Abstract
Background: The incidence of colorectal cancer (CRC) is decreasing in individuals >50 years due to organised screening but has increased for younger individuals. We characterized symptoms and their timing before diagnosis in young individuals. Methods: We identified all patients diagnosed with CRC between 1990–2017 in British Columbia, Canada. Individuals <50 years (n = 2544, EoCRC) and a matched cohort >50 (n = 2570, LoCRC) underwent chart review to identify CRC related symptoms at diagnosis and determine time from symptom onset to diagnosis. Results: Across all stages of CRC, EoCRC presented with significantly more symptoms than LoCRC (Stage 1 mean ± SD: 1.3 ± 0.9 vs. 0.7 ± 0.9, p = 0.0008; Stage 4: 3.3 ± 1.5 vs. 2.3 ± 1.7, p < 0.0001). Greater symptom burden at diagnosis was associated with worse survival in both EoCRC (p < 0.0001) and LoCRC (p < 0.0001). When controlling for cancer stage, both age (HR 0.87, 95% CI 0.8–1.0, p = 0.008) and increasing symptom number were independently associated with worse survival in multivariate models. Conclusions: Patients with EoCRC present with a greater number of symptoms of longer duration than LoCRC; however, time from patient reported symptom onset was not associated with worse outcomes.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in Canada [1]. While the incidence of CRC has decreased in older patients (≥50 years of age, LoCRC), there has been a rise in the incidence of CRC in patients <50 years of age (EoCRC) [2,3,4]. The decrease in incidence of CRC in older patients has been primarily attributed to organised screening removing pre-malignant polpys [1,5]. Current screening guidelines in Canada recommend screening for CRC begin at the age of 50 [6,7]. Recently, in response to the rising incidence of early-onset CRC, American organisations, such as the American Cancer Society and US Preventive Services Task Force, have updated their screening guidelines and recommend screening beginning at age 45 in average-risk adults [6].
As younger patients are excluded from screening programs and there is less awareness about the risk of CRC in young patients, disease in this population may not be detected until patients are more symptomatic and later in their disease course [8,9,10,11]. Possible explanations include patient-related factors, such as lack of knowledge about symptoms which may contribute to a longer time to diagnosis [12,13]. Provider-related factors have also been shown to play a role in delaying diagnosis where, despite younger patients presenting with symptoms that should prompt investigation of CRC, there may be a low suspicion among health care providers [14,15]. Given these issues, our objectives were to (1) characterize the pattern of symptoms at presentation in EoCRC compared to LoCRC, (2) identify differences in the number of symptoms at presentation and whether this associates with survival, and (3) assess if EoCRC patients experience a longer time to diagnosis compared to LoCRC and how this impacts survival.
2. Materials and Methods
2.1. Patient Population
Cancer care in the Canadian province of British Columbia (population ~5 million) is publicly funded and administered through 6 regional cancer centres under the guidance of BC Cancer. Within BC Cancer, the Gastrointestinal Cancer Outcomes Unit (GICOU) prospectively collects demographic, disease, treatment, and outcome data on all patients with CRC. After receiving institutional review board approval, the GICOU database was queried to identify patients diagnosed with CRC between 1990–2017. Patients diagnosed with CRC before age of 50 were deemed early-onset (EoCRC), while other patients were categorized as late-onset (LoCRC). There were 2544 in the EoCRC cohort and 27,616 in the LoCRC.
All individuals in the EoCRC cohort, and a matched cohort of LoCRC patients who were diagnosed between 1 January 2015–31 December 2016 were included in a retrospective chart review (Table S1). The LoCRC subset was chosen from more recent dates of diagnosis to ensure maximum availability of hospital records to conduct our analysis and to ensure a minimum 5 years of follow up for comparison. We selected 18 symptoms associated with different stages of CRC, including early stage (anemia, change in bowel habits, decreased stool calibre, diarrhea, constipation, bloody stools); locally advanced (urinary symptoms, palpable mass, low back pain, tenesmus, nausea/vomiting, abdominal pain); and advanced/metastatic disease (night sweats, weight loss, fatigue, fever, ascites, anorexia) and ascertained if they were reported and how long before diagnosis they were reported. We included asymptomatic stool testing for occult blood and screening colonoscopy as “incidental” diagnoses.
2.2. Statistical Methods
Prevalence of symptoms and complications at presentation or during the index operation including obstruction, perforation and hemorrhage was compared with the Chi-square or Fisher’s exact test as appropriate and an odds ratio with 95% confidence interval was calculated. The number of symptoms at diagnosis grouped by stage was compared between EoCRC and LoCRC using Welch’s t test. Kaplan–Meyer curves were used to summarize overall survival and compared via the log-rank test. Hazard ratios for overall survival of each symptom were calculated by comparing survival of each listed symptom with bloody stools as the reference since it was the most common. The duration of symptoms was calculated from the time of symptom onset until the date of pathologic diagnosis based on chart review. The difference in median symptom duration prior to diagnosis was compared using the Mann–Whitney test.
After all included variables were shown to satisfy the proportional hazards assumption, a Cox-proportional hazard model for overall survival was created using a forward likelihood-ratio inclusion method with p < 0.05 probability for stepwise inclusion and p > 0.10 probability for exclusion. Variables assessed included age, diagnosis era, stage at diagnosis, number of symptoms and duration of symptoms (categorical as 0–90 days, 91–180 days and 181+ days). A total of 3344 patients with all values available were analyzed.
3. Results
3.1. Baseline Characteristics
The baseline demographics of EoCRC and LoCRC are shown in Table 1. There is a slightly higher preponderance of males in the LoCRC cohort (EoCRC 51% vs. LoCRC 58%, p < 0.0001). The median age at diagnosis for the EoCRC cohort is 44 years, and 76 years for LoCRC.

Table 1.
Baseline characteristics of individuals diagnosed with colorectal cancer between 1990–2017 in British Columbia, Canada compared between those less than 50 (EoCRC) or 50 years and older (LoCRC) with colorectal cancer. The absolute number of individuals (n), proportion of the specified cohort (%) and p values are shown.
The baseline demographics of the subgroup of patients reviewed for presence of symptoms compared to the whole group are shown in Table S1. There was a significant difference in the date of diagnosis and clinical stage.
3.2. Symptom Distribution at Time of Cancer Diagnosis
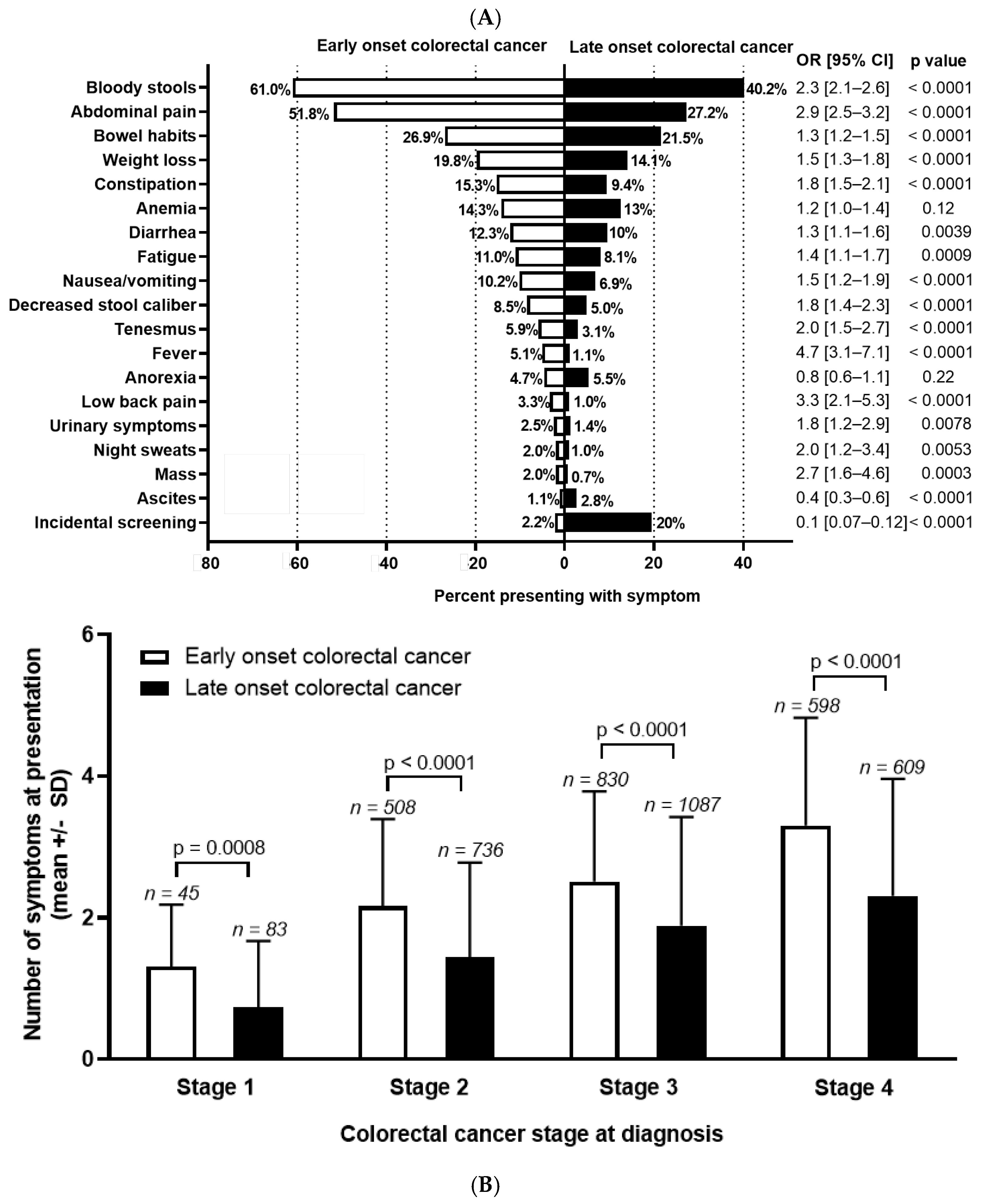
Among patients who underwent chart reviews, sufficient history was present in 1992 EoCRC and 2504 LoCRC to assign symptoms preceding diagnosis. Differences in symptoms are presented in Figure 1A. The most common symptoms at presentation are bloody stools (EoCRC 61.0% vs. LoCRC 40.2%; OR 2.3 (95% CI 2.1–2.6), p < 0.0001); abdominal pain (EoCRC 51.8% vs. LoCRC 27.2%; OR 2.9 (95% CI 2.5–3.2), p < 0.0001); and change in bowel habits (EoCRC 26.9% vs. LoCRC 21.5%; OR 1.3 (95% CI 1.2–1.5)), p < 0.0001). When comparing symptoms between groups, EoCRC more commonly presented with nearly all symptoms. This is with the exception of anemia and anorexia, which were not significantly different, and ascites (p < 0.0001) which was more common in LoCRC. Diagnosis through screening for EoCRC was low, as expected, at 2.2%, mainly occurring in individuals with hereditary cancers or inflammatory bowel disease. For LoCRC, this was significantly higher at 19.6% (OR 0.09, 95% CI 0.07–0.12, p < 0.0001).
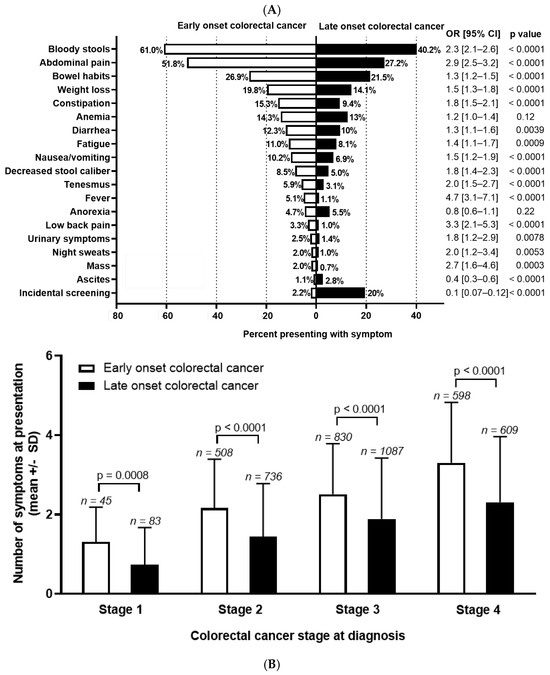
Figure 1.
(A) Symptom distribution and (B) number at diagnosis for early-onset (patients under 50) and late-onset (patients 50 years or older) with colorectal cancer. Odds ratio (OR) and 95% confidence interval (CI) shown to the right of the graph and calculated using Fisher’s exact test. Stage 0 and undefined stage excluded from analysis. Symptom number at each stage of colorectal cancer calculated with unpaired t test.
Next, we compared the total number of symptoms by age, stratified for stage at diagnosis (Figure 1B). As stage increased, the number of symptoms at presentation increased in both groups (p < 0.0001 for both EoCRC and LoCRC). At all stages, EoCRC presented with significantly more symptoms than LoCRC (mean number of symptoms ± SD for Stage 1 EoCRC 1.3 ± 0.9 vs. LoCRC 0.7 ± 0.9, p < 0.001; Stage 2 EoCRC 2.2 ± 1.2 vs. LoCRC 1.4 ± 1.3, p < 0.001; Stage 3 EoCRC 2.5 ± 1.3 vs. LoCRC 1.9 ± 1.5, p < 0.001; Stage 4 EoCRC 3.3 ± 1.5 vs. LoCRC 2.3 ± 1.7, p < 0.001).
3.3. Association of Number of Symptoms at Diagnosis with Survival
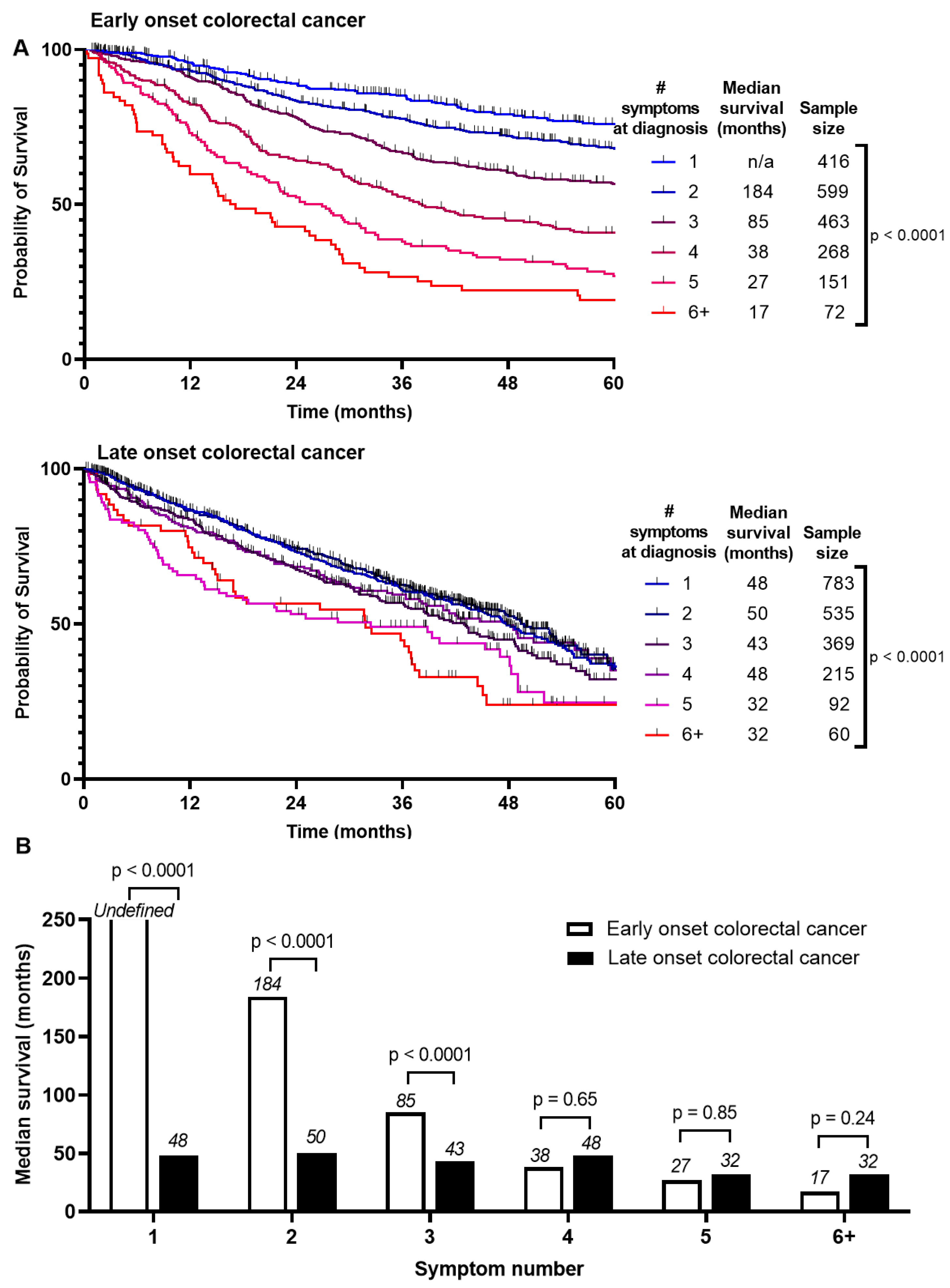
Survival between EoCRC and LoCRC was divided into Stage 1–3 and Stage 4 CRC (Figure S2). Median survival is higher for EoCRC than LoCRC if presenting with Stage 1–3 (190 months vs. 53 months, p < 0.0001). There was no difference if presenting with Stage 4 (18 months vs. 17 months, p = 0.12). We asked whether these survival differences are associated with number of symptoms at presentation (Figure 2A). For EoCRC, median survival is shortened with increased number of symptoms at presentation from 184 months if presenting with 2 symptoms, 85 months if presenting with 3, and 17 months if presenting with 6 or more symptoms (p < 0.0001) (Figure 2B). Similar results were noted for LoCRC, to a lesser magnitude (p < 0.0001). There was little or no difference in disease free survival (Figure S3).
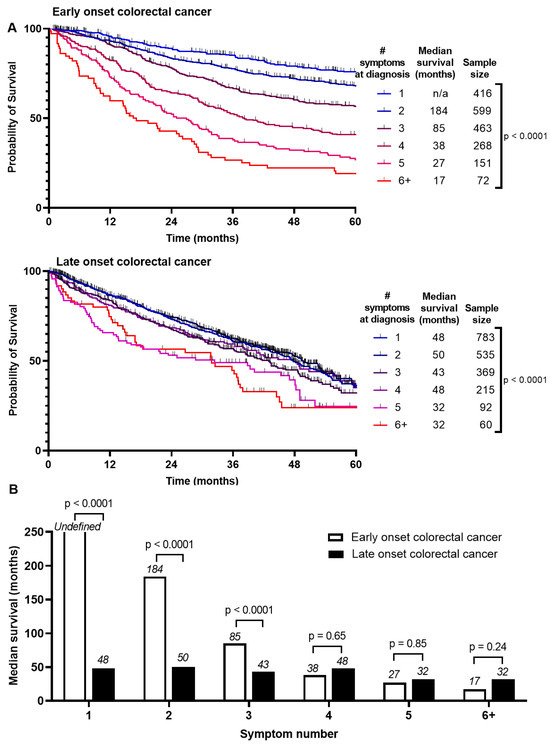
Figure 2.
Comparison of survival by (A) number of symptoms at presentation of patients with early onset (patients under 50) and late onset (patients 50 years or older) with colorectal cancer and (B) summary of median survivals based on number of symptoms at presentation and log-rank test comparing entire survival curve.
Next, we asked whether this difference in survival is due to higher symptom burden being associated with later stage at diagnosis and therefore a worse prognosis. We conducted separate subgroup analyses based on stage at diagnosis in early (stage 1–3) and metastatic (stage 4) CRC (Figure S4). Regardless of whether EoCRC present with early or metastatic disease, the pattern persists where greater symptom burden at presentation, is associated with worse survival (p < 0.0001). This same finding was also seen in LoCRC for both early (p = 0.001) and metastatic stage at diagnosis (p = 0.02). The magnitude of this difference is smaller than for the EoCRC cohort.
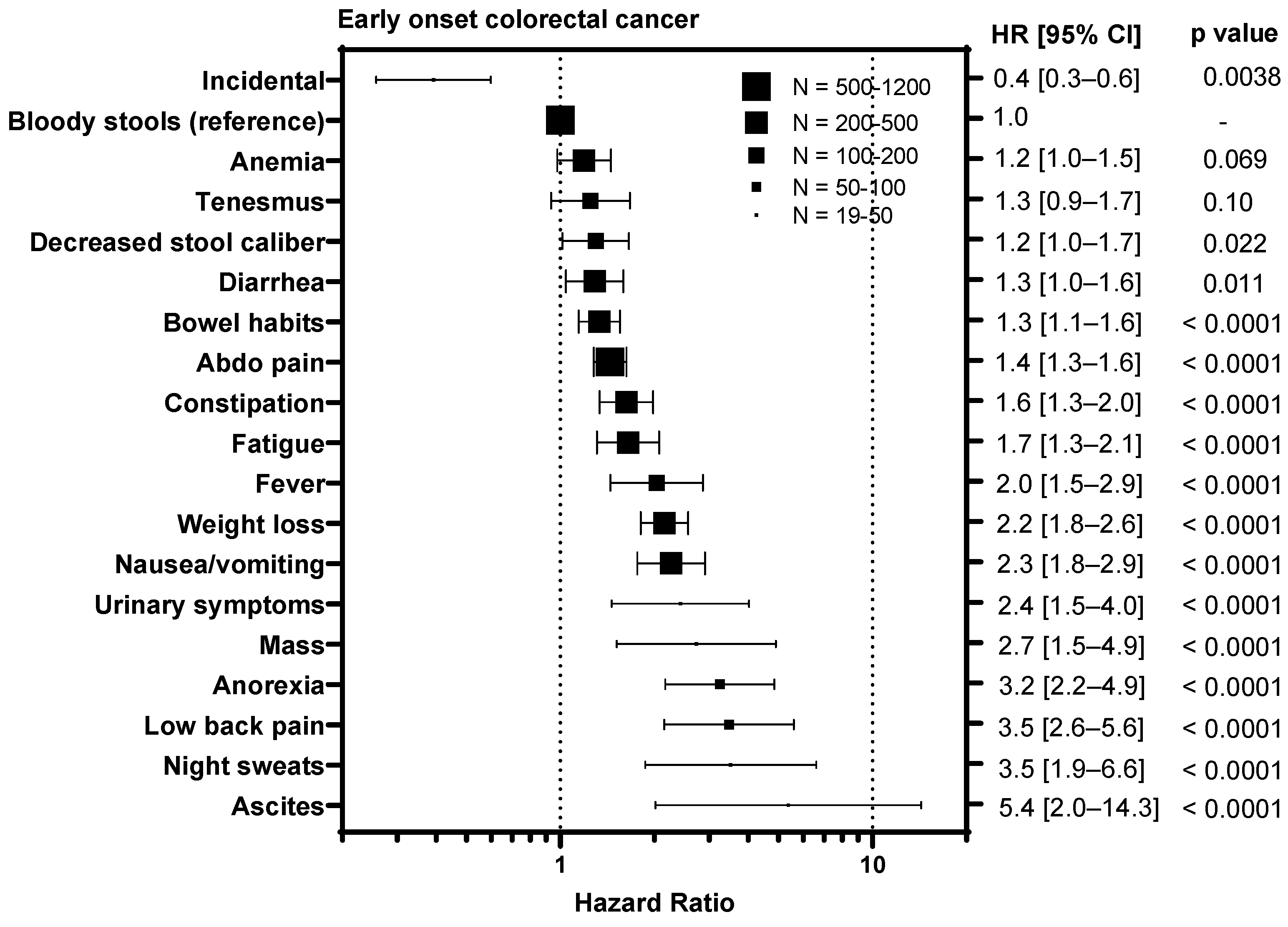
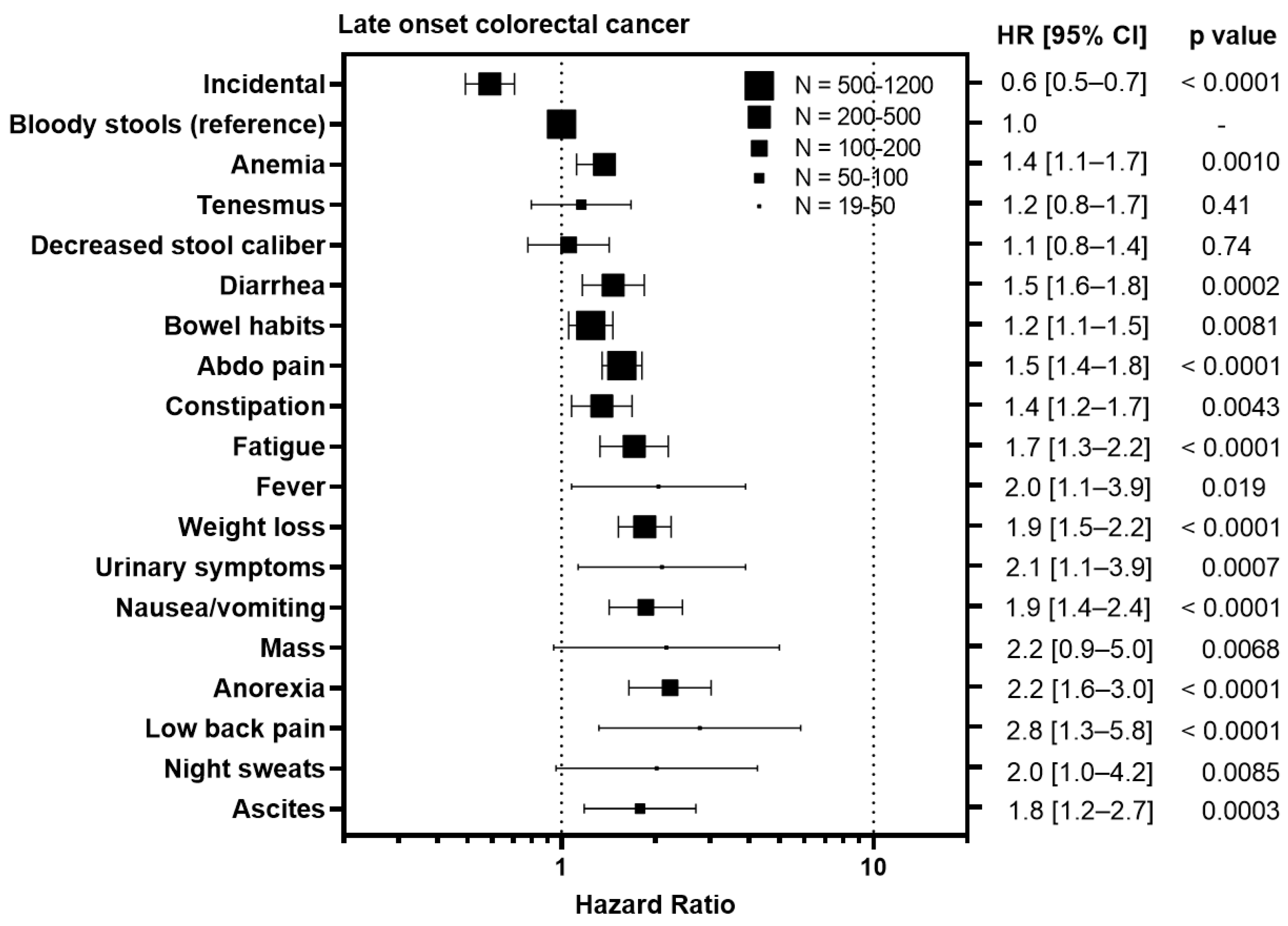
3.4. Symptoms Associated with Advanced Stage Predict Worse Survival
To understand whether certain symptoms are associated with a worse prognosis, we calculated hazard ratios for each symptom compared to bloody stools, the most prevalent symptom (Figure 3). Diagnosis of CRC incidentally during screening colonoscopy/FIT test was associated with an improved outcome in EoCRC with a HR of 0.4 (95% CI 0.3–0.6, p = 0.0038) and LoCRC showing a HR 0.6 (95% CI 0.5–0.7, p < 0.0001). In contrast, symptoms associated with metastatic disease were associated with worse outcomes for both EoCRC and LoCRC. These included ascites (EoCRC HR 5.4, 95% CI 2.0–14.3, p < 0.0001; LoCRC 1.8, 95% CI 1.2–2.7, p = 0.0003); night sweats (EoCRC HR 3.5, 95% CI 1.9–6.6, p < 0.0001; LoCRC 2.0, 95% CI 1.0–4.2, p = 0.0085); fever (EoCRC HR 4.7, 95% CI 3.1–7.1, p < 0.0001; LoCRC 2.8, 95% CI 1.3–5.8, p < 0.0001); and anorexia (EoCRC HR 3.2, 95% CI 2.2–4.9, p < 0.0001; LoCRC 2.2, 95% CI 1.6–3.0, p < 0.0001). In contrast, symptoms associated with local tumor growth including tenesmus (EoCRC HR 1.3, 95% CI 0.9–1.7, p = 0.10; LoCRC 1.2, 95% CI 0.8–1.7, p = 0.41); decreased stool caliber (EoCRC HR 1.2, 95% CI 1.0–1.7, p = 0.022; 1.1, 95% CI 0.8–1.7, p = 0.74); and diarrhea (EoCRC HR 1.3, 95% CI 1.0–1.6, p = 0.011; LoCRC 1.5, 95% CI 1.6–1.8, p = 0.0002) are less or not associated with outcome.
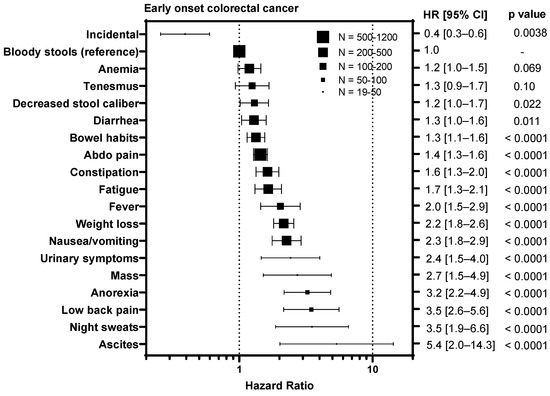
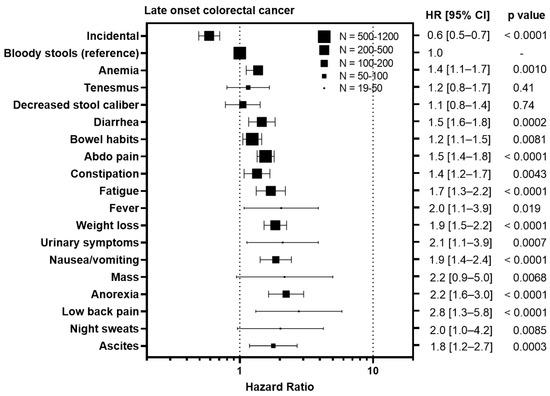
Figure 3.
Impact of symptoms associated with colorectal cancer relative on overall survival to bloody stools in patients with early onset (patients under 50) and late onset (patients 50 years or older) with colorectal cancer. HR = hazard ratio.
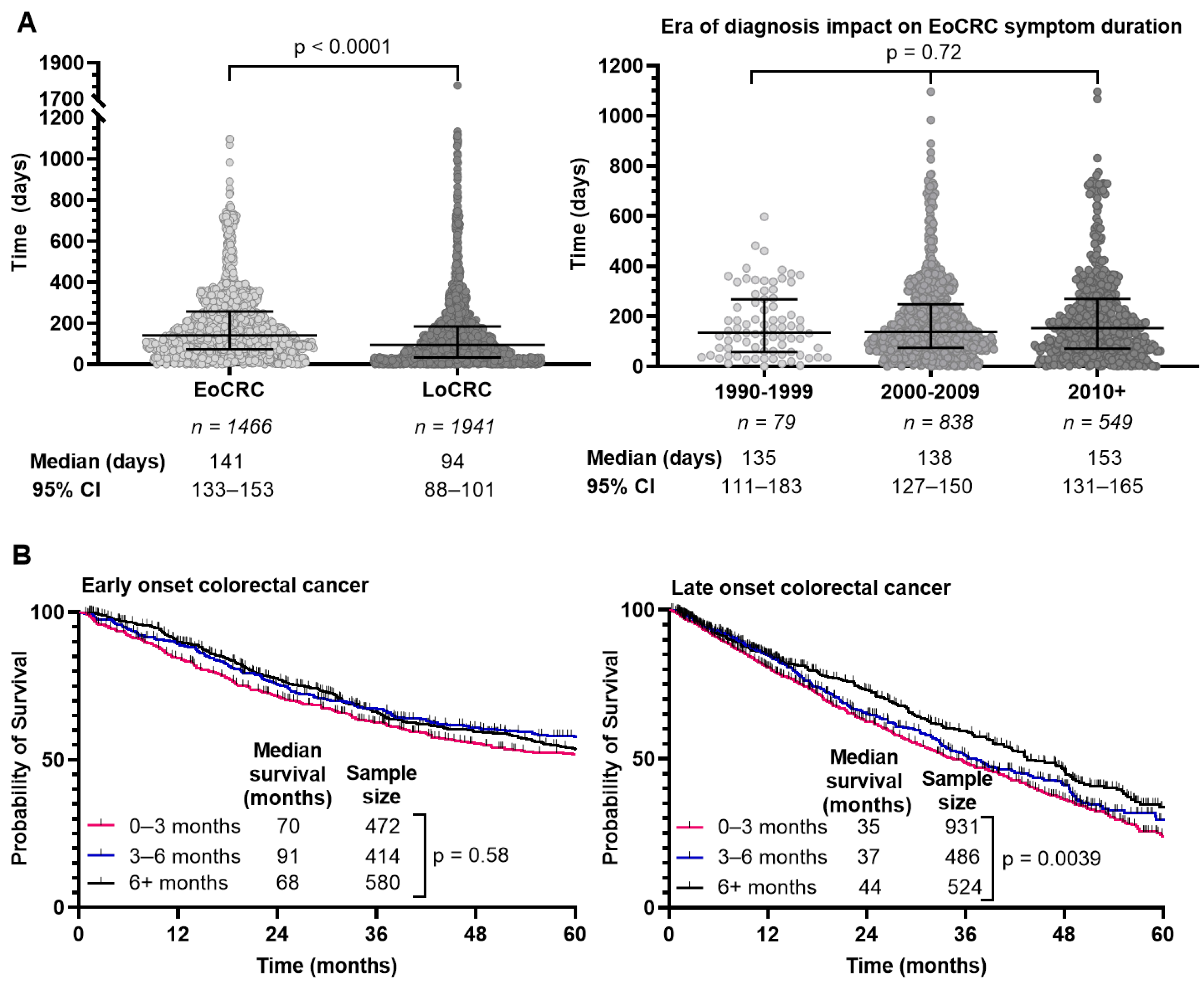
3.5. Time from Symptom Onset to Diagnosis Is Longer for EoCRC
We determined the interval from symptom onset to diagnosis through retrospective chart review where able and summarized the results in Figure 4A. EoCRC had a median interval of 143 days (95% CI 134–154) compared to 95 days (95% CI 88–101) in LoCRC (p < 0.0001). We hypothesized that delay in diagnosis of EoCRC may be mitigated in those diagnosed more recently given increased awareness of the rising incidence of CRC in younger individuals. We separated the population based on diagnosis year and evaluated the interval from symptom onset to diagnosis (Figure 4A, right). In EoCRC, a more recent diagnosis did not result in shorter duration of symptoms before diagnosis. If diagnosed after 2010, median time to diagnosis was 156 days (95% CI 137–169); between 2000–2009 the median time was 138 days (95% CI 126–151); and between 1990–1999 the median time was 134 days (95% CI 103–183) (p = 0.72).
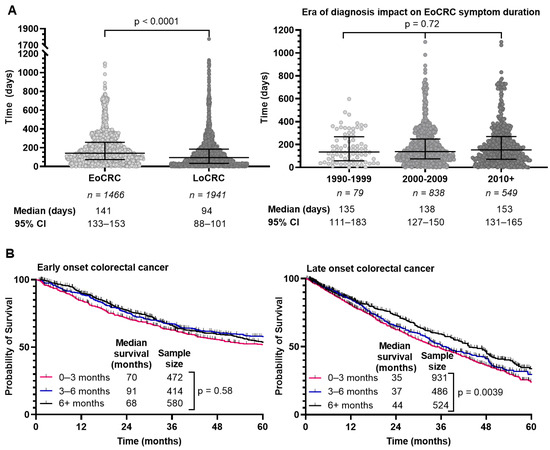
Figure 4.
Duration of symptoms prior to colorectal cancer diagnosis (A), survival of patients with early-onset (patients under 50) and late-onset (patients 50 years or older) colorectal cancer by duration of symptoms (B). Duration of symptoms for each individual excluding inflammatory bowel disease compared using Kruskal–Wallis test (A).
Given the prolonged time to diagnosis in EoCRC compared to LoCRC, we asked whether that affected survival. We compared survival for EoCRC with symptom duration between 0–3, 3–6 and 6+ months (Figure 4B). For EoCRC, there was no difference in survival, with a median survival of 70 months for symptom duration 0–3 months, 91 months for 3–6 months, and 68 months for over 6 months (p = 0.58). In the LoCRC cohort, there was a difference in outcome based on symptom duration, with a median survival of 35 months for symptoms lasting 0–3 months, 37 months for 3–6 months, and 44 months for over 6 months (p = 0.0039).
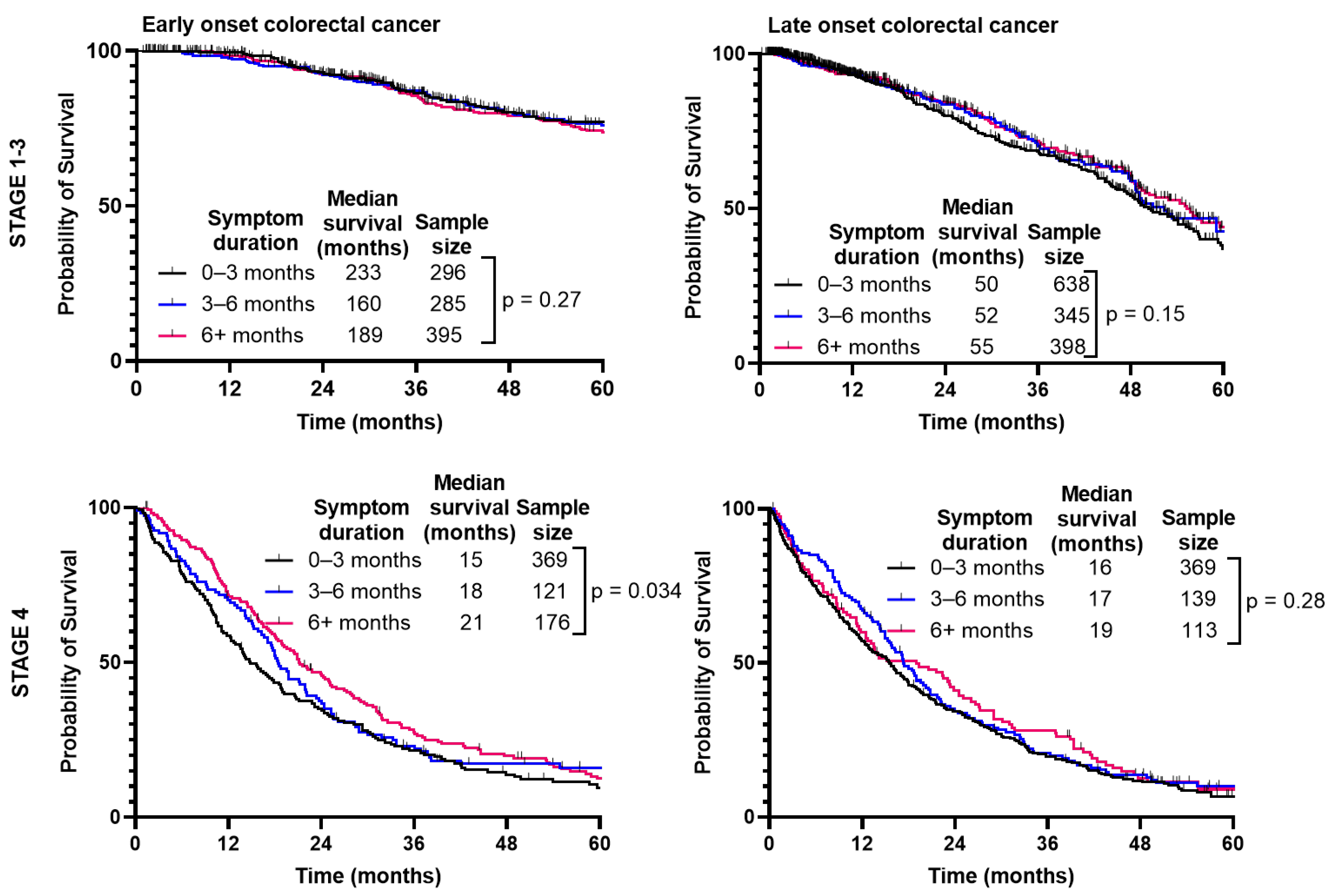
When separated by CRC stage (Figure 5), there was no difference in median survival when comparing duration of symptoms for Stage 1–3 (p = 0.27); however, there was a slight improvement paradoxically with longer symptom duration for Stage 4 (p = 0.034). With LoCRC, there was no difference in survival for Stage 1–3 (p = 0.15) or Stage 4 (p = 0.28).
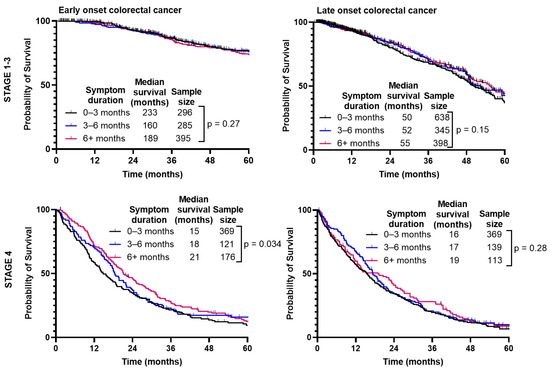
Figure 5.
Survival of patients with early-onset (patients under 50) and late-onset (patients 50 years or older) colorectal cancer is not affected by duration from symptom onset to time of diagnosis.
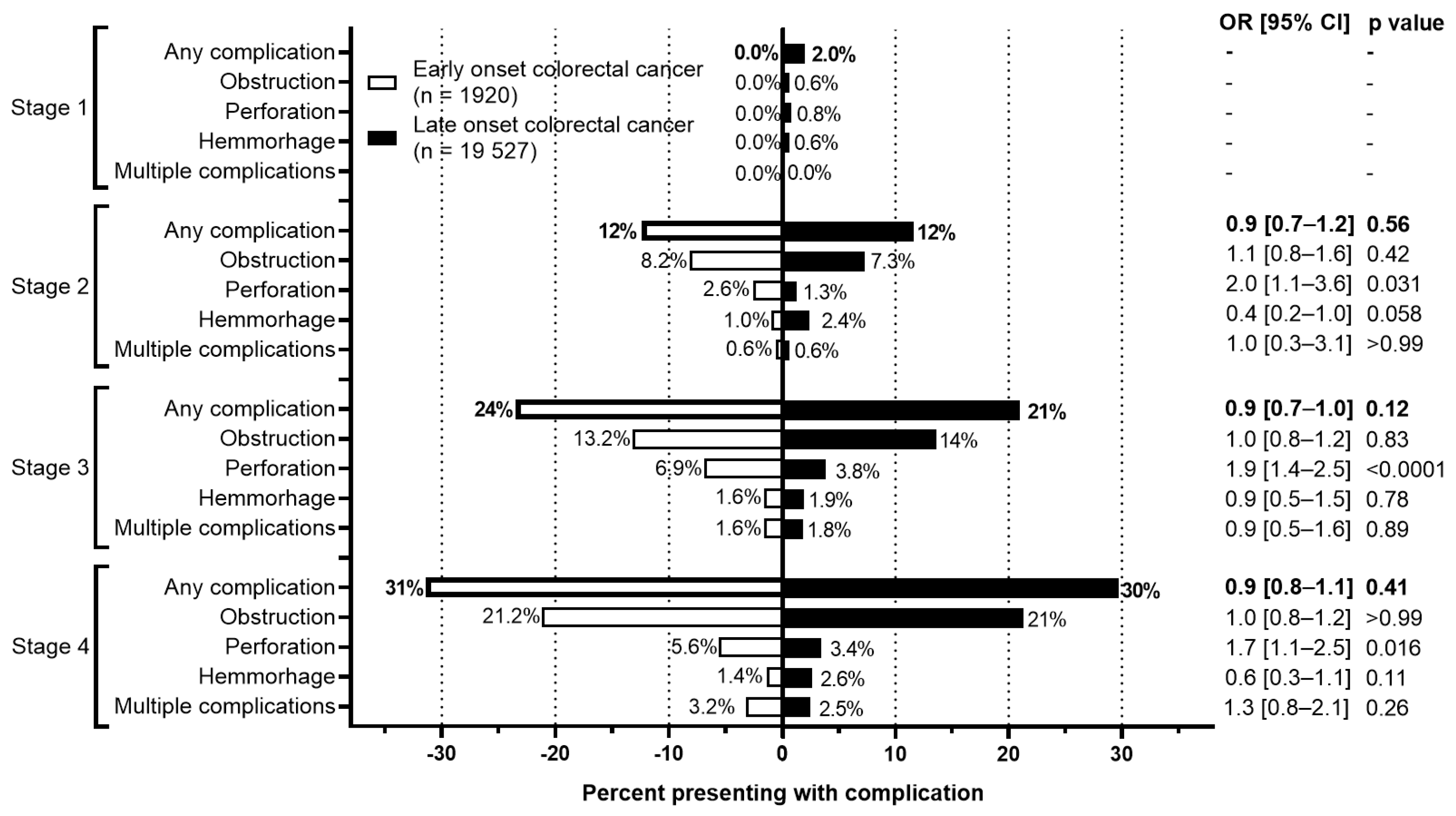
The rate of complications at presentation or during the initial surgery between EoCRC and LoCRC were also evaluated (Figure 6). For both cohorts, more advanced stages were associated with higher rates of complications. For EoCRC, this increased from 0% at stage 1 to 31.4% at stage 4. Similarly, for LoCRC, this increased from 2.0% at stage 1 to 29.7% at stage 4. Between both EoCRC and LoCRC, however, there is no difference in the rate of complications at any stage of presentation.
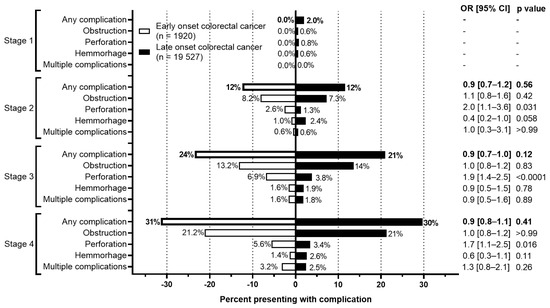
Figure 6.
Rate of complications by stage of colorectal cancer. Odds ratio calculated using Fisher’s exact test.
3.6. Multivariate Analysis Shows Symptom Number, Age, and Stage of CRC Diagnosis Are Independent Predictors of Survival
To account for confounding variables within our known dataset we performed a multivariate analysis that assessed the impact of stage at diagnosis, age, duration of symptoms and symptom burden on outcome (Table S2). We noted that age < 50 years old (HR 0.87, 95% CI 0.78–0.96, p = 0.0080) and fewer number of symptoms at diagnosis are independent predictors of improved overall survival. However, duration of symptoms did not impact survival and did not meet criteria for inclusion in the model (see methods).
4. Discussion
Our study reviewed patterns of presentation, diagnostic delays, and outcomes in EoCRC. Multiple studies have shown early-onset patients experience diagnostic delays and diagnosis at more advanced stages of CRC [8,16,17]. As individuals <50 fall outside screening programs in most countries, it is imperative to understand the constellation of symptoms they present with. Our study demonstrates that EoCRCs present at more advanced stages (although the difference is small in absolute numbers, it is significant due to the size of the database), have more symptoms at diagnosis, and experience diagnostic delays, however in our dataset these delays did not result in worse survival. Furthermore, the number of symptoms at presentation plays a role in determining the survival of all individuals with CRC, but to a much greater extent in EoCRC. This persists after controlling for confounding variables with multivariate analysis.
The pattern of symptoms at presentation is similar between EoCRC and LoCRC. The three most common symptoms are bloody stools, abdominal pain and change in bowel habits, consistent with prior studies [18,19,20]. Several prospective studies [21,22] and a systematic review [23] to determine the positive predictive value of CRC-type symptoms found that these three symptoms individually and in combination are effective at predicting CRC. While it is important to recognize that these three symptoms may be indicative of other benign diseases, placing CRC on the differential is imperative, especially in younger individuals.
Our group found EoCRC has improved survival compared to LoCRC, similar to the work of others [10,24,25,26]. Despite this, the interval duration from symptom onset to diagnosis was longer in EoCRC, in accordance with multiple studies [27,28]. Multiple reasons for this have been postulated, including lack of recognition of symptom significance and denial on both the part of the clinician and patient [4,29]. We have also shown in this study, that duration of symptoms does not impact survival significantly, which is a key finding. In this case, what is driving this survival advantage in EoCRC? We found a strong predictor of survival is the symptom burden at diagnosis and noted that young patients presented with more symptoms. This may be a reflection of a more aggressive tumor causing rapid growth for patients with symptoms, while other young patients may have slower growing tumors and their pre-morbid health may be protective and improve the median survival of the overall cohort.
Our study has important limitations. First, symptoms were collected through retrospective chart review of a pre-defined list of symptoms, some of which may not have been documented, and therefore our analysis may under-represent symptom burden. In addition, our selection of the LoCRC cohort from more recent years (2015–2016) could have introduced a survival bias relative to the EoCRC cohort (1990–2017), as there have been advancements in awareness, screening adherence, and treatment. Second, the duration of symptoms is subject to recall bias as this information was collected from documents at the time of diagnosis, limiting the interpretation of the absolute median days from symptom onset to diagnosis. Third, we found that LoCRC had a rate of 20% detection of cancer through screening. The uptake in BC’s Colon Screening Program in 2019 shows a participation rate of 30–39%, depending on age and a recent randomized study suggested uptake in a clinical trial was only 42% [1]. Overall, uptake in screening programs is low, and with this study we demonstrate the benefit to survival of identifying CRC prior to development of symptoms.
In conclusion, we demonstrate the importance of recognizing symptoms associated with CRC. While symptom burden was associated with survival, we did not note delays in CRC diagnosis as a predictor of survival or complications during initial presentation. With the rising incidence of EoCRC, more work is needed to understand how best to identify these patients early, so they have the best chance of cure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol31040158/s1, Figure S1: Consort diagram of subset cohort of patients younger than 50 (EoCRC) and 50 years or older (LoCRC) with colorectal cancer. Table S1. Demographic characteristics of all individuals ≥ 50 yo and a matched subset of the ≥50 yo cohort (LoCRC). Figure S2. Median survival of patients younger than 50 (EoCRC) and 50 years or older (LoCRC) by stage of cancer at presentation. Figure S3. Disease free survival of patients with early-onset (patients under 50) and late-onset (patients 50 years or older) colorectal cancer by number of symptoms at presentation. Figure S4. Survival of patients with early-onset (patients under 50) and late-onset (patients 50 years or older) with colorectal cancer by number of symptoms at presentation, separated by stage of CRC at presentation. Figure S5. Median survival of patients younger than 50 (EoCRC) and 50 years or older (LoCRC) by symptom number at presentation and stage of diagnosis of colorectal cancer. Table S2. Multivariate analysis of the listed variables controlling for age, diagnosis era, stage at diagnosis, number of symptoms and duration of symptoms (categorical as 0–90, 91–180 and 181+ days).
Author Contributions
V.A.B. conceptualization, methodology, data curation, formal analysis, writing—original draft. A.A.A. conceptualization, methodology, data curation, formal analysis, writing—review and editing. E.B. methodology, formal analysis, writing—review and editing. G.K.L. methodology, formal analysis, writing—review and editing. C.J.B. formal analysis, writing—review and editing. F.D. formal analysis, writing—review and editing. S.G. formal analysis, writing—review and editing. H.C.S. formal analysis, writing—review and editing. J.M.L. conceptualization, methodology, data curation, formal analysis, funding, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was made possible by philanthropic funding from the BC Cancer Foundation. Jonathan Loree Loree has a Michael Smith Health Professional Investigator Award that supports his work.
Institutional Review Board Statement
Data sharing and institutional ethics approval were obtained from the University of British Columbia REB # H19-00988. As this was a retrospective analysis, the need for patient consent was waived by the University of British Columbia REB.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Public Health Agency of Canada. Canadian Cancer Statistics 2019. [Online]. Available online: https://www.canada.ca/en/public-health/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-39-no-8-9-2019/notice-canadian-cancer-statistics-2019.html (accessed on 11 September 2022).
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control CCC 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Kalyta, A.; De Vera, M.A.; Peacock, S.; Telford, J.J.; Brown, C.J.; Donnellan, F.; Gill, S.; Loree, J.M. Canadian Colorectal Cancer Screening Guidelines: Do They Need an Update Given Changing Incidence and Global Practice Patterns? Curr. Oncol. 2021, 28, 1558–1570. [Google Scholar] [CrossRef]
- Canadian Task Force on Preventive Health. Recommendations on screening for colorectal cancer in primary care. CMAJ Can. Med. Assoc. J. 2016, 188, 340–348. [Google Scholar] [CrossRef]
- Scott, R.B.; Rangel, L.E.; Osler, T.M.; Hyman, N.H. Rectal cancer in patients under the age of 50 years: The delayed diagnosis. Am. J. Surg. 2016, 211, 1014–1018. [Google Scholar] [CrossRef]
- Connell, L.C.; Mota, J.M.; Braghiroli, M.I.; Hoff, P.M. The Rising Incidence of Younger Patients with Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr. Treat. Options Oncol. 2017, 18, 23. [Google Scholar] [CrossRef]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef]
- Taggarshe, D.; Rehil, N.; Sharma, S.; Flynn, J.C.; Damadi, A. Colorectal cancer: Are the ‘young’ being overlooked? Am. J. Surg. 2013, 205, 312–316; discussion 316. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.B.; Maggard, M.A.; Livingston, E.H.; Yo, C.K. Colorectal cancer in the young. Am. J. Surg. 2004, 187, 343–348. [Google Scholar] [CrossRef]
- Langenbach, M.R.; Schmidt, J.; Neumann, J.; Zirngibl, H. Delay in treatment of colorectal cancer: Multifactorial problem. World J. Surg. 2003, 27, 304–308. [Google Scholar] [CrossRef]
- Olivo, R.; Ratnayake, S. Colorectal cancer in young patients: A retrospective cohort study in a single institution. ANZ J. Surg. 2019, 89, 905–907. [Google Scholar] [CrossRef]
- Parekh, A.; Hochheimer, C.J.; Espinoza, J.M.; Karlitz, J.J.; Lewis, C.L.; Wani, S.; Patel, S.G. Primary Care Provider Knowledge and Practice in Risk Assessment for Early Age Onset Colorectal Cancer: Opportunities for Improvement. J. Cancer Prev. 2021, 26, 298–303. [Google Scholar] [CrossRef]
- Di Leo, M.; Zuppardo, R.A.; Puzzono, M.; Ditonno, I.; Mannucci, A.; Antoci, G.; Raucci, A.R.; Patricelli, M.G.; Elmore, U.; Tamburini, A.M.; et al. Risk factors and clinical characteristics of early-onset colorectal cancer vs. late-onset colorectal cancer: A case-case study. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1153–1160. [Google Scholar] [CrossRef]
- Castelo, M.; Sue-Chue-Lam, C.; Paszat, L.; Kishibe, T.; Scheer, A.S.; Hansen, B.E.; Baxter, N.N. Time to diagnosis and treatment in younger adults with colorectal cancer: A systematic review. PLoS ONE 2022, 17, e0273396. [Google Scholar] [CrossRef]
- Dozois, E.J.; Boardman, L.A.; Suwanthanma, W.; Limburg, P.J.; Cima, R.R.; Bakken, J.L.R.; Vierkant, R.A.M.; Aakre, J.A.B.; Larson, D.W. Young-onset colorectal cancer in patients with no known genetic predisposition: Can we increase early recognition and improve outcome? Medicine 2008, 87, 259–263. [Google Scholar] [CrossRef]
- Cercek, A.; Chatila, W.K.; Yaeger, R.; Walch, H.; Fernandes, G.D.S.; Krishnan, A.; Palmaira, L.; Maio, A.; Kemel, Y.; Srinivasan, P.; et al. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. J. Natl. Cancer Inst. 2021, 113, djab124. [Google Scholar] [CrossRef]
- Riaz, R.; Masood, N.; Benish, A. Red flag symptoms: Detailed account of clinicopathological features in young-onset colorectal cancer. Intest. Res. 2017, 15, 203–207. [Google Scholar] [CrossRef]
- Thompson, M.R.; Perera, R.; Senapati, A.; Dodds, S. Predictive value of common symptom combinations in diagnosing colorectal cancer. Br. J. Surg. 2007, 94, 1260–1265. [Google Scholar] [CrossRef]
- Rasmussen, S.; Haastrup, P.F.; Balasubramaniam, K.; Elnegaard, S.; Christensen, R.D.; Storsveen, M.M.; Søndergaard, J.; Jarbøl, D.E. Predictive values of colorectal cancer alarm symptoms in the general population: A nationwide cohort study. Br. J. Cancer 2019, 120, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Astin, M.; Griffin, T.; Neal, R.D.; Rose, P.; Hamilton, W. The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br. J. Gen. Pract. 2011, 61, e231–e243. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.-J.; Ping, J. Clinicopathological Features and Survival Outcomes of Colorectal Cancer in Young Versus Elderly: A Population-Based Cohort Study of SEER 9 Registries Data (1988–2011). Medicine 2015, 94, e1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Ping, J.; Li, Y.; Adell, G.; Arbman, G.; Nodin, B.; Meng, W.-J.; Zhang, H.; Yu, Y.-Y.; Wang, C.; et al. The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci. Rep. 2015, 5, 10645. [Google Scholar] [CrossRef] [PubMed]
- Kolarich, A.; George, T.J.; Hughes, S.J.; Delitto, D.; Allegra, C.J.; Hall, W.A.; Chang, G.J.; Tan, S.A.; Shaw, C.M.; Iqbal, A. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer 2018, 124, 3510–3519. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.A.; Feingold, D.L.; Forde, K.A.; Arnell, T.; Jang, J.H.; Whelan, R.L. Colorectal cancer in patients under 50 years of age: A retrospective analysis of two institutions’ experience. World J. Gastroenterol. 2013, 19, 5651–5657. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated with Longer Duration of Symptoms or Time to Diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737.e3. [Google Scholar] [CrossRef]
- Mitchell, E.; Macdonald, S.; Campbell, N.C.; Weller, D.; Macleod, U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: A systematic review. Br. J. Cancer 2008, 98, 60–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).