Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges
Abstract
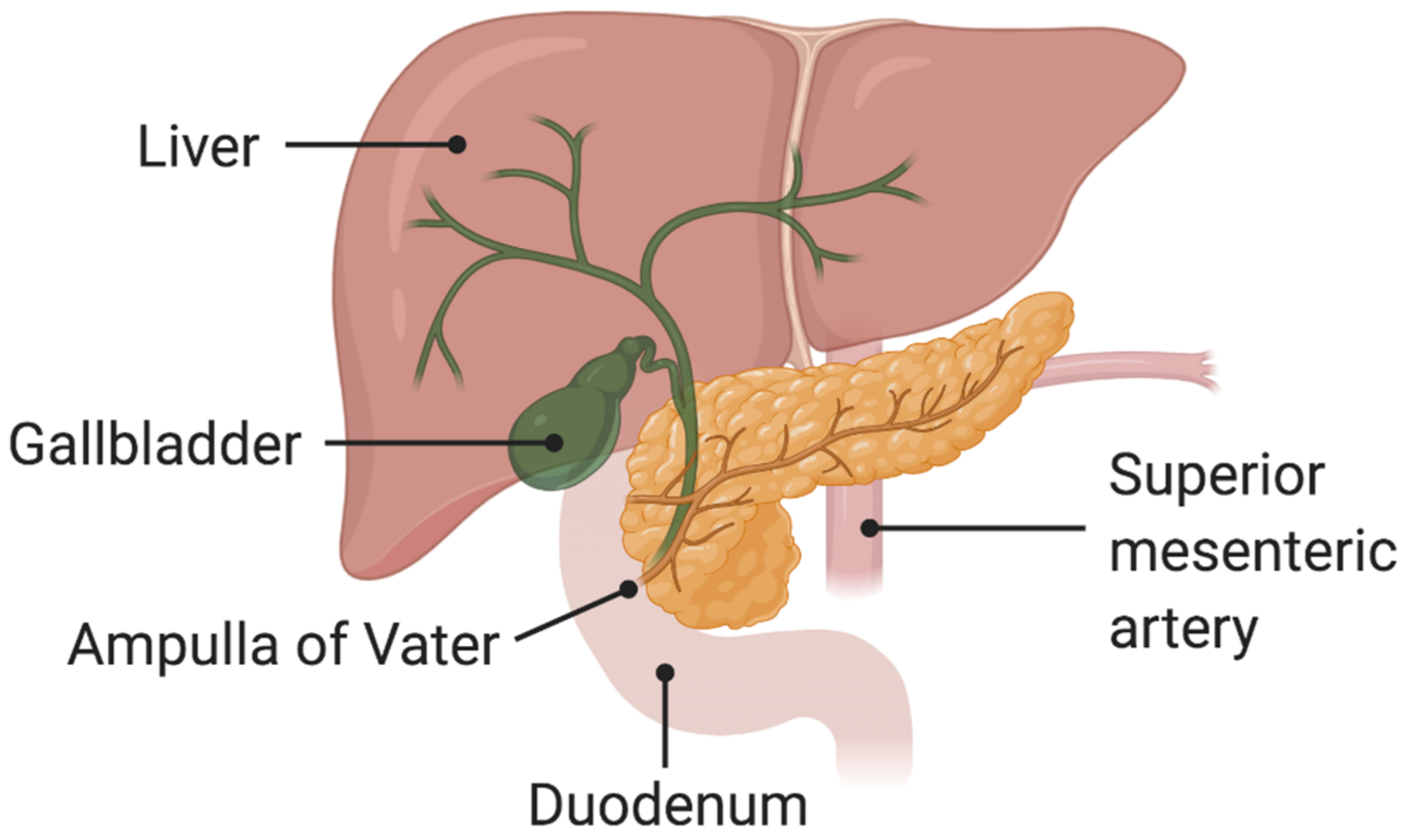
:1. Introduction
2. Histological Features
3. Genetic and Molecular Features
4. Clinical Features, Differential Diagnosis and Staging
5. Surgery
6. Medical Treatment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahn, D.H.; Bekaii-Saab, T. Ampullary Cancer: An Overview. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng-Pywell, R.; Reddy, S. Ampullary Cancer. Surg. Clin. N. Am. 2019, 99, 357–367. [Google Scholar] [CrossRef]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.-P.; Zhou, H. Pathogenesis of carcinoma of the papilla of Vater. J. Hepato-Biliary-Pancreat. Surg. 2004, 11, 301–309. [Google Scholar] [CrossRef]
- Alessandrino, F.; Ivanovic, A.M.; Yee, E.U.; Radulovic, D.; Souza, D.; Mortele, K.J. MDCT and MRI of the ampulla of Vater. Part I: Technique optimization, normal anatomy, and epithelial neoplasms. Abdom. Imaging 2015, 40, 274–291. [Google Scholar] [CrossRef]
- Perone, J.A.; Riall, T.S.; Olino, K. Palliative Care for Pancreatic and Periampullary Cancer. Surg. Clin. N. Am. 2016, 96, 1415–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albores-Saavedra, J.; Schwartz, A.M.; Batich, K.; Henson, D.E. Cancers of the ampulla of vater: Demographics, morphology, and survival based on 5625 cases from the SEER program. J. Surg. Oncol. 2009, 100, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Futakawa, N.; Yamagata, S.; Wada, Y.; Kuroda, A.; Muto, T.; Esaki, Y. Different Clinicopathologic Findings in Two Histologic Types of Carcinoma of Papilla of Vater. Jpn. J. Cancer Res. 1994, 85, 161–166. [Google Scholar] [CrossRef]
- Bosman, F.; Carnerio, F.; Hruban, R.; Theise, N. WHO Classification of Tumors of the Digestive System, 4th ed.; IARC: Lyon, France, 2010. [Google Scholar]
- Pea, A.; Riva, G.; Bernasconi, R.; Sereni, E.; Lawlor, R.; Scarpa, A.; Luchini, C. Ampulla of Vater carcinoma: Molecular landscape and clinical implications. World J. Gastrointest. Oncol. 2018, 10, 370–380. [Google Scholar] [CrossRef]
- Talamini, M.A.; Moesinger, R.C.; Pitt, H.A.; Sohn, T.A.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann. Surg. 1997, 225, 590–600. [Google Scholar] [CrossRef]
- Beghelli, S.; Orlandini, S.; Moore, P.S.; Talamini, G.; Capelli, P.; Zamboni, G.; Falconi, M.; Scarpa, A. Ampulla of vater cancers: T-stage and histological subtype but not Dpc4 expression predict prognosis. Virchows Arch. 2002, 441, 19–24. [Google Scholar] [CrossRef]
- Roh, Y.-H.; Kim, Y.-H.; Lee, H.-W.; Kim, S.-J.; Roh, M.-S.; Jeong, J.-S.; Jung, G.-J. The clinicopathologic and immunohistochemical characteristics of ampulla of Vater carcinoma: The intestinal type is associated with a better prognosis. Hepatogastroenterology 2007, 54, 1641–1644. [Google Scholar] [PubMed]
- Carter, J.T.; Grenert, J.P.; Rubenstein, L.; Stewart, L.; Way, L.W. Tumors of the ampulla of vater: Histopathologic classification and predictors of survival. J. Am. Coll. Surg. 2008, 207, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Veronese, N.; Nottegar, A.; Riva, G.; Pilati, C.; Mafficini, A.; Stubbs, B.; Simbolo, M.; Mombello, A.; Corbo, V.; et al. Perineural Invasion is a Strong Prognostic Moderator in Ampulla of Vater Carcinoma: A Meta-analysis. Pancreas 2019, 48, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kohler, I.; Jacob, D.; Budzies, J.; Lehmann, A.; Weichert, W.; Schulz, S.; Neuhaus, P.; Röcken, C. Phenotypic and Genotypic Characterization of Carcinomas of the Papilla of Vater Has Prognostic and Putative Therapeutic Implications. Am. J. Clin. Pathol. 2011, 135, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.S.; Choi, D.W.; Choi, S.H.; Heo, J.S.; You, D.D.; Lee, H.G. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J. Surg. Oncol. 2012, 105, 266–272. [Google Scholar] [CrossRef]
- Perkins, G.; Svrcek, M.; Bouchet-Doumenq, C.; Voron, T.; Colussi, O.; Debove, C.; Merabtene, F.; Dumont, S.; Sauvanet, A.; Hammel, P.; et al. Can we classify ampullary tumours better? Clinical, pathological and molecular features. Results of an AGEO study. Br. J. Cancer 2019, 120, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, M.D.; Balci, S.; Ohike, N.; Xue, Y.; Kim, G.E.; Tajiri, T.; Memis, B.; Coban, I.; Dolgun, A.; Krasinskas, A.M.; et al. Ampullary carcinoma is often of mixed or hybrid histologic type: An analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod. Pathol. 2016, 29, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.K.; Jamieson, N.B.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Chou, A.; Pinese, M.; Humphris, J.L.; Jones, M.D.; Toon, C.; et al. Histomolecular Phenotypes and Outcome in Adenocarcinoma of the Ampulla of Vater. J. Clin. Oncol. 2013, 31, 1348–1356. [Google Scholar] [CrossRef]
- Ang, D.C.; Shia, J.; Tang, L.H.; Katabi, N.; Klimstra, D.S. The Utility of Immunohistochemistry in Subtyping Adenocarcinoma of the Ampulla of Vater. Am. J. Surg. Pathol. 2014, 38, 1371–1379. [Google Scholar] [CrossRef]
- Zhou, H.; Schaefer, N.; Wolff, M.; Fischer, H.P. Carcinoma of the ampulla of Vater: Comparative histologic/immunohistochemical classification and follow-up. Am. J. Surg. Pathol. 2004, 28, 875–882. [Google Scholar] [CrossRef]
- Nappo, G.; Galvanin, J.; Gentile, D.; Capretti, G.; Pulvirenti, A.; Bozzarelli, S.; Rimassa, L.; Spaggiari, P.; Carrara, S.; Petitti, T.; et al. Long-term outcomes after pancreatoduodenectomy for ampullary cancer: The influence of the histological subtypes and comparison with the other periampullary neoplasms. Pancreatology 2021, 21, 950–956. [Google Scholar] [CrossRef]
- Asano, E.; Okano, K.; Oshima, M.; Kagawa, S.; Kushida, Y.; Munekage, M.; Hanazaki, K.; Watanabe, J.; Takada, Y.; Ikemoto, T.; et al. Phenotypic characterization and clinical outcome in ampullary adenocarcinoma. J. Surg. Oncol. 2016, 114, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.; Carey, F.A.; Pratt, N.R.; Steele, R.J. The colorectal adenoma–carcinoma sequence. J. Br. Surg. 2002, 89, 845–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Six, H.; Olafsson, S.; Ellis, P.; Osborne, R.J.; Sanders, M.A.; Moore, L.; Georgakopoulos, N.; Torrente, F.; Noorani, A.; Goddard, M.; et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature 2019, 574, 532–537. [Google Scholar] [CrossRef]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Ising, M.E.; Evans, N.R.; Phillips, N.J.; Lai, J.; Guzman, M.A. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: Evidence to date and future perspectives. Expert Opin. Investig. Drugs 2021, 30, 317–324. [Google Scholar] [CrossRef]
- Massa, A.; Varamo, C.; Vita, F.; Tavolari, S.; Peraldo-Neia, C.; Brandi, G.; Rizzo, A.; Cavalloni, G.; Aglietta, M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers 2020, 12, 2308. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; de Oliveira, M.L.; Dutkowski, P.; Clavien, P.-A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Wood, L.D.; Suzuki, M.; Takai, E.; Totoki, Y.; Kato, M.; Luchini, C.; Arai, Y.; Nakamura, H.; Hama, N.; et al. Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. Cancer Cell 2016, 29, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingras, M.-C.; Covington, K.R.; Chang, D.K.; Donehower, L.A.; Gill, A.J.; Ittmann, M.M.; Creighton, C.J.; Johns, A.L.; Shinbrot, E.; Dewal, N.; et al. Ampullary Cancers Harbor ELF3 Tumor Suppressor Gene Mutations and Exhibit Frequent WNT Dysregulation. Cell Rep. 2016, 14, 907–919. [Google Scholar] [CrossRef] [Green Version]
- Morini, S.; Perrone, G.; Borzomati, D.; Vincenzi, B.; Rabitti, C.; Righi, D.; Castri, F.; Manazza, A.D.; Santini, D.; Tonini, G.; et al. Carcinoma of the Ampulla of Vater: Morphological and Immunophenotypical Classification Predicts Overall Survival. Pancreas 2013, 42, 60–66. [Google Scholar] [CrossRef]
- Mafficini, A.; Amato, E.; Cataldo, I.; Rusev, B.C.; Bertoncello, L.; Corbo, V.; Simbolo, M.; Luchini, C.; Fassan, M.; Cantù, C.; et al. Ampulla of Vater carcinoma: Sequencing analysis identifies TP53 status as a novel independent prognostic factor and potentially actionable ERBB, PI3K, and WNT pathways gene mutations. Ann. Surg. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Liu, W.; Sadowska, J.; Zhen, L.; Borsu, L.; Arcila, M.E.; Won, H.H.; Shah, R.H.; Berger, M.F.; Vakiani, E.; et al. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2). Mod. Pathol. 2015, 28, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Romiti, A.; Barucca, V.; Zullo, A.; Sarcina, I.; di Rocco, R.; d’Antonio, C.; Latorre, M.; Marchetti, P. Tumors of ampulla of Vater: A case series and review of chemotherapy options. World J. Gastrointest. Oncol. 2012, 4, 60–67. [Google Scholar] [CrossRef]
- Chu, P.G.; Schwarz, R.E.; Lau, S.K.; Yen, Y.; Weiss, L.M. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: Application of CDX2, CK17, MUC1, and MUC2. Am. J. Surg. Pathol. 2005, 29, 359–367. [Google Scholar] [CrossRef]
- Sessa, F.; Furlan, D.; Zampatti, C.; Carnevali, I.; Franzi, F.; Capella, C. Prognostic factors for ampullary adenocarcinomas: Tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch. 2007, 451, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Chang, L.; Chang, M.; Chang, H.M.; Chang, F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Santini, D.; Zagami, M.; Vincenzi, B.; Verzì, A.; Morini, S.; Borzomati, D.; Coppola, R.; Antinori, A.; Magistrelli, P.; et al. COX-2 expression of ampullary carcinoma: Correlation with different histotypes and clinicopathological parameters. Virchows Arch. 2006, 449, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Tang, K.; Sun, F.-B.; Sui, L.-L.; Xu, G. Partial Resection of the Pancreatic Head and Duodenum for Management of Carcinoma of the Ampulla of Vater: A Case Report. Anticancer Res. 2016, 36, 1319–1324. [Google Scholar]
- Panzeri, F.; Crippa, S.; Castelli, P.; Aleotti, F.; Pucci, A.; Partelli, S.; Zamboni, G.; Falconi, M. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World J. Gastroenterol. 2015, 21, 7970–7987. [Google Scholar] [CrossRef]
- Askew, J.; Connor, S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB 2013, 15, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.-N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef]
- Kim, S.J.; An, S.; Kang, H.J.; Kim, J.Y.; Jang, M.A.; Lee, J.H.; Song, K.-B.; Hwang, D.W.; Cho, H.; Kim, S.C.; et al. Validation of the eighth edition of the American Joint Committee on Cancer staging system for ampulla of Vater cancer. Surgery 2018, 163, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Yamamoto, Y.; Sugiura, T.; Okamura, Y.; Ito, T.; Ashida, R.; Ohgi, K.; Uesaka, K. The Prognostic Relevance of the New 8th Edition of the Union for International Cancer Control Classification of TNM Staging for Ampulla of Vater Carcinoma. Ann. Surg. Oncol. 2019, 26, 1639–1648. [Google Scholar] [CrossRef]
- De Castro, S.M.; van Heek, N.T.; Kuhlmann, K.F.; Busch, O.R.; Offerhaus, G.J.; van Gulik, T.M.; Obertop, H.; Gouma, D.J. Surgical management of neoplasms of the ampulla of Vater: Local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery 2004, 136, 994–1002. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Jeng, Y.-M.; Ho, C.-M.; Hu, R.-H.; Chang, H.-P.; Tien, Y.-W. Survival After Pancreaticoduodenectomy for Ampullary Cancer is not Affected by Age. World J. Surg. 2010, 34, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Asano, E.; Kushida, Y.; Kamada, H.; Mori, H.; Suzuki, Y. Factors Influencing Lymph Node Metastasis in Patients with Ampullary Adenocarcinoma. Dig. Surg. 2014, 31, 459–467. [Google Scholar] [CrossRef]
- Okano, K.; Hirao, T.; Unno, M.; Fujii, T.; Yoshitomi, H.; Suzuki, S.; Satoi, S.; Takahashi, S.; Kainuma, O.; Suzuki, Y. Postoperative infectious complications after pancreatic resection. Br. J. Surg. 2015, 102, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Suzuki, Y. Influence of bile contamination for patients who undergo pancreaticoduodenectomy after biliary drainage. World J. Gastroenterol. 2019, 25, 6847–6856. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Liu, X.L.; Jiang, G.Y.; Wu, D.; Maher, H.; Cai, X.J. Minimally invasive pancreaticoduodenectomy for periampullary disease: A comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC Gastroenterol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappo, G.; Gentile, D.; Galvanin, J.; Capretti, G.; Ridolfi, C.; Petitti, T.; Spaggiari, P.; Carrara, S.; Gavazzi, F.; Repici, A.; et al. Trans-duodenal ampullectomy for ampullary neoplasms: Early and long-term outcomes in 36 consecutive patients. Surg. Endosc. 2019, 34, 4358–4368. [Google Scholar] [CrossRef]
- Woo, S.M.; Ryu, J.K.; Lee, S.H.; Lee, W.J.; Hwang, J.H.; Yoo, J.W.; Park, J.K.; Kang, G.H.; Kim, Y.-T.; Yoon, Y.B. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J. Gastroenterol. Hepatol. 2009, 24, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Horaguchi, J.; Takasawa, O.; Obana, T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: A prospective and histopathologically controlled study. Gastrointest. Endosc. 2007, 66, 740–747. [Google Scholar] [CrossRef]
- Howe, J.R.; Klimstra, D.S.; Moccia, R.D.; Conlon, K.C.; Brennan, M.F. Factors Predictive of Survival in Ampullary Carcinoma. Ann. Surg. 1998, 228, 87–94. [Google Scholar] [CrossRef]
- Partelli, S.; Crippa, S.; Capelli, P.; Neri, A.; Bassi, C.; Zamboni, G.; Barugola, G.; Falconi, M. Adequacy of Lymph Node Retrieval for Ampullary Cancer and Its Association with Improved Staging and Survival. World J. Surg. 2013, 37, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Morak, M.J.; van der Gaast, A.; Incrocci, L.; van Dekken, H.; Hermans, J.J.; Jeekel, J.; Hop, W.C.; Kazemier, G.; van Eijck, C.H. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: A prospective randomized controlled trial. Ann. Surg. 2008, 248, 1031–1041. [Google Scholar] [CrossRef]
- Nassour, I.; Hynan, L.S.; Christie, A.; Minter, R.M.; Yopp, A.C.; Choti, M.A.; Mansour, J.C.; Porembka, M.; Wang, S.C. Association of Adjuvant Therapy with Improved Survival in Ampullary Cancer: A National Cohort Study. J. Gastrointest. Surg. 2017, 22, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Vollmer, C.M., Jr.; Behrman, S.W.; Allegrini, V.; Aversa, J.; Ball, C.G.; Barrows, C.E.; Berger, A.C.; Cagigas, M.N.; Christein, J.D.; et al. Role of Adjuvant Multimodality Therapy After Curative-Intent Resection of Ampullary Carcinoma. JAMA Surg. 2019, 154, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Bolm, L.; Ohrner, K.; Nappo, G.; Rückert, F.; Zimmermann, C.; Rau, B.M.; Petrova, E.; Honselmann, K.C.; Lapshyn, H.; Bausch, D.; et al. Adjuvant therapy is associated with improved overall survival in patients with pancreatobiliary or mixed subtype ampullary cancer after pancreatoduodenectomy—A multicenter cohort study. Pancreatology 2020, 20, 433–441. [Google Scholar] [CrossRef]
- Koprowski, M.A.; Sutton, T.L.; Brinkerhoff, B.T.; Grossberg, A.; Sheppard, B.C.; Mayo, S.C. Oncologic outcomes in resected ampullary cancer: Relevance of histologic subtype and adjuvant chemotherapy. Am. J. Surg. 2021, 221, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 577–588. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.A.; Weisbrod, A.B.; Dillhoff, M.; Schmidt, C.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Current management and emerging therapies. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 439–449. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Tober, N.; Nigro, M.C.; Mosca, M.; Palloni, A.; Abbati, F.; Frega, G.; de Lorenzo, S.; Tavolari, S.; et al. Second-line Treatment in Advanced Biliary Tract Cancer: Today and Tomorrow. Anticancer. Res. 2020, 40, 3013–3030. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Brandi, G.; Frega, G.; Rizzo, A. Second-line FOLFOX chemotherapy for advanced biliary tract cancer. Lancet Oncol. 2021, 22, e285. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [Green Version]
- Morganti, S.; Tarantino, P.; Ferraro, E.; D’Amico, P.; Duso, B.A.; Curigliano, G. Next Generation Sequencing (NGS): A Revolutionary Technology in Pharmacogenomics and Personalized Medicine in Cancer. Adv. Exp. Med. Biol. 2019, 1168, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kwong, L.N.; Javle, M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr. Treat. Options Oncol. 2016, 17, 58. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100337. [Google Scholar]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangio-carcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: “And Yet It Moves!”. Cancer Treat. Res. Commun. 2021, 27, 100335. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Frega, G.; Ricci, A.D.; Palloni, A.; Abbati, F.; de Lorenzo, S.; Deserti, M.; Tavolari, S.; Brandi, G. Anti-EGFR Monoclonal Antibodies in Advanced Biliary Tract Cancer: A Systematic Review and Meta-analysis. In Vivo 2020, 34, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, A.; Brandi, G. TRK inhibition in cholangiocarcinoma: Trying to teach an old dog new tricks. Cancer Treat. Res. Commun. 2021, 27, 100351. [Google Scholar] [CrossRef]
| Author (Reference) | Intestinal-Type Ampullary Cancer (Percentage) | Pancreaticobiliary-Type Ampullary Cancer (Percentage) | Mixed-Type Ampullary Cancer (Percentage) |
|---|---|---|---|
| Yachida [34] | APC (49%) | KRAS (67%) | |
| TP53 (39%) | TP53 (67%) | ||
| KRAS (39%) | SMAD4 (20%) | ||
| CTNNB1 (26%) | CTNNB1 (15%) | ||
| ARID2 (18%) | ERBB3 (14%) | ||
| Gingras [35] | TP53 (64%) | TP53 (71%) | KRAS (49%) |
| KRAS (46%) | KRAS (65%) | APC (50%) | |
| APC (41%) | SMAD4 (18%) | TP53 (41%) | |
| PIK3CA (26%) | CDKN2A (16%) | SMARCA4 (27%) | |
| SMAD4 (20%) | PIK3CA (13%) | PIK3CA (23%) |
| T | TX: primary tumor cannot be assessed |
| T0: no evidence of primary tumor | |
| T1a: limited to sphincter of Oddi | |
| T1b: invasion into duodenal submucosa | |
| T2: invasion into duodenal muscularis propria | |
| T3a: invasion into pancreas ≤ 0.5 cm | |
| T3b: invasion into pancreas > 0.5 cm | |
| T4: involvement of celiac or superior mesenteric artery | |
| N | NX: lymph nodes cannot be assessed |
| N0: no lymph node involvement | |
| N1: metastasis in 1–3 lymph nodes | |
| N2: metastasis in 4 or more lymph nodes | |
| M | MX: distant metastasis cannot be assessed |
| M0: no distant metastasis | |
| M1: distant metastasis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, A.; Dadduzio, V.; Lombardi, L.; Ricci, A.D.; Gadaleta-Caldarola, G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Curr. Oncol. 2021, 28, 3393-3402. https://doi.org/10.3390/curroncol28050293
Rizzo A, Dadduzio V, Lombardi L, Ricci AD, Gadaleta-Caldarola G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Current Oncology. 2021; 28(5):3393-3402. https://doi.org/10.3390/curroncol28050293
Chicago/Turabian StyleRizzo, Alessandro, Vincenzo Dadduzio, Lucia Lombardi, Angela Dalia Ricci, and Gennaro Gadaleta-Caldarola. 2021. "Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges" Current Oncology 28, no. 5: 3393-3402. https://doi.org/10.3390/curroncol28050293
APA StyleRizzo, A., Dadduzio, V., Lombardi, L., Ricci, A. D., & Gadaleta-Caldarola, G. (2021). Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Current Oncology, 28(5), 3393-3402. https://doi.org/10.3390/curroncol28050293





