Case report
A 48-year-old woman experienced an episode of cardiac decompensation due to a third-degree atrioventricular block. Normal systolic and diastolic functions of both ventricles were documented. A dual-chamber pacemaker was implanted and eliminated the patient’s symptoms. Two years later, the patient was hospitalised due to biventricular heart failure and wide QRS diomyopathy which was documented by echocardiogtachycardias. Pacemaker follow-up revealed a continu- raphy (Figure 1, Figure 2 and Figure 3). Granulomatous giant cell myocarditis ous deterioration of the pacing threshold and of the (GCM) was diagnosed following endomyocardial biopsy evoked action potential combined with a congestive car- (Figure 4 and Figure 5). Since the patient declined evaluation for heart transplantation, immunosuppressive treatment with cyclosporine, azathioprine and prednisolone was established. This resulted in subjective improvement and regression of inflammation and granulomas, but was accompanied by progressive renal failure. Echocardiography showed a remarkable recovery of left and right ventricular function (Figure 6). Thepatient’s condition has remained stable for the past 4 years.
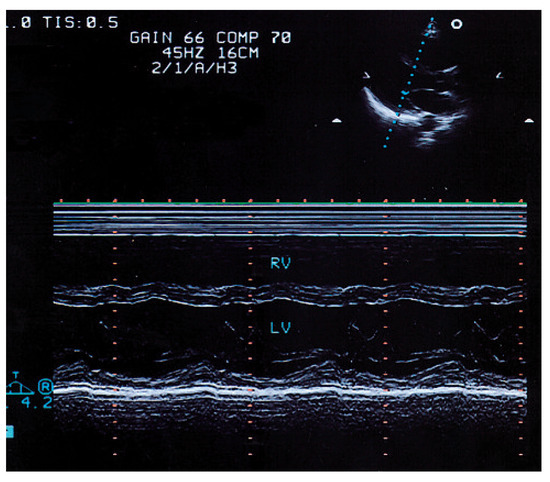
Figure 1.
Parasternal long axis view with M-mode echocardiogram showing depressed contraction in the septal and posterior wall as a sign of global left ventricular systolic dysfunction.RV = right ventricle; LV = left ventricle; IVS = interventricular septum; PW = posterior wall.
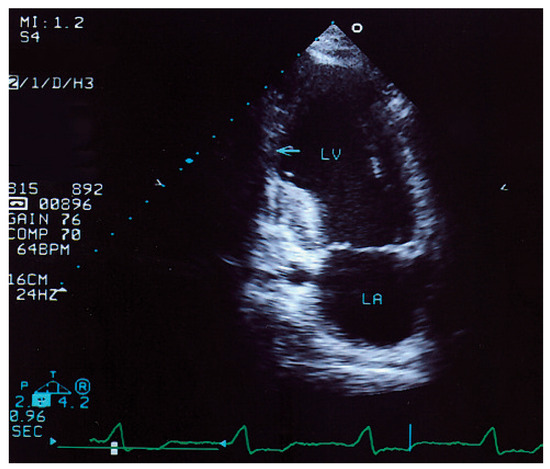
Figure 2.
Apical two-chamber view shows an inferior aneurysm (←) that has a thinned acinetic wall with possible proximal thrombus formation. The thickened proximal inferior wall has subendocardial sclerosis with bright echos. LV = left ventricle; LA = left atrium.
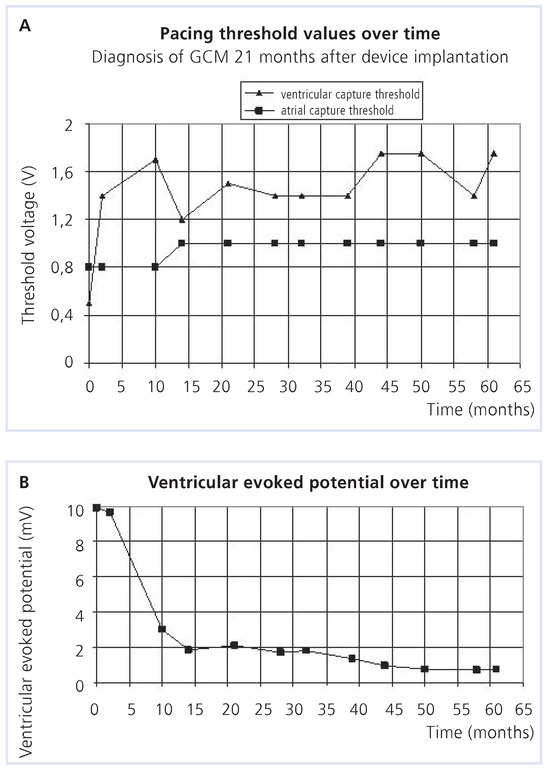
Figure 3.
Pacemaker follow-up.
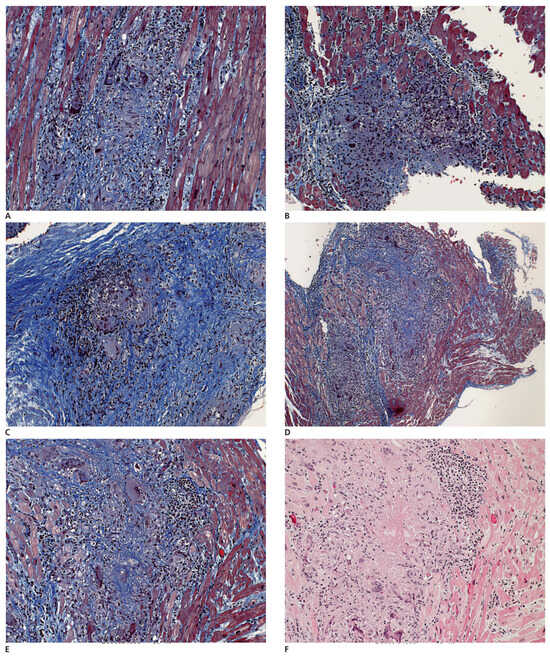
Figure 4.
A–F Different biopsy fragments showing extensive destruction of the myocardium by poorly granuloma-like granulation tissue composed of irregularly distributed giant cells, inflammatory cells and fibrous tissue. F A central areactive necrosis is present.
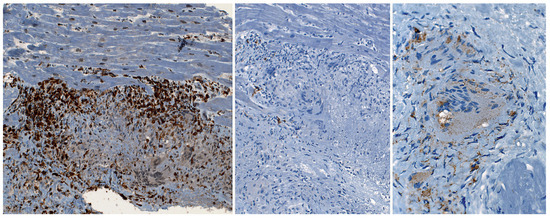
Figure 5.
Massive T-cell accumulation – in equal amounts helper (CD4) and suppressor (CD8) T-cells – in the periphery of the granuloma. No B-cell (CD20) infiltration (B), few histiocytes/macrophages (CD68) within the granulation tissue. The giant cells are of histiocyte/macrophage origin (CD68 positive).
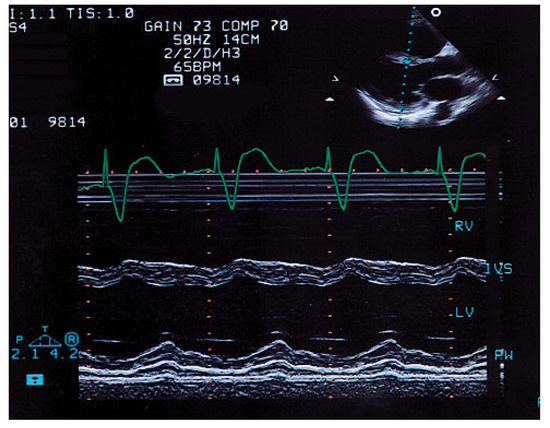
Figure 6.
The M-mode echocardiogram in the parasternal long axis view elevenmonths later documents normalisation of left ventricular function with good contractility in the interventricular septum (IVS) and the posterior wall (PW). RV = right ventricle; LV = left ventricle; IVS = interventricular septum; PW = posterior wall.
Conflicts of Interest
There is no conflict of interest.
References
- McCarthy, R.E., 3rd.; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T., Jr.; Berry, G.J.; Shabetai, R.; for the multicenter Giant Cell Myocarditis Study Group Investigators. Idiopathic giant-cell myocarditis – natural history and treatment. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0