Abstract
Most cultivated potatoes are tetraploid, and the tuber is the main economic part that is consumed due to its calorific and nutritional values. Recent trends in climate change led to the frequent occurrence of heat and drought stress in major potato-growing regions worldwide. The optimum temperature for tuber production is 15–20 °C. High-temperature and water-deficient conditions during the growing season result in several morphological, physiological, biochemical, and molecular alterations. The morphological changes under stress conditions may affect the process of stolon formation, tuberization, and bulking, ultimately affecting the tuber yield. This condition also affects the physiological responses, including an imbalance in the allocation of photoassimilates, respiration, water use efficiency, transpiration, carbon partitioning, and the source–sink relationship. The biochemical responses under stress conditions involve maintaining ionic homeostasis, synthesizing heat shock proteins, achieving osmolyte balance, and generating reactive oxygen species, ultimately affecting various biochemical pathways. Different networks that include both gene regulation and transcription factors are involved at the molecular level due to the combination of hot and water-deficient conditions. This article attempts to present an integrative content of physio-biochemical and molecular responses under the combined effects of heat and drought, prominent factors in climate change. Taking into account all of these aspects and responses, there is an immediate need for comprehensive screening of germplasm and the application of appropriate approaches and tactics to produce potato cultivars that perform well under drought and in heat-affected areas.
1. Introduction
Potato (Solanum tuberosum spp. tuberosum L.) is the third most important food crop after rice and wheat, with increasing popularity in terms of human consumption. Its annual production was 388.19 million tons (MT) in the year 2019, which is expected to increase further in several regions [1]. Potato is a popular staple vegetable in many countries. Potato is also a staple food in European countries, adding carbohydrates to the human diet and nutrients and minerals [2]. As the human population continues to rise, the accessibility of food may emerge as a major concern on a worldwide scale, and thus potato can help to provide food and nutritional security [3]. This crop is also vital in light of ongoing climate change, which is already exerting intense pressure on the human population’s food and grain supply. In the coming decades, climate change will become a major rising problem for governments and policymakers to devise ways to combat the adverse effects of climate change and ensure food and nutritional security [4].
About 10,000 years ago, the domestication of potato took place in the highlands of the Andes in South America [5]. In the early 16th century, explorers from European countries, such as Spain, England, and the Netherlands, introduced potatoes to Europe [6]. Potatoes are grown extensively in two primary regions. The first zone is between 45° N and 57° N, where potato is cultivated as a summer crop; while the second zone is the subtropical lowlands between the latitudes of 23° N and 34° N, where potato is cultivated during the winter [7]. In the subtropical and tropical regions, the potato is grown as a winter crop where the night temperatures remain below 22 °C. However, a temperature below 20 °C is usually required for tuberization in potato [8]. The term “tropicalization” refers to the process of breeding and developing suitable production techniques for vegetable crops that may be grown at lower latitudes [9].
The importance of potatoes in securing food and nutritional security was identified by the Food and Agriculture Organization (FAO) of the United Nations when it declared the year 2008 as the “The International Year of the Potato” [10]. This initiative was pursued to attract the world’s attention toward the importance of potatoes and their more significant role in food and nutritional security in nonconventional areas. Most developing countries are on the Asian and African continents, where the production and demand for potatoes have increased in recent years [11]. The tropicalization of the potato crop is vital, as potato serves as a cheap source of energy and nutrition. The potato can help overcome food and nutritional insecurity and contribute to improving economic growth [12].
Environmental factors such as heat, drought, salinity, flood, and cold are the major causes of adverse effects on the growth, development, and productivity of horticultural crops [13]. Abiotic stressors are the major cause of crop loss on a global scale, since they can reduce the average yield of most crops by more than 50 percent [14]. The rise in global temperature is a threat to agriculture in general. The increase in temperature poses significant abiotic stress for crop plants that adversely affects their survival, adjustment, and performance [12]. Under such a changing climate scenario, potato cultivars need to be developed that can thrive under high temperatures and give reasonably good production and productivity [15].
Heat stress negatively affects the plant’s growth, and developmental, biochemical, and physiological processes, leading to a reduction in yield and productivity. The critical developmental stage affected by heat stress is the reproductive and bulking stages [16]. Similarly, plant response to drought stress is detrimental and impacts morpho-physiological, anatomical, and biochemical parameters. Climate change is detrimental to tuber and root vegetable crops, which are also considered staple foods in many countries. It has been predicted that there will be a decrease in global potato production by 18–32% due to global warming by the middle of this century [12,17]. Therefore, to cope with this climate change problem and ensure food security, it is essential to understand the responses of crops to climate change. Understanding the mechanism of plant response to abiotic stresses could be a viable strategy for developing crop varieties through selection, breeding, and biotechnological approaches, with the goal of developing varieties tolerant to heat or/and drought stress [18]. Regions in tropical areas suffer from unprecedented seasonal heat and drought stress [19,20]. These stresses lead to a detrimental effect on physiological and biochemical mechanisms in the plant that ultimately hamper the growth and development of potato plants [21] and reduce yields and tuber quality [22]. The potato crop originated in borderline subtropical/alpine climates and performed best in places with warm days and cool nights. Potato production in tropical and sub-tropical regions is challenging. However, potato breeders and physiologists are trying to develop thermo-insensitive varieties which may be suitable for tropical regions [23].
Potato cultivation is expanding to non-traditional regions with water-deficient conditions and facing heat stress. In addition, heat and drought spells are becoming more frequent in temperate zones [12,16]. Thus, an integrated approach is required to understand better the morphological, physiological, biochemical, and molecular network at the whole-plant level pertaining to drought and heat stress tolerance. This is then used to develop potato cultivars suitable for abiotic stress conditions. This review highlights various physiological, biochemical, and molecular aspects and responses of potato plants under tropical conditions where the potato plant is exposed to several types of abiotic stresses.
2. Production and Productivity of Potato Affected by Heat and Drought Stress
Potato crop species are highly prone to different abiotic (high-temperature stress, drought, salinity, and mineral stress) and biotic stresses (insect and pest attacks) [13,24,25,26]. Heat stress is a significant issue for temperate countries and potato production locations in the semi-arid Middle East and the Sub-Saharan, subtropical, and tropical regions [27]. Temperature is the most critical uncontrollable factor affecting potato growth, development, production, and productivity. Tropical areas experience high-temperature stress, where plants undergo several anatomical, morphological, physiological, biochemical, and molecular changes. Growth and development are seriously affected, leading to a substantial decrease in potato production [16]. Due to high temperatures, the environment of the tropical region alters the morphological features and the physiological and developmental processes of potato plants. For instance, high temperatures may cause a reduction in the leaf area index, specific leaf area, size and number of leaves, and canopy development, an increase in the plant’s lateral branching and height, and a decrease in the number and size of tubers. The alarming rate of increase in temperature due to climate change causes more frequent heat stress to plants during the summer and high night temperatures in the winter, which hampers crop yield and quality in any region of the world [27]. Heat stress mediates imbalances in source–sink activity, allocation of photoassimilates, necrosis, and malformation of tubers [27]. Furthermore, the soil temperature in which the potato is grown affects the process of stolon formation, tuberization, and bulking, ultimately reducing the tuber yield [28,29].
High-temperature and water stress conditions affect the yield and quality of potato tubers, where the severity, duration, and timing of both heat and water stress adversely affect sprout emergence, stolon formation, tuberization, and final yield of the potato tubers [30]. The potato tuber yield depends on tuber bulking [29], which occurs in the late stage of growth. The production may decrease due to bulking reduction, which is affected by heat and water-deficient conditions [28]. As per Obiero et al. [30], the high-temperature treatment affects the whole plant’s dry matter and potato tuber yield. They reported that high-temperature treatment (30 °C) compared to a control (temperature of 22 °C) before and after tuber initiation leads to 45% smaller tubers (less than 2.5 cm diameter).
Planting time (spring and autumn) affects potato yield [31]. High temperatures in the subtropical climate, particularly during the spring season, are more detrimental than autumn because of the combination of low humidity (higher atmospheric evaporative demand) and higher temperatures. An average yield reduction of 68% and 42% was observed in spring and autumn plantings [22]. This difference may also be due to water availability during the spring and autumn seasons [32]. Under tropical conditions, high-temperature stress negatively impacts potato tuber quality and yield through the inhibition of the transport of photoassimilates to the developing stolons [12]. It was reported by Fleisher et al. [33] that the optimum temperature for photosynthesis and biomass accumulation in potato is 20 °C. Additionally, it was reported that the optimum daily mean temperature might be as low as 13 °C [12]. An increase of every 5 °C above the optimum temperature causes a reduction in the photosynthetic rate by 25%, which ultimately affects biomass accumulation and, later, the sink activity [15,30,34].
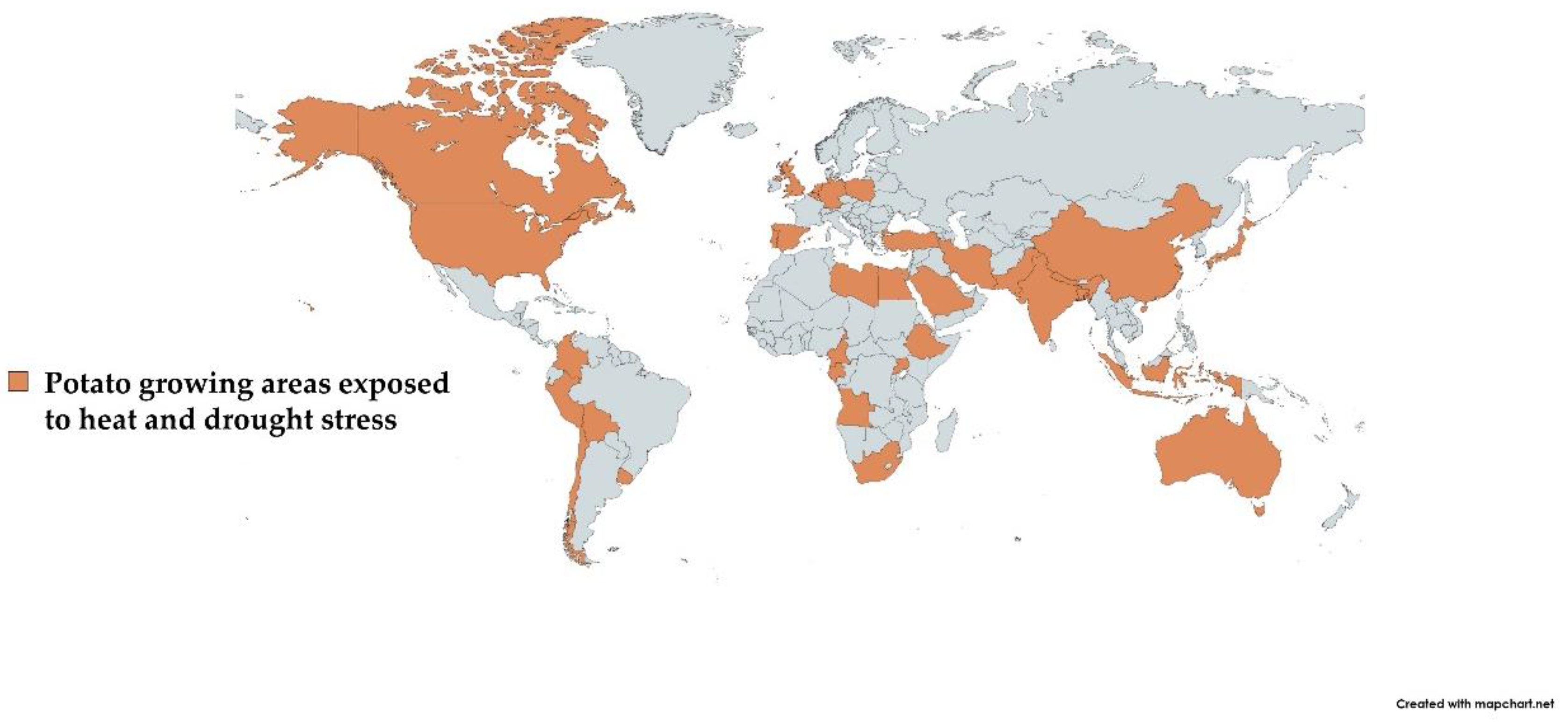
As previously documented, potato production decreases due to heat and drought stress in most East African countries [35]. Additionally, Jarvis et al. [36] anticipated about a 15% reduction in potato yield in Africa by 2030. Across Asia, India and China are at constant drought and heat stress risk. Moreover, periods of high temperatures and drought are becoming more frequent in Central and Western Europe. US potato production was also severely affected due to drought and heat stress during the last 2–3 years. In Mediterranean regions, the problem of dry spells in potato cultivation is also a major concern [37]. Likewise, the major potato-growing areas of the world under consistent risk of drought and heat stress are highlighted in Figure 1.
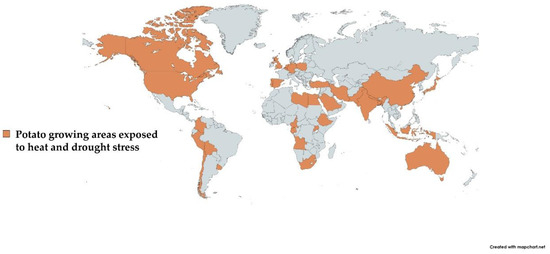
Figure 1.
The area shown in brown color depicts the major potato-growing areas affected due to heat and drought stress.
3. Physiological Changes and Responses of Potato Plants under Heat and Drought Conditions
The changing climate in temperate, subtropical, and tropical regions affects the physiological process of the potato plant [38]. As potato is now adopted for the tropical region, it faces high-temperature and drought stress. Thus, potato plants grown under these conditions may have to acclimate to the situation and alter their growth and development process; however, the final response depends mainly on the intensity of high-temperature and/or drought stress [39]. The adverse effects of the combination of high-temperature and drought conditions lead to a reduction in canopy mass of the potato plant, reduction in photosynthesis and water use efficiency, chlorophyll degradation (Table 1), and an acceleration of leaf senescence [40]. The average optimum daily temperature conditions for potato stolon formation and tuberization are 15–20 °C [8,27,41]. Temperatures above 22 °C have an adverse effect on vegetative, as well as on the reproductive, growth of the potato plant [33,42]. The tolerance to high-temperature stress in potato depends mainly on some critical parameters such as the genotype, developmental stage at which it is exposed, level of stress faced, and the ability to form stolons, initiate tubers, and bulk [15,30,34]. Other physiological aspects such as anatomical features (morphology of vascular bundles), total biomass production, respiration, transpiration, carbon partitioning, and the source–sink relationship are dependent on extrinsic, as well as intrinsic, factors of the potato plant [43,44,45]. Besides physiological responses, biochemical responses are also presented in Table 1.

Table 1.
Physiological and biochemical responses of potato under heat/ drought stress.
3.1. Anatomy and Morphology
The major challenge for the breeder is to make the potato suitable for the tropical climate with maintained production [27]. In this direction, when selecting and developing suitable potato cultivars for these areas, they need to be phenotyped for tolerance/resistance to various abiotic stresses [54]. The tropicalization of potatoes is mainly affected by heat and drought stresses, which are known to alter the anatomical structure and morphology of the potato plant [55,56]. The development of vascular bundles under high-temperature stress may affect the transport of nutrients and photoassimilates in the xylem and phloem, respectively [34]. It was noticed that the cultivars with a reduction and enhancement in the size of the xylem and phloem, respectively, can better withstand high-temperature conditions. The increased size of the phloem enhances the sink capacity to store more photoassimilates and convert them into starch, which ultimately increases tuber yield [34]. In the tropical climate, high temperatures lead to the development of tall plant with small leaves, thin stems, long stolons, increased internodes (elongated internodes), inhibition of tuber development, and reduction in the ratio of tuber fresh weight to total fresh weight [57].
The leaf is the primary source of potato plants that might be affected in many ways under various abiotic stresses [42]. Under heat stress (30 °C), the leaf area of the potato plant was reported to reduce by 35% compared to the control (22 °C). Several wild cultivars of potato are known to be cultivated extensively in altitudes between 2000 to 4000 m (above sea level), where the season is generally represented by long day length, high light intensity, and cooler temperatures. However, the globalization of potato was achieved by different voyagers in different parts of the world. The modern cultivars were selected over time and adopted for long-day conditions. The branching pattern of the shoot in the potato plant was also reported to be affected under high-temperature stress [30]. The growth of lateral shoot branches was affected by heat stress. The number of lateral branches and their diameter were reported to be enhanced under high-temperature stress. The canopy of the potato plant also changes under high temperatures. As the temperature rises over 23 °C, the number of axillary branches increases, resulting in more leaves and a faster rate of senescence. Recent reports suggested that the length of the main branch was reduced under heat stress, which ultimately leads to the stunted growth of the potato plant (Table 1). The change in the ratio of growth hormones such as auxin and gibberellins is responsible for developing lateral and main shoots [58]. Moreover, potato genotypes with the stay-green trait may better alleviate the adverse effects of heat and drought stresses [59].
The root is the important organ responsible for water and mineral nutrient uptake and is highly influenced by abiotic stress [60]. The average optimal temperature required for the growth of roots, tubers, and stolons varies with the growth stages of the plant [8]. The root is the first organ that senses water-deficient conditions and responds accordingly based on physiological, biochemical, and molecular phenomena. The root system architecture and its morphology in potato are significantly affected by both heat and drought stress [61]. The modification of the root architecture occurs by forming more lateral roots and root hairs [62]. High-temperature stress (33 °C) also delays adventitious root initiation in potato. Water-deficient conditions affect potato root system morphology by increasing the proliferation of lateral roots, root thickness, inhibition of root elongation, and increasing root hair formation [63]. Potato cultivars with a deep root architecture may prove beneficial in combating both drought and high-temperature stress.
3.2. Role of Photoperiod
The photoperiod substantially affects the potato’s developmental stages, from the emergence through tuber initiation phases. Short photoperiods (10–12 h) enhance the tuber initiation compared to longer photoperiods (14–18 h). Moreover, it has been previously documented that the photoperiod has little or no effect after tuber initiation. Thereafter, temperature plays a crucial role in the tuberization process [31]. Higher temperatures are inhibitory to tuberization, irrespective of photoperiod, viz., short-day and long-day conditions, although the adverse effect is much more significant in long photoperiods. Higher temperatures affect the partitioning of the assimilates by reducing the amount delivered to tubers and enhancing the content of other parts. These effects are observed in both long and short photoperiods. The longer photoperiods coupled with heat and drought stress adversities might significantly delay and reduce tuberization along with excessive vegetative growth of the haulm [31,33]. Thus, potato crops face a higher yield reduction in heat and drought stress-affected areas encountering shorter day lengths (mainly subtropical lowlands).
3.3. Carbon Partitioning and Source–Sink Relationship
Potato is an indeterminate crop with respect to its growth habit, where the vegetative growth can continue even after flowering and tuber formation; this reduces the sink ability and activity [33]. Drought and heat stress also affect the source–sink relation, carbon partitioning, and potato tuber development [64]. Environmental stress often impacts these parameters, which affects the photosynthetic rate, xylem and phloem transport, sugar metabolism, and photoassimilate diversion away from sink tissues [43,64,65]. Any stress during the early stages is particularly damaging because it decreases carbon assimilation, reduces partitioning, and ultimately impacts tuberization, bulking, and final tuber production [28]. The relationship between the source and sink can be disturbed by high-temperature stress, which further delays the process of tuberization and can result in tuber necrosis and deformities. When the temperature rises above the optimum, the photoassimilates are translocated away from the tuber [66].
As potato originated in the hills of the Andes, it has the inherent ability to tuberize in temperature ranges between 14 °C to 22 °C. Nonetheless, the tuber formation remained limited to the particular temperature (14 °C and 22 °C), and accelerated under short-day conditions [67,68]. It is now well understood that there is more shoot growth with more consumption of assimilates under high temperatures and, as a result, the tuber yield is drastically reduced [69,70]. In this context, physiologically efficient potato cultivars capable of early tuberization (even at relatively high temperatures and extended photoperiods) and a higher rate of photoassimilates being translocated to developing tubers may be beneficial. Variable photoperiods influence tuberization, which may be a micro-evolutionary indicator of the differential transduction of cell-to-cell signalling molecules under the spatial and temporal expression of regulatory genes involved in tuberization [71]. According to reports, the optimal conditions for potato tuberization are high irradiance, low temperature, and a short photoperiod. It is anticipated that regulatory genes encoding transcriptional activators or cell-to-cell communication molecules change more rapidly than structural genes. Increasingly, future studies will focus on identifying the putative regulatory elements in signal transduction pathways driving potato tuberization [72]. In the signal transduction pathway, the leaves perceive an adequate environmental cue, which is mediated by phyB and gibberellins, and then produce a systemic signal that is sent to the underground stolons to induce tuberization [73]. phyB-mediated perception of gibberellic acid (GA) response is controlled by a novel arm repeat photoperiod-responsive 1 protein (PHOR1), which is believed to be a general component of GA-signalling pathways [74,75]. Gibberellins and cytokinins are the two most important phytohormones that regulate the formation of potato tubers. GA also plays a function in the photoperiodic control of tuberization, and its endogenous levels are regulated by sucrose and abscisic acid (ABA). However, at high concentrations, it inhibits tuber induction in potato [76,77]. Efforts in this direction are necessary because they can compensate for the crop cycle being shortened due to high temperatures by utilizing the tropical region’s prevailing long photoperiod.
3.4. Tuber Development
Potato grown under long days with high temperatures may face delayed stolon and tuber initiation, with a reduction in the partitioning of photoassimilates to the developing tubers, which ultimately reduces the bulking (the size) and dry matter content (the quality) of tubers [12,78]. Potato is generally vulnerable to high-temperature stress, and the yield of the tuber is inversely proportional to the temperature. The optimal temperature for the growth and development of the haulm of potato plants is 20–25 °C, whereas the ideal temperature for tuberization and tuber growth is 15–20 °C [70]. The higher temperature during the potato growth stages leads to higher yield losses and more incidence of deterioration in the quality of tubers. High temperatures above 20 °C interfere with the partitioning of assimilates into the potato tubers, resulting in a low tuber yield. Lower tuber productivity under tropical regions with high temperatures leads to an increase in higher sugar retention in leaves, indicating that the translocation of photoassimilates is poor for the sinks (tubers) [12,79]. One of the reported reasons for this is the distorted phloem due to high temperatures, which adversely affects the source–sink relationship (mobilization of photoassimilates to tubers) [34]. Studies on tuber growth and development suggest that, under high temperatures, there is a reduction in the translocation of photoassimilates to the tubers and thus a declining production and productivity of potato. Accumulation of photoassimilates in the tuber tissues increases cell expansion and ultimately causes massive deposition of starch and storage proteins, thereby making strong storage sinks during the tuber development and bulking phases [80].
3.5. Photosynthesis
Many studies suggest a reduction in photosynthetic efficiency and tuber yield under high-temperature and drought stress [70,79,80]. In other studies, it was reported that temperatures of more than 30 °C completely inhibit photosynthesis in potato. Wahid [39] reported that photosynthesis is the most sensitive process under elevated temperatures. Under high temperatures, there is impairment in photosynthetic machinery, and other related physiological functions are often inhibited. The decrease in the photosynthesis rate caused by heat stress is associated with an increase in non-photorespiratory activities [39]. The key enzyme of carboxylation is RuBisCo, which is inhibited by high-temperature stress. Salvucci et al. [81] suggested that the reduction in the activity of RuBisCo under high-temperature stress might be due to the inhibition of Rubisco activation via a rapid and direct effect on RuBisCo activase (RCA). The heat stress leads to the denaturation and aggregation of the RCA protein, which further fails to activate Rubisco. Moreover, under heat stress, the fluidity of thylakoid increases, which leads to a reduction in the electron transport in the photosystem II (PS II) and its dislodging [78].
The investigations linking RuBisCo (ribulose-1,5-bisphosphatase) with photosynthesis reveal that, at elevated temperatures, soluble proteins such as RuBisCo and RuBisCo-binding proteins are reduced [82]. In leaves, the content of RuBisCo under elevated temperatures was found to correlate with the decrease in the photosynthetic rate of potato crop [47]. RuBisCo concentration was found to decrease under high temperatures supplemented by a corresponding decrease in its affinity for carbon dioxide. The effect of topicalization on potato could result in heat injury, protein synthesis suppression, and a loss of membrane integrity [83]. Under heat stress conditions, the respiration rate increases in proportion to the decrease in photosynthesis, and the combined result of these two processes is a decrease in net photosynthesis [84].
Carboxylation is catalyzed by ribulose bisphosphate carboxylase/oxygenase (RuBisCo), which can constitute up to 50% of the soluble protein in a leaf. Along with carboxylation, the RuBisCo protein is also involved in oxygenase activity. Heat stress can affect both carboxylation and oxygenation processes. High-temperature stress can enhance the oxygenase activity of RuBisCo, which leads to an increase in the production of H2O2, which is toxic to plant cells [85]. Meanwhile, photosynthesis was significantly affected under drought stress, mediated by the inactivity of stomatal and photosystem components [78]. Water-deficient conditions lead to a decrease in the quantum yield (Fv/Fm), electron transport rate, and photochemical quenching (Qp) [79]. In addition, the stomatal closure under water-deficient conditions reduces CO2 availability, which hampers the photosynthetic rate. The decrease in carboxylation efficiency and activity of RuBisCo was also reported under water stress conditions. Therefore, further study must be conducted with an emphasis on integrating features that can improve the efficacy of essential activities such as photosynthesis and respiration. This may be effective for the modulation of components that can also balance the source–sink features in order to achieve higher tuber yields with improved quality characteristics under tropical conditions [85].
3.6. Senescence
The leaf senescence of the plant was enhanced when exposed to heat, particularly at the time prior to or at the maturity stage of the plant, due to loss of chloroplastic integrity and chlorophyll synthesis, inhibition of PSII-mediated electron flow, and destruction of antenna pigments [86]. The recent report also suggested the same, where cultivars sensitive toward both high-temperature and water stress conditions show retarded sprout emergence, root growth, and low water potential, resulting in desiccation and early senescence [55].
3.7. Respiration
One of the critical characteristics affecting potato plant growth under heat stress conditions is mitochondrial respiration. When the temperature rises above the optimal temperature, gross photosynthesis is reduced, but normal respiration and photorespiration rates increase significantly [87,88]. The high respiration rate under elevated temperatures contributes to lowering potato yields [30,33]. Under high temperatures, the stored starch in the developing potato tuber is also utilized for respiration, contributing to a substantial decrease in the production and productivity of potato tubers [39,89]. Additionally, because of the high temperature, the rapid development pace results in a shortening of the entire growth cycle. In aggregate, the plant receives less time to collect photoassimilates, resulting in decreased yields [90].
3.8. Transpiration
Transpiration is another parameter that is strongly affected by heat and drought conditions in the potato plant. Due to rising temperatures, potato plants experience water stress as the plant’s transpiration rate increases, leading to greater demand for water from the soil. This raises the water requirement of potato crops [15]. If the water is not limiting, the high-temperature conditions result in a higher rate of CO2 assimilation, but this usually leads to a significant increase only in above-ground biomass production, with no net enhancement of photoassimilates portioned into the tubers [47]. The combination of heat and drought stress affects the transpiration rate significantly by affecting leaf area, root-to-shoot ratio, the orientation of the leaf, leaf thickness, leaf surface characteristics, and distribution of stomata on the leaf [55]. Growing potatoes in the tropical region will be challenging, where the plant will confer higher biomass production with lower tuber production due to improper allocation of photoassimilates.
4. Biochemical Changes and Responses of Potato Plants
The climate-related changes in temperate in subtropical and tropical regions have affected various biochemical events and processes in plants. The potato plant perceives abiotic stress such as heat and drought and responds dynamically by shifting its sugar and starch metabolism, ionic and osmolyte balance, synthesis of heat shock proteins, homeostasis of ROS, and other biochemical pathways [91,92,93]. Under heat and drought stress, the biochemical response includes accumulating reactive oxygen species (ROS), damage to cell membranes, electrolyte leakage, degradation of nucleic acids, and denaturation of proteins and enzymes [94]. The potato plant develops various strategies to mitigate the harmful effect of drought and heat stress to defend against damages caused by ROS and reactive nitrogen species [95]. The enzymatic (superoxide dismutase, ascorbate peroxidase, peroxidase, and catalase) and non-enzymatic (glutathione, ascorbate, polyphenols, vitamins, carotenoids) defence mechanisms effectively scavenge the ROS generated under heat and drought stress in potato [65,96]. Some of the key biochemical changes and responses in variable environments are as follows.
4.1. Carbohydrate Metabolism
The optimum temperature for photosynthesis and sugar and starch metabolism in potato leaves is about 24 °C [8]. Carbohydrate metabolism was reported to be severely affected by high-temperature stress, resembling one main condition in the tropical region [45]. During tuber development, sucrose produced by physiologically and photosynthetically active source leaves is transported to the developing tubers via the phloem [97]. The change in carbohydrate metabolism due to high-temperature exposure usually causes an increase in the levels of reducing sugars in the tubers. This ultimately affects the processing-related quality of potato tubers, as higher levels of reducing sugars adversely affect the chipping quality of processed potato products, even in processing-grade cultivars [98]. Starch synthase is the rate-limiting enzyme responsible for starch synthesis and its deposition in tubers [99]. Under high temperatures, the activity of the starch synthase enzyme decreases, resulting in a slower rate of starch deposition and a slower rate of tuber growth [99]. It was also reported that increased gibberellins due to high temperatures reduces starch synthase activity in developing tubers, preventing the sucrose from re-partitioning away from the tuber. Furthermore, enhanced levels of gibberellin synthesis due to high temperatures also influence tuber initiation and their further development [100].
During the nighttime, the plant’s sucrose is transported from the leaf and gets stored in sink tissue (tuber). Sucrose phosphate synthase (SPS) is the enzyme that controls photoassimilate partitioning by catalyzing the synthesis of sucrose, which ultimately contributes to the osmotically active driving force for phloem translocation [101]. SPS activity was increased under high temperatures in potato, whereas its activity is suppressed in tomato (>40 °C/25 °C, day/night) [53,102]. Metabolites are found to increase when potato is cultivated under high temperatures [103]. Under heat stress, hexose increases significantly in leaves and conversely decreases in tubers [47]. On the other hand, when sucrose and starch levels in the tuber were estimated, both metabolites were shown to decrease significantly in both the tuber and leaves of the potato plant under high-temperature stress. This suggests that the efficiency of sucrose-to-starch conversion in tubers is diminished as a result of decreasing sink strength [47]. Along with the metabolites, it was discovered that some hazardous compounds increased in concentration under high-temperature circumstances. For instance, the level of steroidal glycoalkaloid increased, resulting in bitterness in the tuber [104]. Plant breeders should consider this case when they develop potatoes for tropical climates.
4.2. Ionic and Osmolyte Balance
Potato plants grown in the tropical region are exposed to heat and drought stress, causing certain organic compounds of low molecular mass to accumulate. These compounds are referred to as compatible osmolytes [105,106]. In abiotic stress conditions, the potato plant accumulates various osmolytes such as sugar, sugar alcohol (mannitol and sorbitol), proline, and glycine betaine, which also confers tolerance to heat and drought stress [53,63]. They all help maintain the osmotic balance of the cell. The presence of compatible osmolytes helps cope with the high-temperature and drought stress by scavenging ROS generated due to stressful conditions [107]. Furthermore, these osmolytes also stabilize the protein structure, acting like molecular chaperones [108].
Glycine betaine is a soluble-compatible solute that plays an essential role in tolerance against high-temperature stress [109]. A higher concentration of glycine betaine was reported in a heat-tolerant cultivar of potato (Kufri Surya) compared to susceptible cultivars such as Kufri Chipsona 3 [53]. Accumulation of proline leads to protein stabilization. Due to its metal chelator properties, it acts as a molecular chaperone or chemical protein chaperone. It also acts as an antioxidative defense molecule that scavenges reactive oxygen species (ROS) and has signaling behavior to activate specific gene functions that are crucial for plant recovery from abiotic stresses [110,111]. Additionally, it acts as a supply of carbon, nitrogen, and energy during periods of water-deficient conditions [49]. As a result, biosynthesis of osmolytes is controlled, especially during times of stress, so that they can help plants grow and develop in the tropical climate.
4.3. Heat Shock Proteins (HSPs)
Heat shock proteins (HSPs) are major proteins which act as molecular chaperones and are the key components responsible for protein folding, assembly, translocation, and degradation under abiotic stress conditions [112]. As chaperones, these proteins prevent the irreversible aggregation of other proteins and play a role in the refolding of proteins under heat stress conditions [113]. The synthesis and accumulation of HSPs under heat stress in the plant have been shown to provide tolerance against high-temperature stress [114]. HSPs are categorized into six different families based on their molecular weight and sequence homology, which include Hsp110 (>100 kDa), Hsp100 (90–100 kDa), Hsp90 (80–90 kDa), Hsp70 (66–78 kDa), Hsp60 (50–60 kDa), and Hsp20 (15–39 kDa) [115]. Hsp20s in potatoes (StHsp20) are sensitive and positively regulated under heat stress and they provide thermotolerance to potato plants [50]. Hsp90s are reported as a highly conserved molecular chaperone among all the HSPs, where it is distributed in the cytoplasm, chloroplasts, and mitochondria [116]. Earlier reports revealed that heat shock cognate 70 (HSc70) expression was increased in the Désirée cultivar of potato and positively correlated with the improved tolerance and tuber yield in heat stress conditions [51]. Savić et al. [117] suggested that electrolyte leakage assay in combination with immunoblot measurement of HSP accumulation under heat stress conditions could be a reliable method for screening potato genotypes for heat tolerance and identifying heat-tolerant potato cultivars. Other reports also suggest that the exogenous application of phytohormones such as salicylic acid in potato microplants showed a higher expression of HSPs that provide tolerance to high-temperature stress [118]. Thus, HSPs can play a significant role in maintaining the growth and development of potato plants and tubers under tropical and subtropical conditions. Therefore, this area holds promising aspects with respect to the ability of potato plants to cope with high-temperature stress.
4.4. ROS, RNS, RSS, and Antioxidant System
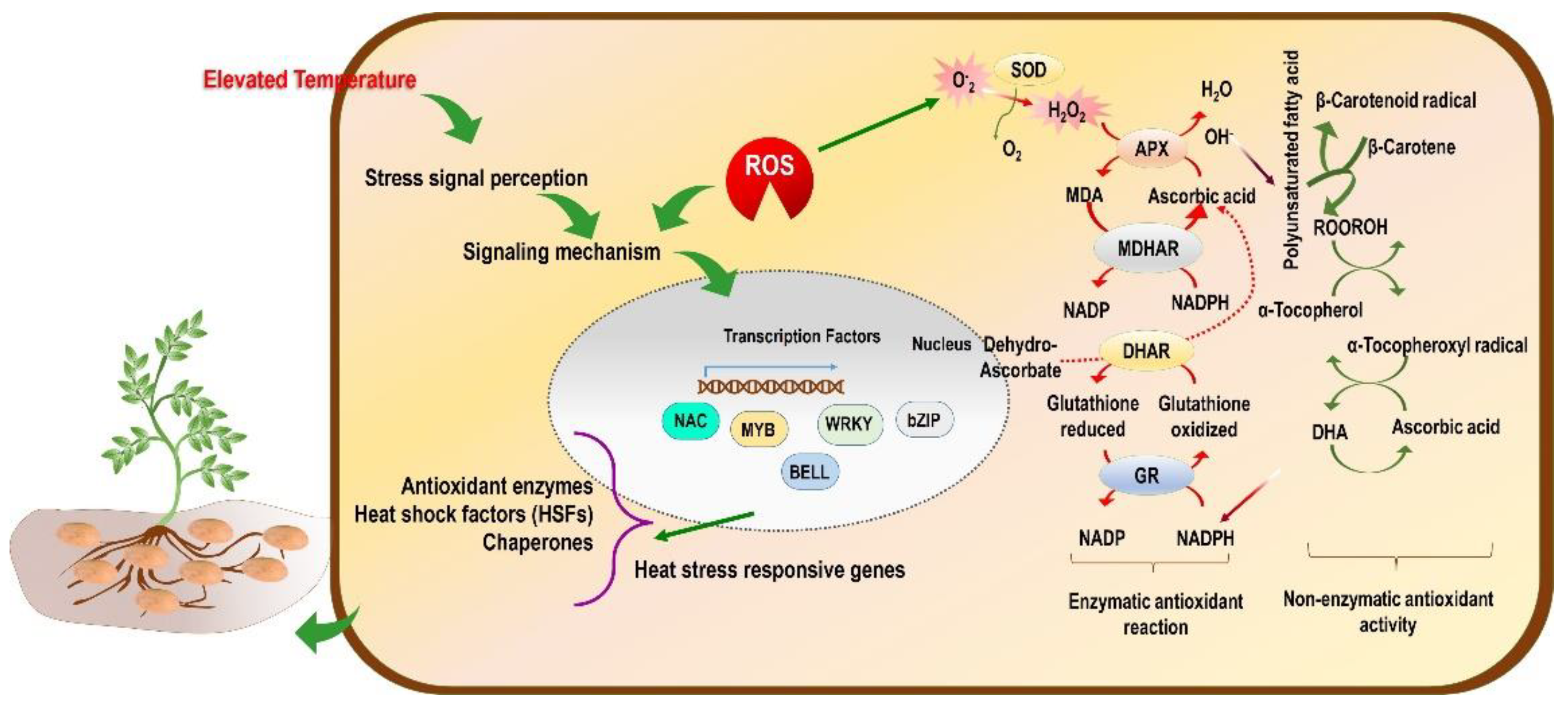
Under heat stress, reactive oxygen species (ROS) accumulate, which causes severe oxidative damage to the plant, thus inhibiting growth and development-related activities [119]. When ROS production increases more than the cellular scavenging capacity, there is an imbalance in redox homeostasis, resulting in more membrane damage (Figure 2). Consequently, more electrolytes are leaked and disrupt the functioning of the cell [40]. Besides the ROS, there are other reactive molecules which originate from other elements, such as nitrogen and sulphur, and they are referred to as reactive nitrogen species (RNS) [120] and reactive sulphur species (RSS) [121]. Overall, the reactive species viz. ROS, RNS, and RSS cause oxidative stress-like conditions in the plant or plant part.
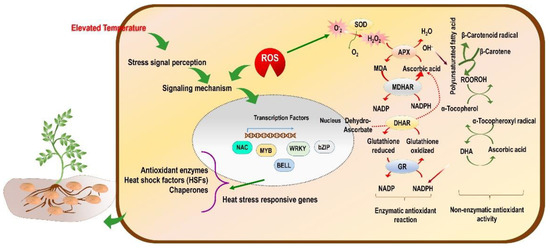
Figure 2.
Conceptual model of the effect of high-temperature stress (tropicalization) on molecular mechanisms and ROS-mediated regulation of enzymatic and non-enzymatic antioxidant activity in potato plants.
The ROS include hydroperoxyl radical (HO2•), superoxide anion (O2•−), alkoxy radical (RO•), and hydroxyl radical (•OH), and also non-radical molecules such as hydrogen peroxide (H2O2) and singlet oxygen(1O2). Likewise, RNS include nitric oxide (NO) [120] and RSS include thiol, disulphide, sulfenic acid, thiosulfinate, and thiosulfonate [122]. They also act as potential signalling molecules under oxidative stress conditions. The enzymatic components are involved in scavenging different types of reactive species formed in the plant under drought and heat stress conditions [120,123]. The antioxidant enzymes such as SOD, CAT, and APX were reported to increase significantly under heat and drought stress conditions (Table 1). The potato plant can cope with the increased levels of reactive species in the cellular system [55,124]. In addition to this, it was reported by Arora et al. [125] that O2•− can react with NO, and this leads to the production of peroxynitrite (ONOO–), which is a powerful oxidant and is involved in post-translational modification of protein through tyrosine nitration. Researchers need to decipher whether the mitigation of oxidative stress is caused due to ROS, RNS, and RSS in potato grown in non-conventional tropical areas. This strategy will help design potato plants that are more suitable and adaptable to the new environment.
5. Approaches for Adaptation of Potato Plant
Potato plants have insufficient abiotic stress tolerance due to the limited genetic diversity of present potato cultivars [126]. Thus, in order to withstand varied environmental stressors, the current commercial cultivars must be modified accordingly. However, little is known about the distinct molecular pathways involved in potato tuberization in response to heat stress. Understanding the role and network of different genes involved in heat stress resistance requires a full characterization from an agrobiotechnology standpoint. Under a changing climate, sustainable potato production necessitates an interdisciplinary strategy that includes innovative technologies, molecular biology, agronomy, breeding, and agrometeorology.
5.1. Agrometeorology-Based Crop Modelling and Agronomic Practices
With the advances in climatology and information technology, it is now simple to analyze genotypes by environmental interactions and create crop models [127]. A comprehensive model of the growth and development of potatoes called LINTUL-POTATO was published in 1994. The mechanistic model recreated the early stages of crop development, such as emergence and leaf growth, as well as the process of light absorption, which continued until extinction [128]. The aforementioned model was enhanced and improved with unique computations to investigate tuber quality features such as tuber size distribution and dry matter concentration in relation to crop environment and management, known as LINTUL-POTATO-DSS [127]. Statistical analysis and records of weather trends may be able to assist us in locating new places that are ideally suited for potato cultivation. Similarly, the DSSAT SUBSTOR-Potato model [129] was used to simulate potato tuber yield in Indian conditions, suggesting that, if planting is postponed beyond November, all of the cultivars in the experiment (Kufri Jyoti, Kufri Pukhraj, and Kufri Himalini) are likely to see a significant decrease in tuber production [130,131]. Out of these cultivars, Kufri Pukhraj was projected to remain a viable cultivar under subtropical conditions until 2050 and can be planted until the first week of December. Modulating potato planting time by using different cropping models might be a strategy to understand the exact date of sowing in tropical areas.
Potato cultivation under changing climatic scenarios requires improved potato cultivation techniques. Appropriate agronomic practices such as planting date, soil management, tillage, mulching, irrigation, intercropping, and superior genotypes tolerant to high temperature and drought can contribute to higher yields [49,132]. Aeration, infiltration, and nutrient uptake are all things that can be improved by tilling the soil. The soil’s moisture and temperature are directly related to the yield and quality of potatoes, and these factors can be controlled by the use of organic and biodegradable mulches [133]. Rykaczewska [134] demonstrated that two weeks of heat and drought stress during the flowering phase of potato plants can diminish their yield by more than 35%, which is also a factor for the development of secondary tuberization. Due to the shallow nature of the root architecture, potato plants are susceptible to drought [135]. Therefore, improved irrigation techniques such as drip irrigation can increase potato output and quality traits under abiotic stress. Moreover, plants exposed to moderate temperatures over a brief period of time can build a memory for stress tolerance, allowing them to live at temperatures where normal plants cannot, this process is known as acquired thermotolerance [51]. The acclimatization of potato plants to 25 °C for two hours is connected with changes in the expression of several heat shock proteins, modifications to the cell wall, abnormalities in hormonal signalling, and chromatin remodelling. Later, when these plants were exposed to 40 °C, their growth was superior to that of control plants [51]. In addition to the adaptation, which includes the development of heat-tolerant cultivars, adjusting plant and harvesting time might help to shift the location of where potato production will be higher. In certain tropical highland regions, potato growing is limited to higher zones (Puna and Pramo zones of Andes; Nilgiri hills of southern India) [27,136].
5.2. Exploring Genetic Diversity and Breeding
Due to the fact that thermotolerance is a multigenic trait, it is imperative that appropriate methods be utilized in order to evaluate the genetic diversity in both inherent and acquired tolerance [136,137]. Due to their resilience to pests and diseases and their ability to adapt to harsh climates, wild potato species are of great interest to potato breeders [138]. Wild potato species inhabit a wide variety of settings and could be anticipated to have varying genetic degrees of stress tolerance [137]. Several wild potato species can withstand high temperatures, including S. kurtzianum, S. sogarandinum, S. chacoense, S. stoloniferum, S. demissum, and S. berthaultii, and these wild potato species can be utilized in breeding programs to create heat-tolerant lines [139]. The selection of superior phenotypes or parents is the most important phase in breeding schemes for crop improvement. According to Rykaczewska [134], the impacts of heat stress are more detrimental during the early growth phases. Nevertheless, with the recurrent method of selection, the frequency of desirable alleles can be increased by selecting the superior genotypes over the base population. Knowledge of the molecular pathways involved in heat tolerance can serve as a foundation for producing high-yielding, stress-tolerant cultivars through molecular breeding, transgenic, and genome-editing techniques.
5.3. Molecular and Transgenic Approaches
A complex biochemical pathway and gene network contributes to the plant’s tolerance to drought and heat stress. Most significantly, tuberization is impaired considerably in potatoes when heat and drought stress are combined, which is mediated by various transcription factors [140]. The molecular network of various transcription factors, hormones, and signalling molecules is involved in coping with climatic conditions and changes when potato plants grow under tropical conditions [55]. The increased expression of starch-degrading genes under drought and heat stress is one of the detrimental factors involved in the degradation of potato tuber quality in terms of carbohydrate metabolism [46]. The tuberization signal in potato is highly dependent on StSP6A, an orthologue of the Arabidopsis protein FLOWERING LOCUS T (FT). StSP6A is highly regulated by elevated temperatures, and it also affects the accumulation of photoassimilates in the tuber in the form of starch [141]. The tropicalization of potato will involve various interactions, including at the gene level. The future implications of the interactions that can assist in coping with the harsh environment and resist the negative impact of such an environment need the attention of researchers.
The transgenics approach has been used to develop tolerance against drought and heat stress [40]. The genes such as C-repeat Binding Factors/Dehydration-responsive element binding (CBF/DREB) play an essential role in signal transduction and gene expression under heat and drought stress in potato plants [52]. They reported that overexpression of the ScCBFI gene in transgenic Solanum tuberosum from Solanum commersonii results in better root development and plant growth under water-deficient conditions (Table 2). Targeting such genes in potato plants can prove beneficial in terms of making the potato plant suitable for growing in tropical and subtropical regions with minimum effect on its production and productivity. Other reports on transgenic potatoes showed an increase in the level of ascorbate in the plant, providing tolerance against water and salt stress. The tolerance was achieved by overexpression of the Arabidopsis thaliana Dehydroascorbate Reductase gene (AtDHAR1) in the transgenic potato plant, with 4.5 times more DHAR activity in comparison to the wild type [142].

Table 2.
Role of the different genes influencing various traits during the process of subtropicalization.
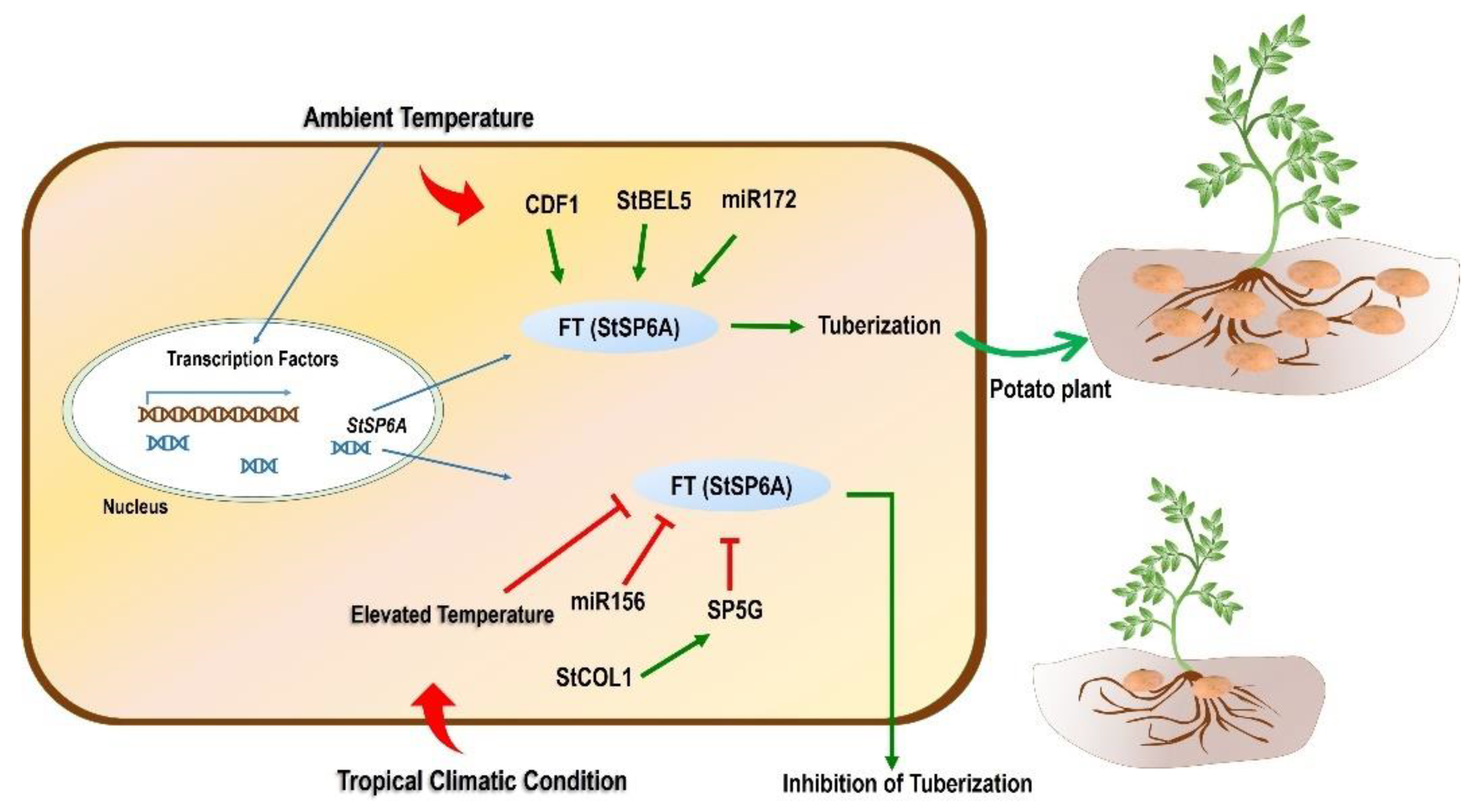
As discussed earlier in this section, SP6A is an essential gene that is involved in temperature-mediated tuberization in potato. The favorable condition triggers the process of tuberization by perceiving the signal (StSP6A) from leaves and transporting it to stolons. The tuberization was reported to be suppressed when StSP6A was silenced using RNAi-based downregulation of this gene, while its upregulation promoted the tuberization [141]. Kloosterman et al. [148] identified CYCLING DOF FACTOR 1 (CDF1), a transcription factor required for the maturity and initiation of tuber development under long-day conditions. CDF1 was found to involve direct regulation of SP6A expression. An illustration related to the regulation of the SP6A gene is presented and described in Figure 3.
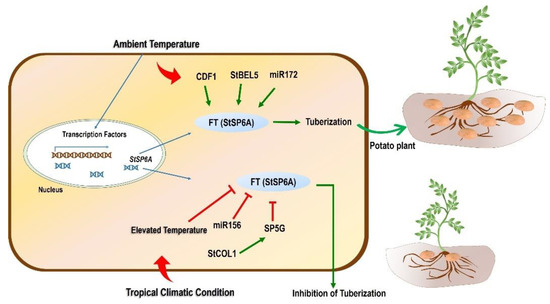
Figure 3.
Schematic model of the effect of tropicalization on tuberization. Temperature plays an important role in the expression of essential genes responsible for tuberization. The master regulator for tuberization in potato is the FT(StSP6A) gene responsible for tuberization under normal/ambient temperature conditions. Different TFs and miRNA also control the process of tuberization through an StSP6A-mediated mechanism. TFs such as CDF1 and StBEL5 upregulate the expression of StSP6A, whereas miR172 also upregulates this gene, leading to tuberization. However, when the potato is grown in tropical conditions and is subjected to high temperatures, tuberization is inhibited. An FT family member, SP5G downregulates the expression of the StSP6A gene. The former SP5G is reported to be regulated positively by StCOL1 expression. Moreover, miR156 also negatively controls the expression of StSP6A, which ultimately inhibits tuberization. CONSTANS-LIKE protein 1: StCOL1; CYCLING DOF FACTOR 1: StCDF1; BELLRINGER-1 LIKE 5: StBEL5.
TFs such as WRKY, AP2, NAC, DREB, bZIP, and BELL are involved in the activation/inactivation of various metabolic pathways, plant hormones, and transcriptional and post-transcriptional regulation under heat and drought stress (Figure 2; Table 2). In potato plants, 70 and 21 TFs were differentially expressed after short (6 h) and prolonged (3 days) exposure to heat, respectively [103]. Under short heat exposure, 14 TFs were upregulated and 56 TFs were downregulated significantly, whereas prolonged heat exposure to potato plants showed upregulation and downregulation of 7 and 14 TFs, respectively. The analysis of TFs and the network of the genes in potato helps to gain insight into the molecular level, working in terms of mechanisms and responses under heat and drought stress conditions. These parameters mentioned above help to provide an essential knowledge base for designing potato breeding programs and strategies suitable for tropical and other non-conventional areas on the globe.
5.4. CRISPR Approach for Molecular Insight and Functional Gene Analysis
Plant genome editing (GE) has advanced rapidly in recent years, opening up new opportunities and intriguing possibilities for both fundamental research and plant breeding. The type II CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9)-mediated GE system from Streptococcus pyogenes has been widely used by plant scientists. It consists of two parts: the RNA-guided DNA endonuclease SpCas9 and a single-guide RNA (sgRNA) [149]. It looks for a 5′-NGG-3′ PAM (protospacer-adjacent motif) in the genome, which triggers melting of nearby DNA and a search for a spacer sequence that matches the 5′ end of a sgRNA. This results in a double-stranded DNA break (DSB) about 3bp upstream of the PAM, which is caused by the combined activity of the RuvC and HNH endonuclease domains [150]. NHEJ (Non-homologous end joining) DNA repair is activated upon a DSB, which can lead to small indels at the breaking site, which results in gene knockout through frameshift mutations [151]. While most research has focused on the generation of loss-of-function alleles, novel CRISPR tools, such as the CRISPR-mediated base-editing system, which allow precise base conversion without the need for donor DNA or the development of a DSB, have recently been created [152]. There have so far been two types of base editors (BEs) discovered and developed: adenine base editors (ABEs) and cytosine base editors (CBEs). Both of these types of base editors (BEs) are composed of an RNA-independent Cas9 with impaired DNA cleavage activity, typically nCas9 (nickase Cas9) for plant crop applications, and a catalytic domain associated with adenine or cytosine deamination, respectively [153,154].
GE has a lot of potential for improving crops, but it has not been fully realized in clonally propagated tetraploids such as the potato, despite its widespread use. In potatoes, CRISPR/Cas experiments have increased tuber starch quality, carotenoid biosynthesis, glycoalkaloids, and enzymatic browning [155,156,157]. Functional mutants were generated to investigate phenotypic and herbicide tolerance differences [158]. Furthermore, the efficacy of Cas9 prime editing and base-editing tools for tolerance to herbicides in potato has been successfully established by researchers [159,160]. Additionally, self-compatible regenerants were generated via Agrobacterium or virus-induced genome editing using Cas9 (VIGE) [161,162]. However, there are several challenges to CRISPR implementation in potato.
Only a few potato cultivars are responsive to transformation; others must be examined in tissue culture for regeneration and transformation. Protoplast transformation and regeneration from leaf protoplasts might result in somaclonal variation, which can negatively influence crop growth and development. Transformation techniques such as agrobacterium, biolistic or particle bombardment, and the floral-dip method have been used regularly in potato [163]. For CRISPR/Cas in potato, sgRNA dicot-origin promoters such as Arabidopsis (AtUp)/potato (StU6p)/U3p and plant promoters such as CaMV 35S are the most often used in protoplasts and Agrobacterium-mediated transformation [164]. Furthermore, the genetically complex tetraploid potato crop, on the other hand, makes it challenging to utilize the A. tumefaciens-mediated technique to deliver ribonucleoprotein (RNP) complexes and to remove Cas9 from the crop genome through backcrossing or selfing [165]. Potato breeders are actively striving to re-invent the crop to speed up progress in understanding the complex genetic characteristics such as quality, yield, and tolerance to stress. CRISPR/Cas-mediated GE will be a game changer in terms of genetic improvement, opening up new opportunities for developing a stronger potato breeding pipeline.
6. Conclusions
The potato plant is vulnerable to high-temperature and water-deficient conditions. Therefore, the high-temperature and drought stress in potato growth and development lead to alterations in morphological, physiological, biochemical, and molecular responses. Tuberization is one physiological process that is adversely affected by high-temperature and water-deficient conditions. In addition, there is a change in shoot growth, reduction in the root-to-shoot ratio, and increase in the incident of senescence. The biochemical changes in potato plants under tropical conditions include accumulation of osmolytes such as proline and glycine betaine, decreased starch accumulation in tubers, increased reducing sugar content, synthesis of HSPs, generation of ROS, and activation of various protective and scavenging mechanisms. The network of genes and TFs are involved in response to stressful conditions at the molecular level. Tuberization is a complex multigenic trait induced under short-day and low-temperature conditions via the interactions of various hormones, genes, and TFs. Various TFs such as WRKY, NAC, DREB, ERF, and others play a role in coping with the heat and drought stress. This role of the StSP6A gene is very important, as it is responsible for tuberization. Moreover, its expression is temperature-dependent. These aforementioned traits and genes need to be explored by physiologists and breeders to develop tolerant cultivars of potato. The selection of early maturing germplasm is also crucial in breeding programs of potato for short-day conditions. The use of agrometeorology-based crop modelling and agronomic practices might be useful under climate change and for particular areas where the potato crops are grown. Exploring several wild potato species and their genetic diversity with regard to which can withstand high temperatures and drought can be utilized in breeding programs to create heat and drought-tolerant lines. Modern genomic tools to develop climate-resilient, transgenic, and non-transgenic cultivars might be employed for enhanced drought and heat stress tolerance. Various approaches and their integration are the best possible way to pave the way forward to enhance the production and productivity in the developing nations of tropical regions. These strategies will also be a step forward in fulfilling global food and nutritional security.
Author Contributions
M.K.L. and R.K.T.: Conceived and designed the article, manuscript writing. D.K., S.S.C., P.R., R.K. and A.D.: wrote the draft manuscript. A.J., S.M. and S.D.: provided conceptual diagrams and tables. S.K.L., A.K., V.P., M.P.S. and B.S.: reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable as no new data were generated or analyzed during this study.
Acknowledgments
The authors also acknowledge the Director, ICAR-CPRI, Shimla, for providing constant support, assistance, and guidance.
Conflicts of Interest
The authors have no conflict of interest to declare relevant to this article’s content.
References
- FAOSTAT. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 14 March 2019).
- Lal, M.K.; Kumar, A.; Kardile, H.B.; Raigond, P.; Changan, S.S.; Thakur, N.; Dutt, S.; Tiwari, R.K.; Chourasia, K.N.; Kumar, D.; et al. Biofortification of Vegetables. Adv. Agri-Food Biotechnol. 2020, 105–129. [Google Scholar] [CrossRef]
- Lutaladio, N.B.; Castaldi, L. Potato: The Hidden Treasure. J. Food Compos. Anal. 2009, 22, 491–493. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of Emerging Implications of Climate Change on Food Production Systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- Stadel, C. Cultivated Landscapes of Native Amazonia and the Andes; Oxford University Press: Oxford, MI, USA, 2005; Volume 25. [Google Scholar]
- Beales, H.L.; Salaman, R.N. The History and Social Influence of the Potato; Cambridge University Press: Cambridge, UK, 1950; Volume 1. [Google Scholar]
- Hijmans, R.J. Global Distribution of the Potato Crop. Am. J. Potato Res. 2001, 78, 403–412. [Google Scholar] [CrossRef]
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kawar, P.G.; Patil, V.U.; Kardile, H.B. Key Players Associated with Tuberization in Potato: Potential Candidates for Genetic Engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.L. The Vegetabe Crops Agribusiness in Brazil: Current Status and Future Trends. In Proceedings of the Embrapa Hortaliças-Resumo em Anais de Congresso (ALICE), Embrapa, Brazil, 2012. [Google Scholar]
- Staiger, C. International Year of the Potato. Pharm. J. 2008, 281, 743–744. [Google Scholar]
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer International Publishing: New York, NY, USA, 2019; pp. 3–35. ISBN 9783030286835. [Google Scholar]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging Roles of Melatonin in Mitigating Abiotic and Biotic Stresses of Horticultural Crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Abiotic Stress Effects on Performance of Horticultural Crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Muthoni, J.; Kabira, J.N. Potato Production in the Hot Tropical Areas of Africa: Progress Made in Breeding for Heat Tolerance. J. Agric. Sci. 2015, 7, 220–227. [Google Scholar] [CrossRef]
- Hancock, R.D.; Morris, W.L.; Ducreux, L.J.M.; Morris, J.A.; Usman, M.; Verrall, S.R.; Fuller, J.; Simpson, C.G.; Zhang, R.; Hedley, P.E.; et al. Physiological, Biochemical and Molecular Responses of the Potato (Solanum Tuberosum L.) Plant to Moderately Elevated Temperature. Plant Cell Environ. 2014, 37, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J. The Effect of Climate Change on Global Potato Production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Li, X.Q. Physiological and Growth Responses of Potato Cultivars to Heat Stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Battisti, D.S.; Naylor, R.L. Historical Warnings of Future Food Insecurity with Unprecedented Seasonal Heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Longmei, N.; Gill, G.K.; Zaidi, P.H.; Kumar, R.; Nair, S.K.; Hindu, V.; Vinayan, M.T.; Vikal, Y. Genome Wide Association Mapping for Heat Tolerance in Sub-Tropical Maize. BMC Genom. 2021, 22, 154. [Google Scholar] [CrossRef]
- Singh, B.; Kukreja, S.; Goutam, U. Impact of Heat Stress on Potato (Solanum Tuberosum L.): Present Scenario and Future Opportunities. J. Hortic. Sci. Biotechnol. 2020, 95, 407–424. [Google Scholar] [CrossRef]
- Steyn, J.M.; du Plessis, H.F.; Fourie, P.; Hammes, P.S. Yield Response of Potato Genotypes to Different Soil Water Regimes in Contrasting Seasons of a Subtropical Climate. Potato Res. 1998, 41, 239–254. [Google Scholar] [CrossRef]
- Bonierbale, M.W.; Amoros, W.R.; Salas, E.; de Jong, W. Potato Breeding. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2019; pp. 163–217. ISBN 9783030286835. [Google Scholar]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Mangal, V.; Altaf, M.A.; Sharma, S.; Singh, B.; Kumar, M. Insight into Melatonin-Mediated Response and Signaling in the Regulation of Plant Defense under Biotic Stress. Plant Mol. Biol. 2021, 1, 385–399. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and Molecular Insights on Wheat Responses to Heat Stress. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef]
- Lal, M.K.; Sharma, N.; Adavi, S.B.; Sharma, E.; Altaf, M.A.; Tiwari, R.K.; Kumar, R.; Kumar, A.; Dey, A.; Paul, V.; et al. From Source to Sink: Mechanistic Insight of Photoassimilates Synthesis and Partitioning under High Temperature and Elevated [CO2]. Plant Mol. Biol. 2022, 1, 1–20. [Google Scholar] [CrossRef]
- Peng, J.; Manevski, K.; Kørup, K.; Larsen, R.; Zhou, Z.; Andersen, M.N. Environmental Constraints to Net Primary Productivity at Northern Latitudes: A Study across Scales of Radiation Interception and Biomass Production of Potato. Int. J. Appl. Earth Obs. Geoinf. 2021, 94, 102232. [Google Scholar] [CrossRef]
- Ávila-Valdés, A.; Quinet, M.; Lutts, S.; Martínez, J.P.; Lizana, X.C. Tuber Yield and Quality Responses of Potato to Moderate Temperature Increase during Tuber Bulking under Two Water Availability Scenarios. Field Crops Res. 2020, 251, 107786. [Google Scholar] [CrossRef]
- Lizana, X.C.; Avila, A.; Tolaba, A.; Martinez, J.P. Field Responses of Potato to Increased Temperature during Tuber Bulking: Projection for Climate Change Scenarios, at High-Yield Environments of Southern Chile. Agric. Meteorol. 2017, 239, 192–201. [Google Scholar] [CrossRef]
- Obiero, C.O.; Milroy, S.P.; Bell, R.W. Importance of Whole Plant Dry Matter Dynamics for Potato (Solanum Tuberosum L.) Tuber Yield Response to an Episode of High Temperature. Environ. Exp. Bot. 2019, 162, 560–571. [Google Scholar] [CrossRef]
- Haverkort, A.J. Ecology of Potato Cropping Systems in Relation to Latitude and Altitude. Agric. Syst. 1990, 32, 251–272. [Google Scholar] [CrossRef]
- Cantore, V.; Wassar, F.; Yamaç, S.S.; Sellami, M.H.; Albrizio, R.; Stellacci, A.M.; Todorovic, M. Yield and Water Use Efficiency of Early Potato Grown under Different Irrigation Regimes. Int. J. Plant Prod. 2014, 8, 409–428. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Timlin, D.J.; Reddy, V.R. Temperature Influence on Potato Leaf and Branch Distribution and on Canopy Photosynthetic Rate. Agron. J. 2006, 98, 1442–1452. [Google Scholar] [CrossRef]
- Paul, S.; Das, M.K.; Baishya, P.; Ramteke, A.; Farooq, M.; Baroowa, B.; Sunkar, R.; Gogoi, N. Effect of High Temperature on Yield Associated Parameters and Vascular Bundle Development in Five Potato Cultivars. Sci. Hortic. 2017, 225, 134–140. [Google Scholar] [CrossRef]
- Adhikari, U.; Nejadhashemi, A.P.; Woznicki, S.A. Climate Change and Eastern Africa: A Review of Impact on Major Crops. Food Energy Secur. 2015, 4, 110–132. [Google Scholar] [CrossRef]
- Jarvis, A.; Ramirez-Villegas, J.; Campo, B.V.H.; Navarro-Racines, C. Is Cassava the Answer to African Climate Change Adaptation? Trop. Plant Biol. 2012, 5, 9–29. [Google Scholar] [CrossRef]
- Hill, D.; Nelson, D.; Hammond, J.; Bell, L. Morphophysiology of Potato (Solanum Tuberosum) in Response to Drought Stress: Paving the Way Forward. Front. Plant Sci. 2021, 11, 2258. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Hareau, G.; Wolf, J. Climate Change Impact on Global Potato Production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Wahid, A. Physiological Implications of Metabolite Biosynthesis for Net Assimilation and Heat-Stress Tolerance of Sugarcane (Saccharum Officinarum) Sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Handayani, T.; Watanabe, K. The Combination of Drought and Heat Stress Has a Greater Effect on Potato Plants than Single Stresses. Plant Soil Environ. 2020, 66, 175–182. [Google Scholar] [CrossRef]
- Muthoni, J.; Shimelis, H. Heat and Drought Stress and Their Implications on Potato Production under Dry African Tropics. Aust J. Crop Sci. 2020, 14, 1405–1414. [Google Scholar] [CrossRef]
- Timlin, D.; Rahman, S.M.L.; Baker, J.; Reddy, V.R.; Fleisher, D.; Quebedeaux, B. Whole Plant Photosynthesis, Development, and Carbon Partitioning in Potato as a Function of Temperature. Agron. J. 2006, 98, 1195–1203. [Google Scholar] [CrossRef]
- Aliche, E.B.; Prusova-Bourke, A.; Ruiz-Sanchez, M.; Oortwijn, M.; Gerkema, E.; van As, H.; Visser, R.G.F.; van der Linden, C.G. Morphological and Physiological Responses of the Potato Stem Transport Tissues to Dehydration Stress. Planta 2020, 251, 45. [Google Scholar] [CrossRef]
- Singh, J.; Pandey, P.; James, D.; Chandrasekhar, K.; Achary, V.M.M.; Kaul, T.; Tripathy, B.C.; Reddy, M.K. Enhancing C3 Photosynthesis: An Outlook on Feasible Interventions for Crop Improvement. Plant Biotechnol. J. 2014, 12, 1217–1230. [Google Scholar] [CrossRef]
- Lafta, A.M.; Lorenzen, J.H. Effect of High Temperature on Plant Growth and Carbohydrate Metabolism in Potato. Plant Physiol. 1995, 109, 637–643. [Google Scholar] [CrossRef]
- Schafleitner, R.; Gutierrez Rosales, R.O.; Gaudin, A.; Alvarado Aliaga, C.A.; Martinez, G.N.; Tincopa Marca, L.R.; Bolivar, L.A.; Delgado, F.M.; Simon, R.; Bonierbale, M. Capturing Candidate Drought Tolerance Traits in Two Native Andean Potato Clones by Transcription Profiling of Field Grown Plants under Water Stress. Plant Physiol. Biochem. 2007, 45, 673–690. [Google Scholar] [CrossRef]
- Hastilestari, B.R.; Lorenz, J.; Reid, S.; Hofmann, J.; Pscheidt, D.; Sonnewald, U.; Sonnewald, S. Deciphering Source and Sink Responses of Potato Plants (Solanum Tuberosum L.) to Elevated Temperatures. Plant Cell Environ. 2018, 41, 2600–2616. [Google Scholar] [CrossRef]
- Paul, S.; Bose, I.; Gogoi, N. Morphophysiological Responses: Criteria for Screening Heat Tolerance in Potato. Curr. Sci. 2016, 111, 1226–1231. [Google Scholar] [CrossRef]
- Monneveux, P.; Ramírez, D.A.; Pino, M.T. Drought Tolerance in Potato (S. Tuberosum L.). Can We Learn from Drought Tolerance Research in Cereals? Plant Sci. 2013, 205–206, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-Wide Analysis of the Potato Hsp20 Gene Family: Identification, Genomic Organization and Expression Profiles in Response to Heat Stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Trapero-Mozos, A.; Morris, W.L.; Ducreux, L.J.M.; McLean, K.; Stephens, J.; Torrance, L.; Bryan, G.J.; Hancock, R.D.; Taylor, M.A. Engineering Heat Tolerance in Potato by Temperature-Dependent Expression of a Specific Allele of HEAT-SHOCK COGNATE 70. Plant Biotechnol. J. 2018, 16, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.T.; Ávila, A.; Molina, A.; Jeknic, Z.; Chen, T.H.H. Enhanced in Vitro Drought Tolerance of Solanum Tuberosum and Solanum Commersonii Plants Overexpressing the ScCBF1 Gene. Cienc. Investig. Agrar. 2013, 40, 171–184. [Google Scholar] [CrossRef]
- Aien, A.; Khetarpal, S.; Singh, M.P. Higher glycinebetaine and antioxidant enzymes activity are associated with high temperature tolerance in potato investigating rice resilience to high Night Temperature and Elevated CO2 Under Warming Climate View Project National Innovations in Climate Res. Indian J. Plant Physiol. 2011, 16, 285. [Google Scholar]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.M.; Goloubinoff, P. The Heat Shock Response in Moss Plants Is Regulated by Specific Calcium-Permeable Channels in the Plasma Membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with Drought: Stress and Adaptive Responses in Potato and Perspectives for Improvement. Front. Plant Sci. 2015, 6, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Farooq, M.; Gogoi, N. Influence of High Temperature on Carbon Assimilation, Enzymatic Antioxidants and Tuber Yield of Different Potato Cultivars. Russ. J. Plant Physiol. 2016, 63, 339–345. [Google Scholar] [CrossRef]
- Minhas, J.S. Potato: Production Strategies under Abiotic Stress. In Improving Crop Resistance to Abiotic Stress; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 2, pp. 1155–1167. ISBN 9783527328406. [Google Scholar]
- Kumlay, A.M. Combination of the Auxins NAA, IBA, and IAA with GA3 Improves the Commercial Seed-Tuber Production of Potato (Solanum Tuberosum L.) under in Vitro Conditions. Biomed. Res. Int. 2014, 2014, 439259. [Google Scholar] [CrossRef]
- Rolando, J.L.; Ramírez, D.A.; Yactayo, W.; Monneveux, P.; Quiroz, R. Leaf Greenness as a Drought Tolerance Related Trait in Potato (Solanum Tuberosum L.). Environ. Exp. Bot. 2015, 110, 27–35. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ahmad, P. Role of Mineral Nutrients in Abiotic Stress Tolerance: Revisiting the Associated Signaling Mechanisms. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–285. ISBN 9780128164518. [Google Scholar]
- Joshi, R.; Karan, R.; Singla-Pareek, S.L.; Pareek, A. Ectopic Expression of Pokkali Phosphoglycerate Kinase-2 (OsPGK2-P) Improves Yield in Tobacco Plants under Salinity Stress. Plant Cell Rep. 2016, 35, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Nibau, C.; Gibbs, D.J.; Coates, J.C. Branching out in New Directions: The Control of Root Architecture by Lateral Root Formation. New Phytol. 2008, 179, 595–614. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; Taylor, M.A.; Dodd, I.C.; White, P.J. Climate Change and Consequences for Potato Production: A Review of Tolerance to Emerging Abiotic Stress. Potato Res. 2017, 60, 239–268. [Google Scholar] [CrossRef]
- Viola, R. Roberts a G, Haupt S, Gazzani S, Hancock RD, Marmiroli N, Machray GC, Oparka KJ. Tuberization in Potato Involves a Switch from Apoplastic to Symplastic Phloem Unloading. Plant Cell 2001, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Ahsan Altaf, M.; Shahid, R.; Kumar, R.; Mohsin Altaf, M.; Kumar, A.; Ullah Khan, L.; Saqib, M.; Azher Nawaz, M.; Saddiq, B.; Bahadur, S.; et al. Phytohormones Mediated Modulation of Abiotic Stress Tolerance and Potential Crosstalk in Horticultural Crops. J. Plant Growth Regul. 2022, 1–27. [Google Scholar] [CrossRef]
- Steward, F.C.; Moreno, U.; Roca, W.M. Supplement 2: Growth, Form and Composition of Potato Plants as Affected by Environment. Ann. Bot. 1981, 1–45. [Google Scholar] [CrossRef]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of Agricultural Crops to Free-Air CO2 Enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Navarro, C.; Prat, S. Flowering and Tuberization: A Tale of Two Nightshades. Trends Plant Sci. 2014, 19, 115–122. [Google Scholar] [CrossRef]
- Kim, Y.U.; Seo, B.S.; Choi, D.H.; Ban, H.Y.; Lee, B.W. Impact of high temperatures on the marketable tuber yield and related traits of potato. Eur. J. Agron. 2017, 89, 46–52. [Google Scholar] [CrossRef]
- Rykaczewska, K. The Impact of High Temperature during Growing Season on Potato Cultivars with Different Response to Environmental Stresses. Am. J. Plant Sci. 2013, 04, 2386–2393. [Google Scholar] [CrossRef]
- Sarkar, D. The Signal Transduction Pathways Controlling in Planta Tuberization in Potato: An Emerging Synthesis. Plant Cell Rep. 2008, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, J.F.; Virgós-Soler, A.; Prat, S. Control of Photoperiod-Regulated Tuberization in Potato by the Arabidopsis Flowering-Time Gene CONSTANS. Proc. Natl. Acad. Sci. USA 2002, 99, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lin, Z.; Huang, L.; Damaris, R.N.; Li, M.; Yang, P. A CONSTANS-LIKE Gene of Nelumbo Nucifera Could Promote Potato Tuberization. Planta 2021, 253, 65. [Google Scholar] [CrossRef] [PubMed]
- Amador, V.; Monte, E.; García-Martínez, J.L.; Prat, S. Gibberellins Signal Nuclear Import of PHOR1, a Photoperiod-Responsive Protein with Homology to Drosophila Armadillo. Cell 2001, 106, 343–354. [Google Scholar] [CrossRef]
- Altaf, M.A.; Sharma, N.; Singh, J.; Samota, M.K.; Sankhyan, P.; Singh, B.; Kumar, A.; Naz, S.; Lal, M.K.; Tiwari, R.K.; et al. Mechanistic Insights on Melatonin-Mediated Plant Growth Regulation and Hormonal Cross-Talk Process in Solanaceous Vegetables. Sci. Hortic. 2023, 308, 111570. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, Z.; Hua, T.; Ashraf, U.; Hamoud, Y.A.; Alaa, A.A.; Xiangru, T.; Meiyang, D.; Zaiman, W.; Shenggang, P. Nitrogen Deep Placement Combined with Straw Mulch Cultivation Enhances Physiological Traits, Grain Yield and Nitrogen Use Efficiency in Mechanical Pot-Seedling Transplanting Rice. Rice Sci. 2022, 29, 89–100. [Google Scholar] [CrossRef]
- Abeytilakarathna, P.D. Factors Affect to Stolon Formation and Tuberization in Potato: A Review. Agric. Rev. 2021, 43, 91–97. [Google Scholar] [CrossRef]
- Wolf, S.; Olesinski, A.A.; Rudich, J.; Marani, A. Effect of High Temperature on Photosynthesis in Potatoes. Ann. Bot. 1990, 65, 179–185. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E. Cracking and Fracture Properties of Potato (Solanum Tuberosum L.) Tubers and Their Relation to Dry Matter, Starch, and Mineral Distribution. J. Sci. Food Agric. 2019, 99, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, N.J.G.; de Bruijn, S.M.; Koot-Gronsveld, E.A.M.; Visser, R.G.F.; Vreugdenhil, D.; van der Plas, L.H.W. Developmental Changes of Enzymes Involved in Conversion of Sucrose to Hexose-Phosphate during Early Tuberisation of Potato. Planta 1997, 202, 220–226. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Mechanism for Deactivation of Rubisco under Moderate Heat Stress. Physiol. Plant 2004, 122, 513–519. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.J. Genetic Improvements of Tolerance to High Temperature. In Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; Haworth Press Inc.: New York, NY, USA, 2005; pp. 277–300. [Google Scholar]
- Cao, Z.; Yao, X.; Liu, H.; Liu, B.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Comparison of the Abilities of Vegetation Indices and Photosynthetic Parameters to Detect Heat Stress in Wheat. Agric. Meteorol. 2019, 265, 121–136. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of High Temperature on Photosynthesis and Related Gene Expression in Poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Sharma, D.K.; Ottosen, C.O. Heat Stress and Recovery of Photosystem II Efficiency in Wheat (Triticum Aestivum L.) Cultivars Acclimated to Different Growth Temperatures. Environ. Exp. Bot. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Nölke, G.; Houdelet, M.; Kreuzaler, F.; Peterhänsel, C.; Schillberg, S. The Expression of a Recombinant Glycolate Dehydrogenase Polyprotein in Potato (Solanum Tuberosum) Plastids Strongly Enhances Photosynthesis and Tuber Yield. Plant Biotechnol. J. 2014, 12, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.B.; Theeuwen, T.P.J.M.; Oortwijn, M.; Visser, R.G.F.; van der Linden, C.G. Carbon Partitioning Mechanisms in POTATO under Drought Stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, L.; Elferjani, R.; Soolanayakanahally, R.; Kagale, S.; Pahari, S.; Kulkarni, M.; Wahab, J.; Bizimungu, B. Drought-Induced Regulatory Cascades and Their Effects on the Nutritional Quality of Developing Potato Tubers. Genes 2020, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ahmad, S.; Abbas, G.; Fatima, Z.; Hussain, S.; Ahmed, M.; Khan, M.A.; Khan, A.; Fahad, S.; Nasim, W.; et al. Modeling the Impact of Climate Warming on Potato Phenology. Eur. J. Agron. 2022, 132, 126404. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Mushtaq, A.; Ali, S.; Muhammad, H.M.D.; Saddiq, B.; Ahmad, R.; Zulfiqar, F.; Hayat, F.; Tiwari, R.K.; Lal, M.K.; et al. Foliar Application of Ascorbic Acid Enhances Growth and Yield of Lettuce (Lactuca Sativa) under Saline Conditions by Improving Antioxidant Defence Mechanism. Funct. Plant Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Mandal, S.; Gupta, S.K.; Anand, U.; Ghorai, M.; Mundhra, A.; Rahman, M.d.H.; Ray, P.; Mitra, S.; Ray, D.; et al. Role of Polyamines in Molecular Regulation and Cross-Talks Against Drought Tolerance in Plants. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Lal, M.K.; Kumar, A.; Altaf, M.A.; Devi, R.; Mangal, V.; Naz, S.; Altaf, M.M.; Dey, A.; et al. Melatonin-Polyamine Interplay in the Regulation of Stress Responses in Plants. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Ahmed, S.; Zhou, X.; Pang, Y.; Jin, L.; Bao, J. Improving Starch-Related Traits in Potato Crops: Achievements and Future Challenges; Wiley-Blackwell: Hoboken, NJ, USA, 2018; Volume 70, ISBN 8657186971932. [Google Scholar]
- Kumar, D.; Ezekiel, R. Developmental Changes in Sugars and Dry Matter Content of Potato Tuber under Sub-Tropical Climates. Sci. Hortic. 2006, 110, 129–134. [Google Scholar] [CrossRef]
- Edwards, A.; Fulton, D.C.; Hylton, C.M.; Jobling, S.A.; Gidley, M.; Rössner, U.; Martin, C.; Smith, A.M. A Combined Reduction in Activity of Starch Synthases II and III of Potato Has Novel Effects on the Starch of Tubers. Plant J. 1999, 17, 251–261. [Google Scholar] [CrossRef]
- Lovell, P.H.; Booth, A. Effects of Gibberellic Acid on Growth, Tuber Formation and Carbohydrate Distribution in Solanum Tuberosum. New Phytol. 1967, 66, 525–537. [Google Scholar] [CrossRef]
- Huber, S.C.; Israel, D.W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine Max Merr.) Leaves. Plant Physiol. 1982, 69, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Altaf, M.M.; Kumar, R.; Naz, S.; Kumar, A.; Alam, P.; Tiwari, R.K.; Lal, M.K.; Ahmad, P. Melatonin: First-Line Soldier in Tomato under Abiotic Stress Current and Future Perspective. Plant Physiol. Biochem. 2022, 185, 188–197. [Google Scholar] [CrossRef]
- Liu, B.; Kong, L.; Zhang, Y.; Liao, Y. Gene and Metabolite Integration Analysis through Transcriptome and Metabolome Brings New Insight into Heat Stress Tolerance in Potato (Solanum Tuberosum L.). Plants 2021, 10, 103. [Google Scholar] [CrossRef]
- Dimenstein, L.; Lisker, N.; Kedar, N.; Levy, D. Changes in the Content of Steroidal Glycoalkaloids in Potato Tubers Grown in the Field and in the Greenhouse under Different Conditions of Light, Temperature and Daylength. Physiol. Mol. Plant Pathol. 1997, 50, 391–402. [Google Scholar] [CrossRef]
- Gao, H.J.; Yang, H.Y.; Bai, J.P.; Liang, X.Y.; Lou, Y.; Zhang, J.L.; Wang, D.; Zhang, J.L.; Niu, S.Q.; Chen, Y.L. Ultrastructural and Physiological Responses of Potato (Solanum Tuberosum L) Plantlets to Gradient Saline Stress. Front. Plant Sci. 2015, 5, 787. [Google Scholar] [CrossRef]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Ranjeet, R. Kumar Protection against Heat Stress in Wheat Involves Change in Cell Membrane Stability, Antioxidant Enzymes, Osmolyte, H2O2 and Transcript of Heat Shock Protein. Int. J. Plant Physiol. Biochem. 2012, 4, 83–91. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The Role of Glycine Betaine in the Protection of Plants from Stress: Clues from Transgenic Plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Kumar, A.; Tiwari, R.K.; Lal, M.K.; Singh, B. An Insight into Microbes Mediated Heavy Metal Detoxification in Plants: A Review. J. Soil Sci. Plant Nutr. 2021, 41, 1–23. [Google Scholar] [CrossRef]
- Park, C.J.; Seo, Y.S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant Heat-Shock Proteins: A Mini Review. J. King Saud Univ. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.; Kim, Y. Performance Optimization of a Hybrid Cooler Combining Vapor Compression and Natural Circulation Cycles. Int. J. Refrig. 2009, 32, 800–808. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of Plant Heat-Shock Proteins and Molecular Chaperones in the Abiotic Stress Response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Ye, M.; Wang, D.; Chen, Q. Evolutionary History of the Heat Shock Protein 90 (Hsp90) Family of 43 Plants and Characterization of Hsp90s in Solanum Tuberosum. Mol. Biol. Rep. 2020, 47, 6679–6691. [Google Scholar] [CrossRef]
- Savić, J.; Dragićević, I.; Pantelić, D.; Oljača, J.; Momčilović, I. Expression of Small Heat Shock Proteins and Heat Tolerance in Potato (Solanum Tuberosum L.). Arch. Biol. Sci. 2012, 64, 135–144. [Google Scholar] [CrossRef]
- Rudić, J.; Pantelić, D.; Oljača, J.; Momčilović, I. Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants. Horticulturae 2022, 8, 372. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant Responses of Wheat Plants under Stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant Enzymes Regulation in Plants in Reference to Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive Oxygen Species or Reactive Sulfur Species: Why We Should Consider the Latter. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Slusarenko, A.J. The Biology of Reactive Sulfur Species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic Insights on Melatonin-Mediated Drought Stress Mitigation in Plants. Physiol. Plant 2021, 172, 1212–1226. [Google Scholar] [CrossRef]
- Paul, S.; Gogoi, N.; Sarma, B.; Baroowa, B. Biochemical Changes in Potato under Elevated Temperature. Indian J. Plant Physiol. 2014, 19, 36–42. [Google Scholar] [CrossRef]
- Arora, D.; Jain, P.; Singh, N.; Kaur, H.; Bhatla, S.C. Mechanisms of Nitric Oxide Crosstalk with Reactive Oxygen Species Scavenging Enzymes during Abiotic Stress Tolerance in Plants. Free Radic. Res. 2016, 50, 291–303. [Google Scholar] [CrossRef]
- Shin, D.; Moon, S.J.; Han, S.; Kim, B.G.; Park, S.R.; Lee, S.K.; Yoon, H.J.; Lee, H.E.; bin Kwon, H.; Baek, D.; et al. Expression of StMYB1R-1, a Novel Potato Single MYB-like Domain Transcription Factor, Increases Drought Tolerance. Plant Physiol. 2011, 155, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, A.J.; Franke, A.C.; Steyn, J.M.; Pronk, A.A.; Caldiz, D.O.; Kooman, P.L. A Robust Potato Model: LINTUL-POTATO-DSS. Potato Res. 2015, 58, 313–327. [Google Scholar] [CrossRef]
- Kooman, P.L.; Haverkort, A.J. Modelling Development and Growth of the Potato Crop Influenced by Temperature and Daylength: LINTUL-POTATO.LINTUL-POTATO. In Potato Ecology and Modelling of Crops under Conditions Limiting Growth, Proceedings of the Second International Potato Modeling Conference, Wageningen, The Netherlands, 17–19 May 1994; Haverkort, A.J., MacKerron, D.K.L., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 41–59. [Google Scholar] [CrossRef]
- Griffin, T.S.; Johnson, B.S.; Ritchie, J.T. A Simulation Model for Potato Growth and Development: Substor-Potato Version 2.0; Michigan State University, Department of Crop and Soil Sciences: East Lansing, MI, USA, 1993. [Google Scholar]
- Goswami, B.; Hussain, R.; Kumar, P.; Saikia, U.; Banarjee, S. Impact assessment of climate change on potato productivity in Assam using SUBSTOR-Potato model. J. Agrometeorol. 2018, 20, 105–109. [Google Scholar] [CrossRef]
- Kumar, R.; Kaundal, P.; Tiwari, R.K.; Siddappa, S.; Kumari, H.; Lal, M.K.; Naga, K.C.; Sharma, S.; Sagar, V.; Kumar, M. Establishment of a One-Step Reverse Transcription Recombinase Polymerase Amplification Assay for the Detection of Potato Virus S. J. Virol. Methods 2022, 307, 114568. [Google Scholar] [CrossRef]
- Struik, P.C. Responses of the Potato Plant to Temperature. Potato Biol. Biotechnol. Adv. Perspect. 2007, 367–393. [Google Scholar] [CrossRef]
- Sharma, E.K.; Tiwari, R.K.; Lal, M.K.; Sharma, E.; Tiwari, R.K.; Devi, R.; Mishra, U.N.; Thakur, R.; Gupta, R.; Dey, A.; et al. Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics. Int. J. Mol. Sci. 2022, 23, 11305. [Google Scholar] [CrossRef]
- Rykaczewska, K. The Effect of High Temperature Occurring in Subsequent Stages of Plant Development on Potato Yield and Tuber Physiological Defects. Am. J. Potato Res. 2015, 92, 339–349. [Google Scholar] [CrossRef]
- Boguszewska-Mańkowska, D.; Zarzyńska, K.; Nosalewicz, A. Drought Differentially Affects Root System Size and Architecture of Potato Cultivars with Differing Drought Tolerance. Am. J. Potato Res. 2020, 97, 54–62. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Verhagen, A. Climate Change and Its Repercussions for the Potato Supply Chain. Potato Res. 2008, 51, 223–237. [Google Scholar] [CrossRef]
- Smillie, R.M.; Hetherington, S.E.; Ochoa, C.; Malagamba, P. Tolerances of Wild Potato Species from Different Altitudes to Cold and Heat. Planta 1983, 159, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Naga, K.C.; Subhash, S.; Sagar, V. Continuous and Emerging Challenges of Silver Scurf Disease in Potato. Int. J. Pest Manag. 2020, 1–13. [Google Scholar] [CrossRef]
- Guedes, M.L.; Haynes, K.G.; Vinyard, B.T.; Pinto, C.A.B.P. Heat Tolerance in Diploid Wild Potato Species In Vitro. Am. J. Potato Res. 2019, 96, 294–302. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Pérez-Sacristán, B.; Blanco-Navarro, I.; Blázquez-García, S. Comparative In Vitro Bioaccessibility of Carotenoids from Relevant Contributors to Carotenoid Intake. J. Agric. Food Chem. 2007, 55, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of Flowering and Storage Organ Formation in Potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- Eltayeb, A.E.; Yamamoto, S.; Habora, M.E.E.; Yin, L.; Tsujimoto, H.; Tanaka, K. Transgenic Potato Overexpressing Arabidopsis Cytosolic AtDHAR1 Showed Higher Tolerance to Herbicide, Drought and Salt Stresses. Breed Sci. 2011, 61, 3–10. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The Roles of Polyamines during the Lifespan of Plants: From Development to Stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lehretz, G.G.; Sonnewald, S.; Hornyik, C.; Corral, J.M.; Sonnewald, U. Post-Transcriptional Regulation of FLOWERING LOCUS T Modulates Heat-Dependent Source-Sink Development in Potato. Curr. Biol. 2019, 29, 1614–1624.e3. [Google Scholar] [CrossRef] [PubMed]
- Abelenda, J.A.; Cruz-Oró, E.; Franco-Zorrilla, J.M.; Prat, S. Potato StCONSTANS-Like1 Suppresses Storage Organ Formation by Directly Activating the FT-like StSP5G Repressor. Curr. Biol. 2016, 26, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Networks Involved in Drought Stress Response and Tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Luan, S. ABA Signal Transduction at the Crossroad of Biotic and Abiotic Stress Responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Kloosterman, B.; Abelenda, J.A.; Gomez, M.D.M.C.; Oortwijn, M.; de Boer, J.M.; Kowitwanich, K.; Horvath, B.M.; van Eck, H.J.; Smaczniak, C.; Prat, S.; et al. Naturally Occurring Allele Diversity Allows Potato Cultivation in Northern Latitudes. Nature 2013, 495, 246–250. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Kim, D.; Bae, S.; Park, J.; Kim, E.; Kim, S.; Yu, H.R.; Hwang, J.; Kim, J.-I.; Kim, J.S. Digenome-Seq: Genome-Wide Profiling of CRISPR-Cas9 off-Target Effects in Human Cells. Nat. Methods 2015, 12, 237–243. [Google Scholar] [CrossRef]
- Puchta, H. The Repair of Double-Strand Breaks in Plants: Mechanisms and Consequences for Genome Evolution. J. Exp. Bot. 2005, 56, 1–14. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, R.K.; Zhao, K. Base Editing in Crops: Current Advances, Limitations and Future Implications. Plant Biotechnol. J. 2020, 18, 20–31. [Google Scholar] [CrossRef]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted Nucleotide Editing Using Hybrid Prokaryotic and Vertebrate Adaptive Immune Systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable Base Editing of T to G C in Genomic DNA without DNA Cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Bánfalvi, Z.; Csákvári, E.; Villányi, V.; Kondrák, M. Generation of Transgene-Free PDS Mutants in Potato by Agrobacterium-Mediated Transformation. BMC Biotechnol. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Sevestre, F.; Facon, M.; Wattebled, F.; Szydlowski, N. Facilitating Gene Editing in Potato: A Single-Nucleotide Polymorphism (SNP) Map of the Solanum Tuberosum L. Cv. Desiree Genome. Sci. Rep. 2020, 10, 2045. [Google Scholar] [CrossRef]
- Zheng, Z.; Ye, G.; Zhou, Y.; Pu, X.; Su, W.; Wang, J. Editing Sterol Side Chain Reductase 2 Gene (StSSR2) via CRISPR/Cas9 Reduces the Total Steroidal Glycoalkaloids in Potato. All Life 2021, 14, 401–413. [Google Scholar] [CrossRef]
- Veillet, F.; Kermarrec, M.P.; Chauvin, L.; Chauvin, J.E.; Nogué, F. CRISPR-Induced Indels and Base Editing Using the Staphylococcus Aureus Cas9 in Potato. PLoS ONE 2020, 15, e0235942. [Google Scholar] [CrossRef] [PubMed]
- Veillet, F.; Chauvin, L.; Kermarrec, M.P.; Sevestre, F.; Merrer, M.; Terret, Z.; Szydlowski, N.; Devaux, P.; Gallois, J.L.; Chauvin, J.E. The Solanum Tuberosum GBSSI Gene: A Target for Assessing Gene and Base Editing in Tetraploid Potato. Plant Cell Rep. 2019, 38, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaundal, P.; Arjunan, J.; Sharma, S.; Chakrabarti, S.K. Development of a Visual Detection Method for Potato Virus S by Reverse Transcription Loop-Mediated Isothermal Amplification. 3 Biotech 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Uranga, M.; Aragonés, V.; Selma, S.; Vázquez-Vilar, M.; Orzáez, D.; Daròs, J.A. Efficient Cas9 Multiplex Editing Using Unspaced SgRNA Arrays Engineering in a Potato Virus X Vector. Plant J. 2021, 106, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaundal, P.; Tiwari, R.K.; Siddappa, S.; Kumari, H.; Chandra Naga, K.; Sharma, S.; Kumar, M. Rapid and Sensitive Detection of Potato Virus X by One-Step Reverse Transcription-Recombinase Polymerase Amplification Method in Potato Leaves and Dormant Tubers. Mol. Cell. Probes 2021, 58, 101743. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, D.; Jogam, P.; Allini, V.R.; Abbagani, S.; Alok, A. The Present and Potential Future Methods for Delivering CRISPR/Cas9 Components in Plants. J. Genet. Eng. Biotechnol. 2020, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Gaoa, Y.K.; Yuan, L.; Zhang, Q.X. Plant Genome Editing Made Efficient and Easy: Targeted Mutagenesis Using the CRISPR/Cas System. Acta Hortic. 2017, 1185, 209–213. [Google Scholar] [CrossRef]
- Koltun, A.; Corte, L.E.D.; Mertz-Henning, L.M.; Gonçalves, L.S.A. Genetic Improvement of Horticultural Crops Mediated by CRISPR/Cas: A New Horizon of Possibilities. Hortic. Bras. 2018, 36, 290–298. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).