Abstract
Background: We aim to ascertain prognostic factors in the current management of anal cancer within this study. Methods: We reviewed the management and outcomes of anal cancer cases over a seven-year period, inclusive (2016–2023). The primary objectives were to assess the demographic characteristics, clinical presentation, and outcomes of all anal cancer patients within our institution. Kaplan–Meier survival analysis was used to estimate survival differences between cohorts, with statistical significance determined using log-rank testing. Cox proportional hazards regression was utilised to identify prognostic factors. Cox regression hazard ratios were reported along with confidence intervals and p-values. Results: The median follow-up time for the study was 29.8 months. Seventy-five patients with anal cancer were included in this study, with 88% (66/75) being squamous cell carcinoma (SCC) and the majority having regional disease (82.7% (62/75)). The median age at diagnosis was 63.4 years (36–94). There was a female preponderance (57.3% (43/75)). In total, 84% (63/75) underwent definitive chemoradiation (dCRT), with 7/63 (11.1%) requiring a salvage abdomino-perineal resection (APR) for residual or recurrent disease. Adverse prognostic indicators include those with T4 disease hazard ratio = 3.81, (95% CI 1.13–12.83, * p = 0.04), poorly differentiated tumour disease HR = 3.37, (95% CI 1.13–10.02, * p = 0.04), having N2 nodal status HR = 5.03, (95% CI 1.11–22.8, * p = 0.04), and having metastatic disease at diagnosis HR = 5.8, (95% CI 1.28–26.42, * p = 0.02). Conclusion: Presenting characteristics including stage, nodal, and differentiation status remain key prognostic indicators in those diagnosed with anal malignancy.
1. Introduction
Anal cancer remains a rare disease, accounting for only about 2% of all gastrointestinal tract (GIT) malignancies [1,2]. Despite this, its diagnosis carries significant clinical, psychological, and public health implications. Anal cancer is largely classified into two subtypes: squamous cell carcinoma (SCC) and adenocarcinoma [1,3]. SCC accounts for the majority (>80%) of anal cancers, with adenocarcinoma (~10–20%), anal melanoma, and basal cell carcinoma occurring less frequently (<3%) [4]. Both SCC and adenocarcinoma share common risk factors, such as human immunodeficiency virus (HIV) infection, history of receptive anal intercourse, and immunosuppression [5,6,7].
There are many challenges in diagnosing anal cancer. Patients may initially present with symptoms such as discomfort, bleeding, or a lump in the anal area, all of which overlap with common, benign conditions [8,9]. Additionally, a large proportion of these neoplasms occur in socio-economically disadvantaged populations, with delays in seeking medical attention compounding issues [9,10,11].
Treatment strategies for anal cancer have evolved slowly over time, especially when compared with colon or rectal neoplasms [12,13]. There is a well-established emphasis on organ preservation in contrast with other GIT malignancies where surgery is the first-line treatment [10,14,15]. This has been the standard of care for anal cancer (anal SCC) since the Nigro protocol of the 1970’s with the first-line option for locoregional anal cancer being chemotherapy with mitomycin-C and 5-flourouracil concurrent with radiotherapy, despite associated toxicities [16,17,18,19,20].
The incidence of anal cancer continues to steadily rise, proportionately with the HPV epidemic [1,21,22,23,24,25]. At time of diagnosis, most cases of anal cancer are at a localised stage with only 5–8% having distant metastases [15,26]. There are high curative rates associated with locoregional anal SCC treated with definitive chemoradiotherapy (dCRT), and survival rates have improved over recent years [15,27]. However, this is heavily influenced by patient-related factors including gender, socioeconomic status, HIV and human papillomavirus (HPV) status, and disease-related factors including tumour size, differentiation, and nodal involvement [11,28].
Nuances in these factors are difficult to clarify and a number of patients with anal cancer still require salvage abdomino-perineal resection (APR) following dCRT due to inadequate response to treatment or disease recurrence [29,30]. Thus, there is an emerging role for technology including radiomics and genomics to be used in combination with clinical risk factors to identify early indicators of treatment resistance or recurrence and tailor treatments in anal cancer [31,32].
The aim of this study is to ascertain factors associated with poorer survival in this Irish patient cohort and in doing so, improve survival outcomes for patients with anal cancer.
2. Methods
2.1. Study Design
A retrospective review of all patients being treated for anal cancer during the year 2016 to 2023, inclusive, was performed in an Irish hospital setting. We examined patient demographics, clinical presentation, disease characteristics, treatment modalities, and survival outcomes.
2.2. Data Collection and Ethical Approval
Medical records of all patients diagnosed with anal cancer during the specified seven-year period were identified and reviewed. Data were collected using a structured data collection form specifically designed for this study. Patient information was de-identified and anonymised to ensure privacy and compliance with ethical guidelines. Ethical approval was granted for this study by SJH/TUH Joint Research Ethics Committee, the Institutional Review Board (IRB) at this centre. Patient data were anonymised and maintained confidentially to ensure privacy and comply with relevant data protection regulations.
2.3. Inclusion Criteria
Patients who met the following criteria were included in this review:
- Confirmed diagnosis of anal cancer (primary or recurrence).
- Diagnosis between 1 January 2016 and 31 December 2023.
- Availability of complete medical records, including demographic information, clinical notes, imaging reports, and treatment details.
2.4. Outcomes
The following data variables were collected for each patient:
- Primary outcome
Overall survival (OS) in days censored at last known follow up or date of known death.
- Secondary outcomes
- I.
- Demographic Information including sex, body mass index (BMI), HIV status, and age at diagnosis
- II.
- Clinical Presentation and Histopathological Subtype
- III.
- Treatment Modalities
- IV.
- Determination of any possible statistically significant predictors of censored survival using regression analysis.
2.5. Statistical Analysis
Descriptive statistics, such as means, medians, frequencies, and percentages, were used to summarise patient demographics, clinical presentation, and treatment modalities. Variables are reported as medians and ranges or means and standard deviations. Where dichotomous data are present, these values are reported as a number and a percentage of the total. The number of deaths observed is also reported. Mortality was treated as a time to event response, with data censored at last known follow up or date of known death. Missing values were not imputed. Kaplan–Meier survival curves are reported for covariates which showed statistically significant differences in survival on log-rank testing. Univariate and multivariable Cox proportional hazards models were used for the time to event outcome, taken as mortality or last date of follow up [33]. Hazard ratios were adjusted using covariates deemed clinically significant. Covariates with a low number of events or that made the statistical model unstable were excluded. Multivariable and univariate Cox regression hazard ratios are reported along with confidence intervals and p-values.
3. Results
3.1. Demographic Characteristics
In total, 75 patients were treated for anal cancer between 2016 and 2023. The median follow-up time for the study was 29.8 months. The median age at diagnosis was 63.4 years (range: 36–94). Female sex was more prevalent (57.3% (43/75)). The median BMI was 27.2 (17.3–48). Overall, 10.7% (8/75) were HIV positive patients and 93.8% (45/48) had documented HPV infection on histopathology (Table 1).

Table 1.
Patient characteristics and demographics.
3.2. Clinical Presentation and Histopathological Subtype
Overall, the most common form of referral was from the general practitioner (44% (33/75). Histopathological subtype was predominantly squamous cell carcinoma (SCC) (88% (66/75)). The majority of those presented having locoregional disease (82.7% (62/75)), with 44 patients (58.7%) being staged as node negative disease. Metastatic disease was present in five patients (6.7%) at diagnosis. At the time of diagnosis and subsequent multidisciplinary team (MDT) discussion regarding oncological management, 63 underwent dCRT, 5 received palliative radiation, 3 were palliated, 2 proceeded to APR from outset, and 2 were undergoing MDT assessment.
3.3. Definitive Chemoradiation
The majority of patients underwent dCRT (84% (63/75)), with 71.4% of (45/63) patients having a complete response to dCRT. Of the patients where the MDT decision was to proceed with dCRT, eight did not undergo radiation therapy (and had chemotherapy alone). Those with adenocarcinoma histology (9.3%, 7/75) received neoadjuvant chemoradiation as part of their treatment regimen. The side effects experienced by the 63 patients following dCRT treatment included vulvovaginal and perineal mucositis (7.9% (5/63)), pedal oedema, peripheral neuropathy (4.8% (2/63)), groin abscess, faecal incontinence, radiation dermatitis and/or skin desquamation (14.3% (9/63)), radiation proctitis (7.9% (5/63)), and urinary tract infections (11.11% (7/63)) (Table 1).
3.4. Abdominoperineal Resection (APR) Group
Overall, nine patients underwent APR. Two patients proceeded straight to APR, one for refractory tumour bleeding, and the other having active inflammatory bowel disease and not suitable for radiation treatment. The remaining seven patients underwent dCRT with subsequent salvage APR given the residual tumour. The median age was 51 years (range: 39–79). Six operations were open, while two were laparoscopic. All cases involved anorectal excision, with four en-bloc multi-visceral resections (two vaginectomies, one urethrectomy and one prostatectomy). All resections had clear margins (R0). Six patients required flap reconstruction, all using vertical rectus abdominis myocutaneous (VRAM) flaps. The median number of nodes collected was 10 (3–17), with none having positive histology. Median hospital stay was 25 days.
3.5. Recurrence
The total recurrence rate post-dCRT was 17.46% (11/63). Local recurrence/re-growth was observed in six patients (8%), and distal recurrence was observed in five patients (6.7%). The sites of metastatic deposits were lung (n = 3, 4%), liver (n = 2, 2.7%), and sacral bone involvement (n = 1, 1.3%). Of the 11 patients with recurrence, 7 patients went on to have a salvage APR, with the remaining 4 patients receiving palliative care. Overall, five patients who had recurrence post-dCRT were alive at the time of manuscript preparation (up to 20 April 24). Those who had recurrence (5/11) had a worse survival compared to those who did not recur (10/64) (* p = 0.006) (Table 2).

Table 2.
Univariate Analysis of Variables Associated with Survival and Log-Rank Test p-values.
3.6. Survival Influences
The 1-year and 3-year OS for the entire cohort, as derived from the Kaplan–Meier curve, was 89.7% and 76.1%, respectively (Supplementary Material S2). In those having dCRT with complete response, survival was 93.2% (41/44), while for those requiring a salvage APR, survival was 57.14% (4/7). Expectantly, T4 tumours had a worse survival compared to those with other stages (* p = 0.02), as did those with nodal disease (* p = 0.02), metastases (* p = 0.001), or poor differentiation at the time of diagnosis (* p = 0.002) (Table 2 and Figure 1A–D).
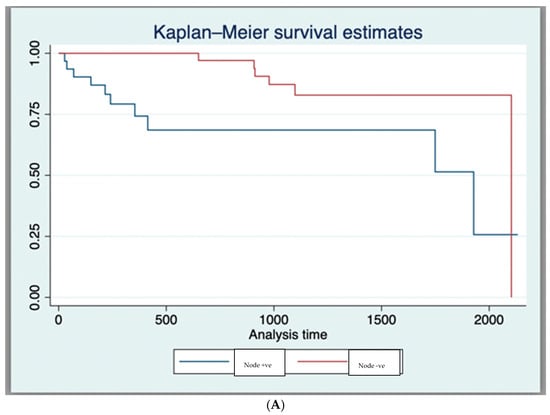
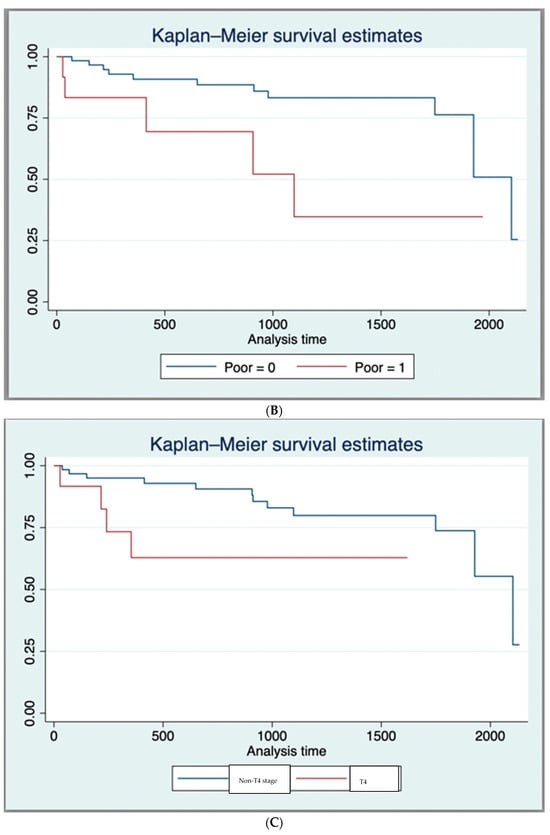
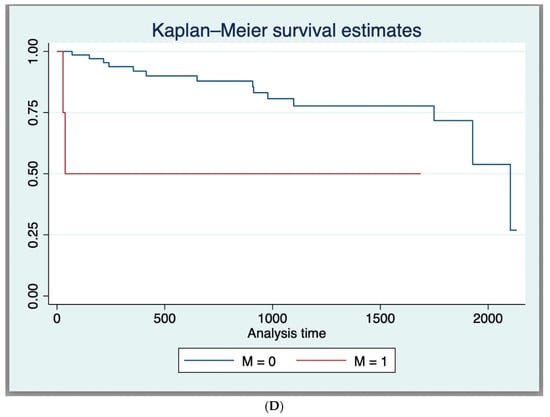
Figure 1.
(A) Kaplan–Meier Survival Curves (Log-Rank Test Results (p < 0.05)) for nodal status. (B) Kaplan–Meier Survival Curves (Log-Rank Test Results (p < 0.05)) for poor histological differentiation. (C) Kaplan–Meier Survival Curves (Log-Rank Test Results (p < 0.05)) for T4 tumour stage. (D) Kaplan–Meier Survival Curves (Log-Rank Test Results (p < 0.05)) for metastasis at diagnosis.
The following factors were associated with better survival on univariate analysis: complete response to dCRT HR = 0.058, (95% CI 0.012–0.28, * p = 0.0001), negative nodal status HR = 0.29, (95% CI 1 0.11–0.82, * p = 0.02). The following factors were associated with poorer survival on univariate analysis: T4 disease HR = 3.81, (95% CI 1.13–12.83, * p = 0.04), poorly differentiated tumour disease HR = 3.37, (95% CI 1.13–10.02, * p = 0.04), having positive nodal status HR = 5.03, (95% CI 1.11–22.8, * p = 0.04), and having metastatic disease at diagnosis HR = 5.8, (95% CI 1.28–26.42, * p = 0.02) (Table 2).
In the multivariable analysis, we examined the impact of several variables on survival, including male sex, tumour stage, nodal status, complete response to dCRT, recurrence, salvage APR, and poorly differentiated histology. The results indicated that, when controlled for all the co-variables listed above, complete response to dCRT (HR = 0.047, (95% CI: 0.004–0.522, * p = 0.013) and N0 status (HR = 0.0017, (95% CI: 0.00002–0.14, * p = 0.005) were found to be statistically significant positive predictors of survival. Male sex HR = 27.5, (95% CI: 2.53–298.78, * p = 0.006) was a statistically significant negative predictor of survival. (Supplementary Material S1).
4. Discussion
As seen in the literature, our retrospective review observed that stage, nodal, and differentiation status remain key prognostic indicators in those diagnosed with anal cancer [28,34,35,36,37]. Lu et al. conducted a multicentre study (11 cancer centres), involving anal cancer patient. Their univariable analysis showed that T stage significantly predicted recurrence-free survival (RFS) ([HR] = 3.03, 95% CI: 1.10–8.37, p = 0.032). Additionally, they found that N stage (HR = 3.05, 95% CI: 1.07–8.74, p = 0.038) was a significant predictor of OS [36]. Anal cancer is more common in elderly cohorts (median age = 63.4 years) and in females (57.3%), which is consistent with existing literature [38,39]. Expectantly, HIV positivity and HPV infection were prevalent in this cohort (in 10.7% and 93.8% of patients, respectively) [40,41,42].
The 3-year OS for the entire cohort was 76.1%, reducing to 57.14% in those requiring salvage APR. This is consistent with survival rates in other international centres [43,44,45].
The majority of patients (n = 63) presented with early disease (<T4), and 44 patients were deemed node negative. This likely reflects effective screening in those with high-risk features, and improved education programmes. A group of HIV care experts has issued the first U.S. federal guidelines designed to prevent anal cancer in people with HIV. These new recommendations advocate for a screening program that utilises high resolution anoscopy to identify and treat precancerous conditions, thereby preventing the development of anal cancer in individuals with HIV [46,47,48]. Expectantly, the predominance of SCC was the main histology of anal cancer, with >80% being well or moderately differentiated, consistent with other reported values [1,22,49].
Screening programmes for anal cancer are essential for early detection and improving survival outcomes, particularly among high-risk populations, working to effectively identify pre-cancerous lesions, such as anal intraepithelial neoplasia (AIN), before they progress to invasive cancer, alongside education and surveillance for high-risk groups [50,51,52]. There is a role for infectious disease clinics to screen high-risk cohorts, including individuals with HIV, HPV, vulvar intraepithelial neoplasia (VIN), and cervical intraepithelial neoplasia (CIN) [47,52]. Our study's findings align with previous research highlighting the importance of early detection in improving prognosis, particularly in patients with localised disease. In the cohort study by Leclerc et al., 700 HIV-positive patients were included. Out of these, 336 patients had at least one proctology visit. Anal cancer was diagnosed in 13 patients. Notably, among the patients who strictly adhered to the screening programmes (4.6%), no cases of AIN or anal cancer were reported [53].
HPV vaccination is critical for HIV-positive individuals and men who have sex with men (MSM), as both groups have a heightened risk of HPV-related anal cancer [41,54]. In HIV-positive individuals, the vaccine significantly reduces the prevalence of persistent HPV infections and associated precancerous lesions, thereby lowering cancer risk [46,54]. Similarly, research indicates that among MSM, HPV vaccination decreases the prevalence of high-risk HPV strains and the incidence of anal intraepithelial neoplasia (AIN), a precursor to anal cancer [55].
dCRT remains the cornerstone of anal cancer treatment, with 84% of patients in this cohort undergoing dCRT, in keeping with international standards [56]. Overall, 68.3% had dCRT alone, with 28.57% (18/63) having an incomplete response and 17.46% (11/63) developing a recurrence. Of these, 11.11% (7/63) underwent salvage APR. The multivariable analysis highlighted key significant predictors of survival. Complete response to dCRT, advanced disease stage at diagnosis (T4), poorly differentiation of the neoplasm, nodal involvement (N2), and metastatic disease were significant factors influencing outcomes. These findings are consistent with other international published results [28,34,35,36,37,57].
Recurrence remains a significant challenge in the management of anal cancer, with 8% of patients experiencing local recurrence and 6.7% developing distant metastases. Our analysis showed that recurrence was a strong predictor of poor survival (p = 0.006), emphasising the need for vigilant post-treatment surveillance and potential adjuvant therapies to mitigate the risk of recurrence. Interestingly, despite the poor prognosis generally associated with recurrent disease, five out of eleven patients with recurrence were still alive at follow-up, suggesting that aggressive management and salvage treatments can provide meaningful survival benefits in select patients.
The use of advanced adjuvants in monitoring the response to dCRT for anal cancer presents promising avenues for improving patient outcomes. Circulating tumour DNA (ctDNA) as a biomarker has shown potential in providing real-time insights into treatment efficacy and detecting minimal residual disease [58,59,60,61]. Our study underscores the importance of comprehensive monitoring, as 11.1% of patients required salvage APR for residual or recurrent disease, highlighting the need for more precise surveillance methods.
Radiomics is another noteworthy advancement in the role of prediction of treatment responses in anal cancer [62,63,64,65]. We have previously shown in a systematic review that radiomics-based risk stratification models were found to provide valuable insights into treatment response and patient outcomes, with all developed signatures demonstrating at least modest accuracy (range AUC: 0.68–1.0) in predicting their primary outcome in anal cancer [32]. The integration of these technologies into routine clinical practice could refine treatment plans and potentially reduce the need for invasive procedures, thereby improving OS rates and quality of life for patients with anal cancer.
We acknowledge that our study has some limitations, especially relating to sample size. This is not unexpected given the relative rarity of this disease. However, the longitudinal follow-up of this cohort has helped identify several critical factors influencing survival. Some findings may be clinically significant even though they are presented as not statistically significant above. These findings reinforce the importance of early detection, precise staging, and aggressive management of residual or recurrent disease following dCRT. Future research should focus on refining therapeutic strategies and optimising surveillance especially in high-risk groups.
5. Conclusions
This study highlights the critical prognostic factors in anal cancer management, including tumour stage, nodal status, and histological differentiation. Continued research into predictive biomarkers and advanced therapeutic approaches is essential to stratifying at-risk patients and optimising care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol31090381/s1, Supplementary Material S1: Multivariable Analysis of Factors Affecting Survival. Supplementary Material S2: Kaplan-Meier survival estimates.
Author Contributions
Conceptualization, H.C.T., N.J.O., B.M.M.C., B.J.M., M.E.K., C.M., J.O.L., C.K. and T.S.T.; methodology, H.C.T. and C.B.; software, D.G.; validation, C.G.; formal analysis, H.C.T. and P.H.M.; investigation, M.E.K. writing—review and editing, M.E.K.; supervision, M.E.K.; project administration, H.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was granted for this study by SJH/TUH Joint Research Ethics Committee, the Institutional Review Board (IRB) at this centre (protocol code 3609 and date of approval: 20 May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analysed in this study.
Acknowledgments
Supported by Joly Leadership Fund, Trinity St. James Cancer Institute.
Conflicts of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors report no conflicts of interest.
References
- Gondal, T.A.; Chaudhary, N.; Bajwa, H.; Rauf, A.; Le, D.; Ahmed, S. Anal Cancer: The Past, Present and Future. Curr. Oncol. 2023, 30, 3232–3250. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Goldblum, J.R. Tumours of the anal canal: Introduction. In WHO Classification of Tumours: Digestive System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Young, A.N.; Jacob, E.; Willauer, P.; Smucker, L.; Monzon, R.; Oceguera, L. Anal Cancer Anal cancer Squamous cell carcinoma Anal canal Adenocarcinoma. Surg. Clin. North Am. 2020, 100, 629–634. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef]
- Kelly, H.; Chikandiwa, A.; Alemany Vilches, L.; Palefsky, J.M.; de Sanjose, S.; Mayaud, P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: A systematic review and meta-analysis. Lancet HIV 2020, 7, e262–e278. [Google Scholar] [CrossRef]
- Clifford, G.M.; Georges, D.; Shiels, M.S.; Engels, E.A.; Albuquerque, A.; Poynten, I.M.; de Pokomandy, A.; Easson, A.M.; Stier, E.A. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int. J. Cancer 2021, 148, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Keilholz, G.; Kranzbühler, H.; Lombriser, N.; Prakash, M.; Vavricka, S.R.; Misselwitz, B. Presenting symptoms predict local staging of anal cancer: A retrospective analysis of 86 patients. BMC Gastroenterol. 2016, 16, 46. [Google Scholar] [CrossRef]
- Chiu, S.; Joseph, K.; Ghosh, S.; Schiller, R.C.D. Reasons for delays in diagnosis of anal cancer and the effect on patient satisfaction Recherche Les raisons des retards de diagnostic du cancer anal et les effets sur la satisfaction des patients. Can. Fam. Physician. 2015, 61, 509–516. [Google Scholar]
- Rao, S.; Guren, M.G.; Khan, K.; Brown, G.; Renehan, A.G.; Steigen, S.E.; Deutsch, E.; Martinelli, E.; Arnold, D. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1087–1100. [Google Scholar] [CrossRef]
- Ali, F.; Ghareeb, A.E.; Jha, A.; Van der Voet, H.; Garg, D.; Jha, M. Anal cancer survival: A socioeconomic analysis. Ann. R. Coll. Surg. Engl. 2021, 103, 191–196. [Google Scholar] [CrossRef]
- Affleck, A.; Koprowski, M.A.; Nabavizadeh, N.; Tsikitis, V.L. The evolution of rectal cancer treatment: The journey to total neoadjuvant therapy and organ preservation. Ann. Gastroenterol. 2022, 35, 226–233. [Google Scholar] [CrossRef]
- Eng, C.; Ciombor, K.K.; Cho, M.; Dorth, J.A.; Rajdev, L.N.; Horowitz, D.P.; Gollub, M.J.; Jacome, A.A.; Lockney, N.A.; Muldoon, R.L.; et al. Anal Cancer: Emerging Standards in a Rare Disease. J. Clin. Oncol. 2022, 40, 2774–2788. [Google Scholar] [CrossRef]
- Congedo, A.; Mallardi, D.; Danti, G.; De Muzio, F.; Granata, V.; Miele, V. An Updated Review on Imaging and Staging of Anal Cancer—Not Just Rectal Cancer. Tomography 2023, 9, 1694–1710. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Nilsson, P.J.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Peiffert, D.; Arnold, D. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother. Oncol. 2014, 111, 330–339. [Google Scholar] [CrossRef]
- Nigro, N.D.; Vaitkevicius, V.K.; Considine, B. Combined therapy for cancer of the anal canal: A preliminary report. Dis. Colon Rectum 1974, 17, 354–356. [Google Scholar] [CrossRef]
- Upadhyay, L.; Hartzell, M.; Parikh, A.R.; Strickland, M.R.; Klempner, S.; Malla, M. Recent Advances in the Management of Anal Cancer. Healthcare 2023, 11, 3010. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B.; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-Term Update of US GI Intergroup RTOG 98-11 Phase III Trial for Anal Carcinoma: Survival, Relapse, and Colostomy Failure with Concurrent Chemoradiation Involving Fluorouracil/Mitomycin Versus Fluorouracil/Cisplatin. J. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Northover, J.; Glynne-Jones, R.; Sebag-Montefiore, D.; James, R.; Meadows, H.; Wan, S.; Jitlal, M.; Ledermann, J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 2010, 102, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Kreimer, A.R.; Coghill, A.E.; Darragh, T.M.; Devesa, S.S. Anal cancer incidence in the United States, 1977–2011: Distinct patterns by histology and behavior. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1548–1556. [Google Scholar] [CrossRef]
- Chelmow, D.; Cejtin, H.; Conageski, C.; Farid, H.; Gecsi, K.; Kesterson, J.; Khan, M.J.; Long, M.; O’Hara, J.S.; Burke, W. Executive Summary of the Lower Anogenital Tract Cancer Evidence Review Conference. Obstet. Gynecol. 2023, 142, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Simard, E.P.; Dorell, C.; Noone, A.M.; Markowitz, L.E.; Kohler, B.; Eheman, C.; Saraiya, M.; Bandi, P.; Saslow, D.; et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J. Natl. Cancer Inst. 2013, 105, 175–201. [Google Scholar] [CrossRef]
- Johnson, L.G.; Madeleine, M.M.; Newcomer, L.M.; Schwartz, S.M.; Daling, J.R. Anal cancer incidence and survival: The Surveilance, epidemiology, and end results experience, 1973–2000. Cancer 2004, 101, 281–288. [Google Scholar] [CrossRef]
- Islami, F.; Ferlay, J.; Lortet-Tieulent, J.; Bray, F.; Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 2017, 46, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.E.; Eng, C. Pharmacotherapy of Anal Cancer. Drugs 2017, 77, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Buchel, E.W.; Finical, S.; Johnson, C. Pelvic Reconstruction Using Vertical Rectus Abdominis Musculocutaneous Flaps. Ann. Plast. Surg. 2004, 52, 22–26. [Google Scholar] [CrossRef]
- Theophanous, S.; Samuel, R.; Lilley, J.; Henry, A.; Sebag-Montefiore, D.; Gilbert, A.; Appelt, A.L. Prognostic factors for patients with anal cancer treated with conformal radiotherapy—A systematic review. BMC Cancer 2022, 22, 607. [Google Scholar] [CrossRef]
- Hagemans, J.A.W.; Blinde, S.E.; Nuyttens, J.J.; Morshuis, W.G.; Mureau, M.A.M.; Rothbarth, J.; Verhoef, C.; Burger, J.W.A. Salvage Abdominoperineal Resection for Squamous Cell Anal Cancer: A 30-Year Single-Institution Experience. Ann. Surg. Oncol. 2018, 25, 1970–1979. [Google Scholar] [CrossRef]
- Shakir, R.; Adams, R.; Cooper, R.; Downing, A.; Geh, I.; Gilbert, D.; Jacobs, C.; Jones, C.; Lorimer, C.; Namelo, W.C.; et al. Patterns and Predictors of Relapse Following Radical Chemoradiation Therapy Delivered Using Intensity Modulated Radiation Therapy With a Simultaneous Integrated Boost in Anal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 329–339. [Google Scholar] [CrossRef]
- Saxena, S.; Jena, B.; Gupta, N.; Das, S.; Sarmah, D.; Bhattacharya, P.; Nath, T.; Paul, S.; Fouda, M.M.; Kalra, M.; et al. Role of Artificial Intelligence in Radiogenomics for Cancers in the Era of Precision Medicine. Cancers 2022, 14, 2860. [Google Scholar] [CrossRef]
- Temperley, H.C.; O’Sullivan, N.J.; Waters, C.; Corr, A.; Mehigan, B.J.; O’Kane, G.; McCormick, P.; Gillham, C.; Rausa, E.; Larkin, J.O.; et al. Radiomics; Contemporary Applications in the Management of Anal Cancer; A Systematic Review. Am. Surg. 2023, 90, 445–454. [Google Scholar] [CrossRef]
- Annesi, I.; Moreau, T.; Lellouch, J. Efficiency of the logistic regression and Cox proportional hazards models in longitudinal studies. Stat. Med. 1989, 8, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Crane, C.H.; Eng, C.; Ajani, J.A. Prognostic factors for squamous cell cancer of the anal canal. Gastrointest. Cancer Res. 2008, 2, 10–14. [Google Scholar] [PubMed] [PubMed Central]
- Iseas, S.; Prost, D.; Bouchereau, S.; Golubicki, M.; Robbio, J.; Oviedo, A.; Coraglio, M.; Kujaruk, M.; Méndez, G.; Carballido, M.; et al. Prognostic Factors of Long-Term Outcomes after Primary Chemo-Radiotherapy in Non-Metastatic Anal Squamous Cell Carcinoma: An International Bicentric Cohort. Biomedicines 2023, 11, 791. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Li, P.; Zhang, T.; Zhou, J.; Ren, Y.; Ding, Y.; Peng, H.; Wei, Q.; You, K.; et al. Clinical characteristics and prognosis of anal squamous cell carcinoma: A retrospective audit of 144 patients from 11 cancer hospitals in southern China. BMC Cancer 2020, 20, 679. [Google Scholar] [CrossRef]
- Mduma, E.; Dharsee, N.; Samwel, K.; Mwita, C.J.; Lidenge, S.J. Clinicopathological Characteristics and Outcomes of Anal Squamous Cell Carcinoma Patients With and Without HIV Infection in Sub-Saharan Africa. JCO Glob. Oncol. 2023, 9, e2200394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, R.A.; Levine, A.M.; Bernstein, L.; Smith, D.D.; Lai, L.L. Changing patterns of anal canal carcinoma in the United States. J. Clin. Oncol. 2013, 31, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Salati, S.A.; Al Kadi, A. Anal cancer—A review. Int. J. Health Sci. 2012, 6, 206–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khandwala, P.; Singhal, S.; Desai, D.; Parsi, M.; Potdar, R. HIV-Associated Anal Cancer. Cureus 2021, 13, e14834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colón-López, V.; Shiels, M.S.; Machin, M.; Ortiz, A.P.; Strickler, H.; Castle, P.E.; Pfeiffer, R.M.; Engels, E.A. Anal Cancer Risk Among People With HIV Infection in the United States. J. Clin. Oncol. 2018, 36, 68–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melbye, M.; Rabkin, C.; Frisch, M.; Biggar, R.J. Changing patterns of anal cancer incidence in the United States, 1940-1989. Am. J. Epidemiol. 1994, 139, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Sawai, K.; Goi, T.; Tagai, N.; Kurebayashi, H.; Morikawa, M.; Koneri, K.; Tamaki, M.; Murakami, M.; Hirono, Y.; Maeda, H. Stage IV anal canal squamous cell carcinoma with long-term survival: A case report. Surg. Case Rep. 2022, 8, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johansson, M.; Axelsson, A.; Haglind, E.; Bock, D.; Angenete, E. Long-term survival after treatment for primary anal cancer- results from the Swedish national ANCA cohort study. Acta Oncol. 2022, 61, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Sebag-Montefiore, D.; Meadows, H.M.; Cunningham, D.; Begum, R.; Adab, F.; Benstead, K.; Harte, R.J.; Stewart, J.; Beare, S.; et al. ACT II study group. Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): A post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 347–356, Erratum in Lancet Oncol. 2017, 18, e196; Erratum in Lancet Oncol. 2020, 21, e518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palefsky, J.M.; Lee, J.Y.; Jay, N.; Goldstone, S.E.; Darragh, T.M.; Dunlevy, H.A.; Rosa-Cunha, I.; Arons, A.; Pugliese, J.C.; Vena, D.; et al. ANCHOR Investigators Group. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N. Engl. J. Med. 2022, 386, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Masiá, M.; Gutiérrez-Ortiz de la Tabla, A.; Gutiérrez, F. Cancer screening in people living with HIV. Cancer Med. 2023, 12, 20590–20603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hillman, R.J.; Berry-Lawhorn, J.M.; Ong, J.J.; Cuming, T.; Nathan, M.; Goldstone, S.; Richel, O.; Barrosso, L.F.; Darragh, T.M.; Law, C.; et al. International Anal Neoplasia Society. International Anal Neoplasia Society Guidelines for the Practice of Digital Anal Rectal Examination. J. Low Genit. Tract. Dis. 2019, 23, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Pessia, B.; Romano, L.; Giuliani, A.; Lazzarin, G.; Carlei, F.; Schietroma, M. Squamous cell anal cancer: Management and therapeutic options. Ann. Med. Surg. 2020, 55, 36–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowak, R.G. Expanding early detection of anal cancer to improve survival. Lancet HIV 2024, 11, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Ekström, A.M.; Ali, T.S.; Salazar, M.; Ahmed, A.; Ali, D.; Haroon, A.; Siddiqi, S. Integration of human papillomavirus associated anal cancer screening into HIV care and treatment program in Pakistan: Perceptions of policymakers, managers, and care providers. BMC Public Health 2023, 23, 1034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barroso, L.F.; Stier, E.A.; Hillman, R.; Palefsky, J. Anal Cancer Screening and Prevention: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infection Guidelines. Clin. Infect. Dis. 2022, 74, S179–S192. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Jacomet, C.; Siproudhis, L.; Abramowitz, L.; Pereira, B.; Buisson, A. Impact of screening programme to prevent anal cancer in high-risk patients with HIV. HIV Med. 2024, 25, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Stier, E.A.; Chigurupati, N.L.; Fung, L. Prophylactic HPV vaccination and anal cancer. Hum. Vaccines Immunother. 2016, 12, 1348–1351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gosens, K.C.M.; van der Zee, R.P.; van Heukelom, M.L.S.; Jongen, V.W.; Cairo, I.; van Eeden, A.; van Noesel, C.J.M.; Quint, W.G.V.; Pasmans, H.; Dijkgraaf, M.G.W.; et al. HPV vaccination to prevent recurrence of anal intraepithelial neoplasia in HIV+ MSM. AIDS 2021, 35, 1753–1764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- PDQ Adult Treatment Editorial Board. Anal Cancer Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Pappou, E.P.; Magruder, J.T.; Fu, T.; Hicks, C.W.; Herman, J.M.; Fang, S.; Wick, E.C.; Safar, B.; Gearhart, S.L.; Efron, J.E. Prognostic and Predictive Clinicopathologic Factors of Squamous Anal Canal Cancer in HIV-Positive and HIV-Negative Patients: Does HAART Influence Outcomes? World J. Surg. 2018, 42, 876–883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Temperley, H.C.; Fannon, T.; O’Sullivan, N.J.; O’Neill, M.; Mac Curtain, B.M.; Gilham, C.; O’Sullivan, J.; O’Kane, G.; Mehigan, B.J.; O’Toole, S.; et al. Assessing Circulating Tumour DNA (ctDNA) as a Biomarker for Anal Cancer Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4005. [Google Scholar] [CrossRef]
- Azzi, G.; Tavallai, M.; Aushev, V.N.; Malashevich, A.K.; Botta, G.P.; Tejani, M.A.; Hanna, D.; Krinshpun, S.; Malhotra, M.; Jurdi, A.; et al. Using Tumor-Informed Circulating Tumor DNA (ctDNA)-Based Testing for Patients with Anal Squamous Cell Carcinoma. Oncologist 2023, 28, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Tessier, A.; Jeannot, E.; Guenat, D.; Debernardi, A.; Michel, M.; Proudhon, C.; Vincent-Salomon, A.; Bièche, I.; Pierga, J.-Y.; Buecher, B.; et al. Clinical Validity of HPV Circulating Tumor DNA in Advanced Anal Carcinoma: An Ancillary Study to the Epitopes-HPV02 Trial. Clin. Cancer Res. 2019, 25, 2109–2115. [Google Scholar] [CrossRef]
- Cabel, L.; Jeannot, E.; Bieche, I.; Vacher, S.; Callens, C.; Bazire, L.; Morel, A.; Bernard-Tessier, A.; Chemlali, W.; Schnitzler, A.; et al. Prognostic Impact of Residual HPV ctDNA Detection after Chemoradiotherapy for Anal Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 5767–5771. [Google Scholar] [CrossRef]
- Brown, P.J.; Zhong, J.; Frood, R.; Currie, S.; Gilbert, A.; Appelt, A.L.; Sebag-Montefiore, D.; Scarsbrook, A. Prediction of outcome in anal squamous cell carcinoma using radiomic feature analysis of pre-treatment FDG PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2790–2799. [Google Scholar] [CrossRef]
- Giraud, N.; Saut, O.; Aparicio, T.; Ronchin, P.; Bazire, L.-A.; Barbier, E.; Lemanski, C.; Mirabel, X.; Etienne, P.-L.; Lievre, A.; et al. MRI-based radiomics input for prediction of 2-year disease recurrence in anal squamous cell carcinoma. Cancers 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Hocquelet, A.; Auriac, T.; Perier, C.; Dromain, C.; Meyer, M.; Pinaquy, J.-B.; Denys, A.; Trillaud, H.; De Senneville, B.D.; Vendrely, V. Pre-treatment magnetic resonance-based texture features as potential imaging biomarkers for predicting event free survival in anal cancer treated by chemoradiotherapy. Eur. Radiol. 2018, 28, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk, K.; Prezzi, D.; Cascino, M.; Kozarski, R.; Gaya, A.; Siddique, M.; Cook, G.J.; Glynne-Jones, R.; Goh, V. MRI heterogeneity analysis for prediction of recurrence and disease free survival in anal cancer. Radiother. Oncol. 2019, 134, 119–126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).