Abstract
There are anatomical limitations in the effective use of distal protection devices, related to the fact that a balloon or stent can only be deployed at a certain distance from the filter. We describe a way to limit the embolic load in a vein graft angioplasty, in a difficult for effective distal protection anatomic situation by doing full balloon inflations on a separate wire in parallel to the deployed FilterWire™; after removal of the FilterWire™, an appropriately sized stent was deployed at low pressure.
Introduction
Due to the increased morbidity and mortality rates following repeat bypass surgery, especially with open but degenerated bypass grafts, percutaneous coronary intervention (PCI) has become the preferred therapeutic strategy for many patients with saphenous vein graft disease.
The embolic protection devices made the saphenous vein graft PCI safer [1,2] but the wide use of these devices has been partly hindered by anatomic limitations in positioning of the device [3,4,5].
We present the case of a patient in which the anatomic limitation of the filter wire positioning, for effective emboli protection, was partly circumvented by dilating the lesion with a large balloon on a separate guidewire parallel to the device, followed by retrieval of the FilterWire™ with the embolic material and finally by implantation of a stent at nominal pressure without any further protection.
Case report
A 67-year-old patient had coronary bypass surgery with saphenous vein grafts, 12 years ago. He had a history of hypertension, insulin dependent diabetes mellitus, hyperlipidaemia on statins and stable effort angina for at least a year.
He presented with a NSTEMI with ST segment depression in the lateral leads and small elevation of myocardial enzymes (CK-MB: 48 U/l). Two weeks after his presentation a left ventriculogram demonstrated hypokinesis of the inferior wall and anterior basilar segment and a global ejection fraction of 40%. Coronary arteriography demonstrated severe diffuse stenoses of the left main, intermediate branch and circumflex artery (probably the culprit site, based on the location of the ECG changes) with complete occlusion of the distal circumflex and occlusion of the right coronary artery. The left anterior descending artery was occluded and was diffusely diseased. The only vein graft that was patent was sequentially anastomosed to the mid and distal segments of the left anterior descending artery (LAD), separated by an occluded intervening part. The vein graft borders were smooth but there was a bulky eccentric 70% stenosis close to the side to side anastomosis (Figure 1A).
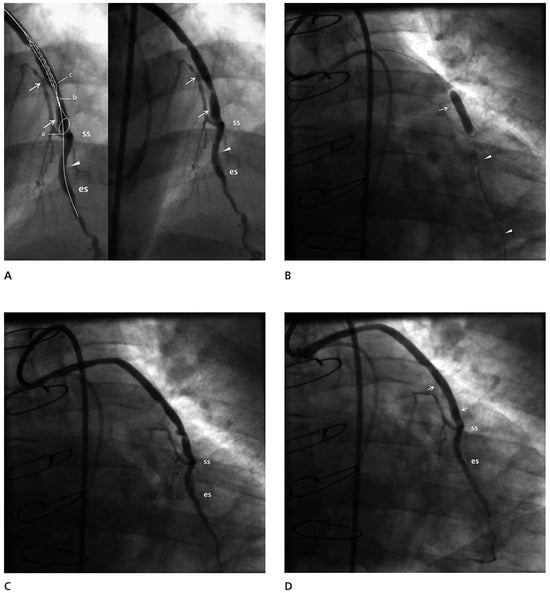
Figure 1.
(A) The vein graft side to side (ss) and end to side (es) anastomoses are marked. The left anterior descending artery is occluded between the two anastomoses (arrowhead) and the distal part is significantly diseased. The vein graft shows a smooth border with the main stenosis, extending between the two arrows. It is obvious that the landing zone is not long enough for the FilterWire™ to provide protection for the proximal part of the left anterior descending artery. This is demonstrated in an inset, in which a cartoon superimposed on the duplicate angiogram demonstrates the positioning of the loop for the achievement of complete emboli protection and the closest distance the stent can be deployed to the loop of the FilterWire™ (ab+bc >19 mm). (B) The balloon on a parallel wire (arrowheads) is inflated covering the whole lesion close to the nitilol loop of the device. The arrow points to proximal shoulder of the lesion. (C) The result of the large balloon inflation was the disruption of the plaque. (D) The post stent implantation result is good with no evidence of protruding atheromatous material and no dissection. The edges of the stent are marked with arrows.
After surgical evaluation the decision was made to proceed with PCI of the whole left coronary artery and vein graft. It was a matter of concern that the vein graft lesion was close to the anastomosis, a fact that precluded the effective use of a FilterWire™ embolic protection device, in protecting the large proximal part of the LAD.
Angioplasty of the distal segment of the LAD followed by angioplasty of the vein graft was performed with a JR4 8F guiding catheter. The angioplasty of the distal LAD was performed successfully and then a FilterWire™ 3.5–5.5 (FilterWire™ EZ System, Boston Scientific Corp) was deployed parallel to the guidewire that extended to the distal LAD, with the FilterWire™ loop proximal to the first of the distal anastomoses. A Sprinter 4/20 balloon (Medtronic, Inc.) was advanced on the guidewire, with its distal tip close to the FilterWire™ loop, closer than it would have been possible if it had been advanced on the FilterWire™ itself (Figure 1B). It was inflated to 18 atm with the intention to disrupt the plaque and get the maximum amount of debris collected during the “protection” phase. The FilterWire™ was properly closed and removed with the guidewire remaining in place. The vein graft plaque was obviously disrupted but the flow remained good (Figure 1C). In the filter there were two solid whitish pieces of the vein graft wall, one large and one small. (Figure 2). A stent Endeavor® Resolute 4/18 (Medronic, Inc.) with a stent balloon/reference artery diameter ratio 1:1, was advanced over the guidewire and deployed at the site of the disrupted plaque at 10 atm. Despite the presence of a small (10–20%) compression of the stent, no further dilatation was performed (Figure 1D). The flow to the LAD through the graft was good with no evidence of branch occlusion or diminished flow.
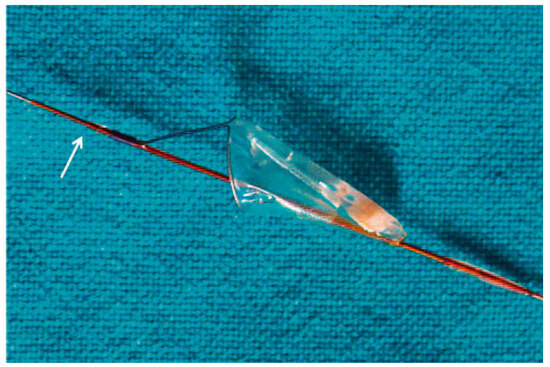
Figure 2.
The FilterWire™ and the atheromatous material that was captured. The arrow points to the balloon stop.
PCI of the left main circumflex with T stenting of the intermediate branch was performed with Endeavor® Resolute (Medtronic, Inc.) stents with good result. The in-hospital stay was uneventful with no CPK or troponin cardiac enzyme elevation.
A follow-up coronary angiogram 10 months later demonstrated full patency of the stents and no obvious progression of the preexisting graft lesions.
Discussion
Traditionally vein graft PCI is considered a high risk procedure due to the increased risk of distal macroem-bolisation and microembolisation with subsequent slow flow or no reflow and periprocedural myocardial infarction. The demonstration in the SVG Angioplasty Free of Emboli Randomised trial (SAFER) of reduced adverse events, primarily a 50% reduction in non-Q wave infarctions (from 13.7 to 7.4%), with the use of a distal balloon occlusion and aspiration system (Percu-Surge GuardWire™, Medtronic, Inc.), ushered a new era in which embolic protection has become the standard of care with vein graft PCI [1].
The efficacy of the distal occlusion system was matched by newer filtering devices that do not occlude anterograde flow but rather strain the effluent so as to prevent particulate embolisation. In the FIRE randomised control trial, the FilterWire™ EX (the first-generation device, distinguished by the absence of the central suspension arm) was as effective as the GuardWire™ (Medtronic, Inc.) in limiting the 30-day incidence of myocardial infarction (9% versus 10%) in patients with vein graft PCI [4]. Although filtering devices offer the potential advantage of perfusion during the intervention, they have similar limitations as far as positioning of the device is concerned. In reviewing the angiographic suitability for distal protection devices, based on the size of the graft (3.5–5.5 mm), or the presence of a landing zone of 2.5 cm from the lesion edge to the anastomosis it was found that 36 to 58% of the cases were not suitable for the effective use of these devices [3,4,5]. To overcome the limitations of the distal protection devices in addressing the “close to the anastomosis lesion PCI”, proximal occlusion devices were developed. These devices proved to be in randomised trials, noninferior to the GuardWire™ and FilterWire™ [6].
In our case, we strongly believed that we needed to use an embolic protection device because of the large area perfused by the retrogradely filled LAD. It is noteworthy that despite the data supporting the use of embolic protection devices, an analysis from the American College of Cardiology (ACC)-National Cardiovascular Data Registry, which included a total of 19 546 SVG PCI from January 2004 through March 2006, showed that embolic protection devices were used in just 22% of patients, a trend that is observed in recent trials [7]. The location of the lesion was close enough to the anastomosis for effective protection of the proximal limb of the vessel with a distal protection device. In the case of the FilterWire™, there is a structural limit to how close the tip of the balloon can get to the lower part of the wire loop and this is 12 mm. The distance of the edge of the stent to the tip of the balloon is 7 mm and so the minimum distance for effective protection for the Endeavor® stent deployment is more than 19 mm (Figure 1A and Figure 2) (personal measurements).
A proximal protection system is ideally designed for this type of lesion location but the system was unavailable to us. That gave us the opportunity to adapt the technique of distal protection to this anatomic subset.
In our case, by advancing the balloon on a parallel wire we were able to get close to the nitilol loop and completely dilate the lesion while at the same time protecting both limbs of the vessel. We used a properly sized balloon which we inflated at high pressure to extrude the maximum part of the plaque and provide space for stent deployment at nominal pressure. In two retrospective studies, an old observation was demonstrated, that undersizing the stent in relation to the reference vessel diameter is associated with a reduction in the frequency of post-procedural creatine kinase-MB elevation without an increase in 1-year events [8,9]. Studies of the production of embolic particles during the different phases of PCI, following the practice of moderate pressure balloon predilatation and high pressure stent deployment, have demonstrated that balloon predilatation does not decrease the particulate debris that follows the additional stenting [10,11]. Direct stenting may decrease the distal embolic load [11] but it is questionable whether it can be reliably implemented without prior knowledge of the composition of the plaque [12]. We assumed that in our case the expansion of a stent of appropriate size at the recommended deployment pressure, in a vessel already dilated with a large balloon at high pressure, could have offered a lower potential for embolisation post-stenting. Whether the proposed changes in technique affect the embolic load post-stenting remains conjectural. In addition the FilterWire™ basket has pores the size of 110 μ that may not completely capture the small particles that are released and the presence of the parallel wire may not permit full apposition of the nitilol loop to the vessel wall. In our case, big fragments were captured and there was no evidence of reduced flow or branch occlusion to suggest that smaller particles were released. We elected not to use a second FilterWire™ in the distal limb, during the stent deployment, because of the need for additional manipulations and the absence of any indication of small particulate debris with the preceding balloon inflation.
The good expansion and apposition of the stent to the vein wall, achieved at low pressure after prior large balloon preparation, may be of importance. Although the use of a relative smaller size stent, as an initial approach, seems to limit the embolic load [9], the long term results, beyond one year are not known; in addition the retrieval of a distal protection device may be complicated by having the loosely applied struts impede the advancement of the retrieval sheath.
In conclusion we present a technique that permits the protective use of the FilterWire™ in cases where the landing zone for the device is inadequate and a proximal protective device is unavailable or inappropriate to use. The inflation of a large sized balloon on a parallel wire close to the protective device, by removing the loosely attached atheromatous material permits the safer deployment of an appropriately sized stent without further embolic protection.
Funding/Potential Competing Interests
No financial support and no other potential conflict of interest relevant to this article were reported.
References
- Baim, D.S.; Wahr, D.; George, B.; Leon, M.B.; Greenberg, J.; Cutlip, D.E.; et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002, 105, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Halkin, A.; Masud, A.Z.; Rogers, C.; Hermiller, J.; Feldman, R.; Hall, P.; Haber; et al. Six-month outcomes after percutaneous intervention for lesions in aortocoronary saphenous vein grafts using distal protection devices: Results from the FIRE trial. Am Heart J. 2006, 151, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Mathew, V.; Lennon, R.J.; Rihal, C.S.; Bresnahan, J.F.; Holmes, D.R., Jr. Applicability of distal protection for aortocoronary vein graft interventions in clinical practice. Catheter Cardiovasc Interv. 2004, 63, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.A.; Dixon, S.R.; Safian, R.D.; O’Neill, W.W. Usefulness of embolic protection devices during saphenous vein graft intervention in a nonselected population. J Interv Cardiol. 2005, 18, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Stolker, J.M.; Frutkin, A.D.; Marso, S.P. Suitability of Saphenous Vein Graft Lesions for the Use of Distal Embolic Protection Devices. J Invasive Cardiol. 2008, 20, 568–570. [Google Scholar] [PubMed]
- Mauri, L.; Cox, D.; Hermiller, J.; Massaro, J.; Wahr, J.; Tay, S.W.; et al. The PROXIMAL trial: Proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: A randomized, prospective, multicenter clinical trial. J Am Coll Cardiol. 2007, 50, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Badhey, N.; Lichtenwalter, C.; deLemos, J.A.; Roesle, M.; Obel, O.; Tayo, A.A.; et al. Contemporary Use of Embolic Protection Devices in Saphenous Vein Graft Interventions: Insights From the Stenting of Saphenous Vein Grafts Trial. Catheter Cardiovasc Interv. 2010, 76, 263–269. [Google Scholar] [CrossRef]
- Iakovou, I.; Dangas, G.; Mintz, G.S.; Mehran, R.; Kobayashi, Y.; Aymong, E.D.; et al. Relation of final lumen dimensions in saphenous vein grafts after stent implantation to outcome. Am J Cardiol. 2004, 93, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Pichard, A.D.; Mintz, G.S.; Kim, S.W.; Lee, S.Y.; Kim, S.Y.; et al. Outcome of undersized Drug-Eluting Stents for percutaneous coronary intervention of saphenous vein graft lesions. Am J Cardiol. 2010, 105, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Ito, H.; Iwakura, K.; Kawano, S.; Kurotobi, T.; Date, M.; et al. Detection and quantification of embolic particles during percutaneous coronary intervention to stable plaque: It correlates to coronary flow dynamics and myocardial damage. Catheter Cardiovasc Interv. 2007, 69, 425–31. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Carere, R.G.; Virmani, R.; Baim, D.; Teirstein, P.S.; Whitlow, P.; et al. Retrieval and Analysis of Particulate Debris After Saphenous Vein Graft Intervention. J Am Coll Cardiol. 1999, 34, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.C.; Houser, S.L.; Aretz, T.; MacNeill, B.D.; Oesterle, S.N.; Palacios, I.F. Significant atheromatous debris following uncomplicated vein graft direct stenting: Evidence supporting routine use of distal protection devices. J Invasive Cardiol. 2002, 14, 636–639. [Google Scholar] [PubMed]
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.