Abstract
Spontaneous regression of metastatic renal cell carcinoma (mRCC) is a rare event, often associated with an activation of innate immunity by various triggers. SARS-CoV-2 infection induces a strong inflammatory response in some patients and a cytokine storm is one of the main causes of severe morbidity and mortality associated with the virus. Here, we describe two cases of patients with histologically and radiologically proven mRCC whose treatment was delayed due to COVID-19 and who experienced spontaneous tumour regression following the infection. Both patients reported here had predominantly pulmonary and mediastinal involvement and underwent nephrectomy. The interval between the diagnosis of COVID-19 and the detection of tumour regression was 3 and 4 months, respectively. Although approved vaccines and other measures are clearly the best way to prevent COVID-19-associated morbidity and mortality in cancer patients, we hypothesize that innate immunity activation by the infection can contribute to tumour regression in special circumstances.
1. Introduction
Spontaneous regression of metastatic renal cell carcinoma (mRCC) is a rare but well described phenomenon. It is thought that spontaneous regression may occur in up to 1% of mRCC cases, especially in patients with clear cell histology after the removal of the primary tumour. Spontaneous regressions in mRCC tend to be long-lasting [1,2,3].
Spontaneous regression of cancer has been reported especially in tumours capable of eliciting strong immune responses, such as renal cell carcinoma, melanoma, and B-cell malignancies. A synthetic attempt has identified a concomitant inflammatory condition as a process providing a link between some reports. The aetiology of these inflammatory responses includes not only severe bacterial or viral infections but also vaccinations followed by febrile reactions [4].
Two cases of spontaneous regression of B-cell lymphomas following COVID-19 have been published [5,6], but, to our knowledge, this is the first description of mRCC regression following SARS-CoV-2 infection.
2. Case Description
2.1. Patient 1
A 71-year-old male with a medical history of type 2 diabetes and prostatic hyperplasia initially presented with weight loss and exertional dyspnoea in August 2020. A CT scan on 28 August 2020 revealed marked mediastinal lymphadenopathy (Figure 1a) and multiple lung nodules as well as a tumour of the right kidney. A cryobiopsy of an intramural bronchial right lung lesion was reported as a metastasis of grade 2 clear cell renal cell carcinoma (RCC). The patient started to develop signs and symptoms of heart failure confirmed by high levels of N-terminal pro-hormone B-type natriuretic peptide and low left ventricular ejection fraction of 25% at the beginning of October 2020 and was admitted for treatment to an internal medicine department on 10 November 2020. On 21 November 2020, he unexpectedly developed abdominal discomfort and diarrhoea. A PCR test for SARS-CoV-2 was positive. Aside from supportive measures, no specific treatment for COVID-19 was administered. As the cardiac failure had improved by this time, he was discharged after the end of the prescribed quarantine period on 1 December 2020. Right nephron-sparing nephrectomy was planned and carried out on 14 January 2021 confirming the histology of clear cell RCC. The patient was then referred for systemic treatment of mRCC.
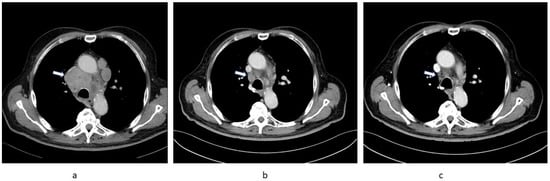
Figure 1.
Sequential chest CT images of Patient 1 showing regression of substantial mediastinal lymphadenopathy (arrow). (a) 28 August 2020, (b) 15 February 2021, (c) 18 May 2021.
At his first oncology appointment he was well, with an Eastern Cooperative Oncology Group (ECOG) performance status of 1. He had a urinary catheter for obstruction due to prostatic hyperplasia. His medication at this time included metformin, spironolactone, furosemide, and metoprolol. Laboratory results were unremarkable except for renal impairment with a glomerular filtration rate (GFR) of 30 mL/min and a moderately increased neutrophil count (8800/µL). A CT scan was ordered to ascertain the extent of the disease prior to planned targeted therapy and was carried out on 15 February 2021; surprisingly, this showed marked regression of all tumour lesions (Figure 1b). Consequently, no treatment was initiated and a follow-up CT scan on 18 May 2021 confirmed ongoing substantial partial regression of metastatic lesions (Figure 1c).
2.2. Patient 2
A 58-year-old male with no significant medical history presented to his general practitioner on 8 March 2021 with fever and headache. A PCR test for SARS-CoV-2 was positive and the patient was started on antibiotics; firstly, clarithromycin and, subsequently, amoxycillin clavulanate for persistent fever. A chest X-ray on 26 March 2021 showed bilateral lung infiltrates. Therapy with prednisone was initiated and by 14 April 2020 the lung infiltrates had regressed. However, the repeat X-ray also revealed multiple bilateral round dense opacities. The patient was still subfebrile and had lost 10 kg of his initial weight of 105 kg over the previous 8 weeks. A CT scan was ordered and carried out on 15 April 2021 showing post-COVID-19 lung parenchymal changes but also a left renal mass and multiple lung lesions consistent with metastatic cancer (Figure 2a). On 9 June 2021, an uncomplicated left nephrectomy was carried out, with histology reported as poorly differentiated clear cell carcinoma with sarcomatoid features.
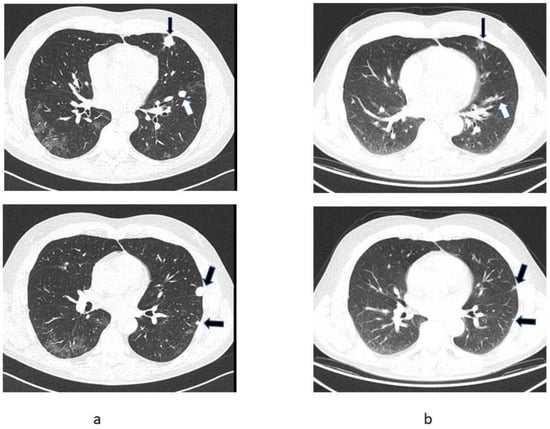
Figure 2.
Sequential chest CT images of Patient 2 showing partial regression of pulmonary metastases (arrows). (a) CT scan of 15 April 2021, (b) CT-registered images from PET/CT scan of 20 July 2021.
At his first oncology appointment on 26 June 2021, the patient was asymptomatic except for mild abdominal discomfort after the nephrectomy. He was taking no regular medication. The physical examination was unremarkable except for mild hypertension. Blood tests showed renal insufficiency with a GFR of 26 mL/min, anaemia (haemoglobin 10.1 mg/dL), mildly increased C-reactive protein (25 mg/L) and increased platelet count (464,000/µL). As a patient with poor prognostic features and sarcomatoid histology, therapy with ipilimumab and nivolumab was contemplated, but a PET/CT was carried out first to assess the current disease extent. The examination on 22 July 2021 showed a marked reduction in lung metastases (Figure 2b). The patient has received no systemic antineoplastic treatment and remains closely followed by an oncologist.
3. Discussion
SARS-CoV-2 infection manifests in some patients with a strong inflammatory response with high levels of cytokines such as interleukin (IL)-6 and interferon (INF)-α, and, in severe cases, also IL-2 and tumour necrosis factor [7,8]. The cytokines and chemokines induce T helper 1 (TH1) cell-polarized response attracting monocytes and T lymphocytes to the lungs [8]. An overwhelming cytokine response to SARS-CoV-2 is associated with severe morbidity and mortality. In particular, IL-6 may trigger acute respiratory distress syndrome and is a target for therapeutic intervention with the monoclonal antibody against the IL-6 receptor tocilizumab [9]. The complement system, another component of innate immunity, is also activated in patients with severe COVID-19 infection resulting in a hypercoagulable state [10,11].
Innate immunity has also been used therapeutically in mRCC in the form of the now largely obsolescent cytokine therapy with IL-2 and INF-α. High-dose IL-2, in particular, can induce a durable, complete tumour response in a small number of patients with mRCC [12,13]. Most current immunotherapy protocols emphasize the activation of specific T cells using inhibitors of the programmed cell death protein 1 pathway or the cytotoxic T lymphocyte antigen 4 receptor, producing durable responses in a proportion of patients, even after the treatment is discontinued [14,15]. However, there is an ongoing research effort to mobilize innate immunity to change immunosuppressive tumour microenvironment using indoleamine 2,3-dioxygenase inhibitors, CD40 agonists, and many other approaches [16].
The two patients reported here had predominant pulmonary and mediastinal involvement and, thus, the conditions were favourable for colocalization of the strong innate SARS-CoV-2-induced immune response [8] and specific antitumour immunity. In addition, in both patients, cytoreductive nephrectomy was carried out. The interval between the diagnosis of COVID-19 and the detection of tumour regression was 3 and 4 months in the two patients, respectively. In Patient 1, the response was ongoing after 6 months. There is evidence that both patients had cleared the virus, although we do not know the precise timepoint when this happened. Confirmatory negative tests were not required according to relevant contemporary guidelines. The respective patients tested negative by PCR for SARS-CoV-2 three and six months after the initial detection when testing was carried out in the context of planned medical procedures.
Antibacterial therapy, including fluoroquinolones, may possess certain anticancer, as well as immunomodulatory, activity [17,18]. Clarithromycin may potentiate the effect of tyrosine kinase inhibitors in chronic myeloid leukemia [19]. Anticancer properties of amoxycillin have not been reported, and antibiotic treatment may cause adverse changes in gut microbiota and is associated with the decreased efficacy of cancer immunotherapy [18,20]. Thus, we have no basis to presume that the antimicrobial treatment received by Patient 2 contributed to the tumour regression.
Although in the pandemic environment delays in cancer treatment in general reflect adversely on patient prognosis, in the case of the two reported patients a delay fortuitously allowed us to observe the rare phenomenon. Because spontaneous tumour regression is obviously a rare and unpredictable event, even in the context of the SARS-CoV-2 pandemic, approved vaccines and other preventative measures must be used to prevent morbidity and mortality in cancer patients, including those with potentially immunogenic malignancies. Nevertheless, the question of SARS-CoV-2-induced innate immune response contributing to tumour regression could be retrospectively explored using clinical trial data, where an augmentation of clinical responses after COVID-19 could be potentially detectable, especially in studies investigating immunotherapy.
4. Conclusions
Innate immunity activation by SARS-CoV-2 infection can contribute to tumour regression in rare cases.
Author Contributions
Conceptualization, T.B.; Resources, L.F., J.B., H.J., J.V.; Writing—Original Draft Preparation, T.B.; Writing—Review and Editing, T.B., L.F., J.B., H.J., J.V.; Funding Acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
Publication fee was covered in part by an unrestricted grant by Servier to the First Faculty of Medicine, Prague, Czech Republic.
Informed Consent Statement
The subjects gave their informed consent for inclusion in the case report.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge the kind provision of PET/CT scans by the Department of Nuclear Medicine-PET Centre, Na Homolce Hospital, Prague, and clinical reports by Libuse Novotna (District Hospital Kladno) and Katerina Petraskova (Medicon, Prague).
Conflicts of Interest
The authors declare no conflict of interest regarding the presented work.
References
- Bumpus, H.C. The Apparent Disappearance of Pulmonary Metastasis in a Case of Hypernephroma Following Nephrectomy. J. Urol. 1928, 20, 185–192. [Google Scholar] [CrossRef]
- Jenkins, G.D. Regression of Pulmonary Metastasis Following Nephrectomy for Hypernephroma: Eight Year Follow-up. J. Urol. 1959, 82, 37–40. [Google Scholar] [CrossRef]
- Melichar, B.; Vaněčková, J.; Morávek, P.; Urminská, H.; Podhola, M. Spontaneous regression of renal cell carcinoma lung metastases in a patient with psoriasis. Acta Oncol. 2009, 48, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Behzad, N. Immunology of the Spontaneous Remission of Cancer. Int. J. Cancer Clin. Res. 2019, 6, 112. [Google Scholar] [CrossRef]
- Challenor, S.; Tucker, D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br. J. Haematol. 2021, 192, 415. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Gelardi, F.; Carlo-Stella, C.; Chiti, A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: From the “flare phenomenon” to the “abscopal effect”. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2652–2654. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Zakowski, P.; Tran, H.P.; Smith, E.A.; Gaultier, C.; Marks, G.; Zabner, R.; Lowenstein, H.; Oft, J.; Bluen, B.; et al. Compassionate Use of Tocilizumab for Treatment of SARS-CoV-2 Pneumonia. Clin. Infect. Dis. 2020, 71, 3168–3173. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, J.; Demaria, O.; Vély, F.; Batista, L.; Benmansour, N.C.; Fares, J.; Carpentier, S.; Thibult, M.-L.; Morel, A.; Remark, R.; et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature 2020, 588, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C. Innate and adaptive immune responses to SARS-CoV-2 in humans: Relevance to acquired immunity and vaccine responses. Clin. Exp. Immunol. 2021, 204, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Law, T.M.; Motzer, R.J.; Mazumdar, M.; Sell, K.W.; Walther, P.; O’Connell, M.; Khan, A.; Vlamis, V.; Vogelzang, N.J.; Bajorin, D.F. Phase iii randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995, 76, 824–832. [Google Scholar] [CrossRef]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Frontera, O.A.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Haineala, B.; Zgura, A.; Diaconu, C.; Mehedintu, C.; Bacinschi, X.; Anghel, R.M. Long-term Response After Stopping Immunotherapy in a Patient With Metastatic Renal Cancer. Vivo 2021, 35, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Campesato, L.F.; Weng, C.-H.; Merghoub, T. Innate immune checkpoints for cancer immunotherapy: Expanding the scope of non T cell targets. Ann. Transl. Med. 2020, 8, 1031. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrześniok, D.; Minecka, A.; Rok, J.; Delijewski, M.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin-mediated induction of S-phase cell cycle arrest and apoptosis in COLO829 melanoma cells. Pharmacol. Rep. 2018, 70, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.-G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.M.; Beltrami, G.; Pica, G.; Carella, A.; Catania, G. Clarithromycin potentiates tyrosine kinase inhibitor treatment in patients with resistant chronic myeloid leukemia. Leuk. Lymphoma 2012, 53, 1409–1411. [Google Scholar] [CrossRef]
- Tsikala-Vafea, M.; Belani, N.; Vieira, K.; Khan, H.; Farmakiotis, D. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 106, 142–154. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).