Case report
A 68-year-old woman was admitted for rapidly progressive systemic sclerosis. She suffered from exertional dyspnea and decreased exercise tolerance. Pulmonary function testing showed mild restriction with pulmonary fibrosis on high resolution computer tomography (CT). Dilatation of the right ventricle (RV) and right atrium (RA) (
Figure 1
A) with severe tricuspid regurgitation (TR) was detected by transthoracic echocardiography. Transoesophageal echocardiography was performed in search for intra-cardiac shunts, whereby the right upper (RUPV) and right lower pulmonary vein (RLPV) could be depicted (
Figure 1
B). Of the left pulmonary veins, only the left lower (LLPV) could be suspected to drain into the left atrium (LA) (
Figure 1
C,D), and no connection between the left upper pulmonary vein (LUPV) and the LA superior to the left atrial appendage (LAA) (arrowheads on
Figure 1
D) could be imaged. Additionally, a large patent foramen ovale with spontaneous right-to-left shunt was found (PFO) (
Figure 1
B). Using angiographic CT, false drainage of the left upper pulmonary vein (LUPV) (
Figure 2
A,B) via the brachiocephalic vein into the superior vena cava could be shown. Cardiac catheterisation revealed normal coronary arteries and normal pulmonary artery pressure. In the context of a slight cyanosis (arterial oxygen saturation of 82%), and keeping in mind the possibly relevant function of the PFO as a release valve for the right heart volume overload (false pulmonary vein connection with secondary tricuspid regurgitation), it was closed transcutaneously by a 35 mm Amplatzer device (
Figure 2
C). The patient was dismissed in good clinical condition.
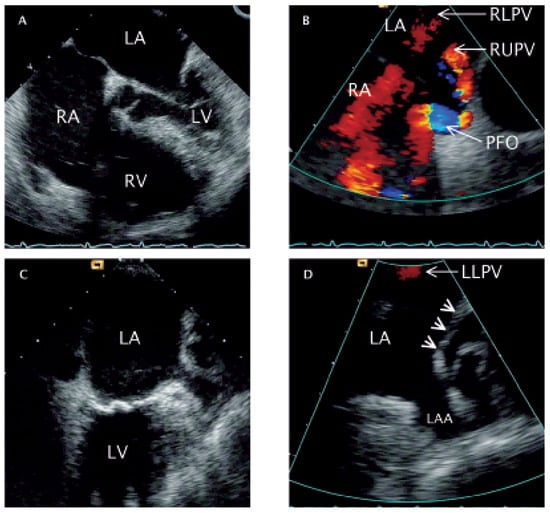
Figure 1.
Transoesophageal echocardiography. A Four-chamber-view obtained at 0° rotation depicting right heart dilatation. B Longitudinal (90°) colour Doppler view of the two atria (caudal: left side of the image; cranial: right side of the image) showing correct drainage of the RUPV and RLPV as red flow velocity signals. In addition, a blue/turbulent flow velocity signal (right-left-shunt) is depictedat the site of the PFO. C Longitudinal (90°) two-chamber-view (anterior: right side of the image) with an enlarged coronary sinus (posterior: left side of the image). D Colour Doppler close-up image of panel C showing the LAA and the absence of a structural opening for the drainage of the LUPV (arrow heads). LLPV: flow velocity signal of the left lower pulmonary vein. RA = right atrium; RV = right ventricle; LA = left atrium; LV = left ventricle; RUPV = right upper pulmonary vein; RLPV =right lower pulmonary vein; PFO = patent foramen ovale; LAA = left atrial appendage; LLPV = left lower pulmonary vein; LUPV = left upper pulmonary vein.
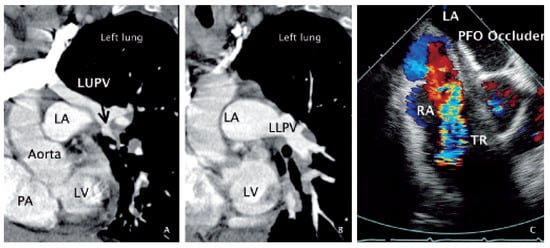
Figure 2.
Angiographic computed tomography and transoesophageal echocardiography. A Oblique section through the LV, PA, aorta andLA imaging the LUPV (arrow) as it does not drain into the LA, but is located alongside the lateral LA wall, and connects to a big vessel in the upper left partof the panel (brachiocephalic vein). B Oblique section at identical angle as in panel A but taken at a slightly more caudal position depicting the LLPV and its drainage into the LA. C Transversal (30°) plane colour Doppler view showing the LA and RA with the implanted PFO occluder to the left and right of the inter-atrial septum, a cross-section through the aortic root an the extensive and turbulent flow velocity signal of the severe tricuspid regurgitation.LV = left ventricle; LA = left atrium; PA = pulmonary artery trunk; LUPV = left upper pulmonary vein; LLPV = left lower pulmonary vein; RA = right atrium;PFO = patent foramen ovale; TR = tricuspid regurgitation
After twelve days, the patient was readmitted in cardiogenic shock because of right heart failure, which was thought to be related to worsened right heart volume overload following PFO closure. Therefore and after three days of medically refractory right heart failure, the patient underwent operative correction of the aberrant pulmonary vein with reinsertion into the left atrial appendage. The Amplatzer occluder was extracted and the PFO surgically occluded. The tricuspid valve was reconstructed. Recovery was protracted due to left ventricular (LV) diastolic dysfunction. One month postoperatively, the patient died from multiple organ failure of unknown cause. The performance of an autopsy was not permitted by the patient’s relatives.

Discussion
This case is interpreted as chronic right heart volume overload due to anomalous pulmonary vein connection with resulting tricuspid insufficiency leading to aggravating right heart volume overload. Although right heart failure did manifest itself following PFO closure only delayed, PFO’s function as a volume overload release valve had likely been more relevant than its negative effect on arterial oxygen saturation. The delayed recovery from surgical repair of the defects with LV diastolic dysfunction again suggests the beneficial effect of the PFO as a release valve. However, now it would have served as a pressure release valve in the presence of raised RV filling pressure (impaired RV systolic function due to severe TR), unless it was closed again surgically, the fact of which lead to a leftward shift of the interventricular septum, ie, constrained LV filling. In addition, augmented RV cardiac output due to tricuspid valve repair led to augmented filling of the LV, thereby challenging the diastolic capacity of the systemic ventricle. It is unlikely that the patient died from cardiac causes.
© 2006 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.