Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment Protocol with Atezolizumab Plus Bevacizumab
2.3. Therapeutic Efficacy
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
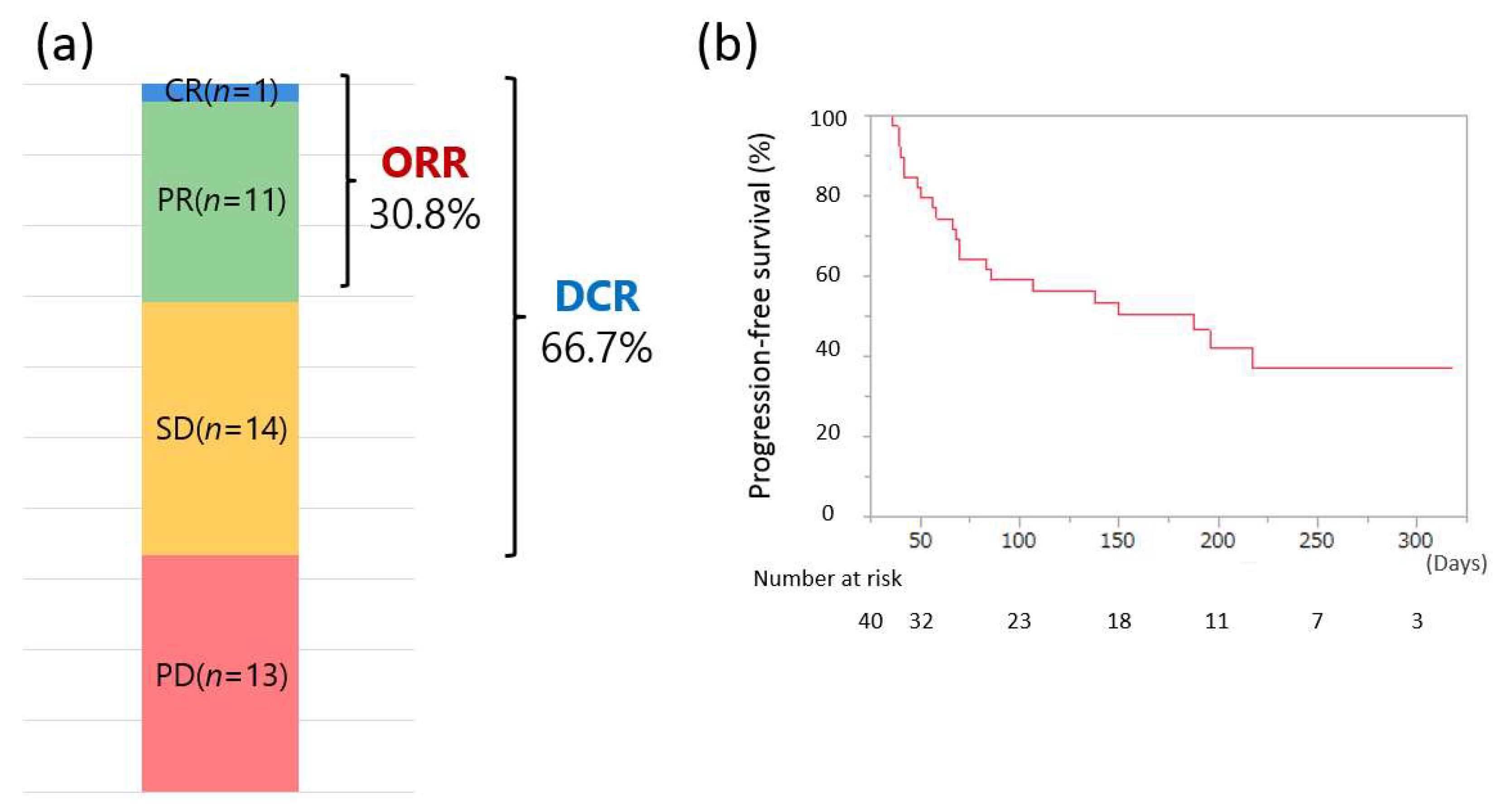
3.2. Therapeutic Response and Progression-Free Survival
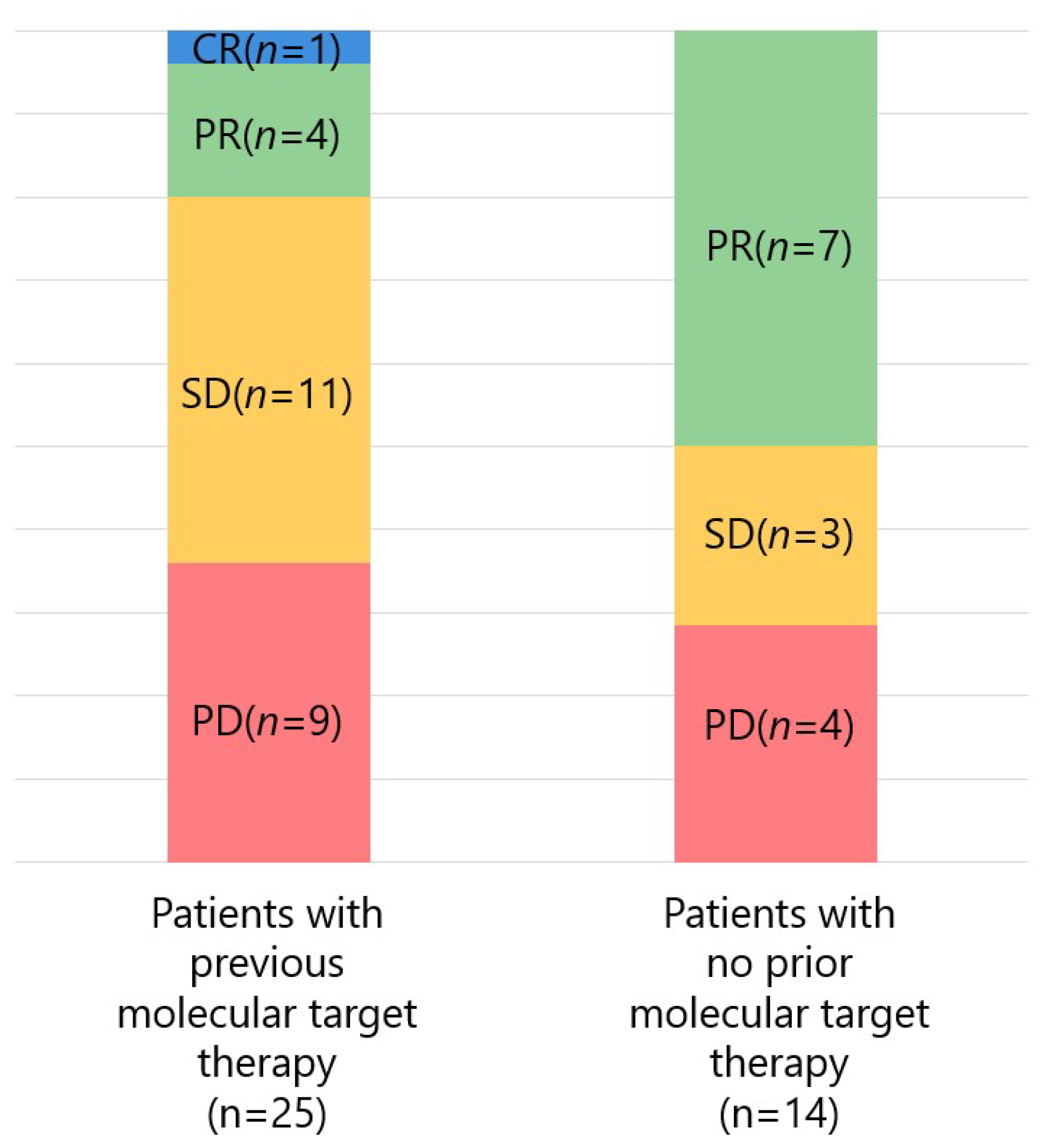
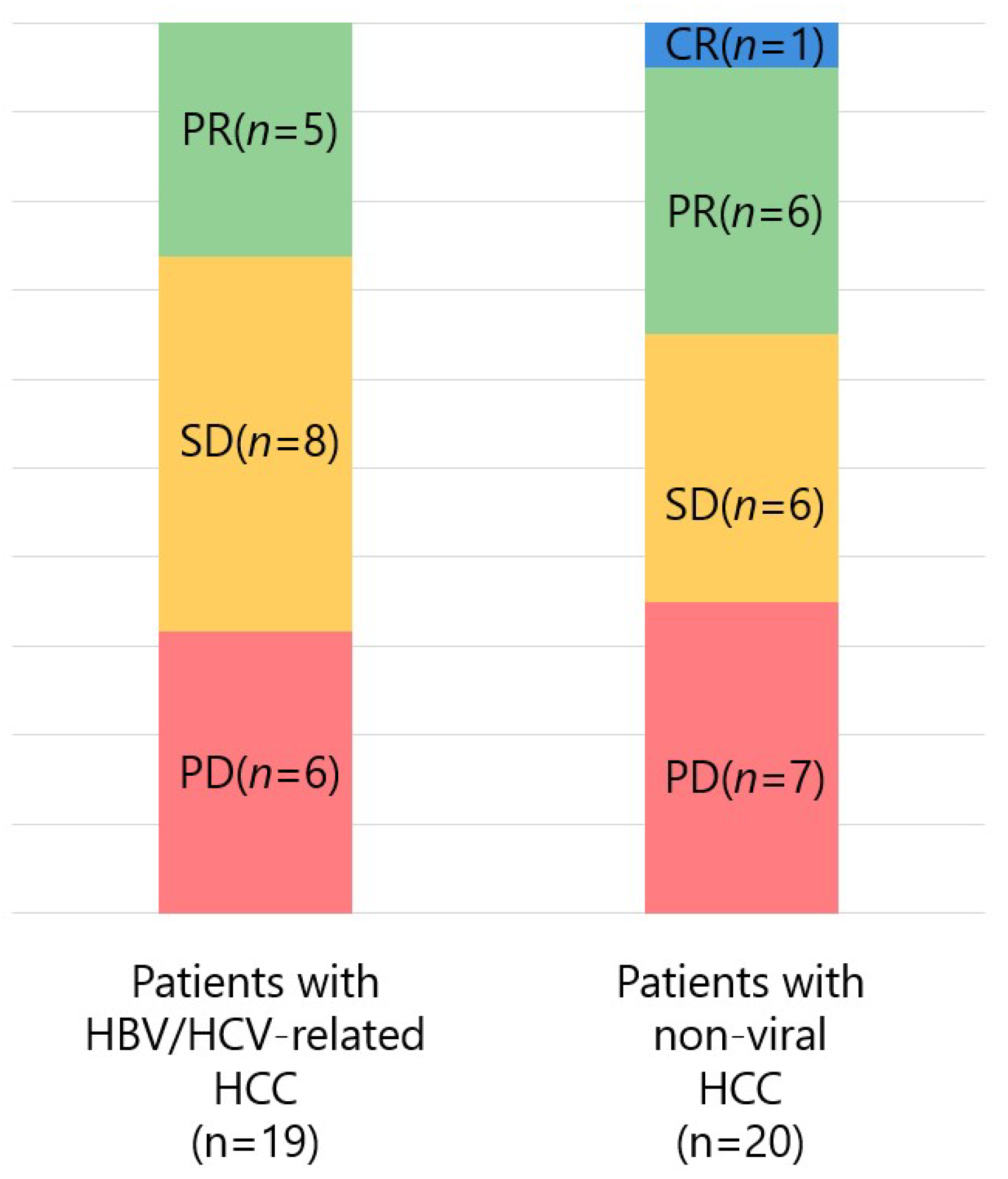
3.3. Therapeutic Response by Prior Molecular-Targeted Therapy and Etiology
3.4. Adverse Events
3.5. Comparison of Patients Who Did and Did Not Achieve Disease Control by Atezo/Bev Threapy
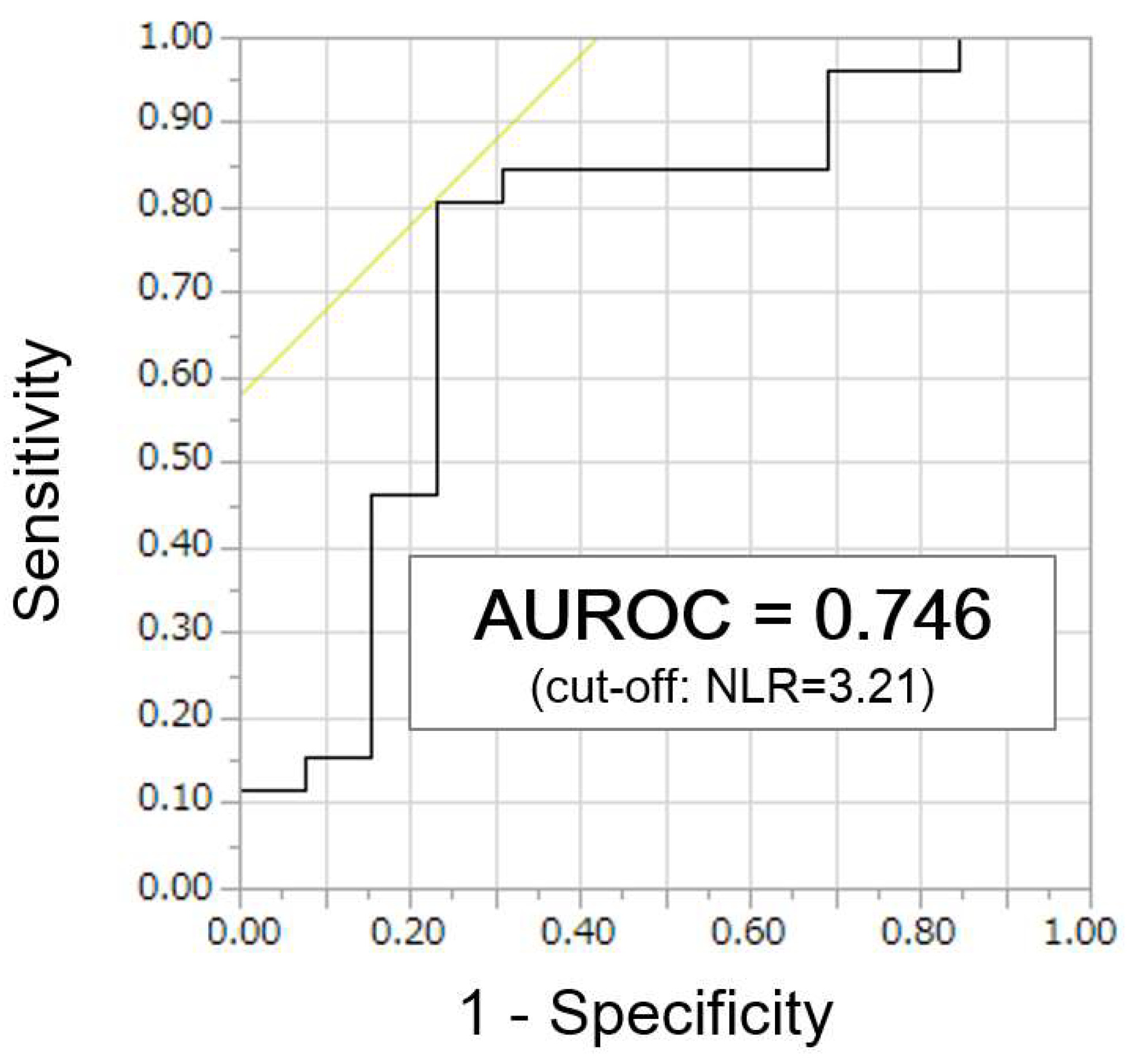
3.6. Identification of NLR Cut-Off Values to Predict Disease Control
3.7. Comparison of Patients with High NLR and Low NLR
3.8. Response Rate and Progression-Free Survival by Pretreatment NLR Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, S.; Eso, Y.; Okada, H.; Takai, A.; Takahashi, K.; Seno, H. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers 2020, 12, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. New Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep. 2021, e1464. [Google Scholar] [CrossRef]
- Ando, Y.; Kawaoka, T.; Kosaka, M.; Shirane, Y.; Johira, Y.; Miura, R.; Murakami, S.; Yano, S.; Amioka, K.; Naruto, K.; et al. Early Tumor Response and Safety of Atezolizumab Plus Bevacizumab for Patients with Unresectable Hepatocellular Carcinoma in Real-World Practice. Cancers 2021, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267. [Google Scholar] [CrossRef]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilen, M.A.; Dutcher, G.M.A.; Liu, Y.; Ravindranathan, D.; Kissick, H.T.; Carthon, B.C.; Kucuk, O.; Harris, W.B.; Master, V.A. Association Between Pretreatment Neutrophil-to-Lymphocyte Ratio and Outcome of Patients With Metastatic Renal-Cell Carcinoma Treated With Nivolumab. Clin. Genitourin. Cancer 2018, 16, e563–e575. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Satake, H.; Ogata, M.; Hatachi, Y.; Inoue, K.; Hamada, M.; Yasui, H. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: A multicenter retrospective study. Oncotarget 2018, 9, 34520–34527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Min, G.-T.; Li, Y.-M.; Yao, N.; Wang, J.; Wang, H.-P.; Chen, W. The pretreatment neutrophil-lymphocyte ratio may predict prognosis of patients with liver cancer: A systematic review and meta-analysis. Clin. Transplant. 2017, 32, e13151. [Google Scholar] [CrossRef] [PubMed]
- Mouchli, M.; Reddy, S.; Gerrard, M.; Boardman, L.; Rubio, M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma.” Review article. Ann. Hepatol. 2021, 22, 100249. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C., IV; Luyten, E.J.; Mahadev, S.; Witkowski, P.; Siegel, A.B.; Brown, R.S., Jr.; Emond, J.C. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Farid, S.; Malik, H.Z.; Young, A.L.; Toogood, G.J.; Lodge, J.P.A.; Prasad, K.R. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J. Surg. 2008, 32, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zhang, J.; Zhu, Q.; Qin, L.; Yao, W.; Lei, B.; Shi, W.; Yuan, S.; Tahir, S.A.; Jin, J.; et al. Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection. Transl. Oncol. 2014, 7, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.-L.; Luo, J.; Chen, M.-S.; Li, J.-Q.; Shi, M. Blood Neutrophil-to-lymphocyte Ratio Predicts Survival in Patients with Unresectable Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. J. Vasc. Interv. Radiol. 2011, 22, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, H. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007, 7, 4. [Google Scholar] [PubMed]
- Kuang, D.-M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.-Y.; Zheng, L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers 2021, 13, 1607. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 40 |
|---|---|
| Age (years, range) | 70.5 (53–82) |
| Sex (male/female) | 35/5 |
| Etiology (HBV/HCV/non-B non-C) | 6/13/21 |
| BCLC stage (B/C) | 21/19 |
| Treatment history (naïve/recurrence) Treatment prior to Atezo/Bev Surgery TACE Lenvatinib Sorafenib Regorafenib Ramucirumab Radiation therapy | 6/34 7 11 11 2 1 1 1 |
| Aspartate aminotransferase (IU/L) | 39.5 (15–192) |
| Alanine aminotransferase (IU/L) | 28.0 (11–110) |
| Platelets (×104/μL) | 14.4 (4.2–28.1) |
| Child-Pugh score (5A/6A/7B) | 26/12/2 |
| ALBI score | −2.53 (−3.16–−1.45) |
| Modified ALBI grade (1/2a/2b) | 16/12/12 |
| α-fetoprotein (ng/mL) | 19.0 (1.4–57063) |
| Des-γ-carboxy prothrombin (mAU/mL) | 136 (12–177443) |
| FIB-4 index | 3.57 (1.49–11.7) |
| Neutrophil-to-lymphocyte ratio (NLR) | 2.56 (0.39–14.0) |
| Platelet-to-lymphocyte ratio (PLR) | 125 (27.1–351) |
| Lymphocyte-to-monocyte ratio (LMR) | 2.79 (0.86–5.41) |
| Adverse Events | Any Grade (%) | Grade ≥ 3 (%) |
|---|---|---|
| Hypertension | 17 (42.5%) | 3 (7.5%) |
| Proteinuria | 16 (40.0%) | 6 (15.0%) |
| Edema | 15 (37.5%) | 1 (2.5%) |
| Fever | 13 (32.5%) | - |
| Fatigue | 11 (27.5%) | - |
| Pruritus | 10 (25.0%) | - |
| Decreased appetite | 7 (17.5%) | 1 (2.5%) |
| Hand-foot skin reaction | 5 (12.5%) | - |
| Nasal bleeding | 5 (12.5%) | 1 (2.5%) |
| Rash | 4 (10.0%) | - |
| Thyroid function abnormality | 4 (10.0%) | - |
| Stomatitis | 4 (10.0%) | - |
| Characteristics | Patients with CR/PR/SD (n = 26) | Patients with PD (n = 13) | p Value |
|---|---|---|---|
| Age (years) | 68.8 (7.38) | 70.1 (6.46) | 0.621 |
| Sex (male/female) | 23/3 | 11/2 | 0.735 |
| Etiology (viral/non-viral) | 12/14 | 6/7 | 1.000 |
| BCLC stage (B/C) | 14/12 | 7/6 | 1.000 |
| Treatment history with MTA (yes/no) | 16/10 | 9/4 | 0.637 |
| Aspartate aminotransferase (IU/L) | 53.4 (43.0) | 46.7 (24.1) | 0.964 |
| Alanine aminotransferase (IU/L) | 38.7 (27.0) | 36.4 (20.1) | 0.777 |
| Platelets (×104/μL) | 16.0 (5.96) | 14.2 (5.23) | 0.465 |
| Child-Pugh score (5A/6A or 7B) | 18/8 | 7/6 | 0.345 |
| ALBI score | −2.47 (0.41) | −2.32 (0.37) | 0.205 |
| Modified ALBI grade (1/2a or 2b) | 12/14 | 3/10 | 0.163 |
| α-fetoprotein (ng/mL) | 1341 (5305) | 7436 (15772) | 0.022 |
| Des-γ-carboxy prothrombin (mAU/mL) | 12751 (40523) | 2529 (3590) | 0.318 |
| FIB-4 index | 4.34 (3.38) | 4.83 (3.08) | 0.475 |
| Neutrophil-to-lymphocyte ratio (NLR) | 2.47 (1.09) | 4.48 (3.22) | 0.013 |
| Platelet-to-lymphocyte ratio (PLR) | 134 (58.1) | 163 (83.0) | 0.270 |
| Lymphocyte-to-monocyte ratio (LMR) | 2.85 (1.08) | 2.68 (1.36) | 0.551 |
| Characteristics | Patients with High NLR (n = 15) | Patients with Low NLR (n = 24) | p Value |
|---|---|---|---|
| Age (years) | 68.6 (6.54) | 69.7 (7.41) | 0.671 |
| Sex (male/female) | 13/2 | 21/3 | 0.940 |
| Etiology (viral/non-viral) | 8/7 | 11/13 | 0.649 |
| BCLC stage (B/C) | 8/7 | 13/11 | 0.960 |
| Treatment history with MTA (yes/no) | 10/5 | 14/10 | 0.603 |
| Aspartate aminotransferase (IU/L) | 51.5 (40.0) | 51.0 (36.6) | 0.762 |
| Alanine aminotransferase (IU/L) | 38.0 (24.8) | 37.8 (25.1) | 0.977 |
| Platelets (×104/μL) | 16.4 (5.45) | 14.7 (5.90) | 0.411 |
| Child-Pugh score (5A/6A or 7B) | 8/7 | 17/7 | 0.268 |
| ALBI score | −2.32 (0.40) | −2.48 (0.39) | 0.298 |
| modified ALBI grade (1/2a or 2b) | 5/10 | 10/14 | 0.603 |
| α-fetoprotein (ng/mL) | 6235 (14969) | 1584 (5503) | 0.387 |
| Des-γ-carboxy prothrombin (mAU/mL) | 14695 (47306) | 5999 (24510) | 0.312 |
| FIB-4 index | 3.88 (2.39) | 4.83 (3.08) | 0.488 |
| Neutrophil-to-lymphocyte ratio (NLR) | 4.97 (2.69) | 2.00 (0.67) | <0.0001 |
| Platelet-to-lymphocyte ratio (PLR) | 193 (77.3) | 113 (38.5) | 0.002 |
| Lymphocyte-to-monocyte ratio (LMR) | 2.27 (1.20) | 3.13 (1.05) | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eso, Y.; Takeda, H.; Taura, K.; Takai, A.; Takahashi, K.; Seno, H. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma. Curr. Oncol. 2021, 28, 4157-4166. https://doi.org/10.3390/curroncol28050352
Eso Y, Takeda H, Taura K, Takai A, Takahashi K, Seno H. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma. Current Oncology. 2021; 28(5):4157-4166. https://doi.org/10.3390/curroncol28050352
Chicago/Turabian StyleEso, Yuji, Haruhiko Takeda, Kojiro Taura, Atsushi Takai, Ken Takahashi, and Hiroshi Seno. 2021. "Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma" Current Oncology 28, no. 5: 4157-4166. https://doi.org/10.3390/curroncol28050352
APA StyleEso, Y., Takeda, H., Taura, K., Takai, A., Takahashi, K., & Seno, H. (2021). Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma. Current Oncology, 28(5), 4157-4166. https://doi.org/10.3390/curroncol28050352







