Abstract
Chemotherapy as an adjuvant therapy that has largely failed to significantly improve outcomes for aggressive brain tumors; some reasons include a weak blood brain barrier penetration and tumor heterogeneity. Recently, there has been interest in designing effective ways to deliver chemotherapy to the tumor. In this review, we discuss the mechanisms of focused chemotherapies that are currently under investigation. Nanoparticle delivery demonstrates both a superior permeability and retention. However, thus far, it has not demonstrated a therapeutic efficacy for brain tumors. Convection-enhanced delivery is an invasive, yet versatile method, which appears to have the greatest potential. Other vehicles, such as angiopep-2 decorated gold nanoparticles, polyamidoamine dendrimers, and lipid nanostructures have demonstrated efficacy through sustained release of focused chemotherapy and have either improved cell death or survival in humans or animal models. Finally, focused ultrasound is a safe and effective way to disrupt the blood brain barrier and augment other delivery methods. Clinical trials are currently underway to study the safety and efficacy of these methods in combination with standard of care.
1. Introduction
There has been no significant improvement in the survival of patients with primary brain tumors although there has been extensive development of drug delivery methods to the central nervous system (CNS). Therapies such as surgical resection, radiotherapy, and chemotherapy have improved the survival in certain tumors, such as medulloblastoma, while glioblastomas still have a poor prognosis. The different responses to pharmacotherapy may be due to differences in the blood brain barrier penetration and tumor microenvironment. To overcome these barriers, several drug delivery methods, such as nanoparticles, convection enhanced delivery, focused ultrasound, and intranasal delivery have been designed [1]. These drug delivery systems are designed to introduce therapeutic substances to the CNS, while controlling the rate, time, and region of its release.
2. Evolution of Drug Delivery to the Brain
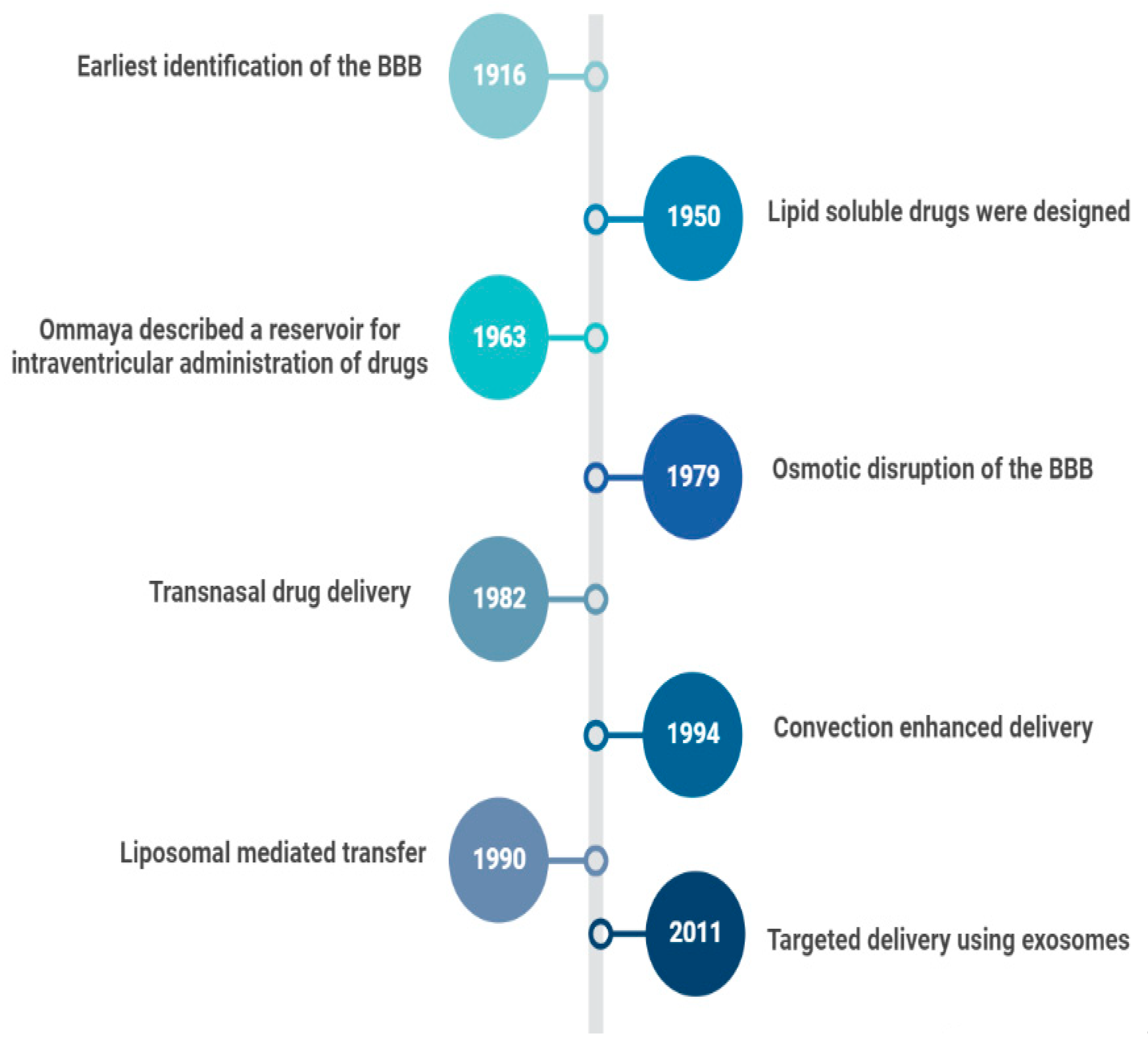
In 1914, it was reported that salvarsan and neosalvarsan both used for syphilis did not enter the brain, via blood, after IV administration [2]. By 1950, lipid soluble drugs, such as tricyclic antidepressants were designed, which traversed the blood brain barrier (BBB) well [3]. The earliest understanding and definition of the BBB was established by McIntosh and Fildes in 1916 [4]. In 1972, the lipid solubility effects on the BBB transport were further explained by Oldendorf [5]. In 1963, the first drug delivery method through the lateral ventricle was developed by Ommaya who designed a reservoir for the administration of intrathecal antibiotics [6]. In 1979, osmotic disruption of the BBB was introduced for drug delivery [7]. In 1982, transnasal drug delivery was identified as a method to bypass the blood brain barrier [8]. By 1994, different transcranial delivery methods started to emerge, such as intracerebral implants and convection enhanced delivery [9,10]. In 1986, BBB mediated transcytosis was used, and antibodies targeting BBB associated receptors to enhance the specific targeting of therapies were developed [11,12]. In 1990, a liposomal mediated transfer was first described as an advance into the history of CNS drug delivery [13]. In 2001, microbubble and ultrasound focused disruption of the BBB was first used as a drug delivery enhancing method [14]. In 2011, the targeted delivery of siRNA to the brain was established using exosome vesicles [15]. Figure 1 is an outline of the history of CNS drug delivery.
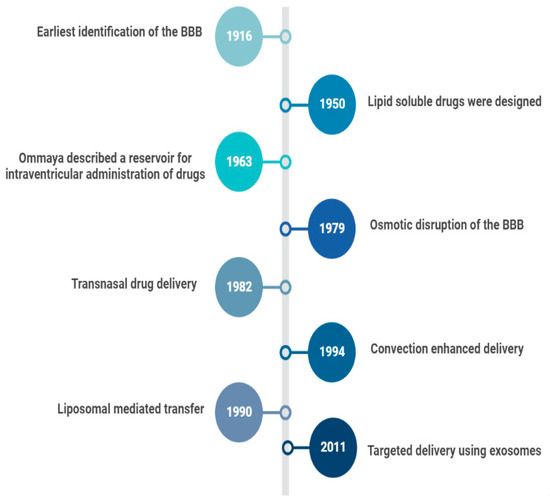
Figure 1.
History of CNS Drug delivery evolution.
3. Methods of Drug Delivery
3.1. Non-Viral Nanoparticles
Nanoparticles have long been used as tools for drug delivery. They can penetrate the leaky tumor capillaries due to their small size, explained by their enhanced permeability and retention effect [16]. Transporter ligands and receptors can direct the uptake of the nanoparticle through the BBB to a specified target [16]. The ligand is not of a therapeutic value, but it facilitates the proper targeting and delivery of the drug [16]. A well-known example is the low-density lipoproteins undergoing transcytosis through brain endothelial cells [17]. In one phase 2 clinical study, investigating enhanced penetration in gliomas, a cell penetrating peptide was conjugated with ANG1005, angiopep-2 paclitaxel conjugate [18]. Angiopep-2 is a ligand fashioned to bind to a low-density lipoprotein receptor-related protein-1 (LRP-1), which enhances LRP-1-mediated transcytosis [18]. In experimental trials, it improved the outcome in glioblastoma mouse models [19].
3.2. Exosomes
Different cells secrete small extracellular vesicles called exosomes. They are stable and can remain in the circulation for a long time. Those isolated from brain endothelial cells regulate the process of transport across the BBB [20]. Exosomes have been utilized to carry small molecules and nucleic acids. In addition to carrying useful materials, exosomes have adhesive proteins on their surface [21]. To combat tumor development, an exosome containing siRNA specific vascular endothelial growth factor (VEGF) was able to release its content into brain tumors of zebrafish. VEGF expression was significantly impacted by the siRNA [22]. Additional studies suggest that brain endothelial derived exosomes may bypass the BBB and noninvasively deliver chemotherapeutic drugs [23].
3.3. Active Transport through Blood-Brain Barrier
Amino acids can cross the BBB using different carriers. Linking drugs to these amino acids can help in the delivery of these drugs. Methotrexate (MTX)-lysine conjugate was designed to improve the permeability of the BBB to methotrexate [24]. Methotrexate was transported using the same mechanism of lysine amino acid transport. Although peptide carriers are promising, the synthesis process and optimization is exceedingly complex. More work must be performed to improve the ease of use before sustained usage can be accomplished. Additionally, prodrugs (esters) are potential BBB drug delivery methods. Furthermore, dimers can be utilized as they can carrier the drug of choice and another versatile compound. Lastly, due to their diversity and optimization, nanoparticles (organic or inorganic) possess a tremendous promise in being coupled with drug delivery [25].
3.4. Microbubble-Enhanced
Microbubble-enhanced delivery is a noninvasive technique, which increases the permeability of the BBB for different treatments. It was found to reduce the expression of different junctional proteins that are responsible for the integrity of the BBB without damaging brain tissue [26]. This technique was reported to increase the local brain concentration of doxorubicin and to enhance drug passage across both the BBB and the blood brain tumor barrier (BBTB) [27]. In early 2000, Hynynen and co-workers used MRI-guided US in combination with a microbubble to open the BBB [28]. Adding the microbubble technique mitigated the brain injury caused by the ultrasound waves. Moreover, Herceptin, the monoclonal antibody used to treat breast cancer brain metastasis was reported to efficiently reach the brain when US and microbubbles were used simultaneously [28].
3.5. Convection Enhanced Delivery (CED)
In the early 1990s, Edward Oldfield and colleagues designed CED [29]. Notably, CED alters the permeability of the BBB and allows for subsequent targeted drug delivery. The drug delivery is localized but invasive [30]. Utilizing CED involves one or more intracranial catheters connected to an external infusion pump, enabling therapeutic substances to be delivered to the target tissues via a pressure gradient. With local infusion, the brain parenchyma receives a higher therapeutic concentration with less systemic side effects [31].
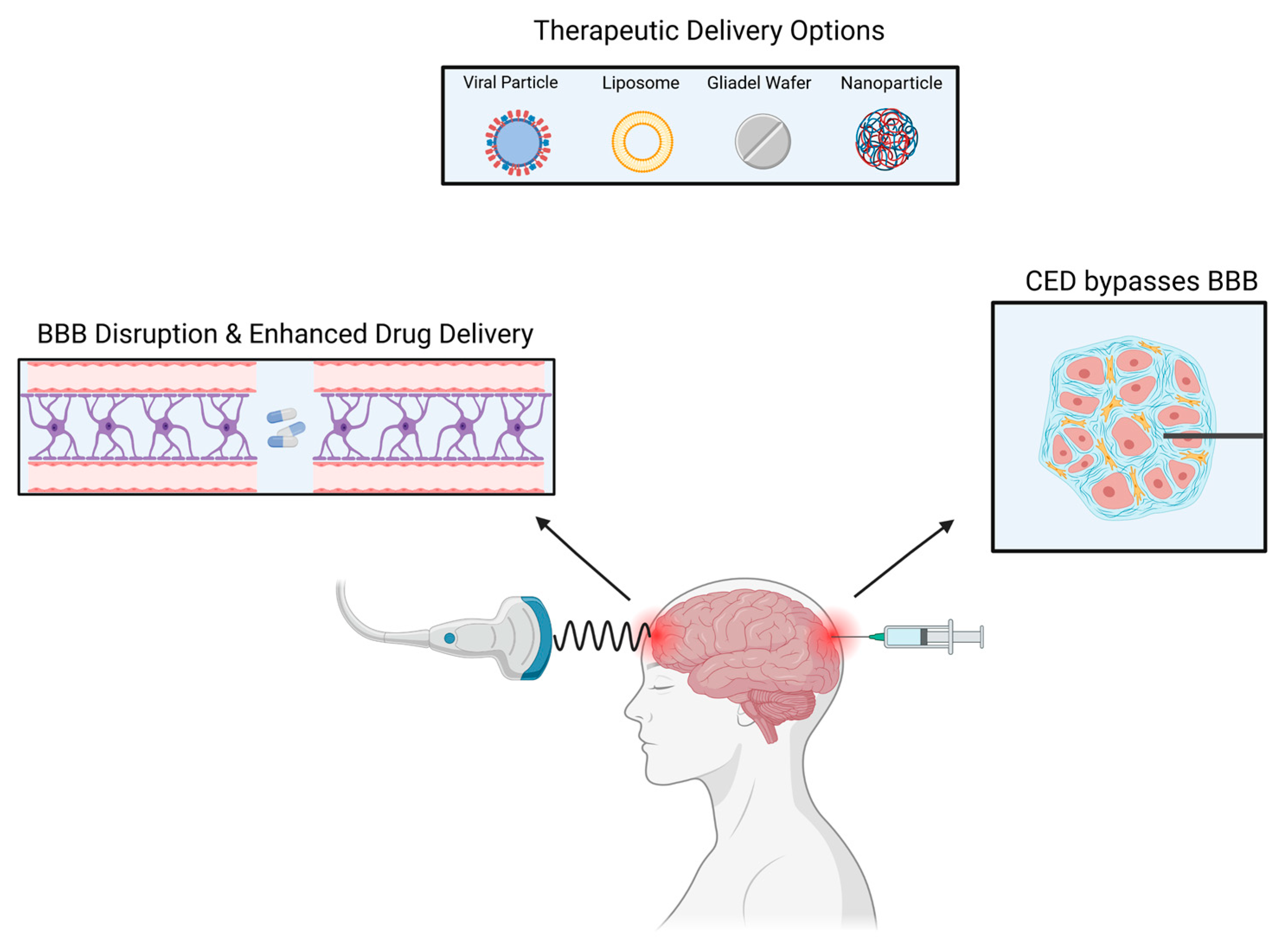
There are currently multiple ongoing clinical trials utilizing CED, most of which are for glioblastoma (GBM) and diffuse intrinsic pontine glioma (DIPG) [32]. In GBM, recurrence mainly occurs in the peritumoral area, therefore, CED utilization is vital for tumor recurrences as it perfuses different areas of the tumor [33]. CED allows for the direct delivery of the drug to the tumor bed resulting in high local concentrations with minimal systemic absorption [34] (Figure 2).
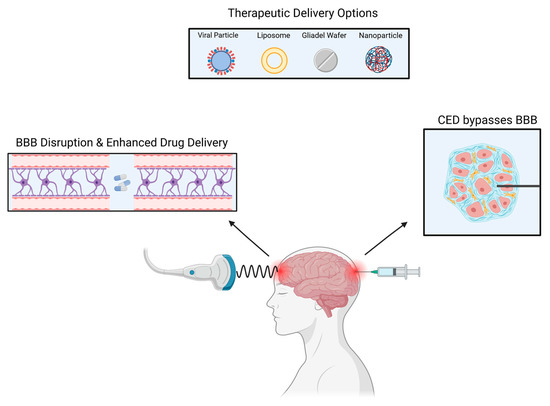
Figure 2.
Several methods can be used to deliver drugs to intracranial tumors.
A wide range of therapeutic elements can be delivered through CED such as chemotherapeutic agents, imaging tracers, proteins, viruses, liposomes, and nanoparticles [35,36,37]. Lastly, in addition to other limiting factors, the ideal CED drug has not yet been found with the best therapeutic index. Due to CED’s heterogeneous pressure gradient, the concentration of drugs in the treated area is not uniform, resulting in an inhomogeneous distribution. There is also an ‘intrinsic’ backflow of solutes and air bubbles adjacent to the catheter due to catheter-induced tissue damage and reflux [38]. A new avenue of research involves MRI coupled CED. The utilization of an MRI is crucial to maintain visual confirmation and to avoid complications. Additionally, to prevent reflex, a device referred to as reflux-resistant infusion cannula ensures reflux induced tissue damage is mitigated [39].
3.6. Laser Interstitial Thermal Therapy (LITT)
Laser interstitial thermal therapy (LITT) utilizes photons for the ablation of tumors to induce subsequent necrosis. LITT can disrupt the impermeability of the BBB—allowing for the enhanced delivery and efficacy of drug therapies [40]. Reportedly, LITT can increase permeability by up to 30 days post-treatment. This increased permeability allows for molecules as large as immunoglobulins to cross the BBB [41]. Notably, LITT has successfully been used in conjunction with doxorubicin to treat glioblastomas [42]. Although LITT has demonstrated a positive impact against forms of metastasis, additional research needs to specifically delineate LITTs impact on additional tumor subtypes [40,43].
3.7. Nanoparticles
It is possible to deliver drugs efficiently using nanoparticles, which can be composed of lipids, polymers, or metallic particles. Nanoparticles can cross the BBB in various ways; through enhanced permeability and retention (EPR), endocytosis, and receptor-mediated transcytosis (Figure 3). Nanoparticles encapsulate drugs to increase the plasma half-life and to enable their entry into the brain parenchyma, as displayed in Figure 1 [44]. Several types of cancers were successfully treated with nanoparticles [45]. In EPR, the nanoparticle utilizes the leaky blood vessel of solid tumors, which can access the tumor locally [46]. Extravasation of the nanoparticle releases the encapsulated drugs into the body slowly. In most organs, nanoparticles cannot cross the normal vasculature, which reduces both peripheral and systemic toxicity [47,48]. Nanoparticles can traverse BBB leakages, making them a potential means of delivering drugs to brain tumors. Nevertheless, during clinical studies, nanoparticles could not reach tumors at therapeutic levels [49,50].
Figure 3.
Enhanced permeability and receptor-mediated transcytosis allow nanoparticles to carry drugs with longer plasma half-lives into the brain parenchyma.
3.8. Intranasal Delivery
An alternative method of overcoming the BBB is intranasal delivery. The nasal cavity allows easy access without interference from the BBB to the brain parenchyma. Drugs are transported from the neuroepithelium of the nasal cavity to the central nervous system, paracellularly, transcellular, and neuronally. However, the use of intranasal medicines is not suitable for all drugs. The bioavailability of lipophilic drugs with a low molecular weight is generally more significant than hydrophilic drugs charged with a charge. For example, improving drug bioavailability with liposomes, cyclodextrins, and nanoparticles is possible. Furthermore, nose delivery of drugs avoids first-pass metabolism, thereby preserving their effectiveness [51].
Clinical trials using intranasal delivery of drugs have only yielded limited results. Malignant gliomas have been treated with perillyl alcohol intranasally. Perillyl alcohol administered four times daily resulted in 45% of cases surviving six months without progression [52]. Non-specificity of drugs can cause toxicity when delivered intranasally. Targeting tumor cells can minimize this toxicity [53]. It is also possible to reduce the toxicity in the surrounding brain tissue by using an intranasal drug delivery with microbubble mediated FUS. When these methods are combined, drug uptake in tumor regions is increased and targeted [54].
3.9. Intra-Arterial Delivery
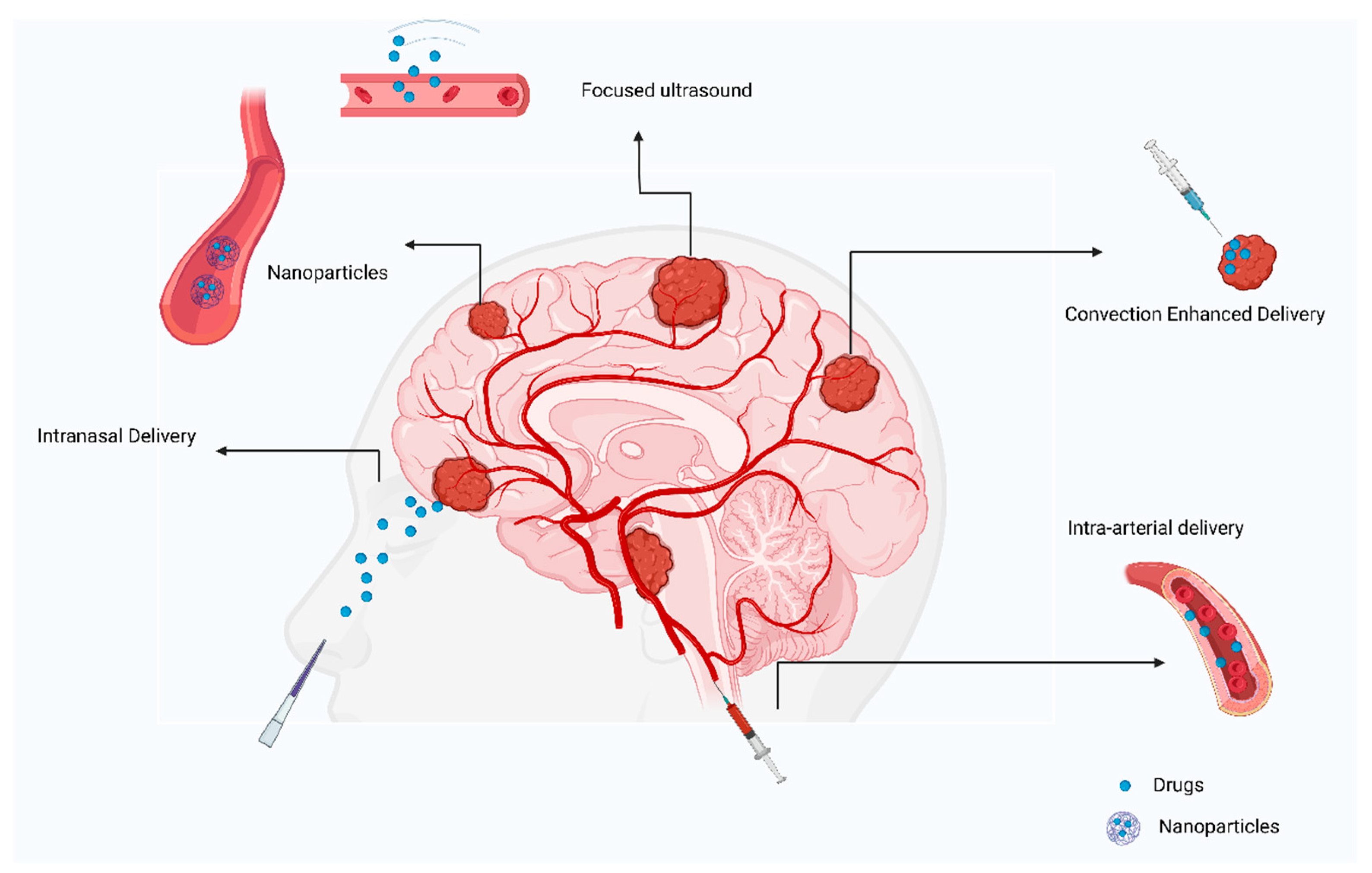
Direct injection of drugs into an artery close to a tumor is referred to as intra-arterial drug delivery [55]. Drugs are released into blood vessels after cannulation in the targeted area (Figure 4). Additionally, hyperosmolar drugs, such as mannitol can be used to open the BBB on a local level [56]. However, the survival rate was not significantly improved in several clinical trials and cases. A group of patients with ependymoma were treated with cetuximab, carmustine, and bevacizumab intra-arterially, with the treatment being successful [57]. GBM patients were treated with intra-arterial drug delivery in several clinical studies. A combination of nimustine, bevacizumab, and carboplatin with other conventional chemotherapies resulted in survival rates ranging from 20 weeks to 10 months [58,59].
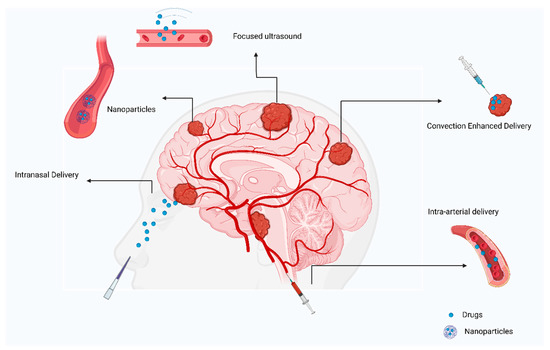
Figure 4.
Review of current approaches to treating primary brain tumors.
4. Pre-Clinical and Current Treatments
In this section, we review pre-clinical and current treatments used in the various focused chemotherapy modalities. The amino acid angiopep-2-paclitaxel conjugate, ANG1005, is readily taken up by the highly expressed low density lipoprotein receptor-related protein (LRP) receptors at the BBB [18,19]. ANG1005 showed a significantly enhanced brain uptake, when compared to paclitaxel alone, in rodent models with brain neoplasms [18,19,60]. There was also an increased treatment effect in humans in an ongoing clinical trial [61]. Similarly, a lysine-methotrexate conjugate showed enhanced brain delivery using the endogenous BBB lysine transport system in an in vitro and pre-clinical rodent study [22]. Rodent studies indicate that gold nanoparticles can serve as a vehicle for targeted hydrophobic drug delivery through the BBB [62,63]. This was demonstrated using doxorubicin loaded gold nanoparticles in pre-clinical studies [64,65]. Pre-clinical rodent studies have also demonstrated doxorubicin delivery using angiopep-2 decorated gold nanoparticles [66].
Polyamidoamine (PAMAM) dendrimers loaded with docetaxel (DTX) have shown a significantly improved glioblastoma cell death and drug delivery in the pre-clinical studies [67]. Similarly, estramustine and podophyllotoxin conjugated onto these dendrimers showed a more effective glioma cell death [68]. A G3-succinamic acid surface dendrimer conjugated with curmurin showed a tumor specific distribution in rats with implanted human glioma cells [69]. Paclitaxel linked onto G3 PAMAM dendrimers showed an increased cell brain tumor cell death and an improved porcine brain endothelial cell permeability [70]. Lastly, tamoxifen and doxorubicin linked to G4 PAMAM dendrimers have demonstrated an increased accumulation within glioma cells [71].
Lipid nanostructures are advantageous due to their non-toxicity when compared to other nanoparticles. A series of pre-clinical studies have demonstrated the successful use of lipid nanostructures in brain tumor models when combined with therapeutic molecules, including carmustine, doxorubicin, etoposide, siRNAs, camptothecin, edelfosine, cytarabine, rhodamine 123, pemetrexed, and miR-21 [72]. Many pre-clinical rodent and animal studies have investigated the use of liposomal drugs [73]. For example, delivery of liposomal temozolomide when combined with focused ultrasound showed improved drug delivery in rodent models of metastatic breast cancer to the brain [74]. There are several liposome drugs that exist on the market, or are currently undergoing clinical trials, which include daunoribucin for pediatric brain tumors [75] and doxorubicin for glioblastoma multiforme [76].
Polymetic nanoparticles have been widely used in clinical medicine. The 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU), or Gliadel® wafer is an FDA approved polyanhydride implant loaded with carmustine used for the treatment of high-grade gliomas, including glioblastoma [77]. This implantable device allows for a sustained, focused chemotherapeutic release, and has improved survival rates in patients with GBM and malignant gliomas [78,79]. Poly lactic-co-glycolic acid (PLGA) is another FDA approved polymer used to encapsulate drugs. Pre-clinical rodent and cell line studies have suggested its efficacy for glioblastoma therapy when loaded with doxorubicin [80,81], bevacizumab [82], and morusin [83]. Similar studies were performed showing an efficacy against gliomas with temozolomide [84,85] and iguratimod [86] loaded with PLGAs. Chitosan is a biodegradable polymer formed by deacetylating chitin. Chitosan-coated nanoparticles containing doxorubicin successfully reduced glioblastoma growth in rodent models [87].
We look forward to further translational research and the outcomes of the several ongoing clinical trials [88]. There also needs to be further research regarding drug-vehicles and drug combinations and which drugs work best with specific mechanisms of delivery, such as focused ultrasound, convection-enhanced delivery, nose-to-brain delivery, and intracranial hydrogel delivery [79].
5. Intra-Arterial vs. Intravenous Access
Both the arterial and venous delivery of chemotherapy have been used since the beginnings of chemotherapy. Venous access preceded arterial by several years with arterial access being first attempted in 1950 [89,90]. Since then, both have had a role in the delivery of chemotherapeutics. Various trials suggested that intra-arterial delivery of chemotherapeutics would allow the administration of higher concentrations of a drug to tumors [91,92]. These trials were often fraught with complications and side effects from the intra-arterial drug administration. In 1992 Shappiro et al. conducted the first randomized trial to better understand venous vs arterial access in the treatment of malignant gliomas [82]. They found that survival was matched between the intravenous and intra-arterial groups, but that intra-arterial administration was associated with more toxicity and tissue necrosis. Under these conditions, intra-arterial administration was neither effective nor safe for the treatment of malignant brain tumors [82]. Other trials have added to our understanding of access in neuro-oncology [93,94,95]. Kochii et al. again found that intra-arterial administration of chemotherapy does not increase survival but was not associated with toxicity when compared to previous studies [93]. These results were supported by another trial with the same conclusions by Silvani et al. In addition, they found that the cost-benefit ratio of intra-arterial administration was not sufficient to consider it worthwhile in continuing. Several trials have found modest, but insignificant increases in survival [94]. In a systematic review by Cheng et al., they again found that across four trials and 460 patients, intra-arterial delivery was not superior to intravenous in terms of efficacy or overall survival [96]. At this point in time, intravenous and intra-arterial delivery of chemotherapeutics appears to be equal. Only if dosing levels and infusion techniques are improved may we see the benefits that intra-arterial delivery claims to have [97].
6. Ultrasound in Focused Blood Brain Barrier Disruption
The blood brain barrier is a complex and highly selective semipermeable system, mostly made up of tight connections between capillary endothelial cells. This modulates the access of peripheral molecules into the brain’s circulation [98,99,100,101]. Vascular smooth muscle cells, pericytes, immunological cells, glial cells, and brain cells are additional crucial BBB supporting cells [98,100]. Notably, lipid molecules of a particular size can passively diffuse through the BBB [100,101,102]. Due to its restrictions on drug delivery and tumor site penetration, the selective specificity of the BBB poses a significant obstacle. Numerous approaches have been investigated in the past with the aim of improving drug transport into brain tumors and transiently disrupting the BBB [102,103,104,105,106]. The use of mannitol, polymeric nano- and microparticles, radiation treatment, and convection-enhanced distribution are some methods which cause chemical disruption [102,103,104,105,106,107,108].
Despite higher drug concentrations at the target, these techniques have drawbacks, such as performing direct cerebral injections that require repeated invasive administrations [102,106,107]. Chemical BBB disruption can result in unpredictably broad BBB disruption, which can have adverse systemic implications, as well as posing a risk to normal brain parenchyma [107]. It has also been demonstrated that radiotherapy can open the BBB, although it can also be temporally unpredictable and can significantly harm healthy brain structures [108]. Outside of research protocols, these BBB opening techniques are rarely used in a neuro-oncology clinical practice. This infrequent use is due to scant clinical evidence for the effectiveness and safety of these therapies. Because of the ongoing demand for efficient BBB penetration methods for targeted tumor therapy, focused ultrasound (FUS) BBB disruption has shown promise as a potential therapeutic role
Focused BBB disruption is a noninvasive, focal, safe, and reversible procedure to treat brain tumors (oligodendroglioma, ependymoma, astrocytoma, and oligoastrocytoma (mixed glioma) [102,109,110,111,112]. Moreover, it has proven its effectiveness in neurological disorders, such as Parkinson’s and Alzheimer’s disease [113,114,115]. This technique overpowers the limitations set by the BBB and enhances the delivery of molecules into the brain that are useful for the treatment of brain disorders. To successfully deliver chemotherapy to the targeted area of the brain, FUS BBB disruption modifies the brain’s protective barrier [102,116]. This is accomplished by transmitting low-frequency ultrasonic waves through an external device to the targeted brain parenchyma [28,111,117,118,119,120]. The use of low frequency ultrasound allows for the reduction in any potential harm to the brain’s permanent tissue [28,111,112].
Low-frequency sound waves are given when lipid-coated perfluorocarbon gas microbubbles are intra-capillary infused [102,121]. In response to FUS sound waves, the BBB is altered due to consequent acoustic cavitation, rapid oscillation, high concentration of waves, and the collapse of microbubbles in the capillary walls [102]. These microbubbles further reduce the frequency of waves, which has an additive effect on BBB disruption [98,100,122,123]. As a result, the barrier becomes more permeable, making it easier for medications and therapeutic molecules to cross the BBB and enter the brain’s targeted regions [109,122]. BBB FUS is temporary and closes after 4 to 8 h, which avoids the BBB being permanently compromised, preventing neurotoxicity-related long-term negative effects [102,109,112].
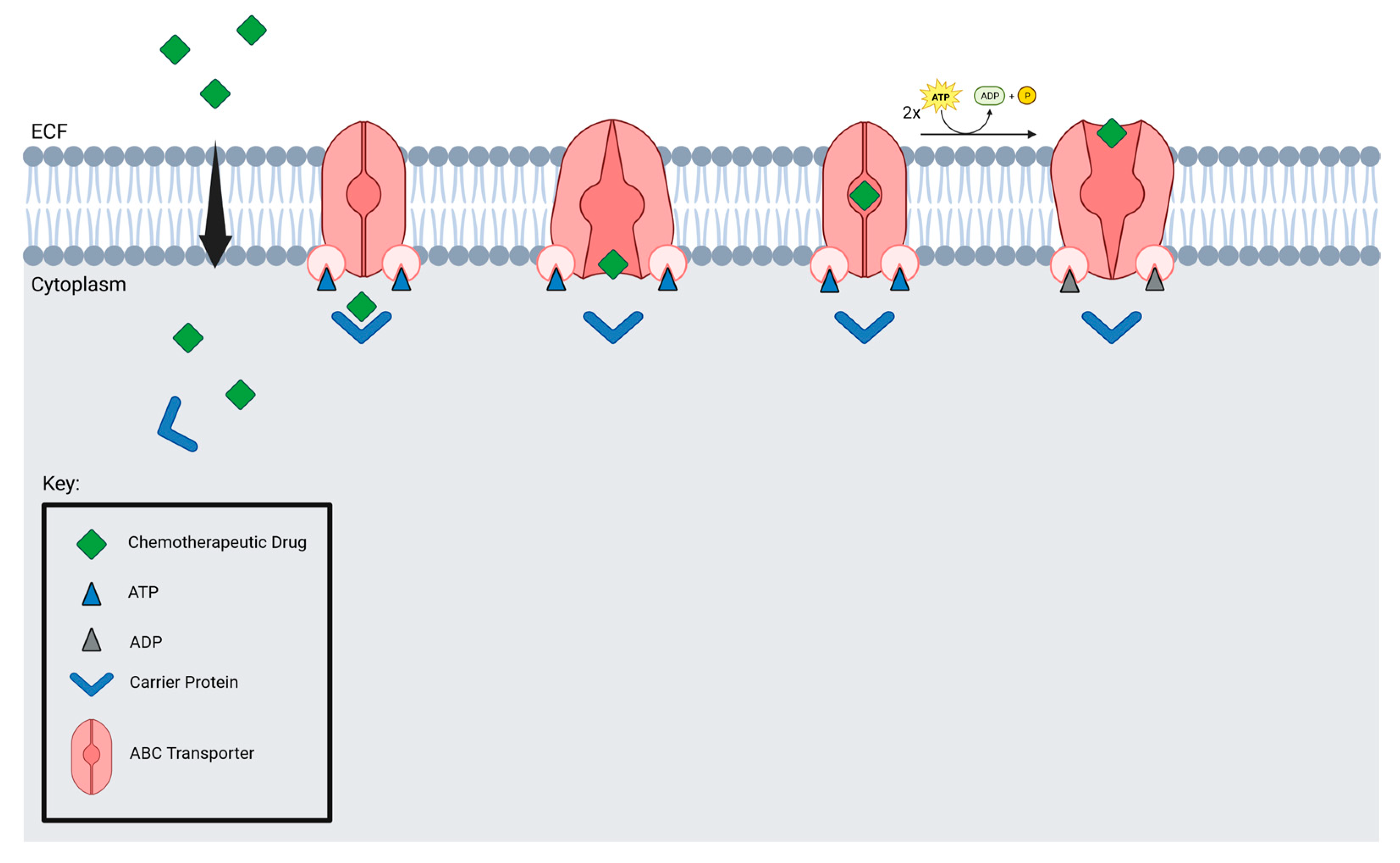
Microbubbles and drugs are delivered to tumors by blood vessels, which are crucial for microbubble delivery. Focused ultrasound may not be suitable for brain tumors with low vessel density. Additionally, efflux transporters prevent drugs from accumulating in the brain. Nevertheless, studies have found that FUS suppresses MDR1, resulting in an increased accumulation of drugs in tumor cells [124]. ABC transporters are found in several brain tumors, including GBM, DIPG, and medulloblastoma (Figure 5).
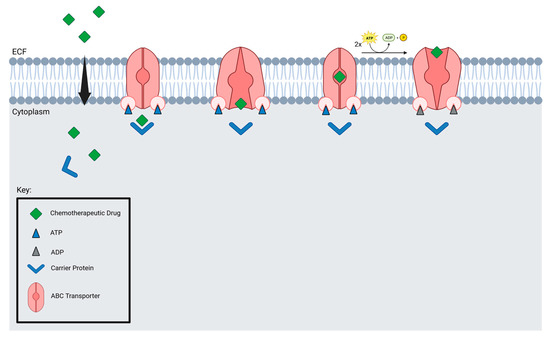
Figure 5.
ABC transporter mechanism of action.
FUS may increase drug accumulation within these tumors. GBM and DIPG can be targeted with a noninvasive drug delivery method, such as FUS. MRI-guided FUS (MRgFUS) was performed for the first time on patients with GBM [111]. Analyzing tissue from two patients, temozolomide concentrations were 1.5 to sevenfold higher in tumors that had been sonicated than in those that had not. All patients tolerated the treatment well [111]. Several clinical studies have examined implanted ultrasound devices: CarThera (SonoCloud) was used in phase I clinical trials in conjunction with carboplatin systemic administration [125]. There are some disadvantages to these implanted ultrasound devices, including the requirement of invasive surgery, their inability to target precisely, and their suitability for superficial brain tumors. Brain cancer might be treated more effectively by combining FUS with immunotherapy. Immune cells cannot pass through the BBB because their adhesion molecules are low on CNS endothelial cells [48].
MRI-guided focused ultrasound allows for a better assessment of tissue characteristics, such as skull thickness. This allows for the precise targeting of different brain and tumor areas [102,110,113]. Prognostically, 70–80% of patients with malignant glioma die within two years of chemotherapy. Due to this dismal statistic, ample resources should be used to attempt to lengthen the survival of diagnosed patients [126]. While administering monoclonal antibodies and chemotherapeutic drugs (methotrexate and doxorubicin) in animal model pre-clinical trials, FUS BBB disruption displayed efficacy and safety [117,127,128,129,130].
According to studies using rat and mouse glioma models, FUS-induced BBB opening was linked to higher tissue TMZ concentrations, which improved tumor control rates and lengthened animal longevity [127,131]. For instance, the BBB opened with FUS, following the injection of TMZ in Fisher rats, implanted with 9-L glioma cells, was related to a higher TMZ CSF/plasma ratio, a lower 7-d tumor progression ratio, and an enhanced survival of TMZ-FUS-treated rats by 38% when compared to the controls [131]. Additionally, focused ultrasound BBB disruption enables viral gene-based therapy, tumor targeting with therapeutic nanoparticles (such as gold nanoparticles for malignant brain tumors), and the tumor’s exposure to US waves have all been further linked to an immunomodulatory role, a secondary to tumor antigen exposure and activation of heat shock proteins, which may stimulate tumor immunogenicity [102,127,132,133].
In 21 patients with recurrent GBM, a single-center trial (NCT02253212) examined the safety and effectiveness of an implantable, low-intensity pulsed ultrasound device with microbubble injection [125,134]. The BBB disruption was assessed using contrast-enhanced T1-weighted brain images, the treatment was found to be safe and without any serious side effects or carboplatin-related neurotoxicity Patients with a documented BBB disruption had a longer progression-free and overall survival time than patients without or with poor BBB disruption [134]. Another recent study by Park et al. (Clinical trial: NCT03712293) is the first study to repeatedly apply MRgFUS to the same target, while administering a chemotherapy regimen to patients with malignant brain tumors, hence demonstrating the viability and safety of repeated temporary BBB disruption using MRgFUS. This study also offers the potential for future research that assess the utility of other adjuvant chemotherapy and immunotherapy treatment deliveries, which were challenging in the past due to the intact BBB.
Studies suggest that around 10% of the pediatric population and 2.5% of adults are diagnosed with brainstem glioma (BSG) [135]. With this prevalence rate, there is a need for an absolute treatment option for brain tumors. Unfortunately, there is no ultimate solution to target and treat malignant tumors yet. While one therapy alone has not shown absolute treatment potential and survival benefits, with the advances in neurosurgical care, multiple adjuvant therapies, when used in conjunction (mostly post-surgical resection), can reduce the overall mortality rate, and lengthen the survival period after therapy [126,136,137]. Although the benefits of surgical resections of brain tumors vary with different age groups and the types of resections, the gross total resection (GTR) has a higher survival rate than a subtotal resection, with a better 5-year cancer-specific survival [109].
With technological advancement in the neurosurgical field, minimally invasive procedures, such as neuroendoscopy and endonasal endoscopic surgery are preferred to open brain surgery, because of the lesser associated complications. Moreover, adjuvant targeted irradiation, after open surgery (craniotomy), is a preferred choice to avoid the neurotoxicity, which was previously seen with whole brain radiation [137]. Overall, the treatment modalities have advanced significantly in the past several decades and now hold potential for improved outcomes with respect to longer survival rates and a better quality of life for patients with brain tumors. With availability of advanced treatment options and the enhancement of targeted drug delivery systems to the tumor vis modalities, such as FUS BBB disruption, we need clinical trials to assess the efficacy of this method in improving the outcomes of patients with brain tumors, while also assessing areas of improvement for a better clinical benefit to the patients.
A current avenue of research is the enhancement of liquid biopsies. Currently, utilizing cerebrospinal fluid (CSF) to detect tumor-derived biomarkers is non-significant. However, using MRgFUS to open the BBB could increase the concentration of tumor-derived biomarkers within the CSF. This would noninvasively allow physicians to monitor the development of a tumor [138]. Moreover, microbubble-enhanced focused ultrasound is a newly developing technology that may disrupt the BBB in a more efficient manner—allowing for the delivery of chemotherapeutic drugs [139].
7. Conclusions
The BBB is a complex barrier that limits options for the treatment of brain tumors. The methods most substantiated by promising data are FUS and CED. FUS allows for noninvasive, local, safe, and most importantly the reversible opening of the BBB. The utilization of FUS can also inactivate an important gene, MDR1, thus increasing the accumulation of chemotherapeutic drugs within cancer cells. Furthermore, FUS can be coupled with MRI imaging to enhance the precise drug targeting of differing brain regions. Lastly, microbubble enhancement with FUS has been used to increase the survivability of patients with GBM. CED is an invasive yet precise form of drug delivery that utilizes pressure gradients. Specifically noted for the ability to perfuse all areas of the GBM tumor, CED can isolate drug delivery without systemic absorption. Lastly, CED is noted for its versatility, allowing vectors such as chemotherapeutic agents, imaging tracers, proteins, viruses, liposomes, and nanoparticles to enter cancer cells. Other strategies that were discussed in this review, such as exosome release, active transport, and nanoparticles have limited studies supporting their efficacy and should be further investigated. To continue the research within chemotherapeutic delivery, novel processes, such as LITT and immunotherapeutic neuro-oncology, should be further explored to enhance the management of intracranial tumors in combination with surgery.
Author Contributions
Conceptualization, B.L.-W. and Y.M.; writing—review and editing, Y.M., S.W., K.P., A.D., J.H., M.R.H.S. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood—Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, N.W. Chlorpromazine in the Treatment of Neuropsychiatric Disorders. JAMA 1954, 155, 18. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.; Fildes, P. The Factors which Govern the Penetration of Arsenic (Salvarsan) and Aniline Dyes into the Brain and their Bearing upon the Treatment of Cerebral Syphilis. Brain 1916, 39, 478–483. [Google Scholar] [CrossRef][Green Version]
- Oldendorf, W.H.; Hyman, S.; Braun, L.; Oldendorf, S.Z. Blood-Brain Barrier: Penetration of Morphine, Codeine, Heroin, and Methadone after Carotid Injection. Science 1972, 178, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Ommaya, K. Subcutaneous Reservoir and Pump for Sterile Access to Ventricular Cerebrospinal Fluid. Lancet 1963, 282, 983–984. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Maravilla, K.R.; Frenkel, E.P.; Rapaport, S.I.; Hill, S.A.; Barnett, P.A. Osmotic Blood-Brain Barrier Disruption. Computerized Tomographic Monitoring of Chemotherapeutic Agent Delivery. J. Clin. Investig. 1979, 64, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Anand Kumar, T.C.; David, G.F.; Sankaranarayanan, A.; Puri, V.; Sundram, K.R. Pharmacokinetics of Progesterone after Its Administration to Ovariectomized Rhesus Monkeys by Injection, Infusion, or Nasal Spraying. Proc. Natl. Acad. Sci. USA 1982, 79, 4185–4189. [Google Scholar] [CrossRef]
- Brem, H.; Tamargo, R.J.; Olivi, A.; Pinn, M.; Weingart, J.D.; Wharam, M.; Epstein, J.I. Biodegradable Polymers for Controlled Delivery of Chemotherapy with and without Radiation Therapy in the Monkey Brain. J. Neurosurg. 1994, 80, 283–290. [Google Scholar] [CrossRef]
- Morrison, P.F.; Laske, D.W.; Bobo, H.; Oldfield, E.H.; Dedrick, R.L. High-Flow Microinfusion: Tissue Penetration and Pharmacodynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R292–R305. [Google Scholar] [CrossRef]
- Pardridge, W.M. Receptor-Mediated Peptide Transport through the Blood-Brain Barrier. Endocr. Rev. 1986, 7, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Buciak, J.L.; Friden, P.M. Selective Transport of an Anti-Transferrin Receptor Antibody through the Blood-Brain Barrier in Vivo. J. Pharmacol. Exp. Ther. 1991, 259, 66–70. [Google Scholar] [PubMed]
- Shibata, S.; Ochi, A.; Mori, K. Liposomes as Carriers of Cisplatin into the Central Nervous System: Experiments with 9L Gliomas in Rats. Neurol. Med. Chir. Tokyo 1990, 30, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Blomley, M.J.K. Science, Medicine, and the Future: Microbubble Contrast Agents: A New Era in Ultrasound. BMJ 2001, 322, 1222–1225. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y. In Vivo Delivery of RNAi with Lipid-Based Nanoparticles. Annu. Rev. Biomed. Eng. 2011, 13, 507–530. [Google Scholar] [CrossRef]
- Georgieva, J.; Hoekstra, D.; Zuhorn, I. Smuggling Drugs into the Brain: An Overview of Ligands Targeting Transcytosis for Drug Delivery across the Blood—Brain Barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef]
- Candela, P.; Gosselet, F.; Miller, F.; Buee-Scherrer, V.; Torpier, G.; Cecchelli, R.; Fenart, L. Physiological Pathway for Low-Density Lipoproteins across the Blood-Brain Barrier: Transcytosis through Brain Capillary Endothelial Cells In Vitro. Endothelium 2008, 15, 254–264. [Google Scholar] [CrossRef]
- Thomas, F.C.; Taskar, K.; Rudraraju, V.; Goda, S.; Thorsheim, H.R.; Gaasch, J.A.; Mittapalli, R.K.; Palmieri, D.; Steeg, P.S.; Lockman, P.R.; et al. Uptake of ANG1005, A Novel Paclitaxel Derivative, Through the Blood-Brain Barrier into Brain and Experimental Brain Metastases of Breast Cancer. Pharm. Res. 2009, 26, 2486–2494. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Gong, M.; Zhang, J. Delivery of a Peptide-Drug Conjugate Targeting the Blood Brain Barrier Improved the Efficacy of Paclitaxel against Glioma. Oncotarget 2016, 7, 79401–79407. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Delaney, C.E.; Tremblay, T.-L.; Sodja, C.; Sandhu, J.K.; Stanimirovic, D.B. Method for Isolation and Molecular Characterization of Extracellular Microvesicles Released from Brain Endothelial Cells. Fluids Barriers CNS 2013, 10, 4. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using Exosomes, Naturally-Equipped Nanocarriers, for Drug Delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fogarty, B.; LaForge, B.; Aziz, S.; Pham, T.; Lai, L.; Bai, S. Delivery of Small Interfering RNA to Inhibit Vascular Endothelial Growth Factor in Zebrafish Using Natural Brain Endothelia Cell-Secreted Exosome Nanovesicles for the Treatment of Brain Cancer. AAPS J. 2017, 19, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Subudhi, B.B. Development and Characterization of Lysine-Methotrexate Conjugate for Enhanced Brain Delivery. Drug Deliv. 2016, 23, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review. Molecules 2018, 23, E1289. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef]
- Park, J.; Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; McDannold, N. Evaluation of Permeability, Doxorubicin Delivery, and Drug Retention in a Rat Brain Tumor Model after Ultrasound-Induced Blood-Tumor Barrier Disruption. J. Control. Release 2017, 250, 77–85. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging—Guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-Enhanced Delivery of Macromolecules in the Brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Van der Jeugd, A.; Leinenga, G.; Evans, H.T.; Janowicz, P.W.; Götz, J. Combined Effects of Scanning Ultrasound and a Tau-Specific Single Chain Antibody in a Tau Transgenic Mouse Model. Brain 2017, 140, 1220–1230. [Google Scholar] [CrossRef]
- De Vries, N.A.; Zhao, J.; Kroon, E.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. P-Glycoprotein and Breast Cancer Resistance Protein: Two Dominant Transporters Working Together in Limiting the Brain Penetration of Topotecan. Clin. Cancer Res. 2007, 13, 6440–6449. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Brady, M.L.; Rodríguez-Ponce, M.I.; Hartlep, A.; Pedain, C.; Sampson, J.H. Convection-Enhanced Delivery of Therapeutics for Brain Disease, and Its Optimization. FOC 2006, 20, E12. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Rothrock, R.J.; Canoll, P.; Bruce, J.N. Convection-Enhanced Delivery for Targeted Delivery of Antiglioma Agents: The Translational Experience. J. Drug Deliv. 2013, 2013, 107573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ksendzovsky, A.; Walbridge, S.; Saunders, R.C.; Asthagiri, A.R.; Heiss, J.D.; Lonser, R.R. Convection-Enhanced Delivery of M13 Bacteriophage to the Brain: Laboratory Investigation. JNS 2012, 117, 197–203. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Lecouteur, R.A.; Higgins, R.J.; Bringas, J.R.; Roberts, B.; Larson, R.F.; Yamashita, Y.; Krauze, M.; Noble, C.O.; Drummond, D.; et al. Canine Model of Convection-Enhanced Delivery of Liposomes Containing CPT-11 Monitored with Real-Time Magnetic Resonance Imaging: Laboratory Investigation. JNS 2008, 108, 989–998. [Google Scholar] [CrossRef]
- Szerlip, N.J.; Walbridge, S.; Yang, L.; Morrison, P.F.; Degen, J.W.; Jarrell, S.T.; Kouri, J.; Kerr, P.B.; Kotin, R.; Oldfield, E.H.; et al. Real-Time Imaging of Convection-Enhanced Delivery of Viruses and Virus-Sized Particles. JNS 2007, 107, 560–567. [Google Scholar] [CrossRef]
- Bidros, D.S.; Vogelbaum, M.A. Novel Drug Delivery Strategies in Neuro-Oncology. Neurotherapeutics 2009, 6, 539–546. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Forsayeth, J.R.; Dickinson, P.J.; Bankiewicz, K.S. Image-Guided Convection-Enhanced Delivery Platform in the Treatment of Neurological Diseases. Neurotherapeutics 2008, 5, 123–127. [Google Scholar] [CrossRef]
- Melnick, K.; Shin, D.; Dastmalchi, F.; Kabeer, Z.; Rahman, M.; Tran, D.; Ghiaseddin, A. Role of Laser Interstitial Thermal Therapy in the Management of Primary and Metastatic Brain Tumors. Curr. Treat. Options Oncol. 2021, 22, 108. [Google Scholar] [CrossRef]
- Salehi, A.; Paturu, M.R.; Patel, B.; Cain, M.D.; Mahlokozera, T.; Yang, A.B.; Lin, T.-H.; Leuthardt, E.C.; Yano, H.; Song, S.-K.; et al. Therapeutic Enhancement of Blood-Brain and Blood-Tumor Barriers Permeability by Laser Interstitial Thermal Therapy. Neuro-Oncol. Adv. 2020, 2, vdaa071. [Google Scholar] [CrossRef] [PubMed]
- Butt, O.H.; Zhou, A.Y.; Huang, J.; Leidig, W.A.; Silberstein, A.E.; Chheda, M.G.; Johanns, T.M.; Ansstas, G.; Liu, J.; Talcott, G.; et al. A Phase II Study of Laser Interstitial Thermal Therapy Combined with Doxorubicin in Patients with Recurrent Glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab164. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Rao, G.; Kew, Y.; Prabhu, S.S. Laser Interstitial Thermal Therapy for Newly Diagnosed and Recurrent Glioblastoma. Neurosurg. Focus 2016, 41, E12. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Kuhlenkamp, J.F.; Jeandidier, E.; Trinh, H.; Ookhtens, M.; Kaplowitz, N. Evidence for Carrier-Mediated Transport of Glutathione across the Blood-Brain Barrier in the Rat. J. Clin. Investig. 1990, 85, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle Technology for Drug Delivery across the Blood-Brain Barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood-Brain Barrier and Blood-Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Amighi, K.; Mathieu, V.; Lefranc, F.; van Gool, S.; de Vleeschouwer, S. Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers 2013, 5, 1020–1048. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Linden, R.; Futuro, D.; Gattass, C.R.; Quirico-Santos, T. Ras Pathway Activation in Gliomas: A Strategic Target for Intranasal Administration of Perillyl Alcohol. Arch. Immunol. Ther. Exp. 2008, 56, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Ozawa, T.; Gryaznov, S.M.; Bollen, A.W.; Lamborn, K.R.; Frey, W.H.; Deen, D.F. New Therapeutic Approach for Brain Tumors: Intranasal Delivery of Telomerase Inhibitor GRN163. Neuro-Oncology 2008, 10, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, X.; Yue, Y.; Raliya, R.; Biswas, P.; Taylor, S.; Tai, Y.; Rubin, J.B.; Liu, Y.; Chen, H. Focused Ultrasound Combined with Microbubble-Mediated Intranasal Delivery of Gold Nanoclusters to the Brain. J. Control. Release 2018, 286, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Ellis, J.A.; Ornstein, E.; Bruce, J.N. Intraarterial Drug Delivery for Glioblastoma Mutiforme: Will the Phoenix Rise Again? J. Neuro-Oncol. 2015, 124, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.E. Novel Therapeutic Delivery Approaches in Development for Pediatric Gliomas. CNS Oncol. 2013, 2, 427–435. [Google Scholar] [CrossRef]
- Stewart, D.J.; Benoit, B.; Hugenholtz, H.; Maroun, J.A.; Russell, N.; Richard, M.T.; Peterson, E.; Dennery, J.; Nabwangu, J.F.; Grahovac, Z.; et al. Cisplatin, Arabinofuranosyl Cytosine, and Caffeine before Radiation for Glioblastomas. In Biology of Brain Tumour; Walker, M.D., Thomas, D.G.T., Eds.; Springer: Boston, MA, USA, 1986; pp. 393–398. [Google Scholar] [CrossRef]
- Newton, H.B.; Slivka, M.A.; Volpi, C.; Bourekas, E.C.; Christoforidis, G.A.; Baujan, M.A.; Slone, W.; Chakeres, D.W. Intra-Arterial Carboplatin and Intravenous Etoposide for the Treatment of Metastatic Brain Tumors. J. Neuro-Oncol. 2003, 61, 35–44. [Google Scholar] [CrossRef]
- Burkhardt, J.-K.; Riina, H.; Shin, B.J.; Christos, P.; Kesavabhotla, K.; Hofstetter, C.P.; Tsiouris, A.J.; Boockvar, J.A. Intra-Arterial Delivery of Bevacizumab after Blood-Brain Barrier Disruption for the Treatment of Recurrent Glioblastoma: Progression-Free Survival and Overall Survival. World Neurosurg. 2012, 77, 130–134. [Google Scholar] [CrossRef]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.-P. Antitumour Activity of ANG1005, a Conjugate between Paclitaxel and the New Brain Delivery Vector Angiopep-2: Antitumour Activity of ANG1005. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef]
- Kumthekar, P.; Tang, S.-C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a Brain-Penetrating Peptide—Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef]
- Day, E.S.; Thompson, P.A.; Zhang, L.; Lewinski, N.A.; Ahmed, N.; Drezek, R.A.; Blaney, S.M.; West, J.L. Nanoshell-Mediated Photothermal Therapy Improves Survival in a Murine Glioma Model. J. Neuro-Oncol. 2011, 104, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ung, C.; Tsoli, M.; Liu, J.; Cassano, D.; Pocoví-Martínez, S.; Upton, D.H.; Ehteda, A.; Mansfeld, F.M.; Failes, T.W.; Farfalla, A.; et al. Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma. Cancers 2021, 13, 1278. [Google Scholar] [CrossRef] [PubMed]
- Morshed, R.A.; Muroski, M.E.; Dai, Q.; Wegscheid, M.L.; Auffinger, B.; Yu, D.; Han, Y.; Zhang, L.; Wu, M.; Cheng, Y.; et al. Cell-Penetrating Peptide-Modified Gold Nanoparticles for the Delivery of Doxorubicin to Brain Metastatic Breast Cancer. Mol. Pharm. 2016, 13, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor Microenvironment Sensitive Doxorubicin Delivery and Release to Glioma Using Angiopep-2 Decorated Gold Nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Swami, R.; Singh, I.; Kulhari, H.; Jeengar, M.K.; Khan, W.; Sistla, R. p-Hydroxy Benzoic Acid-Conjugated Dendrimer Nanotherapeutics as Potential Carriers for Targeted Drug Delivery to Brain: An in Vitro and in Vivo Evaluation. J. Nanopart. Res. 2015, 17, 265. [Google Scholar] [CrossRef]
- Sk, U.H.; Dixit, D.; Sen, E. Comparative Study of Microtubule Inhibitors—Estramustine and Natural Podophyllotoxin Conjugated PAMAM Dendrimer on Glioma Cell Proliferation. Eur. J. Med. Chem. 2013, 68, 47–57. [Google Scholar] [CrossRef]
- Nh, G.; Li, J. Targeted Theranostic Approach for Glioma Using Dendrimer-Based Curcumin Nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 1000393. [Google Scholar] [CrossRef]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of Paclitaxel across Cellular Barriers Using a Dendrimer-Based Nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Jia, X.; Lu, W.-L.; Lou, J.; Wei, Y. A Dual-Targeting Nanocarrier Based on Poly(Amidoamine) Dendrimers Conjugated with Transferrin and Tamoxifen for Treating Brain Gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Odeh, F.; Al-Adaileh, F.; Al-Taher, S.; Jaber, A.M.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Lipid Nanostructures for Targeting Brain Cancer. Heliyon 2021, 7, e07994. [Google Scholar] [CrossRef]
- Vieira, D.; Gamarra, L. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood–Brain Barrier. IJN 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Huang, X.; Wang, J.; Cai, F.; Zhao, P.; Yan, F. Targeted Delivery of Liposomal Temozolomide Enhanced Anti-Glioblastoma Efficacy through Ultrasound-Mediated Blood—Brain Barrier Opening. Pharmaceutics 2021, 13, 1270. [Google Scholar] [CrossRef] [PubMed]
- Lippens, R.J.J. Liposomal Daunorubicin (Daunoxome) In Children with Recurrent or Progressive Brain Tumors. Pediatric Hematol. Oncol. 1999, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Panigrahi, M.; Das, P. Brain Tumor and Gliadel Wafer Treatment. Indian J. Cancer 2011, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xing, W.; Shao, C.; Yang, C.; Wang, Z. The Role of Gliadel Wafers in the Treatment Of Newly Diagnosed GBM: A Meta-Analysis. DDDT 2015, 9, 3341. [Google Scholar] [CrossRef]
- Iuchi, T.; Inoue, A.; Hirose, Y.; Morioka, M.; Horiguchi, K.; Natsume, A.; Arakawa, Y.; Iwasaki, K.; Fujiki, M.; Kumabe, T.; et al. Long-Term Effectiveness of Gliadel Implant for Malignant Glioma and Prognostic Factors for Survival: 3-Year Results of a Postmarketing Surveillance in Japan. Neuro-Oncol. Adv. 2022, 4, vdab189. [Google Scholar] [CrossRef]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-Loaded PLGA Nanoparticles for the Chemotherapy of Glioblastoma: Towards the Pharmaceutical Development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Eivazi, N.; Rahmani, R.; Paknejad, M. Specific Cellular Internalization and PH-Responsive Behavior of Doxorubicin Loaded PLGA-PEG Nanoparticles Targeted with Anti EGFRvIII Antibody. Life Sci. 2020, 261, 118361. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced Anti-Angiogenic Effects of Bevacizumab in Glioblastoma Treatment upon Intranasal Administration in Polymeric Nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mohamed, M.S.; Mizuki, T.; Maekawa, T.; Sakthi Kumar, D. Chlorotoxin Modified Morusin—PLGA Nanoparticles for Targeted Glioblastoma Therapy. J. Mater. Chem. B 2019, 7, 5896–5919. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Meng, X.; Zhao, C.; Yang, Y.; Liu, G. Development of Transferrin-Modified Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Glioma Therapy. Anti-Cancer Drugs 2019, 30, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Duwa, R.; Emami, F.; Jeong, J.-H.; Yook, S. Enhanced Caspase-Mediated Abrogation of Autophagy by Temozolomide-Loaded and Panitumumab-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles in Epidermal Growth Factor Receptor Overexpressing Glioblastoma Cells. Mol. Pharm. 2020, 17, 4386–4400. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.; Faming, W.; Hongyan, Z.; Mengmeng, T.; Hang, S.; Liudi, Y. Iguratimod Encapsulated PLGA-NPs Improves Therapeutic Outcome in Glioma, Glioma Stem-like Cells and Temozolomide Resistant Glioma Cells. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102101. [Google Scholar] [CrossRef]
- Yang, C.-L.; Chen, J.-P.; Wei, K.-C.; Chen, J.-Y.; Huang, C.-W.; Liao, Z.-X. Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle. Nanomaterials 2017, 7, 85. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Klopp, C.T.; Alford, T.C.; Bateman, J.; Berry, G.N.; Winship, T. Fractionated Intra-Arterial Cancer. Chemotherapy with Methyl Bis Amine Hydrochloride; A Preliminary Report. Ann. Surg. 1950, 132, 811–832. [Google Scholar] [CrossRef]
- Goodman, L.S. Nitrogen Mustard Therapy: Use of Methyl-Bis(Beta-Chloroethyl)Amine Hydrochloride and Tris(Beta-Chloroethyl)Amine Hydrochloride for Hodgkin’s Disease, Lymphosarcoma, Leukemia and Certain Allied and Miscellaneous Disorders. JAMA 1946, 132, 126. [Google Scholar] [CrossRef]
- Madajewicz, S.; West, C.R.; Park, H.C.; Ghoorah, J.; Avellanosa, A.M.; Takita, H.; Karakousis, C.; Vincent, R.; Caracandas, J.; Jennings, E. Phase II Study—Intra-Arterial Bcnu Therapy for Metastatic Brain Tumors. Cancer 1981, 47, 653–657. [Google Scholar] [CrossRef]
- Greenberg, H.S.; Ensminger, W.D.; Seeger, J.F.; Kindt, G.W.; Chandler, F.; Doan, K.; Dakhil, S.R. Intra-Arterial BCNU Chemotherapy for the Treatment of Malignant Gliomas of the Central Nervous System: A Preliminary Report. Cancer Treat. Rep. 1981, 65, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, W.R.; Green, S.B.; Burger, P.C.; Selker, R.G.; VanGilder, J.C.; Robertson, J.T.; Mealey, J.; Ransohoff, J.; Mahaley, M.S. A Randomized Comparison of Intra-Arterial versus Intravenous with or without Intravenous 5-Fluorouracil, for Newly Diagnosed Patients with Malignant Glioma. J. Neurosurg. 1992, 76, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Kochi, M.; Kitamura, I.; Goto, T.; Nishi, T.; Takeshima, H.; Saito, Y.; Yamamoto, K.; Kimura, T.; Kino, T.; Tada, K.; et al. Randomized Comparison of Intra-arterial Versus Intravenous Infusion of ACNU for Newly Diagnosed Patients with Glioblastoma. J. Neuro-Oncol. 2000, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Eoli, M.; Salmaggi, A.; Erbetta, A.; Fariselli, L.; Boiardi, A. Intra-Arterial ACNU and Carboplatin versus Intravenous Chemotherapy with Cisplatin and BCNU in Newly Diagnosed Patients with Glioblastoma. Neurol. Sci. 2002, 23, 219–224. [Google Scholar] [CrossRef]
- Imbesi, F.; Marchioni, E.; Benericetti, E.; Zappoli, F.; Galli, A.; Corato, M.; Ceroni, M. A Randomized Phase III Study: Comparison between Intravenous and Intraarterial ACNU Administration in Newly Diagnosed Primary Glioblastomas. Anti-Cancer Res. 2006, 26, 553–558. [Google Scholar]
- Chen, W.; Wu, Q.; Mo, L.; Nassi, M. Intra-Arterial Chemotherapy Is Not Superior to Intravenous Chemotherapy for Malignant Gliomas: A Systematic Review and Meta-Analysis. Eur. Neurol. 2013, 70, 124–132. [Google Scholar] [CrossRef]
- Su, Y.S.; Ali, R.; Feroze, A.H.; Li, G.; Lawton, M.T.; Choudhri, O. Endovascular Therapies for Malignant Gliomas: Challenges and the Future. J. Clin. Neurosci. 2016, 26, 26–32. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood—Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Daneman, R. The Blood-Brain Barrier in Health and Disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood—Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Deeken, J.F.; Löscher, W. The Blood-Brain Barrier and Cancer: Transporters, Treatment, and Trojan Horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper. Surg. 2020, 19, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the Blood—Brain Tumor Barrier for Effective Glioblastoma Treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the Treatment of Glioblastoma Multiforme: Current State-of-the-Art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of Molecularly Targeted Therapy to Malignant Glioma, a Disease of the Whole Brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Arvanitis, C.D.; Wong, C.S.F.; Ferraro, G.B.; Jain, R.K. Emerging Strategies for Delivering Antiangiogenic Therapies to Primary and Metastatic Brain Tumors. Adv. Drug Deliv. Rev. 2017, 119, 159–174. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to Improve Delivery of Anticancer Drugs across the Blood—Brain Barrier to Treat Glioblastoma. Neuro-Oncology 2016, 18, 27–36. [Google Scholar] [CrossRef]
- Etame, A.B.; Diaz, R.J.; Smith, C.A.; Mainprize, T.G.; Hynynen, K.; Rutka, J.T. Focused Ultrasound Disruption of the Blood-Brain Barrier: A New Frontier for Therapeutic Delivery in Molecular Neurooncology. FOC 2012, 32, E3. [Google Scholar] [CrossRef]
- Meng, Y.; Suppiah, S.; Mithani, K.; Solomon, B.; Schwartz, M.L.; Lipsman, N. Current and Emerging Brain Applications of MR-Guided Focused Ultrasound. J. Ther. Ultrasound 2017, 5, 26. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. Safety and Feasibility of Multiple Blood-Brain Barrier Disruptions for the Treatment of Glioblastoma in Patients Undergoing Standard Adjuvant Chemotherapy. J. Neurosurg. 2021, 134, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.-Q.; Gwinn, R.; Witt, J.; et al. Safety and Efficacy of Focused Ultrasound Thalamotomy for Patients With Medication-Refractory, Tremor-Dominant Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 1412. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Salas, C.; Fernández-Rodríguez, B.; Pineda-Pardo, J.A.; Rodríguez-Rojas, R.; Obeso, I.; Hernández-Fernández, F.; del Álamo, M.; Mata, D.; Guida, P.; Ordás-Bandera, C.; et al. Blood-Brain Barrier Opening with Focused Ultrasound in Parkinson’s Disease Dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood—Brain Barrier Opening in Alzheimer’s Disease Using MR-Guided Focused Ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Meng, Y.; Suppiah, S.; Surendrakumar, S.; Bigioni, L.; Lipsman, N. Low-Intensity MR-Guided Focused Ultrasound Mediated Disruption of the Blood-Brain Barrier for Intracranial Metastatic Diseases. Front. Oncol. 2018, 8, 338. [Google Scholar] [CrossRef]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; McDannold, N. Multiple Sessions of Liposomal Doxorubicin Delivery via Focused Ultrasound Mediated Blood—Brain Barrier Disruption: A Safety Study. J. Control. Release 2015, 204, 60–69. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular Mechanisms of the Blood-Brain Barrier Opening Induced by Ultrasound in Presence of Microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.-Y.; Han, M.; Choi, J.; Ahn, S.; Lee, T.; Chang, Y.; Park, J. Localized Down-Regulation of P-Glycoprotein by Focused Ultrasound and Microbubbles Induced Blood-Brain Barrier Disruption in Rat Brain. Sci. Rep. 2016, 6, 31201. [Google Scholar] [CrossRef]
- Chen, H.; Konofagou, E.E. The Size of Blood—Brain Barrier Opening Induced by Focused Ultrasound Is Dictated by the Acoustic Pressure. J. Cereb. Blood Flow Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef]
- Liu, H.-L.; Fan, C.-H.; Ting, C.-Y.; Yeh, C.-K. Combining Microbubbles and Ultrasound for Drug Delivery to Brain Tumors: Current Progress and Overview. Theranostics 2014, 4, 432–444. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meairs, S.; Alonso, A. Ultrasound, Microbubbles and the Blood—Brain Barrier. Prog. Biophys. Mol. Biol. 2007, 93, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.-Z.; McDannold, N. Effects on P-Glycoprotein Expression after Blood-Brain Barrier Disruption Using Focused Ultrasound and Microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical Trial of Blood-Brain Barrier Disruption by Pulsed Ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.; Jordão, J.F.; O’Reilly, M.A.; Markham, K.; Weng, Y.-Q.; Foust, K.D.; Kaspar, B.K.; Hynynen, K.; Aubert, I. Targeted Delivery of Self-Complementary Adeno-Associated Virus Serotype 9 to the Brain, Using Magnetic Resonance Imaging-Guided Focused Ultrasound. Hum. Gene Ther. 2012, 23, 1144–1155. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted Delivery of Doxorubicin to the Rat Brain at Therapeutic Levels Using MRI-Guided Focused Ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive Localized Delivery of Herceptin to the Mouse Brain by MRI-Guided Focused Ultrasound-Induced Blood—Brain Barrier Disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef]
- Mei, J.; Cheng, Y.; Song, Y.; Yang, Y.; Wang, F.; Liu, Y.; Wang, Z. Experimental Study on Targeted Methotrexate Delivery to the Rabbit Brain via Magnetic Resonance Imaging-Guided Focused Ultrasound. J. Ultrasound Med. 2009, 28, 871–880. [Google Scholar] [CrossRef]
- Wei, K.-C.; Chu, P.-C.; Wang, H.-Y.J.; Huang, C.-Y.; Chen, P.-Y.; Tsai, H.-C.; Lu, Y.-J.; Lee, P.-Y.; Tseng, I.-C.; Feng, L.-Y.; et al. Focused Ultrasound-Induced Blood—Brain Barrier Opening to Enhance Temozolomide Delivery for Glioblastoma Treatment: A Preclinical Study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hua, M.-Y.; Yang, H.-W.; Huang, C.-Y.; Chu, P.-C.; Wu, J.-S.; Tseng, I.-C.; Wang, J.-J.; Yen, T.-C.; Chen, P.-Y.; et al. Magnetic Resonance Monitoring of Focused Ultrasound/Magnetic Nanoparticle Targeting Delivery of Therapeutic Agents to the Brain. Proc. Natl. Acad. Sci. USA 2010, 107, 15205–15210. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Xu, Z.; Sheehan, J.P. Focused Ultrasound-Aided Immunomodulation in Glioblastoma Multiforme: A Therapeutic Concept. J. Ther. Ultrasound 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood—Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21 (Suppl. 5), v1–v100. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, S.; Li, J.; Cao, H.; Huang, J.; Fan, F.; Cheng, L.; Liu, Z.; Cheng, Q. The Survival Benefits of Surgical Resection and Adjuvant Therapy for Patients With Brainstem Glioma. Front. Oncol. 2021, 11, 566972. [Google Scholar] [CrossRef]
- Mathieu, D. Radiosurgery after Craniotomy. In Progress in Neurological Surgery; Kim, D.G., Lunsford, L.D., Eds.; S. Karger AG: Basel, Switzerland, 2012; Volume 25, pp. 221–227. [Google Scholar] [CrossRef]
- Rincon-Torroella, J.; Khela, H.; Bettegowda, A.; Bettegowda, C. Biomarkers and Focused Ultrasound: The Future of Liquid Biopsy for Brain Tumor Patients. J. Neuro-Oncol. 2022, 156, 33–48. [Google Scholar] [CrossRef]
- Schoen, S.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards Controlled Drug Delivery in Brain Tumors with Microbubble-Enhanced Focused Ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).