Abstract
En bloc resection is typically performed to treat giant cell tumors of bone (GCTB), particularly when curettage can be challenging owing to extensive bone cortex destruction with soft tissue extension. Few reports have addressed the clinical outcomes after reoperation for local recurrence in patients with GCTB who underwent en bloc resection. In this multicenter retrospective study, we investigated local recurrence, distant metastasis, malignant transformation, mortality, and limb function in patients treated for local recurrence following en bloc resection for GCTB. Among 205 patients who underwent en bloc resection for GCTB of the extremities between 1980 and 2021, we included 29 with local recurrence. En bloc resection was performed for large tumors with soft tissue extension, pathological fractures with joint invasion, complex fractures, and dispensable bones, such as the proximal fibula and distal ulna. Local re-recurrence, distant metastasis, malignant transformation, and mortality rates were 41.4% (12/29), 34.5% (10/29), 6.9% (2/29), and 6.9% (2/29), respectively. The median Musculoskeletal Tumor Society score was 26 (interquartile range, 23–28). The median follow-up period after surgery for local recurrence was 70.1 months (interquartile range, 40.5–123.8 months). Local recurrence following en bloc resection for GCTB could indicate an aggressive GCTB, necessitating careful follow-up.
1. Introduction
A giant cell tumor of bone (GCTB) is an intermediate-grade primary bone tumor. Approximately 5% of all primary bone tumors are GCTB [1]; moreover, GCTBs commonly occur in the distal femur, proximal tibia, and distal radius [1]. GCTB have a high local recurrence rate (median, 20%) [2], 1–9% of GCTBs develop distant metastases [3,4,5,6,7,8], and approximately 2.4% of GCTBs develop malignant transformation (secondary malignant GCTB) [9,10]. The prognosis of malignant GCTB remains poor, with a reported mortality rate of 42–70% [11,12,13,14]. Curettage is the mainstay of treatment for preserving good limb function; however, it is associated with a relatively high local recurrence rate (median, 20%) [2]. En bloc resection (resection of a large bulky tumor virtually without dissection) should be considered in cases of extensive cortical destruction with extensive soft tissue involvement [15,16]. GCTB often extends close to the joints, necessitating resection of the joints and reconstruction with prostheses or allografts in extremities other than the proximal fibula and distal ulna [15,16]. En bloc resection and reconstruction with a prosthesis or allograft can reduce local recurrence rates (2–13%) compared to curettage; however, the postoperative function is poor, leading to more frequent complications, such as loosening of the prosthesis, fracture of the allograft, and joint subluxation [16,17,18]. Because tourniquets cannot be used for GCTB in the proximal femur or proximal humerus, the amount of bleeding is greater than that at other extremity sites. Hence, preoperative embolization is required [16,17].
Few reports have examined clinical outcomes after reoperation for local recurrence in patients with GCTB following en bloc resection [19,20,21,22,23]. Therefore, we performed a three-center retrospective study to investigate the rates of local recurrence, distant metastasis, malignant transformation, mortality, and limb function after reoperation for local recurrence of GCTB in the extremities following en bloc resection.
2. Materials and Methods
Among 620 patients with histologically diagnosed GCTB of the extremities treated at the authors’ institutions between January 1980 and December 2021, 29 patients with local recurrence after en bloc resection were retrospectively analyzed (Figure 1). The following data were collected from the patient’s medical records: age, sex, tumor site, Campanacci stage at presentation [24], lung metastasis at presentation, pathological fracture at presentation, denosumab administration before the first en bloc resection and before surgery for local recurrence, previous surgery at another hospital, time from the first en bloc resection to local recurrence, site of local recurrence (bone or soft tissue), surgical procedure for local recurrence, local re-recurrence, time from surgery for local recurrence to local re-recurrence, distant metastasis, malignant transformation, tumor death, postoperative follow-up period, oncological outcome (whether or not the tumor was observed at the final follow-up, whether or not the patient died of the tumor), Musculoskeletal Tumor Society (MSTS) score [25], surgery-related complications, and denosumab-related complications (Table 1 and Table 2).
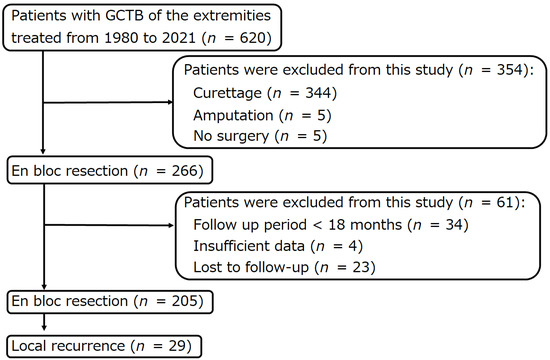
Figure 1.
Flow diagram of patients with giant cell tumors of bones of the extremities, who were treated at three institutions between 1980 and 2021.

Table 1.
Patients’ characteristics.

Table 2.
Patients’ outcomes.
Initial en bloc resection was performed for large tumors with soft tissue extension, joint involvement, complex fractures, or dispensable bones, such as the proximal fibula or distal ulna [26]. Overall, 4 patients underwent no reconstruction, 13 underwent allograft reconstruction, 8 underwent prosthetic reconstruction, 2 underwent free vascularized fibula graft reconstruction, 1 underwent arthrodesis by translocating the ipsilateral ulna as a vascularized graft [27], and 1 underwent reconstruction of the quadriceps tendon with mesh (Table 1). Local recurrence occurred in the bone and soft tissue of 11 (37.9%) and 18 (62.1%) patients, respectively. Curettage for local recurrence was performed in 3 of 29 patients (10.3%) with moderate cortical thinning and well-maintained bone structure [28,29]. Curettage was performed through a large cortical bone window using a sharp curette, and all visible tumors were removed [28,29]. Next, curettage was performed via the cavity using a high-speed bar, followed by washing with saline to remove the entire tumor [28,29]. Phenol was applied to the cavity border using a cotton-tipped applicator and neutralized with alcohol. Subsequently, the tumor cavity was filled with polymethyl methacrylate (PMMA) bone cement (Table 1). En bloc resection for local recurrence was performed in 25 of the 29 patients (86.2%), of whom 19 did not require any reconstruction, 4 underwent reconstruction with a prosthesis, and 2 underwent reconstruction with an allograft. Amputation was performed in one of the 29 patients (3.4%) with massive local recurrence surrounding the neurovasculature of the popliteal fossa (Table 1).
Denosumab was used to downstage the tumor by promoting the shrinkage of extraosseous lesions, tumor hardening, and osteosynthesis of pathological fractures (Table 1). Prior to the initial en bloc resection, two patients received denosumab (120 mg) subcutaneously, once weekly 1 one month, followed by once monthly for a total of 2–9 doses, depending on the clinical benefit, surgical plan, and clinical trial protocol (6 months). No denosumab was administered postoperatively. The patient received oral calcium (500 mg/day) and vitamin D (≥400 IU/day) supplements to prevent hypocalcemia. In addition, two patients received 1 or 29 doses of denosumab before surgery for local recurrence. En bloc resection was performed after the administration of denosumab (Table 1).
Patients underwent follow-up examinations (radiography of the tumor area and computed tomography of the chest) every 4 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Local recurrence, lung metastasis, malignant transformation, and treatment-related complications were also recorded.
Statistical Analysis
Local recurrence-free survival was defined as the period between surgery for local recurrence and local recurrence or last follow-up. Distant metastasis-free survival was defined as the interval between surgery for local recurrence and distant metastasis or the last follow-up. Malignant transformation-free survival was defined as the interval between surgery for local recurrence and the diagnosis of malignant transformation or the last follow-up. Disease-specific survival was defined as the interval between surgery for local recurrence and death from the disease or last follow-up. Local recurrence-free survival, distant metastasis-free survival, malignant transformation-free survival, and disease-specific survival were evaluated using Kaplan–Meier survival analysis, and survival curves were compared using a log-rank test. The data were analyzed using the JMP 14 software (SAS Institute Inc., Cary, NC, USA).
3. Results
Local recurrence occurred in 12 out of 29 patients (41.4%). In the three patients who underwent curettage, local re-recurrence occurred in two patients (66.7%), whereas in the 26 patients who underwent en bloc resection, local recurrence occurred in 10 patients (38.5%). Five of the 11 patients (45.5%) who had local recurrence in the bone experienced local re-recurrence, whereas 7 out of 18 patients (38.9%) who had local recurrence in the soft tissue experienced local re-recurrence. The median time from surgery for local recurrence to local re-recurrence was 7.5 months (interquartile range [IQR], 4–25.3) (Table 2). The five-year local re-recurrence-free survival after surgery for local recurrence was 58.2% (95% confidence interval (CI): 39.0–75.2) (Figure 2a). Curettage for local re-recurrence was performed in two patients. En bloc resection for local re-recurrence was performed in 10 patients. None of the patients had a third local recurrence.
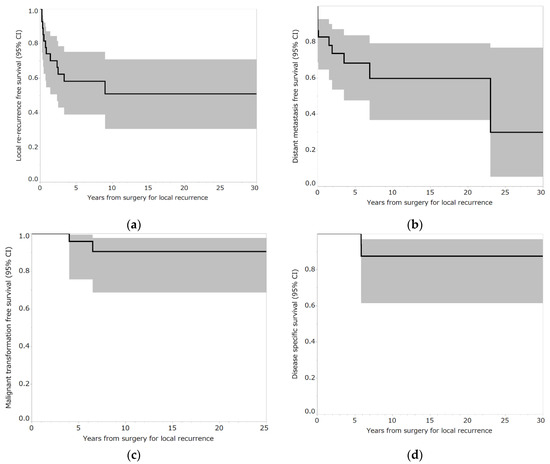
Figure 2.
(a) Local re-recurrence-free survival rates of patients who underwent reoperation for local recurrence following en bloc resection for bone giant cell tumor of the extremities. Shading around the curves represents 95% confidence intervals (CI). (b) Distant metastasis-free survival rates of patients who underwent reoperation for local recurrence following en bloc resection for bone giant cell tumor of the extremities. Shading around the curves represents 95% CI. (c) Malignant transformation-free survival rates of patients who underwent reoperation for local recurrence following en bloc resection for bone giant cell tumor of the extremities. Shading around the curves represents 95% CI. (d) Disease-specific survival rates of patients who underwent reoperation for local recurrence following en bloc resection for bone giant cell tumor of the extremities. Shading around the curves represents 95% CI.
The distant metastasis rate was 34.5% (10 out of 29 patients). Two patients had lung metastases at presentation and two patients exhibited lung metastases at the time of surgery for local recurrence. Five patients had lung metastases and one had iliac metastasis after surgery for local recurrence. The median time from surgery for local recurrence to distant metastasis was 9.5 months (IQR, 0–52.3 months) (Table 2). The five-year distant metastasis-free survival after surgery for recurrence was 68.3% (95% CI: 47.5–83.7) (Figure 2b). Six patients underwent surgery for lung metastases. One patient with iliac metastases underwent curettage (Case 27) (Table 3 and Table 4). Two patients were followed-up for lung metastases. One patient experienced malignant transformation and died of the tumor (case 26) (Table 3 and Table 4).

Table 3.
Details of 29 patients with GCTB of the extremities who experienced local recurrence after en bloc resection.

Table 4.
Details of 29 patients with GCTB of the extremities who experienced local recurrence after en bloc resection.
The malignant transformation rate was 6.9% (2 out of 29 patients). The median time from surgery for local recurrence to malignant transformation was 13.5 months (IQR, 3–24 months) (Table 2). The five-year malignant transformation-free survival rate after surgery for local recurrence was 92.4% (95% CI: 74.1–98.1) (Figure 2c). (Table 3 and Table 4, respectively). One patient underwent external hemipelvectomy but experienced local recurrence and died due to the tumor (Case 5) (Table 3 and Table 4). The other patient underwent amputation and chemotherapy (cisplatin, ifosfamide, doxorubicin, gemcitabine, and docetaxel) but died after developing lung metastases (Case 26) (Table 3 and Table 4). The mortality rate was 6.9% (2 out of 29 patients). The median time from surgery for local recurrence to tumor death was 70 months (IQR: 70–70 months) (Table 2). Five-year disease-specific survival after surgery for local recurrence was 100% (Figure 2d).
The median MSTS score was 26 (IQR: 23–28) (Table 2). Surgical complications included two cases of infection requiring debridement and antibiotics (Cases 5 and 19) (Table 3 and Table 4) and one case of wrist arthropathy requiring arthrodesis (Case 6) (Table 3 and Table 4). One patient presented with an allograft fracture requiring allograft replacement (Case 3) (Table 3 and Table 4). No denosumab-related complications were noted (Table 2). The median follow-up period after the initial en bloc resection was 97 months (IQR, 66.4–141.5 months). The median follow-up period after surgery for local recurrence was 70.1 months (IQR, 40.5–123.8 months) (Table 2).
There were no significant correlations between local recurrence-free survival and variables, such as sex, age, tumor site, Campanacci stage at presentation, lung metastasis at presentation, pathological fracture at presentation, denosumab administration before the first en bloc resection, previous surgery, time from the first en bloc resection to local recurrence, location of local recurrence (bone or soft tissue), surgical method for local recurrence, denosumab administration before surgery for local recurrence, distant metastasis, or malignant transformation (Table 5).

Table 5.
Univariate analysis for local re-recurrence-free survival in patients who experienced local recurrence after en bloc resection for GCTB of the extremities.
4. Discussion
Currently, there are few reports regarding the outcomes of reoperation for local recurrence after en bloc resection [19,20,21,22,23]. In this study, en bloc resection was performed for tumors with large extraosseous lesions, pathologic fractures with joint involvement, complex fractures, or dispensable bones, such as the proximal fibula and distal ulna. The local recurrence rate (41.4%), distant metastasis rate (34.5%), malignant transformation rate (6.9%), and mortality rate (6.9%) after reoperation for local recurrence after en bloc resection were higher than those reported in previous studies (local recurrence rate after initial surgery (median), 20% [2]; distant metastasis rate, 1–9% [3,4,5,6,7,8]; malignant transformation rate (median), 2.4% [9,10] and mortality rate, 1–1.7%) [11,12,13,14]. Therefore, recurrent GCTB after en bloc resection seems to exhibit markedly aggressive behavior and warrants careful follow-up after reoperation (Figure 3).
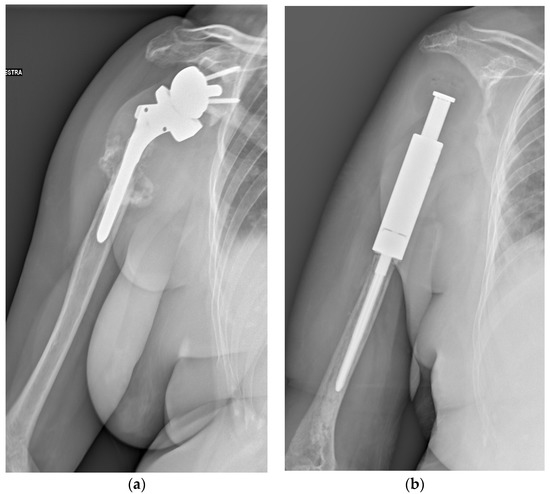
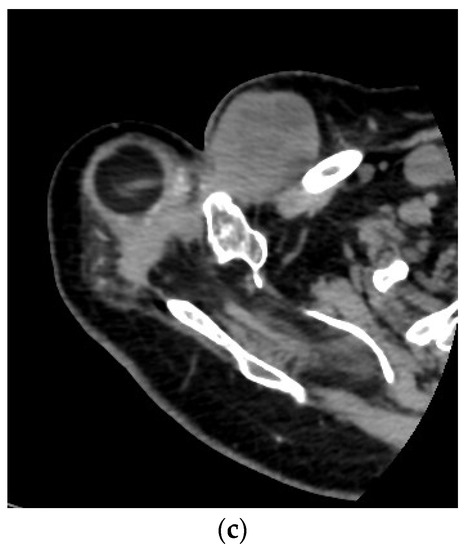
Figure 3.
A 71-year-old female patient underwent en bloc resection and reconstruction with a prosthesis for a bone giant cell tumor of the proximal humerus with a pathological fracture at presentation. Six months postoperatively, she experienced local recurrence and was treated with denosumab for 2 years and 5 months (a). She then underwent tumor resection and revision of prosthesis (b). One year after the revision, she experienced local re-recurrence and underwent tumor resection. One month later, a third local recurrence and lung metastasis were detected, and the biopsy revealed malignant transformation (c). She died of the disease 6 years and 4 months after the first surgery (Case 26, Table 3 and Table 4).
The local recurrence rate following en bloc resection in patients with GCTB is approximately 2–13% [19,20,21,22,23]. Prosser et al. reported that resection was performed in two patients with GCTB with local recurrence after en bloc resection, and local recurrence was observed in one patient (50%) [19]. Klenke et al. reported that 18 patients with GCTB that had locally relapsed after en bloc resection underwent resection, and one (6%) patient experienced local re-recurrence [20]. Niu et al. reported that a patient who experienced local recurrence after en bloc resection had an aggressive course [22]. The patient experienced multiple soft tissue recurrences after en bloc resection of GCTB of the distal ulna. He was treated with denosumab for 6 months and then underwent resection of multiple lesions but experienced multiple extensive soft tissue recurrences shortly after surgery and amputation [22]. A pathological examination revealed no malignant transformation. After the discontinuation of denosumab, multiple lung metastases showed significant progression [22]. Zhang et al. reported that a patient with GCTB of the proximal humerus experienced local recurrence in the soft tissue 18 months after receiving six doses of preoperative denosumab and en bloc resection. The patient remained disease-free for 9 months after undergoing en bloc resection for local soft tissue recurrence [23].
Higher local recurrence rates have been reported in the distal radius, proximal femur, hands, and feet [15,30,31]. The higher recurrence rate in the distal radius can be attributed to the relatively fragile bone quality of the distal radius, with the proximity of the distal radius to the carpal bones and ulna making adequate curettage more challenging [32,33]. The high recurrence rate in the proximal femur can be explained by insufficient curettage owing to the risk of osteonecrosis and fractures [15]. Furthermore, the high recurrence rate in the hand and foot could be attributed to difficulties in opening a large window, resulting in insufficient curettage, which makes it impossible to secure a sufficient field of view [30]. Herein, we detected no significant correlation between tumor site and local recurrence-free survival in patients with local recurrence after en bloc resection. Previous studies have reported that 9–30% of patients with GCTB may exhibit pathological fractures at presentation [31,34,35,36,37,38]. Patients with pathological fractures are often considered to have a more aggressive disease; however, a recent meta-analysis reported that pathological fractures have no significant effect on the local recurrence of GCTB [39]. In the present study, no significant correlation was observed between the presence of pathological fractures and local recurrence-free survival in patients with local recurrence after en bloc resection.
Furthermore, we found no association between the time from initial en bloc resection to local recurrence and local recurrence-free survival. Takeuchi et al. reported the prognoses of 94 patients with recurrence after initial curettage and those of 16 patients with recurrence after initial en bloc resection for GCTB of the extremities (110 patients). Of these, 25 patients experienced a second local relapse, and 6 patients had a third local relapse. The time from the first surgery to the first recurrence in the repeated recurrence group (two and three recurrences) was significantly shorter than that in the single recurrence group (mean 14.1 vs. 28.3 months, respectively; p = 0.016) [40]. Therefore, if the time between the first surgery and local recurrence is less than 24 months, the patient should be followed up carefully, given the high local re-recurrence rate after reoperation.
In GCTB, neoplastic stromal cells are rich in receptor activation of nuclear factor-kappa β (RANK) ligands, which induce receptor activation in RANK-positive osteoclast-like giant cells and their precursors [41,42,43,44,45,46,47]. Denosumab is a fully human monoclonal antibody that inhibits the RANK ligand (RANKL). It suppresses RANK-RANKL interaction and prevents bone destruction caused by giant cell tumors [45,48,49]. Denosumab was approved by the Food and Drug Administration in 2013, following reports of its observed efficacy and safety, although no other drugs have been approved for use in GCTB [50]. Denosumab has also been reported to exert a downstaging effect in less invasive surgery [51]. Currently, denosumab is indicated for the treatment of unresectable GCTB with significant functional impairment after resection [50]. In the case of GCTB with large soft tissue components in close proximity to neurovascular structures, the bony margins formed after denosumab administration can help reduce potential damage to neurovascular structures when the tumor and neurovasculature are dissected [52,53]. Denosumab may also facilitate intraoperative manipulation, prevent inadvertent tumor contamination, and decrease recurrence rates [52,53]. However, it has been reported that preoperative denosumab administration does not reduce the local recurrence rate after en bloc resection [53,54,55]. In this study, no significant correlation was observed between preoperative denosumab administration and local recurrence-free survival in patients with local recurrence after en bloc resection.
The outcome of lung metastases from GCTB varies from spontaneous regression to uncontrolled growth, with eventual death [56]. Mortality rates reported for metastatic GCTB range from 0 to 25% [5,6,7,57,58,59,60,61,62]. Lung metastases were significantly more common in patients with local recurrence [56]. In this study, no significant correlation was observed between the occurrence of lung metastases and local re-recurrence-free survival in patients with local recurrence after en bloc resection. Malignant GCTB is divided into primary and secondary types [9,13]. Primary malignant GCTB is characterized by the simultaneous presence of sarcoma and GCTB at initial diagnosis. Secondary malignant GCTB develops at sites of GCTB previously treated with surgery or radiotherapy (malignant transformation) [13]. Although malignant transformation was found to be significantly more common in patients with local recurrence [10], there was no significant correlation between the occurrence of malignant transformation and local recurrence-free survival in patients with local recurrence after en bloc resection in the present study.
This study had several limitations. First, indication bias for each treatment was associated with the retrospective study design. The small number of patients precluded multivariate analysis, and we could not adjust for confounding factors. Second, there is a possibility of type 2 errors due to the small number of cases. An increase in the number of patients could result in the emergence of factors with significant differences. In the future, a multivariate analysis with a large sample size is necessary. Third, the presence of the H3F3A mutation could only be confirmed in 4 out of 29 patients. Two of the four patients experienced malignant transformation, and no H3F3A mutations were observed in the malignant lesions. Therefore, the H3F3A mutation was not confirmed in the remaining 25 cases. These patients were diagnosed before evaluation. However, these patients were diagnosed by an experienced pathologist specializing in bone tumors.
5. Conclusions
The rates of local recurrence, distant metastasis, malignant transformation, and death after reoperation for local recurrence following en bloc resection are high. Therefore, GCTB that develop local recurrence despite en bloc resection are markedly aggressive and require careful follow-up.
Author Contributions
Conceptualization, S.T., A.F.M. and C.E.; methodology, S.T., A.F.M. and C.E.; formal analysis, S.T., T.M. and C.E.; data curation, S.T., C.E. and S.H.; writing—original draft preparation, S.T. and C.E.; writing—review and editing, A.F.M.; supervision, K.H., A.K., H.F., Y.T., P.S.C. and D.M.D.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of IRCCS Istituto Ortopedico Rizzoli (protocol code 0008286 and date of approval 15 March 2016), Nara Medical University (protocol code 2833 and date of approval 27 November 2020), and HCG Hospital (protocol code EC/330/17/03 and date of approval 4 May 2017).
Informed Consent Statement
Informed consent was obtained from all participants at the IRCCS Istituto Ortopedico Rizzoli and HCG Hospital. The requirement for written consent from participants at Nara Medical University was waived because an “opt-out” process was used and the retrospective nature of the study.
Data Availability Statement
The datasets generated, analyzed, or both during the present study are not publicly available because of privacy issues but are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all the patients and their families.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flanagan, A.M.; Larousserie, F.; O’Donnell, P.G.; Yoshida, A. Giant cell tumour of bone. In Soft Tissue and Bone Tumours. WHO Classification of Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2020; pp. 440–446. [Google Scholar]
- Machak, G.N.; Snetkov, A.I. The impact of curettage technique on local control in giant cell tumour of bone. Int. Orthop. 2021, 45, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Dominkus, M.; Ruggieri, P.; Bertoni, F.; Briccoli, A.; Picci, P.; Rocca, M.; Mercuri, M. Histologically verified lung metastases in benign giant cell tumours--14 cases from a single institution. Int. Orthop. 2006, 30, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Seethalakshmi, V.; Jambhekar, N.A.; Prabhudesai, S.; Merchant, N.; Puri, A.; Agarwal, M. Clinicopathologic Profile of 470 giant cell tumors of bone from a cancer hospital in Western India. Ann. Diagn. Pathol. 2008, 12, 239–248. [Google Scholar] [CrossRef]
- Rock, M.G.; Pritchard, D.J.; Unni, K.K. Metastases from histologically benign giant-cell tumor of bone. J. Bone Jt. Surg. Am. 1984, 66, 269–274. [Google Scholar] [CrossRef]
- Siebenrock, K.A.; Unni, K.K.; Rock, M.G. Giant- cell tumour of bone metastasising to the lungs. A long-term Follow-up. J. Bone Jt. Surg. Br. 1998, 80, 43–47. [Google Scholar] [CrossRef]
- Tubbs, W.S.; Brown, L.R.; Beabout, J.W.; Rock, M.G.; Unni, K.K. Benign giant-cell tumor of bone with pulmonary metastases: Clinical findings and radiologic appearance of metastases in 13 cases. Am. J. Roentgenol. 1992, 158, 331–334. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Leone, G.; Righi, A.; Akahane, M.; Tanzi, P.; Kido, A.; Honoki, K.; Tanaka, Y.; Donati, D.M.; et al. Denosumab does not decrease the risk of lung metastases from bone giant cell tumour. Int. Orthop. 2019, 43, 483–489. [Google Scholar] [CrossRef]
- Palmerini, E.; Picci, P.; Reichardt, P.; Downey, G. Malignancy in giant cell tumor of bone: A review of the literature. Technol. Cancer Res. Treat. 2019, 18, 1533033819840000. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Righi, A.; Mavrogenis, A.F.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; Errani, C. Late Local recurrence of bone giant cell tumors associated with an increased risk for malignant transformation. Cancers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Anract, P.; De Pinieux, G.; Cottias, P.; Pouillart, P.; Forest, M.; Tomeno, B. Malignant giant-cell tumours of bone. clinico-pathological types and prognosis: A review of 29 cases. Int. Orthop. 1998, 22, 19–26. [Google Scholar] [CrossRef]
- Bertoni, F.; Bacchini, P.; Staals, E.L. Malignancy in giant cell tumor of bone. Cancer 2003, 97, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chan, C.M.; Gong, L.; Bui, M.M.; Han, G.; Letson, G.D.; Yang, Y.; Niu, X. Malignancy in giant cell tumor of bone in the extremities. J. Bone Oncol. 2021, 26, 100334. [Google Scholar] [CrossRef]
- Joo, M.W.; Lee, Y.-S.; Park, H.S.; Chung, Y.-G.; Yoon, C. Secondary malignancy in giant cell tumor: A single-center study. Curr. Oncol. 2022, 29, 4068–4080. [Google Scholar] [CrossRef]
- Errani, C.; Ruggieri, P.; Asenzio, M.A.N.; Toscano, A.; Colangeli, S.; Rimondi, E.; Rossi, G.; Longhi, A.; Mercuri, M. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat. Rev. 2010, 36, 1–7. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Kido, A.; Errani, C. Current concepts in the treatment of giant cell tumors of bone. Cancers 2021, 13, 3647. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, Q.; Hao, L.; Ding, Y.; Li, Y.; Xu, H.; Liu, W. Giant cell tumor of the extremity: Retrospective analysis of 621 Chinese patients from one institution. J. Bone Jt. Surg. Am. 2012, 94, 461–467. [Google Scholar] [CrossRef]
- Van der Heijden, L.; Dijkstra, P.D.S.; Campanacci, D.A.; Gibbons, C.L.M.H.; van de Sande, M.A.J. Giant Cell Tumor with Pathologic Fracture: Should We Curette or Resect? Clin. Orthop. Relat. Res. 2013, 471, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Prosser, G.H.; Baloch, K.G.; Tillman, R.M.; Carter, S.R.; Grimer, R.J. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin. Orthop. Relat. Res. 2005, 435, 211–218. [Google Scholar]
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Recurrent giant cell tumor of long bones: Analysis of surgical management. Clin. Orthop. Relat. Res. 2011, 469, 1181–1187. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Shimizu, T.; Oku, N.; Kitagawa, R.; Tsuchiya, H. Total spondylectomy for Enneking stage III giant cell tumor of the mobile spine. Eur. Spine J. 2018, 27, 3084–3091. [Google Scholar] [CrossRef]
- Niu, X.; Yang, Y.; Wong, K.C.; Huang, Z.; Ding, Y.; Zhang, W. Giant cell tumour of the bone treated with denosumab: How has the blood supply and oncological prognosis of the tumour changed? J. Orthop. Translat. 2019, 18, 100–108. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Ma, T.-X.; Qi, D.-W.; Zhao, M.; Hu, T.; Zhang, G.-C. Short-term preoperative denosumab with surgery in unresectable or recurrent giant cell tumor of bone. Orthop. Surg. 2019, 11, 1101–1108. [Google Scholar] [CrossRef]
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-cell tumor of bone. J. Bone Jt. Surg. Am. 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanzi, P.; Leone, G.; Ciani, G.; Righi, A.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; et al. Denosumab for bone giant cell tumor of the distal radius. Orthopedics 2020, 43, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Gulia, A.; Agarwal, M.G.; Reddy, K. Ulnar translocation after excision of a Campanacci Grade-3 giant-cell tumour of the distal radius: An effective method of reconstruction. J. Bone Jt. Surg. Br. 2010, 92, 875–879. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanzi, P.; Leone, G.; Akahane, M.; Tanaka, Y.; Errani, C. Curettage as first surgery for bone giant cell tumor: Adequate surgery is more important than oncology training or surgical management by high volume specialized teams. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 3–9. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Akahane, M.; Honoki, K.; Kido, A.; Tanaka, Y.; Donati, D.M.; Errani, C. Risk factors of fracture following curettage for bone giant cell tumors of the extremities. BMC Musculoskelet. Disord. 2022, 23, 477. [Google Scholar] [CrossRef]
- Hindiskere, S.; Errani, C.; Doddarangappa, S.; Ramaswamy, V.; Rai, M.; Chinder, P.S. Is a short-course of preoperative denosumab as effective as prolonged therapy for giant cell tumor of bone? Clin. Orthop. Relat. Res. 2020, 478, 2522–2533. [Google Scholar] [CrossRef]
- O’Donnell, R.J.; Springfield, D.S.; Motwani, H.K.; Ready, J.E.; Gebhardt, M.C.; Mankin, H.J. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J. Bone Jt. Surg. Am. 1994, 76, 1827–1833. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Shih, H.N.; Hsu, K.Y.; Hsu, R.W. Treatment of giant cell tumor of the distal radius. Clin. Orthop. Relat. Res. 2001, 383, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sheth, D.S.; Healey, J.H.; Sobel, M.; Lane, J.M.; Marcove, R.C. Giant cell tumor of the distal radius. J. Hand Surg. Am. 1995, 20, 432–440. [Google Scholar] [CrossRef]
- McDonald, D.J.; Sim, F.H.; McLeod, R.A.; Dahlin, D.C. Giant-cell tumor of bone. J. Bone Jt. Surg. Am. 1986, 68, 235–242. [Google Scholar] [CrossRef]
- Dreinhöfer, K.E.; Rydholm, A.; Bauer, H.C.; Kreicbergs, A. Giant-cell tumours with fracture at diagnosis. Curettage and acrylic cementing in ten cases. J. Bone Jt. Surg. Br. 1995, 77, 189–193. [Google Scholar] [CrossRef]
- Blackley, H.R.; Wunder, J.S.; Davis, A.M.; White, L.M.; Kandel, R.; Bell, R.S. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J. Bone Jt. Surg. Am. 1999, 81, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Deheshi, B.M.; Jaffer, S.N.; Griffin, A.M.; Ferguson, P.C.; Bell, R.S.; Wunder, J.S. Joint salvage for pathologic fracture of giant cell tumor of the lower extremity. Clin. Orthop. Relat. Res. 2007, 459, 96–104. [Google Scholar] [CrossRef]
- Turcotte, R.E.; Wunder, J.S.; Isler, M.H.; Bell, R.S.; Schachar, N.; Masri, B.A.; Moreau, G.; Davis, A.M.; Canadian Sarcoma Group. Giant Cell Tumor of Long Bone: A Canadian Sarcoma Group Study. Clin. Orthop. Relat. Res. 2002, 379, 248–258. [Google Scholar] [CrossRef]
- Salunke, A.A.; Chen, Y.; Chen, X.; Tan, J.H.; Singh, G.; Tai, B.C.; Khin, L.W.; Puhaindran, M.E. Does pathological fracture affect the rate of local recurrence in patients with a giant cell tumour of bone? A meta-analysis. Bone Jt. J. 2015, 97-B, 1566–1571. [Google Scholar] [CrossRef]
- Takeuchi, A.; Tsuchiya, H.; Niu, X.; Ueda, T.; Jeon, D.-G.; Wang, E.H.M.; Asavamongkolkul, A.; Kusuzaki, K.; Sakayama, K.; Kang, Y.-K. The prognostic factors of recurrent GCT: A Cooperative Study by the Eastern Asian Musculoskeletal Oncology Group. J. Orthop. Sci. 2011, 16, 196–202. [Google Scholar] [CrossRef]
- Atkins, G.J.; Kostakis, P.; Vincent, C.; Farrugia, A.N.; Houchins, J.P.; Findlay, D.M.; Evdokiou, A.; Zannettino, A.C.W. RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J. Bone Miner. Res. 2006, 21, 1339–1349. [Google Scholar] [CrossRef]
- Goldring, S.R.; Roelke, M.S.; Petrison, K.K.; Bhan, A.K. Human giant cell tumors of bone identification and characterization of cell types. J. Clin. Investig. 1987, 79, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, J.; Wood, D.J.; Zheng, M.H. Gene Expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-KappaB in giant cell tumor of bone: Possible involvement in tumor cell-induced osteoclast-like cell formation. Am. J. Pathol. 2000, 156, 761–767. [Google Scholar] [CrossRef]
- Liao, T.S.; Yurgelun, M.B.; Chang, S.-S.; Zhang, H.-Z.; Murakami, K.; Blaine, T.A.; Parisien, M.V.; Kim, W.; Winchester, R.J.; Lee, F.Y.-I. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J. Orthop. Res. 2005, 23, 203–209. [Google Scholar] [CrossRef]
- Roux, S.; Amazit, L.; Meduri, G.; Guiochon-Mantel, A.; Milgrom, E.; Mariette, X. RANK (Receptor Activator of Nuclear Factor Kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am. J. Clin. Pathol. 2002, 117, 210–216. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Cheng, E.Y.; Clohisy, D.R.; Thompson, R.C.; Skubitz, A.P.N. Gene expression in giant-cell tumors. J. Lab. Clin. Med. 2004, 144, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Skubitz, K.M. Giant cell tumour of bone. Curr. Opin. Oncol. 2009, 21, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Branstetter, D.G.; Nelson, S.D.; Manivel, J.C.; Blay, J.-Y.; Chawla, S.; Thomas, D.M.; Jun, S.; Jacobs, I. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin. Cancer Res. 2012, 18, 4415–4424. [Google Scholar] [CrossRef]
- Lau, Y.S.; Sabokbar, A.; Gibbons, C.L.M.H.; Giele, H.; Athanasou, N. Phenotypic and molecular studies of giant-cell tumors of bone and soft tissue. Hum. Pathol. 2005, 36, 945–954. [Google Scholar] [CrossRef]
- Chawla, S.; Henshaw, R.; Seeger, L.; Choy, E.; Blay, J.-Y.; Ferrari, S.; Kroep, J.; Grimer, R.; Reichardt, P.; Rutkowski, P.; et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013, 14, 901–908. [Google Scholar] [CrossRef]
- Rutkowski, P.; Ferrari, S.; Grimer, R.J.; Stalley, P.D.; Dijkstra, S.P.D.; Pienkowski, A.; Vaz, G.; Wunder, J.S.; Seeger, L.L.; Feng, A.; et al. Surgical downstaging in an open-label phase ii trial of denosumab in patients with giant cell tumor of bone. Ann. Surg. Oncol. 2015, 22, 2860–2868. [Google Scholar] [CrossRef]
- Müller, D.A.; Beltrami, G.; Scoccianti, G.; Campanacci, D.A.; Franchi, A.; Capanna, R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J. Surg. Oncol. 2016, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Medellin, M.R.; Fujiwara, T.; Tillman, R.M.; Jeys, L.M.; Gregory, J.; Stevenson, J.D.; Parry, M.; Abudu, A. Prognostic factors for local recurrence in extremity-located giant cell tumours of bone with pathological fracture. Bone Jt. J. 2018, 100-B, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Sahito, B.; Ali, S.M.E.; Kumar, D.; Kumar, J.; Hussain, N.; Lakho, T. Role of denosumab before resection and reconstruction in giant cell tumors of bone: A single-centered retrospective cohort study. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 567–574. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanaka, Y.; Kido, A.; Kawaguchi, M.; Errani, C. Denosumab does not decrease local recurrence in giant cell tumor of bone treated with en bloc resection. Orthopedics 2021, 44, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, W.; Xie, X.; Tu, J.; Huang, G.; Zou, C.; Yin, J.; Wen, L.; Shen, J. Development and validation of a prognostic index to predict pulmonary metastasis of giant cell tumor of bone. Oncotarget 2017, 8, 108054–108063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertoni, F.; Present, D.; Enneking, W.F. Giant-cell tumor of bone with pulmonary metastases. J. Bone Jt. Surg. Am. 1985, 67, 890–900. [Google Scholar] [CrossRef]
- Bertoni, F.; Present, D.; Sudanese, A.; Baldini, N.; Bacchini, P.; Campanacci, M. Giant-cell tumor of bone with pulmonary metastases. Six case reports and a review of the literature. Clin. Orthop. Relat. Res. 1988, 237, 275–285. [Google Scholar] [CrossRef]
- Kay, R.M.; Eckardt, J.J.; Seeger, L.L.; Mirra, J.M.; Hak, D.J. Pulmonary Metastasis of benign giant cell tumor of bone. six histologically confirmed cases, including one of spontaneous regression. Clin. Orthop. Relat. Res. 1994, 302, 219–230. [Google Scholar] [CrossRef]
- Viswanathan, S.; Jambhekar, N.A. Metastatic giant cell tumor of bone: Are there associated factors and best treatment modalities? Clin. Orthop. Relat. Res. 2010, 468, 827–833. [Google Scholar] [CrossRef]
- Cheng, J.C.; Johnston, J.O. Giant cell tumor of bone. Prognosis and treatment of pulmonary metastases. Clin. Orthop. Relat. Res. 1997, 338, 205–214. [Google Scholar] [CrossRef]
- Osaka, S.; Toriyama, M.; Taira, K.; Sano, S.; Saotome, K. Analysis of giant cell tumor of bone with pulmonary metastases. Clin. Orthop. Relat. Res. 1997, 335, 253–261. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).