 633 K; detector temperature: 573 K; amount of injection: 0.2 μL. The inlets were: Split ratio: 1:1; total flow: 55 mL min−1. The oven was held on 323 K for 5 min and subsequently increased to 673 K with 5 K min−1. The standard of ASTM D2887 quantitative calibration mix was supplied by Supelco (Bellefonte, PA, USA). The results were compared to those of standards containing hexane to n-tetratetracontane (C6–C44). The simulated distillation characteristic (SDC) of the sample in terms of percent distillate vs. boiling point was then deduced.
4. Conclusions
In this work, jatropha oil (JO) was esterified using isopropyl alcohol (IPA) with H2SO4 (H) acid catalyst via ultrasound irradiation aiming at reducing its acid value (AV). At the same esterification temperature (TE), an increase of molar ratio of isopropyl alcohol to JO (MIOE) can obviously reduce the AV of esterified JO. For semi-batch esterification conditions at TE = 394.2 K, weight percent of sulfuric acid to sum of sulfuric acid and oil (MH/HO) = 0.94 wt.%, flow rate of IPA/H mixture (QIPA/H) = 1.5 mL/min, and MIOE = 5, the AV of JO can be effectively decreased from 36.12 to about 0.27 mg KOH/g. This low AV of the esterified JO satisfies the Taiwan Biodiesel Standard of a maximum limit of 0.5 mg KOH/g. The values of conversion efficiency of free fatty acid (FFA) via esterification (ηA) and yield of biodiesel of fatty acid isopropyl ester (FAIE) (YF) reach as high as 99.25% and 67.15%, respectively.
With TE higher than the boiling point of water, AV can be reduced effectively, and YF shows drastic growth. A further higher TE than about 434.1 K in this semi-batch process results in rapid evaporation of IPA, thus greatly decreasing the esterification effect on kinematic viscosity (KV) and YF. Further, the findings showed that semi-batch process would be more effective to reduce AV than batch process. Operation of ultrasonic irradiation (UI) assisted semi-batch process for esterification is proper at MIOE = 5 and TE = 394.2 K to provide esterified JO for the following alkali-catalyzed transesterification.
Author Contributions
C.-C.C., S.T. and C.-Y.C. conceived and designed the experiments; C.-C.C., S.T., S.-Y.T., M.-Y.T., M.-C.C., Y.-H.C., M.H. and B.-L.L. performed the experiments; C.-C.C., S.T., M.-H.Y. and C.-Y.C. analyzed the data; C.-Y.C., Y.-H.C. and J.-L.S. contributed reagents/materials/analysis tools; D.-R.J., C.H., C.A.A.-T. and D.V.M. discussed the results and provided suggestion; C.-C.C., M.-H.Y. and C.-Y.C. wrote the paper.
Funding
This research was funded by Ministry of Science and Technology of Taiwan grant numbers: MOST 103-2221-E-002-009- and MOST 104-2221-E-002-003-.
Acknowledgments
The authors would like to thank the Ministry of Science and Technology of Taiwan for the financial support of this study (MOST 103-2221-E-002-009-; MOST 104-2221-E-002-003-).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AV | Acid value (mg KOH/g) |
| BP | Boiling point (K) |
| IV | Iodine value (100g I2/g) |
| KV | Kinematic viscosity (mm2/s) |
| MFFA | Content of free fatty acid FFA (wt.%) |
| MH/HO | Weight percent of sulfuric acid to sum of sulfuric acid and oil (wt.%) |
| MIOE | Molar ratio of isopropyl alcohol to JO (mol/mol) |
| MW | Water content (wt.%) |
| mO | Mass of initial oil (g) |
| MHFT | Mechanical mixing with temperature controlled by employing external thermal heating using water bath of 338 K |
| MHFT-E30S10 | MHFT with tE = 30 min and settling time = 10 min after tE |
| MNAT | Mechanical mixing without temperature control with initial temperature at ambient temperature |
| MNAT-E30S10 | MNAT with tE = 30 min and settling time = 10 min after tE |
| PUI | Power of ultrasonic irradiation (W) |
| QIPA/H | Flow rate of IPA/H mixture (mL/min) |
| R | Carbon chain of fatty acid |
| TE | Esterification temperature (K) |
| tE | Esterification time (min) |
| UNVT | UI with temperature-rise effect without temperature control, while temperature varied from initial ambient temperature |
| UNVT-E30S10 | UNVT with tE = 30 min and settling time = 10 min after tE |
| VO | Volume of oil (mL) |
| YF | Yield of biodiesel of FAIE, = (2 × Integral of proton signal at 4.9 ppm / Integral of proton signal at 2.3 ppm) × 100 (%) |
| ρLO | Density of liquid or oil (kg/m3) |
| ηA | Conversion efficiency of FFA via esterification (%) |
Abbreviation
| ASTM | American standards of testing methods |
| CNS | Chinese National Standards |
| EN | European standards |
| FAIE | Fatty acid isopropyl ester |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid |
| IPA | Isopropyl alcohol |
| IPA/H | Mixture of IPA/H2SO4 |
| JO | Jatropha oil |
| M/H | Mixture of methanol and H2SO4 |
| NMR | Nuclear magnetic resonance |
| SDC | Simulated distillation characteristic |
| UI | Ultrasonic irradiation |
References
- Abdullah, R.; Sianipar, R.N.; Ariyani, D.; Nata, I.F. Conversion of palm oil sludge to biodiesel using alum and KOH as catalysts. Sustain. Environ. Res. 2017, 27, 291–295. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S.A. Comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Kathirvelu, B.; Subramanian, S.; Govindan, N.; Santhanam, S. Emission characteristics of biodiesel obtained from jatropha seeds and fish wastes in a diesel engine. Sustain. Environ. Res. 2017, 27, 283–290. [Google Scholar] [CrossRef]
- Shankar, V.; Jambulingam, R. Waste crab shell derived CaO impregnated Na-ZSM-5 as a solid base catalyst for the transesterification of neem oil into biodiesel. Sustain. Environ. Res. 2017, 27, 273–278. [Google Scholar] [CrossRef]
- Jayed, M.H.; Masjuki, H.H.; Kalam, M.A.; Mahlia, T.M.I.; Husnawan, M.; Liaquat, A.M. Prospects of dedicated biodiesel engine vehicles in Malaysia and Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 220–235. [Google Scholar] [CrossRef]
- Lim, S.; Teong, L.K. Recent trends, opportunities and challenges of biodiesel in Malaysia: An overview. Renew. Sustain. Energy Rev. 2010, 14, 938–954. [Google Scholar] [CrossRef]
- Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Norhasyima, R.S. Comparison of palm oil, Jatropha curcas and Calophyllum inophyllum for biodiesel: A review. Renew. Sustain. Energy Rev. 2011, 15, 3501–3515. [Google Scholar] [CrossRef]
- Sarin, R.; Sharma, M.; Sinharay, S.; Malhotra, R.K. Jatropha–Palm biodiesel blends: An optimum mix for Asia. Fuel 2007, 86, 1365–1371. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Prospects of biodiesel from Jatropha in India: A review. Renew. Sustain. Energy Rev. 2010, 14, 763–771. [Google Scholar] [CrossRef]
- Yang, S.Q. Trend of refinery of toxin plants. Bus. Week Mag. 2007, 1041, 166. (In Chinese) [Google Scholar]
- Romano, S.D.; Sorichetti, P.A. Dielectric Spectroscopy in Biodiesel Production and Characterization; Springer: London, UK, 2011. [Google Scholar]
- Ghanei, R. Improving cold-flow properties of biodiesel through blending with nonedible castor oil methyl ester. Environ. Prog. Sustain. Energ. 2015, 34, 897–902. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energ. 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, L.; Wang, Q.; Ma, H.; Niu, R.; Gao, Z. Synergistic effect of mixed methanol/ethanol on transesterification of waste food oil using p-toluenesulfonic acid as catalyst. Environ. Prog. Sustain. Energy 2015, 34, 1547–1553. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Liu, Y.T.; Nguyen, P.L.; Ismadji, S.; Ju, Y.H. In situ transesterification of Jatropha curcas L. seeds in subcritical solvent system. J. Taiwan Inst. Chem. Eng. 2014, 45, 1516–1522. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, S.C.; Wu, T.Y.; Yang, P.M.; Jhang, S.R.; Lin, J.F. Energy-saving and rapid transesterification of jatropha oil using a microwave heating system with ionic liquid catalyst. J. Taiwan Inst. Chem. Eng. 2015, 49, 72–78. [Google Scholar] [CrossRef]
- Satar, I.; Wan Isahak, W.N.R.; Salimon, J. Characterization of biodiesel from second generation gamma-irradiated Jatropha curcas. J. Taiwan Inst. Chem. Eng. 2015, 49, 85–89. [Google Scholar] [CrossRef]
- Zarei, A.; Amin, N.A.S.; Talebian-Kiakalaieh, A.; Zain, N.A.M. Immobilized lipase-catalyzed transesterification of Jatropha curcas oil: Optimization and modeling. J. Taiwan Inst. Chem. Eng. 2014, 45, 444–451. [Google Scholar] [CrossRef]
- Karthick, K.; Dinesh, C.; Namasivayam, C. Utilization of ZnCl2 activated Jatropha husk carbon for the removal of reactive and basic dyes: Adsorption equilibrium and kinetic studies. Sustain. Environ. Res. 2014, 24, 139–148. [Google Scholar]
- Hsu, S.H.; Huang, C.S.; Chung, T.W.; Gao, S. Adsorption of chlorinated volatile organic compounds using activated carbon made from Jatropha curcas seeds. J. Taiwan Inst. Chem. Eng. 2014, 45, 2526–2530. [Google Scholar] [CrossRef]
- Singh, A.K.; Ferando, S.D.; Hernandez, R. Base-catalyzed fast transesterification of soybean oil using ultrasonication. Energy Fuel 2007, 21, 1161–1164. [Google Scholar] [CrossRef]
- Achten, W.M.J.; Verchot, L.; Franken, Y.J.; Mathijs, E.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Achten, W. Sustainability Evaluation of Biodiesel from Jatropha curcas L.; Katholieke Universiteit Leuven, Groep Wetenschap & Technologie: Heverlee, Belgium, 2010. [Google Scholar]
- Andrade-Tacca, C.A.; Chang, C.C.; Chen, Y.H.; Manh, D.V.; Chang, C.Y.; Ji, D.R.; Tseng, J.Y.; Shie, J.L. Esterification of jatropha oil via ultrasonic irradiation with auto-induced temperature-rise effect. Energy 2014, 71, 346–354. [Google Scholar] [CrossRef]
- Andrade-Tacca, C.A.; Chang, C.C.; Chen, Y.H.; Manh, D.V.; Chang, C.Y. Esterification of jatropha oil by sequential ultrasonic irradiation with auto-induced temperature rise and dosing of methanol and sulfuric acid catalyst. J. Taiwan Inst. Chem. Eng. 2014, 45, 1523–1531. [Google Scholar] [CrossRef]
- Zou, H.; Lei, M. Optimum process and kinetic study of Jatropha curcas oil pre-esterification in ultrasonical field. J. Taiwan Inst. Chem. Eng. 2012, 43, 730–735. [Google Scholar] [CrossRef]
- Miao, X.; Li, R.; Yao, H. Effective acid-catalyzed transesterification for biodiesel production. Energy Convers. Manag. 2009, 50, 2680–2684. [Google Scholar] [CrossRef]
- Patil, P.D.; Deng, S. Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Tiwari, K.A.; Kumar, A.; Raheman, H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenergy 2007, 31, 569–575. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Zhou, H.; Yang, Y.; Chen, M.; Liang, B. Production of biodiesel from Jatropha curcas L. oil. Compt. Chem. Eng. 2009, 33, 1091–1096. [Google Scholar] [CrossRef]
- Lu, H.; Chen, M.; Jiang, W.; Liang, B. Biodiesel processes and properties from Jatropha curcas L. oil. J. Biobased Mater. Bioenergy 2011, 5, 546–551. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Taiwan Environmental Protection Administration (TEPA). Statistic Data of Industrial Waste from Industrial Waste Management System; TEPA: Taipei, Taiwan, 2013.
- Wang, P.S.; Tat, M.E.; Gerpen, J.V. The production of fatty acid isopropyl esters and their use as a diesel engine fuel. J. Am. Oil Chem. Soc. 2005, 82, 845–849. [Google Scholar] [CrossRef]
- Lee, I.; Johnson, L.A.; Hammond, E.G. Use of branched-chain esters to reduce the crystallization temperature of biodiesel. J. Am. Oil Chem. Soc. 1995, 72, 1155–1160. [Google Scholar] [CrossRef]
- Colucci, J.A.; Borrero, E.E.; Alape, F. Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing. J. Am. Oil Chem. Soc. 2005, 82, 525–530. [Google Scholar] [CrossRef]
- Ji, J.; Wang, J.; Li, Y.; Yu, Y.; Xu, Z. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, 411. [Google Scholar] [CrossRef] [PubMed]
- Hakoda, A.; Sakaida, K.; Suzuki, T.; Yasui, A. Determination of the Acid Value of Instant Noodles: Interlaboratory Study. J. AOAC Int. 2006, 89, 1341–1346. [Google Scholar] [PubMed]
- Augustine, A.; Okoro, I.C.; Francis, E.U.; Gilbert, U.; Okuchukwu, O. Comparative assessment of lipids and physicochemical properties of African locust beans and shea nut oils. J. Nat. Sci. Res. 2013, 3, 25–31. [Google Scholar]
- Taiwan Bureau of Standards, Metrology & Inspection (TBSMI). CNS-15072 Taiwan Biodiesel-Fatty Acid Methyl Esters Standards; TBSMI: Taipei, Taiwan, 2007.
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from non-edible oil using sequential combination of microwave and ultrasound. Fuel Process. Technol. 2013, 106, 62–69. [Google Scholar] [CrossRef]
- Veljkovic, V.B.; Avramivic, J.M.; Stamenkovic, O.S. Biodiesel production by ultrasound assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2012, 16, 1193–1209. [Google Scholar] [CrossRef]
- Knothe, G. Review: Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- American Standards of Testing Methods (ASTM). ASTM D6751; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Choudhury, H.A.; Malani, R.S.; Moholkar, V.S. Acid catalyzed biodiesel synthesis from Jatropha oil: Mechanistic aspects of ultrasonic intensification. Chem. Eng. J. 2013, 231, 262–272. [Google Scholar] [CrossRef]
- Manh, D.V.; Chen, Y.H.; Chang, C.C.; Chang, M.C.; Chang, C.Y. Biodiesel production from Tung oil and blended oil via ultrasonic transesterification process. J. Taiwan Inst. Chem. Eng. 2011, 42, 640–644. [Google Scholar] [CrossRef]
- Knothe, G. Analysis of oxidized biodiesel by H-1-NMR and effect of contact area with air. Eur. J. Lipid Sci. Technol. 2006, 108, 493–500. [Google Scholar] [CrossRef]
- TBSMI. CNS-14474 Method of Test for Density and Relative Density of Liquids by Digital Density Meter; TBSMI: Taipei, Taiwan, 2000.
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).