Abstract
Serum carcinoembryonic antigen (CEA) is a tumor marker especially used to follow a patient with colorectal cancer. However, it is non-specific and could be increased in several cancers and some benign conditions. We report the case of a 70-year-old man followed since 2014 for a left colon adenocarcinoma with the persistence of an increased CEA. There was no evidence of recurrence, but a right lobar thyroid nodule without a significantly increased uptake was incidentally discovered on the CT scan of 18F-fluorodeoxyglucose (18F-FDG) PET/CT. We suspected a medullary thyroid carcinoma (MTC) explaining the persistent elevation of CEA. Plasma calcitonin levels were 47 ng/L (N < 10). Fine needle aspiration cytology found atypia of undetermined significance and the patient was reluctant to undergo surgery without any further exploration. We performed a 18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET/CT preoperatively which revealed a punctiform focus of the right thyroid lobe corresponding to a pT1aN1aMxR0 medullary thyroid carcinoma, histopathologically confirmed. This case highlights that despite the potential usefulness of 18F-FDG PET/CT in case of an unknown source of elevated CEA this imaging may be falsely negative as in the case of MTC and should lead to further explorations.
1. Introduction
Serum carcinoembryonic antigen (CEA) is a member of a family of cell surface glycoproteins involved in cell adhesion [1] that is used as a tumor marker. The interest in monitoring CEA post-operatively in colorectal cancer (CRC) was shown in several studies, and it is related to an improved overall survival [2,3]. International guidelines recommend performing CEA testing every three to six months, for at least three years then every 6–12 months at years four and five after initial surgery of a localized CRC [4], and every two to three months in case of metastatic CRC [5].
However, CEA is not specific to CRC and can be increased in several cancers such as pancreatic cancer [6], breast cancer [7,8], lung cancer [9], or medullary thyroid carcinoma (MTC). Moreover, some benign conditions can also present elevated CEA [10,11,12,13,14,15,16,17].
We present the case of persistent CEA elevation after complete remission of CRC and negative 18F-fluorodeoxyglucose (18F-FDG) PET/CT revealing localized MTC diagnoses with 18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET/CT.
2. Case Report
A 70-year-old man was followed since 2014 with a pT2N1M0 left colon adenocarcinoma treated by left hemicolectomy then adjuvant chemotherapy (capecitabine and oxaliplatin). At the end of systemic treatments, carcinoembryonic antigen (CEA) was negative.
In 2015, CEA increased to 47 µg/L (N < 5) and 18F-fluorodeoxyglucose (18F-FDG) PET/CT showed a left adrenal hypermetabolism. The patient underwent a left adrenalectomy revealing a metastasis from the previous primary colon tumor, and post-operative CEA decreased to 5.6 µg/L. During follow-up, CEA gradually increased to 13.5 µg/L then stabilized around 12 µg/L. However, no lesions suspected of recurrence were found on several CT and 18F-FDG PET/CT.
In June 2018, as part of the follow-up, a new 18F-FDG PET/CT was performed in order to detect metastasis. The nuclear medicine physician noticed a right lobar thyroid nodule on the CT of 18F-FDG PET/CT without a significantly increased uptake (Figure 1).
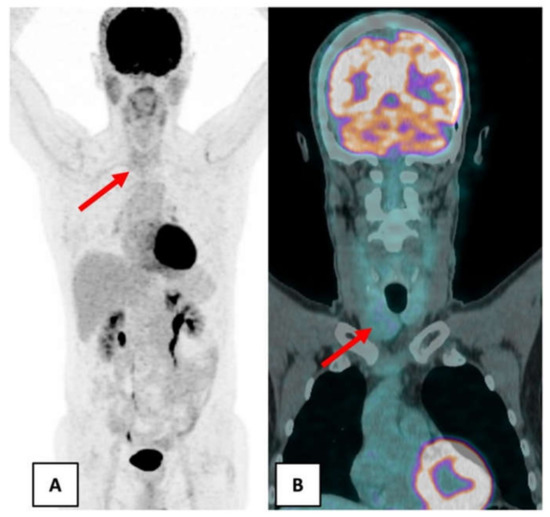
Figure 1.
June 2018 18F-FDG PET/CT showed a right lobar thyroid nodule without significant hypermetabolism (red arrow). (A) Maximum intensity projection. (B) Coronal fusion PET/CT.
In order to explore this nodule, cervical ultrasonography was performed and revealed a 28 × 22 × 36 mm hypoechoic nodule of the right lobe classified EU-TIRADS 5 (high risk of malignancy) [18]. Given the persistent elevation of CEA without secondary localization found and the presence of a thyroid nodule, an MTC was suspected.
Fine needle aspiration (FNA) was performed in this context of thyroid nodule with elevated calcitonin and CEA and found atypia of undetermined significance. Blood calcitonin was increased at 47 ng/L (N < 10). In our center, we do not routinely perform the calcium stimulated calcitonin test.
Since our patient was reluctant to undergo surgery without any further exploration, we decided to perform a 18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET/CT (Figure 2).
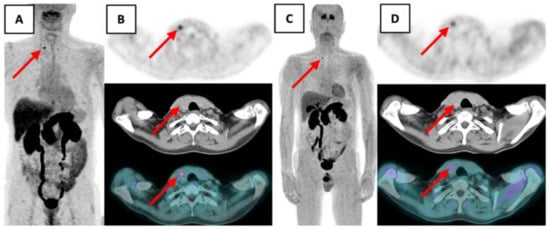
Figure 2.
18F-FDOPA PET/CT showed a punctiform focus of the right thyroid lobe at the superior part of the nodular bulge suspected of MTC (red arrow).(A) Maximum intensity projection 12 min post-injection. (B) Transaxial PET, CT and fusion PET/CT 12 min post injection. (C) Maximum intensity projection 79 min post-injection. (D) Transaxial PET, CT and fusion PET/CT 79 min post injection.
It showed a punctiform focus of the right thyroid lobe at the superior part of the visible nodular bulge with a maximum standardized uptake value (maxSUV) of 5.09 (12 min post-injection), and of 2.71 (79 min post-injection) compatible with a localized MTC.
A hemi-thyroidectomy with ipsilateral lymphadenectomy was performed in December 2018 because the patient wanted to avoid levothyroxine substitution. Histopathology confirmed a 1 cm MTC at the upper third of the right lobe without extrathyroidal extension nor vascular invasion, excised in the healthy zone and associated with a unique lymph node micrometastasis. It was classified pT1aN1aMxR0. One month after surgery, calcitonin was undetectable, and CEA decreased to the normal range at 2.6 µg/L. Genetic testing concluded a sporadic MTC (no REarranged during Transfection (RET) gene mutation).
3. Discussion
MTC is a rare neuroendocrine tumor derived from the C cells of the thyroid accounting for 1 to 2% of thyroid cancer in the United States. Although most MTC are sporadic, 25% are familial with germline mutations of the RET proto-oncogene resulting in multiple endocrine neoplasia (MEN) type 2A, type 2B, or familial medullary thyroid cancer (FMTC) with a phenotype-genotype correlation [19]. Sporadic MTC occurs in the fourth and sixth decades of life and is presented as a solitary nodule located in the upper portion of thyroid lobes [20]. At diagnosis, 70% of patients with a MTC presented as a palpable thyroid nodule have lymph node involvement and 10% have distant metastases.
There are two major prognostic factors at diagnosis: age and stage of the disease [19,21,22]. The 10-year specific survival is 81% in patients at any stage up to 96% in patient with localized MTC [23]. But microcarcinomas (≤1 cm) can also present distant metastases as shown by Kazaure et al. out of 310 patients with microcarcinomas, 5% had distant metastasis at diagnosis [24].
MTC cells are capable of secreting CEA and calcitonin used as a tumor marker. These two markers correlate with a tumor mass. Moreover, CEA and calcitonin doubling time are strong markers of the aggressiveness of MTC [25,26].
Despite the potential usefulness of 18F-FDG PET/CT in case of an unknown source of elevated CEA [27], this imaging may be falsely negative as in the case of a MTC, especially with a mild increase of serum calcitonin [28]. Therefore, after ruling out other non-malignant causes known to increase calcitonin such as renal failure, thyroiditis, sepsis, or other neuroendocrine tumors, an unexplained elevated CEA should lead to a calcitonin measurement in order not to miss a potential MTC [29].
Elevated CEA associated with elevated calcitonin is suggestive of MTC and further thyroid explorations are needed in particular cervical ultrasonography (US) to detect thyroid nodules. But physicians have to keep in mind that US risk stratification systems have been developed against papillary thyroid carcinoma and so there are no US features specifically for MTC [30]. In the same way, a FNA is usually performed to confirm the diagnosis of MTC despite its inconstant sensitivity, less than in other thyroid cancer, with a detection rate of only one-half of MTC [31]. This inconstant sensitivity could be increased by calcitonin measurement in the FNA needle washout fluids [32]. In case of mildly elevated CT, knowing MTC are difficult to diagnose with lack of sensitivity of US and cytology, a calcium stimulated calcitonin test could be performed [33].
In our case, 18F-FDG PET/CT was negative, and FNA cytology was non-contributory since it concluded atypia of undetermined significance, so we performed 18F-FDOPA PET/CT.
18F-FDOPA is a radiolabeled analog of DOPA that binds to an amino acid transporter (LAT1), which is overexpressed by neuroendocrine tumors, and which is converted to 18F-FDODA and stored in vesicles [34]. This imaging is currently the most sensitive and specific modality for detecting residual disease in patients with MTC and increased blood calcitonin levels [35,36], but there is no evidence of its performance to characterize a thyroid nodule.
As up to 6% of patients with sporadic MTC have bilateral foci [37], recommended treatment of localized MTC is total thyroidectomy and dissection of the lymph nodes in the central compartment [19]. Nevertheless, hemithyroidectomy with central neck dissection for sporadic MTC located in only one lobe did not affect the biochemical cure in selected patients [38].
So, given the absence of lymph node involvement shown by ultrasound and 18F-FDOPA PET/CT, blood calcitonin mildly elevated at 47 ng/L and the patient’s reluctance to purchases levothyroxine supplementation, hemithyroidectomy was an option.
4. Conclusions
Persistence of an increased CEA in case of colon adenocarcinoma without any secondary localization leading to the discovery of a MTC has already been reported [39,40,41] but for the first time, we report the contribution of 18F-FDOPA PET/CT in the pre-surgical characterization of a thyroid nodule. Moreover, this case demonstrates that a negative 18F-FDG PET/CT is not enough to exclude thyroid neoplasia, in particular, an indolent MTC and additional investigations are needed.
Author Contributions
Writing—original draft preparation, A.L. and P.H. Writing—review and editing, A.L. Performed imaging exams, C.A. Case analysis, all authors. Conceptualization, J.B. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from the patient for the publication of this case report and related images.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
We have read and understood Current Oncology’s policy on conflicts of interest disclosure and declare that we have none.
References
- Benchimol, S.; Fuks, A.; Jothy, S.; Beauchemin, N.; Shirota, K.; Stanners, C.P. Carcinoembryonic Antigen, a Human Tumor Marker, Functions as an Intercellular Adhesion Molecule. Cell 1989, 57, 327–334. [Google Scholar] [CrossRef]
- Primrose, J.N.; Perera, R.; Gray, A.; Rose, P.; Fuller, A.; Corkhill, A.; George, S.; Mant, D. FACS Trial Investigators Effect of 3 to 5 Years of Scheduled CEA and CT Follow-up to Detect Recurrence of Colorectal Cancer: The FACS Randomized Clinical Trial. JAMA 2014, 311, 263–270. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Shinkins, B.; Pathiraja, I.; Roberts, N.W.; James, T.J.; Mallett, S.; Perera, R.; Primrose, J.N.; Mant, D. Blood CEA Levels for Detecting Recurrent Colorectal Cancer. Cochrane Database Syst. Rev. 2015, 2015, CD011134. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic Colorectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2014, 25, iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and Prognostic Value of Carcinoembryonic Antigen in Pancreatic Cancer: A Systematic Review and Meta-Analysis. OncoTargets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Clinicopathological and Prognostic Significance of Cancer Antigen 15-3 and Carcinoembryonic Antigen in Breast Cancer: A Meta-Analysis Including 12,993 Patients. Dis. Markers 2018, 2018, 9863092. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Morimoto, T.; Nomura, T.; Michishita, S.; Nishimukai, A.; Higuchi, T.; Fujimoto, Y.; Miyagawa, Y.; Kira, A.; Murase, K.; et al. Independent Prognostic Impact of Preoperative Serum Carcinoembryonic Antigen and Cancer Antigen 15-3 Levels for Early Breast Cancer Subtypes. World J. Surg. Oncol. 2018, 16, 26. [Google Scholar] [CrossRef]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic Antigen (CEA) as Tumor Marker in Lung Cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Sekiya, K.; Sakai, T.; Homma, S.; Tojima, H. Pulmonary Tuberculosis Accompanied by a Transient Increase in Serum Carcinoembryonic Antigen Level with Tuberculous Empyema Drainage. Intern. Med. 2007, 46, 1795–1798. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, S.; Takeuchi, Y.; Arai, K.; Oishi, C.; Norose, T.; Yamochi-Onizuka, T.; Kushima, M.; Ota, H.; Imawari, M. Elevation of Carcinoembryonic Antigen Coinciding with Disease Activity of Ulcerative Colitis. Clin. J. Gastroenterol. 2012, 5, 150–154. [Google Scholar] [CrossRef]
- Tang, T.-T.; Cheng, H.-H.; Zhang, H.; Lin, X.-L.; Huang, L.-J.; Zhang, W.; Jiang, S.-P. Hypereosinophilic Obliterative Bronchiolitis with an Elevated Level of Serum CEA: A Case Report and a Review of the Literature. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2634–2640. [Google Scholar]
- Kwon, H.J. Xanthogranulomatous Pancreatitis Mimicking Potentially Malignant Pancreatic Neoplasm: Report of a Case. Ann. Hepato-Biliary-Pancreat. Surg. 2017, 21, 243–246. [Google Scholar] [CrossRef]
- Khan, A.F.; Waqar, S.H.; Raheem, M.; Ali, Z. Mucocele of Appendix with an Elevated Carcinoembryonic Antigen: A Case Report. J. West Afr. Coll. Surg. 2017, 7, 120–127. [Google Scholar] [PubMed]
- Rani, B.S.; Suchitra, M.M.; Srinivasa Rao, P.V.L.N.; Kumar, V.S. Serum Tumor Markers in Advanced Stages of Chronic Kidney Diseases. Saudi J. Kidney Dis. Transpl. 2019, 30, 898–904. [Google Scholar] [CrossRef]
- Matsueda, K.; Otani, T.; Fujioka, Y.; Mizuno, M. A Giant Apocrine Hidrocystoma Associated with Elevated Serum Carcinoembryonic Antigen Levels: A Case Report. J. Med. Case Rep. 2019, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Liu, J.; Huang, S.; Xiong, B. Elevated Carcinoembryonic Antigen in Patients with COVID-19 Pneumonia. J. Cancer Res. Clin. Oncol. 2020, 146, 3385–3388. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Ngu, R.; Royer, B.; Giovanella, L.; Bigorgne, C.; Simo, R.; Carroll, P.; Russ, G. A Multicentre Validation Study for the EU-TIRADS Using Histological Diagnosis as a Gold Standard. Clin. Endocrinol. 2019, 91, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Leboulleux, S.; Baudin, E.; Travagli, J.-P.; Schlumberger, M. Medullary Thyroid Carcinoma. Clin. Endocrinol. 2004, 61, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Matrone, A.; Gambale, C.; Prete, A.; Piaggi, P.; Cappagli, V.; Bottici, V.; Romei, C.; Ciampi, R.; Torregrossa, R.; De Napoli, L.; et al. Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma. Cancers 2020, 13, E94. [Google Scholar] [CrossRef] [PubMed]
- Raue, F.; Bruckner, T.; Frank-Raue, K. Similar stage-dependent survival and outcome in sporadic and hereditary medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2021, dgab326. [Google Scholar] [CrossRef]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the Presentation, Treatment, and Survival of Patients with Medullary Thyroid Cancer over the Past 30 Years. Surgery 2017, 161, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kazaure, H.S.; Roman, S.A.; Sosa, J.A. Medullary thyroid microcarcinoma. Cancer 2012, 118, 620–627. [Google Scholar] [CrossRef]
- Barbet, J.; Campion, L.; Kraeber-Bodéré, F.; Chatal, J.-F.; GTE Study Group. Prognostic Impact of Serum Calcitonin and Carcinoembryonic Antigen Doubling-Times in Patients with Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2005, 90, 6077–6084. [Google Scholar] [CrossRef] [PubMed]
- Laure Giraudet, A.; Al Ghulzan, A.; Aupérin, A.; Leboulleux, S.; Chehboun, A.; Troalen, F.; Dromain, C.; Lumbroso, J.; Baudin, E.; Schlumberger, M. Progression of Medullary Thyroid Carcinoma: Assessment with Calcitonin and Carcinoembryonic Antigen Doubling Times. Eur. J. Endocrinol. 2008, 158, 239–246. [Google Scholar] [CrossRef]
- Fu, L.; Li, W.; Tian, X. 18F-FDG PET-CT in Unknown-Source of Elevated Serum Carcinoembryonic Antigen (CEA) Level. J. Coll. Physicians Surg. Pak. 2018, 28, 910–913. [Google Scholar] [CrossRef]
- Treglia, G.; Villani, M.F.; Giordano, A.; Rufini, V. Detection Rate of Recurrent Medullary Thyroid Carcinoma Using Fluo-rine-18 Fluorodeoxyglucose Positron Emission Tomography: A Meta-Analysis. Endocrine 2012, 42, 535–545. [Google Scholar] [CrossRef]
- Allelein, S.; Ehlers, M.; Morneau, C.; Schwartz, K.; Goretzki, P.E.; Seppel, T.; Feldkamp, J.; Krieg, A.; Knoefel, W.T.; Kuebart, A.; et al. Measurement of Basal Serum Calcitonin for the Diagnosis of Medullary Thyroid Cancer. Horm. Metab. Res. 2018, 50, 23–28. [Google Scholar] [CrossRef]
- Matrone, A.; Gambale, C.; Biagini, M.; Prete, A.; Vitti, P.; Elisei, R. Ultrasound features and risk stratification systems to identify medullary thyroid carcinoma. Eur. J. Endocrinol. 2021, 185, 193–200. [Google Scholar] [CrossRef]
- Trimboli, P.; Treglia, G.; Guidobaldi, L.; Romanelli, F.; Nigri, G.; Valabrega, S.; Sadeghi, R.; Crescenzi, A.; Faquin, W.C.; Bon-giovanni, M.; et al. Detection Rate of FNA Cytology in Medullary Thyroid Carcinoma: A Meta-Analysis. Clin. Endocrinol. 2015, 82, 280–285. [Google Scholar] [CrossRef]
- Trimboli, P.; Cremonini, N.; Ceriani, L.; Saggiorato, E.; Guidobaldi, L.; Romanelli, F.; Ventura, C.; Laurenti, O.; Messuti, I.; Solaroli, E.; et al. Calcitonin Measurement in Aspiration Needle Washout Fluids Has Higher Sensitivity than Cytology in Detecting Medullary Thyroid Cancer: A Retrospective Multicentre Study. Clin. Endocrinol. 2014, 80, 135–140. [Google Scholar] [CrossRef]
- Fugazzola, L.; Di Stefano, M.; Censi, S.; Repaci, A.; Colombo, C.; Grimaldi, F.; Magri, F.; Pagotto, U.; Iacobone, M.; Persani, L.; et al. Basal and stimulated calcitonin for the diagnosis of medullary thyroid cancer: Updated thresholds and safety assessment. J. Endocrinol. Investig. 2021, 44, 587–597. [Google Scholar] [CrossRef]
- Santhanam, P.; Taïeb, D. Role of (18) F-FDOPA PET/CT Imaging in Endocrinology. Clin. Endocrinol. 2014, 81, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Taïeb, D. Positron Emission Tomography Imaging in Medullary Thyroid Carcinoma: Time for Reappraisal? Thyroid Off. J. Am. Thyroid Assoc. 2021, 31, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Shim, S.R.; Jeong, S.Y.; Kim, S.-J. Comparison of 5 Different PET Radiopharmaceuticals for the Detection of Recur-rent Medullary Thyroid Carcinoma: A Network Meta-Analysis. Clin. Nucl. Med. 2020, 45, 341–348. [Google Scholar] [CrossRef]
- Essig, G.F.; Porter, K.; Schneider, D.; Arpaia, D.; Lindsey, S.C.; Busonero, G.; Fineberg, D.; Fruci, B.; Boelaert, K.; Smit, J.W.; et al. Multifocality in Sporadic Medullary Thyroid Carcinoma: An International Multicenter Study. Thyroid 2016, 26, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Yabuta, T.; Fukushima, M.; Inoue, H.; Tomoda, C.; Uruno, T.; Kihara, M.; Higashiyama, T.; Takamura, Y.; et al. Alternative Surgical Strategies and Favorable Outcomes in Patients with Medullary Thyroid Carcinoma in Japan: Ex-perience of a Single Institution. World J. Surg. 2009, 33, 58–66. [Google Scholar] [CrossRef]
- Göksu, S.S.; Göksu, U.A.; Gündüz, S.; Coskun, H.S. Rising CEA Levels in a Patient with Colon Carcinoma: Metachronous Medullary Thyroid Cancer. Int. J. Biol. Markers 2014, 29, e184–e186. [Google Scholar] [CrossRef]
- Martins, A.; Gonçalves, A.; Almeida, T.; Midões, A. Persistent Elevation of Carcinoembryonic Antigen as First Presentation of a Medullary Thyroid Carcinoma. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Chen, S.-W.; Chen, Y.-K. High CEA Levels in a Case of Resected Colorectal Cancer: Delayed Diagnosis of Metachronous Me-dullary Thyroid Cancer. World J. Surg. Oncol. 2017, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).