Abstract
Study Design: Literature review. Objective: This review aims to explore current opioid use trends for surgical management of facial fractures, as well as methods and alternative treatments to decrease opioid use. Methods: Review of selected literature from Medline and Google Scholar. Results: Despite the devastating effects of the opioid epidemic and recent policy changes aimed at reducing unnecessary opioid prescription, opioids remain commonly used for pain management after facial fracture repair. Recently, use of multimodal analgesic therapy has been suggested to decrease opioid dosage utilized for post-operative pain control. Alternatives to medication therapies have been proposed for pain management; however, standardized recommendations for pain management in facial fracture patients remain unclear. Conclusions: Further research is required to establish evidence-based recommendations for pain management in craniofacial fracture repair.
Introduction
The opioid epidemic remains a nationwide public health crisis. Treatment of chronic pain and acute injuries with opioids contributes to the issue, as these prescriptions can lead to dependence, abuse, and overdose. These medications can have significant adverse effects on patient health, and, when abused, there is a high rate of toxicity and psychological harm and a potential for fatality, in the event of overdose []. According to the Drug Enforcement Agency (DEA) and the Centers for Disease Control and Prevention (CDC), rates of opioid prescription and opiate-related deaths exponentially increased throughout the United States from 2001 to 2010 and again from 2013 to 2014 []. From 2015 to 2016, there was a 27% increase in the death rate from opioid overdose, with the projection that these numbers would continue to rise yearly []. While adequate management of pain directly impacts psychological and physiological health as well as quality of life, these benefits must be balanced with the risks of prescribing addictive and potentially deadly medications.
Given this challenge, government officials and medical professionals have worked to decrease unnecessary opioid prescriptions. Strict policies have been enacted on both the national and statewide level regarding prescribing and dispensing controlled substances, as well as strengthening the reporting system for controlled substance abuse [,].
Similarly, researchers across the world have studied methods to decrease narcotic use in numerous facets of medicine. One subset of this research has focused on methods to decrease post-operative pain with limited opioid use, with many focusing on multimodal therapy including co-analgesics. These therapies have the opportunity and potential to decrease opioid dependence and misuse [,].
Over 50% of trauma patients are prescribed narcotics upon discharge, putting this patient population at high risk for opioid misuse and dependence []. Craniomaxillofacial injuries, including skull, maxillary, mandibular, zygomatic, and orbital fractures, constitute approximately 10% of injuries in trauma patients and may require surgical repair []. Although there have been noteworthy changes in the policies for narcotic prescription, opioids continue to be the foundation of post-operative pain management following facial trauma repair []. While Barbarite et al. found a national trend in lower opioid prescriptions between 2014 and 2016 [], an 11-year retrospective review demonstrated that opioid prescribing patterns for craniomaxillofacial trauma have remained relatively stable over time [].
Studies on opioid trends and risk factors for dependence in this unique patient population, however, are very limited. Additionally, there are limited studies on the use of multimodal therapy or non-conventional treatments to manage post-operative pain and decrease opioid use in this patient population. Currently, evidence-based and universal guidelines on pain management in patients suffering from facial fractures are not available. In this article, we aim to provide a comprehensive review of pain management in facial fractures and to discuss alternative modalities that can potentially reduce or eliminate the use of opioids after facial trauma.
Risk Factors for Opioid Use
Understanding risk factors for increased incidence of opioid refill and misuse can help physicians identify appropriate opportunities to use different forms of pain control. A study investigating opioid use in post-surgical trauma patients determined prior substance abuse and history of mental health disorder as independent predictors of opioid refill []. Increased odds of refill were also seen in patients who underwent multiple surgical repairs for their facial fractures or other injuries due to their inciting trauma []. Sagheer et al. also demonstrated that history of opioid or benzodiazepine use as well as obesity was predictors of increased opioid consumption []. Aside from patient history, the mechanism of injury can also be an independent risk factor for opioid prescription, with high impact and traumatic fractures increasing post-operative narcotic usage []. Additionally, patient’s age, specifically younger patients (18–44), and location of injury, specifically lower face trauma, were independently associated with increased rates of opioid prescriptions []. Geographical region, ethnicity, and sex were not predictive variables [,].
Pediatric Opioid Use
Guidance on opioid use is especially limited in the population of pediatric patients suffering from craniomaxillofacial trauma, despite the fact that injured adolescents have a 56% increased risk of substance use disorder []. In a study on adolescent trauma, it was discovered that 20% of adolescents filled 2 opioid prescriptions after 1 year and 13% after 4 years []. A cross-sectional study investigated opioid prescription patterns in trauma patients under 19 years of age both during their emergency department stay and after discharge []. Risk factors for opioid administration after multivariable analysis in this population included age, evening visit, and severe pain score []. Encouragingly, they found a decrease in opioid administration throughout the study period (2006–2015). Alternatively, Som et al. used the MarketScan Commercial Database to investigate the use of opioids after pediatric mandibular fracture repair and found inappropriate opioid prescriptions and prolonged opioid use in this population [].
Limiting Opioid Use
With the widespread opioid crisis throughout the United States, institutions have begun to implement different programs to decrease the rate of opioid prescriptions. A study conducted at NorthShore Center in Illinois implemented an office protocol to offer alternatives to opioids for post-operative pain management for maxillofacial surgery []. They used non-steroidal agents, acetaminophen, and a homeopathic kit 3 days before and up to 7 days after the surgery. They subsequently compared prescription patterns for patients undergoing maxillofacial surgery before and after the implementation of the protocol []. After 2 years, they found a 19% reduction in prescribed and filled opioid prescriptions []. In North Carolina, the Strengthen Opioid Misuse Prevention (STOP) act was employed in 2018 to encourage the reduction of opioid prescriptions. A retrospective chart review at a level 1 trauma center found a statistically significant decrease in average morphine milligram equivalents used in patients with maxillofacial trauma before and after the implementation of the STOP act []. These studies represent the utility and importance of implementing protocols and guidelines into practices with the goal of opioid use limitation.
While limiting opioid use is important, controlling patient pain is also a priority. Therefore, in order to achieve the optimal analgesia with avoiding or limiting the use of addictive substances, several alternative pain algorithms have been suggested. These treatment options include multimodal therapy that focuses on pain control via medications with different mechanisms of action as well as nonpharmaceutical management such as setting expectations with pre-operative counseling. The following review will discuss additional options and therapies that can help reduce the use of opioids.
Other Medications and Multimodal Therapy
One proposed method to limit over-prescribing and opioid misuse in trauma patients is multimodality therapy. The concept of multimodal pain management is to utilize a combination of different classes of analgesic medications in order to maximize synergistic effects while limiting complications []. Previous studies suggest that the use of multimodal analgesia in combination with opioids can minimize the dosage of opioid required for adequate pain control in trauma patients []. Multimodal therapy can include, but is not limited to, acetaminophen, local anesthetics and regional nerve blocks, non-steroidal anti-inflammatory drugs, corticosteroids, gabapentinoids, muscle relaxants, and neuropathic agents [,]. A comprehensive summary of different medications and their potential to decrease opioid usage/prescription is demonstrated in Table 1.

Table 1.
Alternative medications to opioids for reducing pain and rate of opioid use/prescriptions.
Acetaminophen. Acetaminophen is an analgesic and antipyretic that has traditionally been used for mild to moderate pain, although its exact mechanism of action is not well understood. Haines et al. compared the effects of combined acetaminophen and opioid use post-operatively on opioid dosage in elderly trauma patients requiring femur or hip fixation. They found that 16.8% received acetaminophen prior to opioids, 27.5% received a combined acetaminophen-opioid therapy, and 9.2% received opioids without receiving acetaminophen []. Patients who received acetaminophen first had their pain controlled with significantly lower opioid use post-operatively []. In the maxillofacial surgery literature, Morgan et al. found the use of acetaminophen resulting in significantly lower narcotic usage in an isolated facial fracture post-operatively []. Both of these studies, however, assess the immediate postoperative period (24 hours) and cannot comment on the effect of acetaminophen on long-term or persistent opioid use. Combining acetaminophen with an NSAID has also been shown to be effective in managing post-operative pain []. Often, these medications are used in combination, alternating them for around the clock pain control [].
Local Anesthetics. Morgan et al. further demonstrated that lidocaine has similar utility in reducing post-operative opioid usage in facial fracture management. While lidocaine is typically used as a local anesthetic due to its quick onset and short half-life, it can also be used as a general anesthetic []. The utility of perioperative lidocaine to decrease post-operative pain and prevent unnecessary opioid use has been accepted across many surgical specialties []. Lidocaine can be administered in different forms, including topical ointment or spray, as well as an intramuscular or intravenous injection. Zhu et al. compared effects of different modalities of lidocaine administration on postoperative pain relief after closed nasal bone reduction; patients received morphine if their pain was not adequately controlled on the lidocaine alone []. Those patients who had lidocaine administered as a general anesthetic required less morphine when compared to those who received topical injection and lidocaine spray; however, lidocaine infusion was associated with nausea and vomiting []. Jeong et al. investigated the use of topical lidocaine gel compared to traditional injection during intermaxillary fixation on controlling patient’s pain []. They found that patients who had topical lidocaine gel usage had shorter surgeries, decreased pain scores, and decreased arch bar loosening []. Understanding how different administration modalities of lidocaine can reduce pain for patients can help guide ideal methods for their use in multimodal therapy.
While lidocaine may be one of the more common local anesthetics used, there are also several other options. These include, but are not limited to, mepivacaine, bupivacaine, and ropivacaine, which may be used for longer-lasting pain control. In a recent study comparing the effects of lidocaine and bupivacaine for pain control in impacted third molar surgery, the post-operative analgesic efficacy of bupivacaine was demonstrated to be similar to that of lidocaine []. Lidocaine had a quicker onset of anesthesia and lower visual analog score values compared to bupivacaine, although the duration of anesthesia for bupivacaine was longer []. According to a meta-analysis specifically assessing postoperative pain after nasal surgery, however, bupivacaine was found to have better pain control [].
Liposomal Bupivacaine. Liposomal bupivacaine is a longacting form of local anesthetic available as an adjunct for optimal post-operative pain control. A recent literature review investigated the use of liposomal bupivacaine in oral and maxillofacial surgery []. The authors ultimately concluded that this treatment has the potential to limit postoperative pain and opioid use []. To our knowledge there have been no recent prospective studies investigating its use specifically for facial fractures. In the trauma and orthopedic literature, however, randomized control trials showed liposomal bupivacaine reduced the use of opioids [,]. Davidovitch et al. demonstrated liposomal bupivacaine along with intraoperative injection of standard bupivacaine for open reduction internal fixation of ankle fractures provided improved pain relief up to 2 days post-operatively and resulted in reduced opioid use [].
A randomized control trial comparing traditional bupivacaine to liposomal bupivacaine noted improved pain ratings on post-operative day 1 as well as decreased opioid use on postoperative day 1 and 2 for patients in the liposomal bupivacaine group who underwent total knee arthroplasty []. Similar findings were demonstrated by Ma et al. for patients undergoing hip arthroplasty []. In the fracture literature, patients with distal radius fractures demonstrated decreased pain on the same day of surgery with liposomal bupivacaine, but there was no difference between groups on subsequent days, or in total number of opioid pills consumed at the end of the study []. We anticipate liposomal bupivacaine being used more frequently for operative facial fracture management; however, additional prospective studies and clinical trials must be completed in this patient population.
Regional Anesthesia/Nerve Blocks. In regional anesthesia (regional nerve blocks), anesthetic agents (such as ropivacaine or bupivacaine) are injected into a peripheral nerve sheath to inhibit nerve transmission with the goal of preventing or relieving regional pain without affecting the patient’s level of consciousness []. Previous studies have shown nerve blocks can successfully reduce post-operative pain in orthopedic surgeries [,], but recently mandibular nerve block has been introduced for better pain management of facial trauma. A 2021, prospective, double-blind, randomized control trial found patients who underwent mandibular nerve block for mandibular osteotomies had significantly less pain and lower post-operative morphine consumption at 24 hours when compared to the control group []. One case series described successful pain control with mandibular nerve block alone in 2 separate patients with mandibular fractures, without any need for parenteral or oral analgesics throughout the entire post-operative period []. El-Anwar et al. conducted a prospective study including 44 patients with mandibular fractures comparing treatment with general anesthesia to regional anesthesia []. Patients who underwent regional anesthesia found the pain control to be equivalent to general anesthesia, without the risks of airway manipulation or systemic therapy posed by general anesthesia [,]. While more research is needed, these studies provide promising evidence on the utility of mandibular nerve blocks to reduce post-operative pain in facial fractures without, or with reduced, use of opioids.
Non-Steroidal Anti-Inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used to reduce pain and inflammation. They function by decreasing the production of inflammatory mediators such as thromboxane, prostaglandins, and prostacyclin through inhibition of cyclooxygenase enzymes. One study assessed pain management for bimaxillary osteotomy surgery by comparing the efficacy of NSAIDs to acetaminophen for post-operative analgesia []. Patients were given diclofenac sodium intramuscularly for post-operative pain, and the amount and the time of additional doses were recorded and compared to a control group using acetaminophen. The post-operative pain between groups was compared using the visual analog scale []. Another study assessing internal fixation of mandibular fractures compared opioid and NSAID use for post-operative pain. Both drugs reduced pain intensity, but better pain control was obtained in the NSAID group at 24 hours after surgery, further supporting the use of non-opioid analgesics for postoperative pain control []. In addition, Nezafati et al. investigated post-operative pain in reduction of mandibular fractures and demonstrated that administering NSAIDs, specifically ketorolac, immediately before the end of surgery can decrease the pain severity and the need for opioids, post-operatively []. Concerns over post-operative bleeding have limited the use of ketorolac for pain control. However, in a systematic review and meta-analysis of randomized control trials, post-operative bleeding was not increased with ketorolac use compared with controls [].
Non-steroidal anti-inflammatory drugs block both cyclooxygenase (COX)-1 and COX-2, decreasing prostaglandin production, however, there are selective COX-2 inhibitors such as celecoxib and rofecoxib that have also been shown to be effective at decreasing post-operative pain []. A systematic review reports that COX-2 inhibitors, when given pre-operatively reduce both post-operative pain, and analgesic consumption compared with placebo []. Despite the potential for decreased pain, some physicians are hesitant to use NSAIDS or COX-2 inhibitors in patients with fractures due to reports that they delay bone healing. The majority of these claims come from animal studies investigating the rate of bone healing when using NSAIDS [,]. However, there is limited human data that shows NSAIDs impact bone healing. Kim et al. demonstrate that there is no increase in non-union or delayed union rates when using NSAIDs and COX-2 inhibitors for a short period of time []. However, continued use of these medications (>3 weeks) may be associated with increased non-union []. In contrast, Farii et al. completed a meta-analysis of randomized controlled trials which claimed there was an increased risk for non-union in patients who received NSAIDS, but no difference between patients who received medications for a short period of time (<2 weeks) compared to a longer period of time (>4 weeks) [].
Corticosteroids. Corticosteroids are often used for pain management due to their anti-inflammatory effects, with dexamethasone being the most commonly studied in pain management of facial trauma. Oksa et al. conducted a randomized trial of 45 patients with fractures of the mandible and intraoral miniplate fixation, comparing administration of 30 mg of dexamethasone perioperatively vs no pre-operative corticosteroids or placebo administration []. The dexamethasone group had lower visual analog scale scores at 18 hours post-operatively, but there were no differences in post-operative opioid administration, edema, or trismus between the groups []. Another study compared different dosages of dexamethasone, 10 mg and 30 mg, to each other as well as to no corticosteroid administration after zygomatic complex fractures. They found that patients receiving dexamethasone had lower average pain scores with no significant difference between the 2 subgroups receiving dexamethasone []. Similar results were found when using dexamethasone before orbital blowout fracture surgery []. Corticosteroids may be able to reduce pain scores on the visual analog scale; however, further studies are required to demonstrate their role in decreasing or eliminating the need for post-operative opioid prescriptions in facial fracture repairs.
Gabapentinoids. Traditionally used only as an anticonvulsant, gabapentin is an analog of the neurotransmitter GABA (γ-Aminobutyric acid) which has been utilized for postoperative pain management due to its ability to reduce the excitability of cortical nerve cells and modify the perception of the pain. A recent systematic review, including 11 randomized controlled trials, was performed investigating the use of pregabalin, a newer generation gabapentinoid, for cervicofacial surgery. Authors ultimately concluded that administration of pregabalin before surgery can alleviate pain and reduce the need for rescue analgesia, postoperatively []. In a randomized controlled trial investigating pain management for double-jaw surgery, a single dose of pregabalin reduced visual analog scale scores and 24-hour opioid consumption with no additional side effects []. Future studies are necessary to determine the ideal preoperative dosage of gabapentinoids to optimize pain management and reduce post-operative opioid requirements. In a 2007 case report, topiramate, another class of anticonvulsant, was successfully used for treatment of chronic neuropathic pain after an untreated zygomatic fracture; however, further research is required to determine its potential use and safety in facial trauma pain management [].
Melatonin. Melatonin supplements are synthetic analogs of the native hormone responsible for regulating the sleep/wake rhythm by acting on melatonin receptors in the suprachiasmatic nucleus. It has been traditionally used as a preferential sleep-aid, due to its lack of medication dependence. While melatonin is not typically considered to have analgesic effects, one study found its pre-operative use for zygomatic fractures to be better than a placebo in reducing the required opioid dosage [].
Multimodal Therapy
Thus far, individual pharmacologic therapeutics have been discussed. The different location of pharmacological actions can be observed in Figure 1. Using a combination of these therapies may be the best approach to optimal pain management. Multimodal analgesia, as defined by the American College of Surgeons (ACS), is the use of multiple analgesics, regional analgesia, and nonpharmacologic interventions to affect the pain pathway []. This allows for risk mitigation of individual therapies and synergistic pain control. The American Pain Society (APS) published guidelines recommending physicians utilize multimodal therapy in their practice []. Randomized trials showed analgesia combining medications with different mechanisms of action or different administrative techniques has superior pain relief and decreased opioid consumption compared to the use of a single medication [,]. While multimodal therapy is recommended, the ideal combination of treatment components will vary depending on the patient and surgical procedure. American College of Surgeon also focuses on implementing nonpharmacological management of pain such as positioning, calming, and ice []. Multimodal therapy needs to be patient centered and patient specific. Therefore, there is no perfect algorithm to follow for every patient.
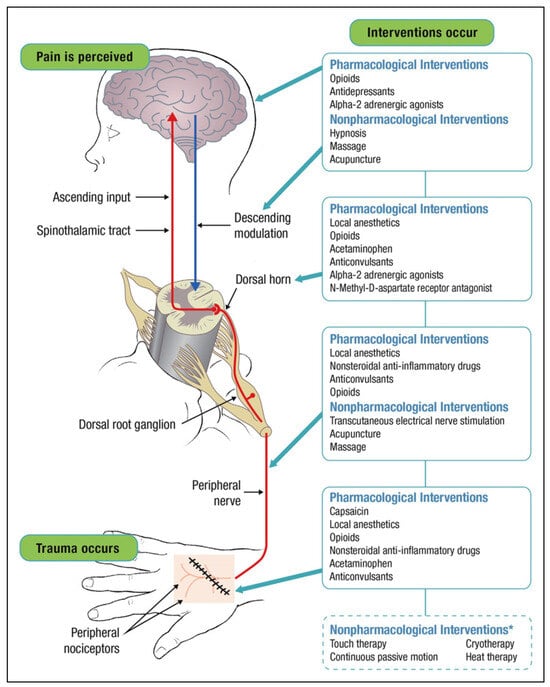
Figure 1.
The pathway where each type of intervention exerts its mechanism of action to relieve pain (image reproduced under the terms of the Creative Commons Attribution License, and with permission from John Wiley & Sons: Multimodal pain management and the future of a personalized medicine approach to pain. AORN J. 2015;101(3):308-318. doi:10.1016/j.aorn.2014.12.009.
Nonpharmacologic Therapies
In addition to traditional pharmacotherapy and multimodal analgesic therapy, there are several nonpharmacologic strategies that have been identified to reduce post-operative pain and potentially decrease opioid usage. These strategies can be categorized into pre-operative (regional anesthesia/nerve blocks and counseling) and post-operative (hilotherapy, acupuncture, virtual reality, hypnosis, and music therapy) interventions. The literature suggests that these therapies, when used as part of a multimodal therapeutic regimen in conjunction with pharmacologic agents, can successfully decrease or eliminate opioid use for postoperative pain management. A comprehensive summary of the impact of utilizing alternative therapies is demonstrated in Table 2.

Table 2.
Alternative therapies to opioids for reducing pain and rate of opioid use/prescriptions.
One potential algorithm for analgesia management in patients with facial fractures is outlined in Figure 2.
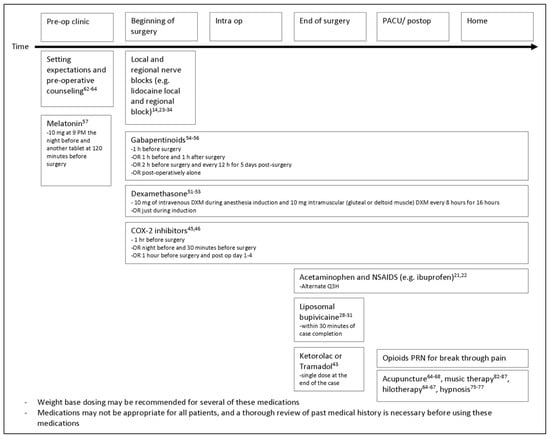
Figure 2.
Proposed algorithm for approaching analgesic management in patients undergoing surgical management for facial fractures.
Pre-operative Counseling. Pre-operative pain counseling has been studied in a few surgical specialties, including orthopedic trauma and general surgery. A cohort study found that orthopedic trauma patients who were informed pre-operatively that they would be prescribed opiates for a maximum of 6 weeks were significantly less likely to require further opioid use after post-operative week 6 when compared to the control group []. A quality improvement study tested the efficacy of pre-operative pain management education on patients undergoing same-day laparoscopic cholecystectomies when compared to patients without preoperative education. The intervention group not only reported less severe pain in the first 24 hours after surgery but also utilized more nonpharmacologic pain management therapies after surgery when compared to the control group []. Furthermore, a systematic review published in 2020 correlated pre-operative education to decreased postsurgical recovery time and acute pain. The authors found that reduced post-operative acute pain associated with preoperative education subsequently decreased the number of post-surgical opioid prescriptions which in theory should decrease the risk of developing chronic post-surgical pain []. Despite these promising results, further research is required to determine the direct benefit of pre-operative pain counseling on post-operative opioid use and dependency in craniomaxillofacial trauma.
Hilotherapy. Hilotherapy is an alternative to conventional post-operative cryotherapy (i.e., ice packs or cold compresses) where 15°C water is continuously circulated through a polyurethane mask applied directly to the surgical site []. This treatment has been primarily utilized in craniofacial surgeries [,,]. One clinical trial looked at patients with unilateral angle fractures of the jaw on the identical post-operative pharmacological pain management regimen and compared use of hilotherapy to traditional cryotherapy []. Their results demonstrated increased efficacy of hilotherapy in reducing soft tissue swelling and pain postoperatively in all facial segments []. Similarly, Modabber et al. found that the pain and edema in patients with unilateral zygomatic bone fractures can be managed more efficiently with the use of hilotherapy, decreasing postoperative recovery time when compared to conventional therapy []. They also discovered that these patients suffer less limitation of eye motility and diplopia, as well as fewer neurological complaints in the immediate post-operative period, as compared to the control group []. Furthermore, a systematic review found that patients also preferred this method to traditional ice-cooling treatment, which the authors attributed to the idea that hilotherapy provides better distribution of temperature to affected areas and increased ease of use, leading to patient compliance compared with conventional therapy [,]. While hilotherapy is not intended to replace typical pharmacologic analgesia, its ability to decrease post-operative pain and reduce recovery time makes it an ideal candidate to incorporate in multimodal therapy. Further investigation into use of hilotherapy as part of multimodal therapy is still needed to determine its longterm effects on pain management in complex facial trauma.
Acupuncture. The ancient Chinese practice of acupuncture consists of inserting thin needles into specific meridian points on the body to balance qi, one’s life force, and thereby relieve pain []. Western medicine attributes the associated pain relief to stimulation of the nerves and other tissues which increases production of the body’s natural opioids including endorphins and enkephalins []. Electrical impulses can also be added to the needles to increase stimulation, a technique referred to as electroacupuncture. In a systematic review of randomized controlled trials, Sun et al. found decreased pain intensity, as quantified by the visual analog score, as well as decreased opioid consumption in the first 8, 24, and 48 hours post-operatively in patients who underwent acupuncture for pain control as compared to control groups []. Similarly, another systematic review and meta-analysis found that post-operative trauma patients who received acupuncture not only had decreased pain and opioid use on post-operative day 1 but also suffered fewer opioid-related side effects []. One case study also reported that use of acupuncture in a patient with preexisting head and neck pain reduced pain by a mean of 3.7 units on a 10-point scale, stating the greater utility of acupuncture for acute rather than psychogenic pain []. The current literature supports the utility of acupuncture therapy in post-operative pain management, leading to a decreased need for pharmacologic intervention and therefore analgesic side effects.
Virtual Reality. Virtual reality (VR) is another technique that has been used for pain management in trauma patients. Virtual reality is thought to alleviate pain by redirecting the senses and conscious attention toward the computerized images, thereby reducing recognition of pain stimuli. In addition to distraction, researchers believe VR may produce neurophysiologic changes related to conditioning and exposure therapies []. Virtual reality has been studied as a treatment for phantom limb pain and as a distraction for children undergoing dental procedures, but it has not yet been studied in post-operative surgical patients [,]. One study utilized a combination of VR and hypnosis for the treatment of physical trauma in hospitalized patients. Patients in the treatment group reported reduced pain scores up to 8 hours after treatment, as compared to patients utilizing standard analgesics or VR without hypnotic induction []. The benefits seen in these patient populations supports future investigation into inclusion of VR in post-surgical multimodal pain management regimen.
Hypnosis. Hypnosis is a widely accepted nonpharmacological technique with 2 main theories regarding its mechanism of pain relief [,,]. Bowers et al. []. and Hilgard et al. []. both support a dissociation process which utilizes hypnotic susceptibility and the patient’s alerted state of consciousness, whereas Chaves proposes the influence of social and cognitive processes on patient compliance, expectations, and role enactment []. Lu et al. found that hypnosis reduced pain by a mean of 4.8 units on a 10-point scale in patients with preexisting head and neck pain, and the authors suggested that hypnosis was more effective in treating psychogenic rather than acute pain []. Based on a review of 29 randomized control trials, however, Kendrick et al. found that hypnosis is at least as effective as comparable adjunct psychological interventions in the treatment of acute procedural pain, with the best results in pain reduction occurring with multiple hypnosis sessions prior to the procedure itself []. Furthermore, brain imaging studies on hypnotized patients have identified reduction in subjective pain intensity in the somatosensory cortex and pain unpleasantness in the anterior cingulate cortex [,]. A 2020 systematic review concluded that mind-body therapies, like hypnosis, moderately reduce pain as well as the required opioid dose in patients who were prescribed opioids for chronic, acute, post-operative, or cancer pain []. While the above support the utility of hypnosis in pain management, further studies must determine the relevance of this modality for post-operative patients in general as well as for facial fracture patients.
Music Therapy. Music therapy has been proposed as a method of pain reduction, believed to function through activation of the reward network in the brain leading to distraction and improved mood rather than direct analgesia []. While no clear mechanism of action is known, music therapy efficacy continues to be explored because of its ease of implementation, low cost, and lack of side effects []. A 2020 systematic review of 9 randomized controlled trials found that music therapy, especially when the music was chosen by the patient, significantly reduced both patient’s anxiety and pain after orthopedic surgery []. Multiple studies have examined the efficacy of music therapy in improving pain outcomes in post-operative patients, including those who underwent orthopedic and facial surgeries. A study of septoplasty patients found that music therapy just before surgery, and up to 2 days post-operatively, significantly reduced pre-and post-operative anxiety as well as postoperative pain between days 0 and 2, as compared to the control group []. Another study of patients undergoing septorhinoplasty confirmed these findings, demonstrating not only lower levels of post-operative pain but also less analgesic medication required for pain control in the music therapy group as compared to the control []. Schneider et al. found that orthopedic surgery patients had a significant reduction in pain scores after listening to music, independent of the duration of listening []. Additionally, when surveyed, all participants stated that they would recommend music therapy to others undergoing surgery []. Seeing as music therapy is a safe and inexpensive method of effectively reducing post-operative pain, further exploration is required to evaluate its potential utility to reduce opioid use in post-operative patients.
Conclusion
Opioids continue to be the foundation of post-operative pain management following facial trauma repair, placing the patients in this population at high risk of opioid misuse and dependence. Various studies have investigated the alternatives to opioid medications in trauma patients and post-operative period with the goal of decreasing the dose and usage of opioids. Several studies have demonstrated promising results on multimodal pain management using a combination of different analgesic medication classes including Tylenol, NSAIDS, local anesthetics, regional nerve blocks, gabapentinoids, melatonin, and corticosteroids. Alternative therapies such as hilotherapy, music therapy, hypnosis, acupuncture, and virtual reality have also been found to be effective in reducing post-operative pain, with subsequent decrease in post-operative opioid usage and prescriptions.
Nevertheless, it is important to note that many of the current studies are retrospective, non-randomized, and have small sample size. Prospective clinical trials on multimodal therapy and nontraditional modalities for pain management in craniomaxillofacial trauma are needed to ultimately develop evidence-based and universal guidelines on pain management in this patient population.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Due to the nature of this project being a literature review and not utilizing patient data, IRB review was not obtained.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Schiller, E.; Goyal, A.; Mechanic, O. Opioid Overdose; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470415/ (accessed on 2 July 2022).
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States. Recommendations and Reports. 2016. Available online: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm (accessed on 3 July 2022).
- Seth, P.; Scholl, L.; Rudd, R.A.; Bacon, S. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018, 67, 349–358. [Google Scholar] [CrossRef]
- Curtis, C.; Scarcella, J.; Viscardi, C.; Samia, A.; Zeri, R.; Guo, Y. Reduction of opioid prescriptions in maxillofacial trauma following North Carolina STOP Act. Craniomaxillofac Trauma Reconstr. 2021, 14, 231–235. [Google Scholar] [CrossRef]
- DEA. The Controlled Substances Act. 25 July 2018. Available online: https://www.dea.gov/drug-information/csa (accessed on 2 July 2022).
- Fortune, S.; Frawley, J. Optimizing pain control and minimizing opioid use in trauma patients. AACN Adv Crit Care 2021, 32, 89–104. [Google Scholar] [CrossRef]
- Drahos, A.L.; Scott, A.M.; Johns, T.J.; Ashley, D.W. Multimodal analgesia and decreased opioid use in adult trauma patients. Am Surg. 2020, 86, 950–954. [Google Scholar] [CrossRef]
- Chaudhary, M.A.; Schoenfeld, A.J.; Harlow, A.F.; et al. Incidence and predictors of opioid prescription at discharge after traumatic injury. JAMA Surg. 2017, 152, 930. [Google Scholar] [CrossRef]
- DiMaggio, C.J.; Avraham, J.B.; Lee, D.C.; Frangos, S.G.; Wall, S.P. The epidemiology of emergency department trauma discharges in the United States. Acad Emerg Med. 2017, 24, 1244–1256. [Google Scholar] [CrossRef]
- Lapidus, J.B.; Santosa, K.B.; Skolnick, G.B.; et al. Opioid prescribing and use patterns in postsurgical facial trauma patients. Plast Reconstr Surg. 2020, 145, 780–789. [Google Scholar] [CrossRef]
- Barbarite, E.; Occhiogrosso, J.; McCarty, J.C.; et al. Opioid prescribing patterns among facial plastic and reconstructive surgeons in the medicare population. Facial Plast Surg Aesthet Med. 2021, 23, 401–404. [Google Scholar] [CrossRef]
- Shah, J.; Lesko, R.P.; Lala, B.; Ricci, J. Trends in opioid prescription for craniomaxillofacial trauma in the United States: An 11-year retrospective study of emergency room and office visits. Surgery. 2021, 170, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sagheer, S.H.; Yan, B.M.; Bovenzi, C.D.; et al. Postoperative opioid-prescribing practices in nasal surgery: A prospective study. Facial Plast Surg Aesthet Med. 2022, 24, 266–270. [Google Scholar] [CrossRef]
- Morgan, A.C.; Davis, G.L.; Mehta, I.H.; Stark, P.; Paap, M.K.; Gosman, A.A. Analysis of narcotic use in isolated facial fractures: Potential targets for a narcotic reduction protocol. J Craniofac Surg. 2021, 32, 1033–1036. [Google Scholar] [CrossRef]
- Bell, T.M.; Raymond, J.; Vetor, A.; et al. Long-term prescription opioid utilization, substance use disorders, and opioid overdoses after adolescent trauma. J Trauma Acute Care Surg. 2019, 87, 836–840. [Google Scholar] [CrossRef]
- Foster, A.A.; Porter, J.J.; Bourgeois, F.T.; Mannix, R. The use of opioids in low acuity pediatric trauma patients. PLoS ONE 2019, 14, e0226433. [Google Scholar] [CrossRef]
- Som, A.; Santosa, K.B.; Skolnick, G.B.; Lapidus, J.B.; Waljee, J.F.; Patel, K.B. Opioid use among adolescents undergoing surgical repair of facial trauma. Plast Reconstr Surg. 2021, 147, 690–698. [Google Scholar] [CrossRef]
- Tatch, W. Opioid prescribing can be reduced in oral and maxillofacial surgery practice. J Oral Maxillofac Surg. 2019, 77, 1771–1775. [Google Scholar] [CrossRef]
- Fortune, S.; Frawley, J. Optimizing pain control and minimizing opioid use in trauma patients. AACN Adv Crit Care. 2021, 32, 89–104. [Google Scholar] [CrossRef]
- Haines, K.L.; Fuller, M.; Antonescu, I.; et al. Underutilization of acetaminophen in older adult trauma patients. Am Surg 2022, 88, 2003–2010. [Google Scholar] [CrossRef]
- Merry, A.F.; Gibbs, R.D.; Edwards, J.; et al. Combined acetaminophen and ibuprofen for pain relief after oral surgery in adults: A randomized controlled trial. Br J Anaesth. 2010, 104, 80–88. [Google Scholar] [CrossRef]
- Michigan Pain Control Optimization Pathway (MPOP). Managing Your Pain after Surgery Without Opioids; University of Michigan: Ann Arbor MI, USA, 2020; Available online: https://www.med.umich.edu/1libr/Surgery/MPOPeducation-ManagingPainWithoutOpioids.pdf (accessed on 12 August 2022).
- Dunn, L.K.; Durieux, M.E. Perioperative use of intravenous lidocaine. Anesthesiology 2017, 126, 729–737. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, J.; Shen, G.; Zhong, T.; Yu, X. Comparison of efficacy outcomes of lidocaine spray, topical lidocaine injection, and lidocaine general anesthesia in nasal bone fractures surgeries: A randomized, controlled trial. Med Sci Mon Int Med J Exp Clin Res. 2018, 24, 4386–4394. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, H.J.; Kwon, H.; Shim, H.S.; Seo, B.F.; Jung, S.N. The use of topical lidocaine gel during intermaxillary fixation procedure. J Craniofac Surg. 2016, 27, e475–e477. [Google Scholar] [CrossRef]
- Velioglu, O.; Calis, A.S.; Koca, H.; Velioglu, E. Bupivacaine vs. lidocaine: A comparison of local anesthetic efficacy in impacted third molar surgery. Clin Oral Investig. 2020, 24, 3539–3546. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Huang, L.; Yu, X.; He, Z. Efficacy and safety of bupivacaine versus lidocaine in local anesthesia of the nasopharynx: A meta-analysis. Am J Rhinol Allergy. 2016, 30, e176–e180. [Google Scholar] [CrossRef]
- Neal, T.W.; Hammad, Y.; Schlieve, T. Liposomal bupivacaine: A literature review of applications in oral and maxillofacial surgery. J Oral Maxillofac Anesth. 2022, 1, 3. [Google Scholar] [CrossRef]
- Wallen, T.E.; Singer, K.E.; Makley, A.T.; et al. Intercostal liposomal bupivacaine injection for rib fractures: A prospective randomized controlled trial. J Trauma Acute Care Surg. 2022, 92, 266–276. [Google Scholar] [CrossRef]
- Ott, M. Efficacy of liposomal bupivacaine in orthopedic procedures in an academic trauma hospital. Ann Musculoskelet Med. 2017, 1, 027–031. [Google Scholar] [CrossRef]
- Davidovitch, R.; Goch, A.; Driesman, A.; Konda, S.; Pean, C.; Egol, K. The use of liposomal bupivacaine administered with standard bupivacaine in ankle fractures requiring open reduction internal fixation: A single-blinded randomized controlled trial. J Orthop Trauma. 2017, 31, 434–439. [Google Scholar] [CrossRef]
- Hubler, C.P.; Bevil, K.M.; Greiner, J.J.; Hetzel, S.J.; Borden, S.B.; Cios, H.A. Liposomal bupivacaine versus standard bupivacaine in the adductor canal for total knee arthroplasty: A randomized, controlled trial. Orthopedics. 2021, 44, 249–255. [Google Scholar] [CrossRef]
- Ma, T.T.; Wang, Y.H.; Jiang, Y.F.; et al. Liposomal bupivacaine versus traditional bupivacaine for pain control after total hip arthroplasty. Medicine 2017, 96, e7190. [Google Scholar] [CrossRef]
- Alter, T.H.; Liss, F.E.; Ilyas, A.M. A prospective randomized study comparing bupivacaine hydrochloride versus bupivacaine liposome for pain management after distal radius fracture repair surgery. J Hand Surg Am. 2017, 42, 1003–1008. [Google Scholar] [CrossRef]
- Folino, T.; Mahboobi, S. Regional Anesthetic Blocks; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wong, S.S.; Chan, W.S.; Fang, C.; et al. Infraclavicular nerve block reduces postoperative pain after distal radial fracture fixation: A randomized controlled trial. BMC Anesthesiol. 2020, 20, 130. [Google Scholar] [CrossRef]
- Joshi, G.; Gandhi, K.; Shah, N.; Gadsden, J.; Corman, S.L. Peripheral nerve blocks in the management of postoperative pain: Challenges and opportunities. J Clin Anesth. 2016, 35, 524–529. [Google Scholar] [CrossRef]
- Bertuit, M.; Rapido, F.; Ly, H.; et al. Bilateral mandibular block improves pain relief and morphine consumption in mandibular osteotomies: A prospective, randomized, doubleblind, placebo-controlled clinical trial. Reg Anesth Pain Med. 2021, 46, 322–327. [Google Scholar] [CrossRef]
- Singh, B.; Bhardwaj, V. Continuous mandibular nerve block for pain relief: A report of two cases. Can J Anesth. 2002, 49, 951–953. [Google Scholar] [CrossRef]
- El-Anwar, M.W.; Hegab, A. Internal fixation of single mandibular fracture under mandibular nerve block. Oral Maxillofac Surg. 2016, 20, 57–61. [Google Scholar] [CrossRef]
- Öncül, A.M.T.; Çimen, E.; Kuçüükyavuz, Z.; Cambazoğlu, M. Postoperative analgesia in orthognathic surgery patients diclofenac sodium or paracetamol? Br J Oral Maxillofac Surg. 2011, 49, 138–141. [Google Scholar] [CrossRef]
- Jain, A.D.; Ravisankar, V.; Siva Bharani, K.; Sudheesh, K.; Tewathia, N. A comparative assessment of postoperative analgesic efficacy of lornoxicam versus tramadol after open reduction and internal fixation of mandibular fractures. Craniomaxillofac Trauma Reconstr. 2017, 10, 171–174. [Google Scholar] [CrossRef]
- Nezafati, S.; Khiavi, R.K.; Mirinejhad, S.S.; Ammadi, D.A.; Ghanizadeh, M. Comparison of pain relief from different intravenous doses of ketorolac after reduction of mandibular fractures. J Clin Diagn Res. 2017, 11, PC06. [Google Scholar] [CrossRef]
- Gobble, R.M.; Hoang, H.L.T.; Kachniarz, B.; Orgill, D.P. Ketorolac does not increase perioperative bleeding. Plast Reconstr Surg. 2014, 133, 741–755. [Google Scholar] [CrossRef]
- Solomon, D.H.; Furst, D.E.; Curtis, M.R. Overview of COX-2 selective NSAIDs. UpToDate. Available online: https://www-uptodate-com.medjournal.hmc.psu.edu:2200/contents/overview-of-cox-2-selective-nsaids?search=OverviewofCOX-2selectiveNSAIDs&source=search_result&selectedTitle=1∼150&usage_type= default&display_rank=1 (accessed on 6 August 2022).
- Straube, S.; Derry, S.; McQuay, H.J.; Moore, R.A. Effect of preoperative Cox-II-selective NSAIDs (coxibs) on postoperative outcomes: A systematic review of randomized studies. Acta Anaesthesiol Scand. 2005, 49, 601–613. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Calori, G.M.; Giannoudis, P. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. Sci World J. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Al-Waeli, H.; Reboucas, A.P.; Mansour, A.; Morris, M.; Tamimi, F.; Nicolau, B. Non-steroidal anti-inflammatory drugs and bone healing in animal models—A systematic review and metaanalysis. Syst Rev. 2021, 10, 201. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.H.; Kim, D.M.; et al. Do nonsteroidal antiInflammatory or COX-2 inhibitor drugs increase the nonunion or delayed union rates after fracture surgery? J Bone Joint Surg. 2021, 103, 1402–1410. [Google Scholar] [CrossRef]
- al Farii, H.; Farahdel, L.; Frazer, A.; Salimi, A.; Bernstein, M. The effect of NSAIDs on postfracture bone healing: A metaanalysis of randomized controlled trials. OTA Int. 2021, 4, e092. [Google Scholar] [CrossRef]
- Oksa, M.; Haapanen, A.; Furuholm, J.; Thore´n, H.; Snäll, J. Effect of perioperative systemic dexamethasone on pain, edema, and trismus in mandibular fracture surgery: A randomized trial. J Craniofac Surg. 2021, 32, 2611–2614. [Google Scholar] [CrossRef]
- Kormi, E.; Thore´n, H.; Sna¨ll, J.; Törnwall, J. The effect of dexamethasone on pain severity after zygomatic complex fractures. J Craniofac Surg. 2019, 30, 742–745. [Google Scholar] [CrossRef]
- Kormi, E.; Sna¨ll, J.; Koivusalo, A.M.; Suominen, A.L.; Thore´n, H.; Törnwall, J. Analgesic effect of perioperative systemic dexamethasone on blowout fracture surgery. J Oral Maxillofac Surg. 2017, 75, 1232–1237. [Google Scholar] [CrossRef]
- Lie´bana-Hermoso, S.; Manzano-Moreno, F.J.; Vallecillo-Capilla, M.F.; Olmedo-Gaya, M.V. Oral pregabalin for acute pain relief after cervicofacial surgery: A systematic review. Clin Oral Investig. 2018, 22, 119–129. [Google Scholar] [CrossRef]
- Ahiskalioglu, A.; Ince, I.; Aksoy, M.; Yalcin, E.; Ahiskalioglu, E.O.; Kilinc, A. Effects of a single-dose of pre-emptive pregabalin on postoperative pain and opioid consumption after doublejaw surgery: A randomized controlled trial. J Oral Maxillofac Surg. 2016, 74, 53.e1. [Google Scholar] [CrossRef]
- Benoliel, R.; Sharav, Y.; Eliav, E. Painful posttraumatic trigeminal neuropathy: A case report of relief with topiramate. Cranio. 2007, 25, 57–62. [Google Scholar] [CrossRef]
- de Carvalho Nogueira, E.F.; de Oliveira Vasconcelos, R.; Teixeira Correia, S.S.; Souza Catunda, I.; Amorim, J.A.; do Egito Cavalcanti Vasconcelos, B. Is there a benefit to the use of melatonin in preoperative zygomatic fractures? J Oral Maxillofac Surg. 2019, 77, 2017.e1. [Google Scholar] [CrossRef]
- ACS Trauma Quality Programs. Best Practices Guidelines For Acute Pain Management In Trauma Patients. 2020. Available online: https://www.facs.org/media/exob3dwk/acute_pain_guidelines.pdf (accessed on 24 July 2022).
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016, 17, 131–157. [Google Scholar] [CrossRef]
- McDaid, C.; Maund, E.; Rice, S.; Wright, K.; Jenkins, B.; Woolacott, N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: A systematic review. NIHR Health Technology Assessment programme: Executive Summaries. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56886/ (accessed on 24 July 2022).
- Elia, N.; Lysakowski, C.; Tramèr, M.R. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patientcontrolled analgesia morphine offer advantages over morphine alone? Anesthesiology 2005, 103, 1296–1304. [Google Scholar] [CrossRef]
- Holman, J.E.; Stoddard, G.J.; Horwitz, D.S.; Higgins, T.F. The effect of preoperative counseling on duration of postoperative opiate use in orthopaedic trauma surgery: A surgeon-based comparative cohort study. J Orthop Trauma. 2014, 28, 502–506. [Google Scholar] [CrossRef]
- O’Donnell, K.F. Preoperative pain management education: A quality improvement project. J PeriAnesthesia Nurs. 2015, 30, 221–227. [Google Scholar] [CrossRef]
- Horn, A.; Kaneshiro, K.; Tsui, B.C.H. Preemptive and preventive pain psychoeducation and its potential application as a multimodal perioperative pain control option: A systematic review. Anesth Analg. 2020, 130, 559–573. [Google Scholar] [CrossRef]
- Barca, I.; Colangeli, W.; Cristofaro, M.G.; et al. Effects of cold therapy in the treatment of mandibular angle fractures: Hilotherm system vs ice bag. Ann Ital Chir. 2016, 87, 411–416. [Google Scholar]
- Bates, A.S.; Knepil, G.J. Systematic review and meta-analysis of the efficacy of hilotherapy following oral and maxillofacial surgery. Int J Oral Maxillofac Surg. 2016, 45, 110–117. [Google Scholar] [CrossRef]
- Modabber, A.; Rana, M.; Ghassemi, A.; et al. Three-dimensional evaluation of postoperative swelling in treatment of zygomatic bone fractures using two different cooling therapy methods: A randomized, observer-blind, prospective study. Trials. 2013, 14, 238. [Google Scholar] [CrossRef]
- Sun, Y.; Gan, T.J.; Dubose, J.W.; Habib, A.S. Acupuncture and related techniques for postoperative pain: A systematic review of randomized controlled trials. Br J Anaesth. 2008, 101, 151–160. [Google Scholar] [CrossRef]
- Wu, M.S.; Chen, K.H.; Chen, I.F.; et al. The efficacy of acupuncture in post-operative pain management: A systematic review and meta-analysis. PLoS ONE. 2016, 11, e0150367. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.P.; Lu, G.P.; Kleinman, L. Acupuncture and clinical hypnosis for facial and head and neck pain: A single crossover comparison. Am J Clin Hypn. 2001, 44, 141–148. [Google Scholar] [CrossRef]
- Gupta, A.; Scott, K.; Dukewich, M. Innovative technology using virtual reality in the treatment of pain: Does it reduce pain via distraction, or is there more to it? Pain Med. 2018, 19, 151–159. [Google Scholar] [CrossRef]
- Kumari, S.; Bahuguna, R.; Garg, N.; Yeluri, R. Immersive and non-immersive virtual reality distraction on pain perception to intraoral injections. J Clin Pediatr Dent. 2021, 45, 389–394. [Google Scholar] [CrossRef]
- Rothgangel, A.; Bekrater-Bodmann, R. Mirror therapy versus augmented/virtual reality applications: Towards a tailored mechanism-based treatment for phantom limb pain. Pain Manag. 2019, 9, 151–159. [Google Scholar] [CrossRef]
- Patterson, D.R.; Jensen, M.P.; Wiechman, S.A.; Sharar, S.R. Virtual reality hypnosis for pain associated with recovery from physical trauma. Int J Clin Exp Hypn. 2010, 58, 288–300. [Google Scholar] [CrossRef]
- Kendrick, C.; Sliwinski, J.; Yu, Y.; et al. Hypnosis for acute procedural pain: A critical review. Int J Clin Exp Hypn. 2016, 64, 75–115. [Google Scholar] [CrossRef]
- Bowers, K.S. Imagination and dissociation in hypnotic responding. Int J Clin Exp Hypn. 1992, 40, 253–275. [Google Scholar] [CrossRef]
- Hilgard, M.T.; Hilgard, E.R.; Hilgard, J.R. Hypnosis in the relief of pain. New York: Brunner/Mazel Publishers, pp. 312. Am J Clin Hypn. 1994, 37, 60. [Google Scholar] [CrossRef]
- Chaves, J. Hypnosis in pain management. In Handbook of Clinical Hypnosis, 2nd ed.; Lynn, S., Rhue, J., Kirsh, I., Eds.; American Psychological Association: Washington, DC, USA, 1993; pp. 511–532. [Google Scholar]
- Hofbauer, R.K.; Rainville, P.; Duncan, G.H.; Bushnell, M.C. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001, 86, 402–411. [Google Scholar] [CrossRef]
- Rainville, P.; Duncan, G.H.; Price, D.D.; Carrier, B.; Bushnell, M.C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science (1979) 1997, 277, 968–971. [Google Scholar] [CrossRef]
- Garland, E.L.; Brintz, C.E.; Hanley, A.W.; et al. Mind-Body therapies for opioid-treated pain: S systematic review and meta-analysis. JAMA Intern Med. 2020, 180, 91. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.R.; Carreiro, S.; Ranney, M.L.; et al. Music as an adjunct to opioid-based analgesia. J Med Toxicol. 2017, 13, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Agres, K.R.; Foubert, K.; Sridhar, S. Music therapy during COVID-19: Changes to the practice, use of technology, and what to carry forward in the future. Front Psychol. 2021, 12, 647790. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Hwang, S.L.; Jiang, P.; Hsiung, N.H. Effect of music therapy on pain after orthopedic surgery—A systematic review and meta-analysis. Pain Pract. 2020, 20, 422–436. [Google Scholar] [CrossRef]
- Gogoularadja, A.; Bakshi, S.S. A randomized study on the efficacy of music therapy on pain and anxiety in nasal septal surgery. Int Arch Otorhinolaryngol. 2020, 24, e232–e236. [Google Scholar] [CrossRef]
- Gö kçek, E.; Kaydu, A. The effects of music therapy in patients undergoing septorhinoplasty surgery under general anesthesia. Braz J Otorhinolaryngol. 2020, 86, 419–426. [Google Scholar] [CrossRef]
- Schneider, M.A. The effect of listening to music on postoperative pain in adult orthopedic patients. J Holist Nurs. 2018, 36, 23–32. [Google Scholar] [CrossRef]
© 2024 by the author. The Author(s) 2024.