Summary
Obstructive thrombus is a rare complication of surgical valve replacement. Current guidelines recommend treatment with repeat surgery or fibrinolysis, especially in critically ill patients, despite a high risk of complication and death linked with these treatments. The treatment of haemodynamically stable patients is, however, less well documented. We describe the rare case of a 40-year-old patient presenting with symptomatic but stable obstruction late after porcine bioprosthetic aortic valve replacement, who was successfully treated with oral anticoagulation. Interruption of oral anticoagulation resulted in rapid recurrence of obstruction, highlighting the importance of long-term anticoagulation in such cases.
Case report
A 40-year-old, otherwise healthy and active man was referred to our cardiology consultation 15 years ago for exercise testing prior to taking out life insurance. The patient had no significant medical history or current complaints. Because of a 3/6 protomesosystolic murmur and a 2/6 diastolic murmur, both located in the aortic region and consistent with aortic valvular disease, the patient underwent transthoracic echocardiography, which revealed a normal left ventricular ejection fraction (LVEF), visually estimated at 65%, as well as an abnormal aortic valve with important thickening of the right coronary and noncoronary cusps, which seemed to be fused. Maximum aortic jet velocity was measured as 3.2 m/s (n = <1.7), with a corresponding mean gradient of 25 mm Hg (n = <10) and a calculated valvular area of 1.8 cm2 or 0.9 cm2/m2 in indexed value, corresponding to mild to moderate valvular stenosis. The examination also revealed mild aortic regurgitation. Because of the absence of symptoms, no particular treatment was prescribed, and a follow-up echocardiogram performed 6 months later showed stability of the valvular disease.
5 years later, the patient was reassessed because of dyspnoea on exertion and one episode of syncope whilst walking up a steep hill. A transthoracic echocardiogram revealed worsening of the aortic stenosis which was now moderate to severe, with a peak flow velocity measured at 3.7 m/s, mean gradient of 40 mm Hg and calculated valvular area of 1.1 cm2 (0.55 cm2/m2) and, because of the persisting symptoms, it was decided to proceed with aortic valve replacement with a semi-stentless porcine bioprosthesis (Shelhigh porcine n° 25), after he had refused mechanical valve implantation because of his numerous sporting activities. Macroscopic examination confirmed a bicuspid valve, and immediate follow-up echocardiography at hospital discharge showed a satisfactory result of surgery with good positioning of the bioprosthesis, a peak jet velocity of 2.8 m/s, mean gradient of 18 mm Hg as well as minimal transvalvular regurgitation and normal LVEF (70%). Thereafter, the patient refused medical or echographic follow-up.
Eight years later, the patient, now aged 54, consulted because of the recent appearance of dyspnoea on exertion after 3–4 flights of stairs. Auscultation revealed a 3/6 aortic murmur, and transthoracic echocardiography showed a normal-looking bioprosthesis but with accelerated peak jet velocity (3.8 m/s), a mean gradient of 35 mm Hg and a calculated effective orifice area (EOA) of 1.3 cm2 (0.65 cm2/m2). LVEF was still normal at 70%. Six months later, the aortic jet velocity had increased to 4.6 m/s with a mean transvalvular gradient of 50 mm Hg and the EOA was 1.1 cm2 (0.55 cm2/m2). Transoesophageal echocardiography (Figure 1) showed mild thickening of the cusps, which could not clearly explain the dysfunction of the prosthesis. There was a suspicion of a superimposed mass located adjacent to the struts in the posterior position (Figure 1, panel B), but visualisation of the cusps was slightly hampered by shadowing and reverberation of the struts. Colour Doppler showed mild prosthetic regurgitation. Because the presentation was not typical of valvular prosthesis degeneration, it was decided to start the patient on empirical oral anticoagulation with acenocoumarol to treat putative obstructing thrombus.
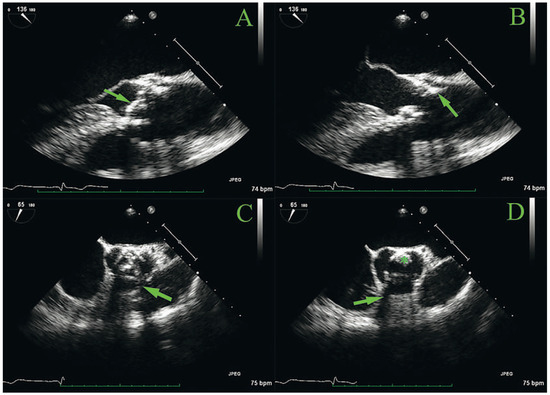
Figure 1.
Transoesophageal echocardiography of the aortic bioprosthesis. A and B: long axis view with cusps in the closed (panel A, arrow) and open (panel B) position; the arrow in panel B points to a possible superimposed mass adjacent to the struts in the posterior position. C and D: short-axis view, also with cusps in the closed (Panel C) and open (panel D) position. Note examples of shadowing of the struts (panels C and D, arrows). The asterisk represents a possible superimposed mass adjacent to the struts in the posterior position.
After 2 months of effective anticoagulation with international normalised ratio (INR) values between 2 and 3, peak velocity and mean gradient had decreased to 3.8 m/s and 34 mm Hg, respectively, and the EOA was 1.6 cm2 (0.8 cm2/m2) Despite resolution of the dyspnoea, the patient was extremely reluctant to continue the anticoagulation, and it was decided to interrupt the treatment and check the echocardiogram 2 months later in order to confirm the benefit of the acenocoumarol and its indication. The follow-up echocardiogram showed once again increased obstruction (peak velocity 4.7 cm/s, mean gradient 55 mm Hg, EOA 1.1 cm2 or 0.55 cm2/m2), without any significant visual changes on two-dimensional imaging. Because of a significant reduction in his physical activity, the patient had remained asymptomatic during these 2 months. He then accepted longterm oral anticoagulation in order to be able to start exercising again, and so far the function of the bioprosthesis remains stable (latest values (the patient then being 56 years old): peak velocity 3.8 cm/s, mean gradient 33 mm Hg, EOA 1.4 cm2 or 0.7 cm2/m2). He remains asymptomatic.
Discussion
Herein we discuss the case of a patient presenting with symptomatic but haemodynamically stable, suspected obstructing thrombus of an aortic porcine bioprosthesis that regressed under oral anticoagulant treatment with a vitamin K antagonist.
Prosthetic valve thrombosis is considered a rare complication, with an estimated incidence of up to 0.13% [1] according to clinical studies, although autopsy studies have showed an incidence of up to 11%. The risk appears to be highest during the first year after implantation, and appears to be similar in mechanical valves under strictly therapeutic anticoagulation and in bioprosthetic valves with or without antiplatelet treatment [2]. Risk factors for valve thrombosis include subtherapeutic anticoagulation amongst holders of mechanical valves and thromboembolic risk factors such as mitral or tricuspid valve position, atrial fibrillation and constitutional or acquired hypercoagulability.
Prosthetic valve thrombosis is classified as either obstructive or nonobstructive depending on the presence of an increased transvalvular gradient. Obstructive thrombosis is often associated with dyspnoea, acute pulmonary oedema, arrhythmia or systemic embolism. Nonobstructive thrombosis often presents with thromboembolism, or is asymptomatic and diagnosed during routine cardiac follow-up. Diagnosis of prosthetic valve thrombosis is challenging and usually requires transoesophageal echocardiography (TOE) or fluoroscopy / computed tomography, in addition to standard transthoracic echocardiography [3]. However, in the aortic position, even with TOE diagnosis may be difficult, as in our patient, owing to shadowing.
According to current guidelines [4], treatment of prosthetic valve thrombosis consists of repeat surgery or fibrinolysis if surgery is deemed to be very high risk, especially in the case of critically ill patients, patients with obstructive thrombi whilst under therapeutic anticoagulation and patients who have suffered from thromboembolism. However, these recommendations are mainly based on data concerning mechanical valves, and these therapeutic measures carry substantial risk, with death rates of up to 38% for fibrinolysis and 46% for surgery, especially when balanced against clinically stable patients, such as ours.
Successful regression of obstructive thrombosis by oral anticoagulation has been described in mitral bioprosthetic valves and, more recently, in a series of 6 of 470 patients with aortic bioprothestic valve replacement [5]. All patients had received initial oral anticoagulation for 3 months postoperatively (phenprocoumon) followed by standard antiplatelet therapy with acetylsalicylic acid 100 mg/day. Six patients were subsequently diagnosed with obstructive thrombi. Of note, all six patients had received porcine valves, and no obstructing thrombi were found in patients with pericardial valves. Five of the six patients were treated with phenprocoumon, which resulted in a return to baseline postoperative mean gradient values after around 4 months.
The clinical presentation was similar in our case and the patient responded well to oral anticoagulation, once again confirming the benefit of such a treatment on obstructive valve thrombosis in the case of aortic bioprostheses. Perhaps the most interesting element in our case was the rapid redevelopment of obstruction after interruption of anticoagulation, followed by an equally rapid improvement in valve function after reinstatement of the treatment (Figure 2), a phenomenon also noted in one patient in the series of Jander et al. [5]. We believe these elements could bring added weight to the recommendation of long-term maintenance of oral anticoagulation, ideally lifelong, in cases of obstructive aortic bioprosthetic thrombi, although more data are needed to completely substantiate such a recommendation.
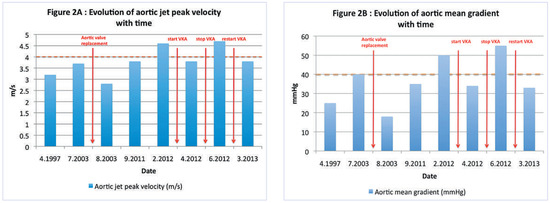
Figure 2.
Graphs demonstrating the evolution of aortic peak velocity (panel A, y-axis) and mean transvalvular gradient (panel B, y-axis) with time (panel A and B, x-axis). Important clinical events are annotated in red. The dashed orange lines represent the cut-off values for severe valvular stenosis. VKA = vitamin K antagonist.
Funding
No financial support.
Conflicts of Interest
No other potential conflict of interest relevant to this article was reported.
References
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000, 36, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Davidson, M.J.; Lamy, A.; Eikelboom, J.W. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009, 374, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves. J Am Soc Echocardiogr. 2009, 22, 975–1014. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012, 33, 2451–2496. [Google Scholar] [PubMed]
- Jander, N.; Kienzle, R.P.; Kayser, G.; Neumann, F.J.; Gohlke-Baerwolf, C.; Minners, J. Usefulness of phenprocoumon for the treatment of obstructing thrombus in bioprostheses in the aortic valve position. Am J Cardiol. 2012, 109, 257–262. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.