Greywater Reuse: Contaminant Profile, Health Implications, and Sustainable Solutions
Abstract
1. Introduction
2. Greywater Composition and Characteristics
2.1. Fats, Oil and Grease (Fog)
2.2. Temperature
2.3. pH Levels
2.4. Total Suspended Solids (TSS)
2.5. Chemical Oxygen Demand (COD)
2.6. Biochemical Oxygen Demand (BOD)
3. Presence of Heavy Metals and Their Dynamics in Greywater
3.1. Lead, Cadmium, Mercury
3.2. Copper and Zinc
3.3. Chromium and Nickel
4. Presence of Organic Contaminants in Grey Wastewater
4.1. Surfactants and Detergents
4.1.1. Anionic Surfactants
4.1.2. Cationic Surfactants
4.1.3. Non-Ionic Surfactants
4.2. Emerging Chemical Contaminants
4.2.1. Pharmaceuticals and Personal Care Products (PPCPs)
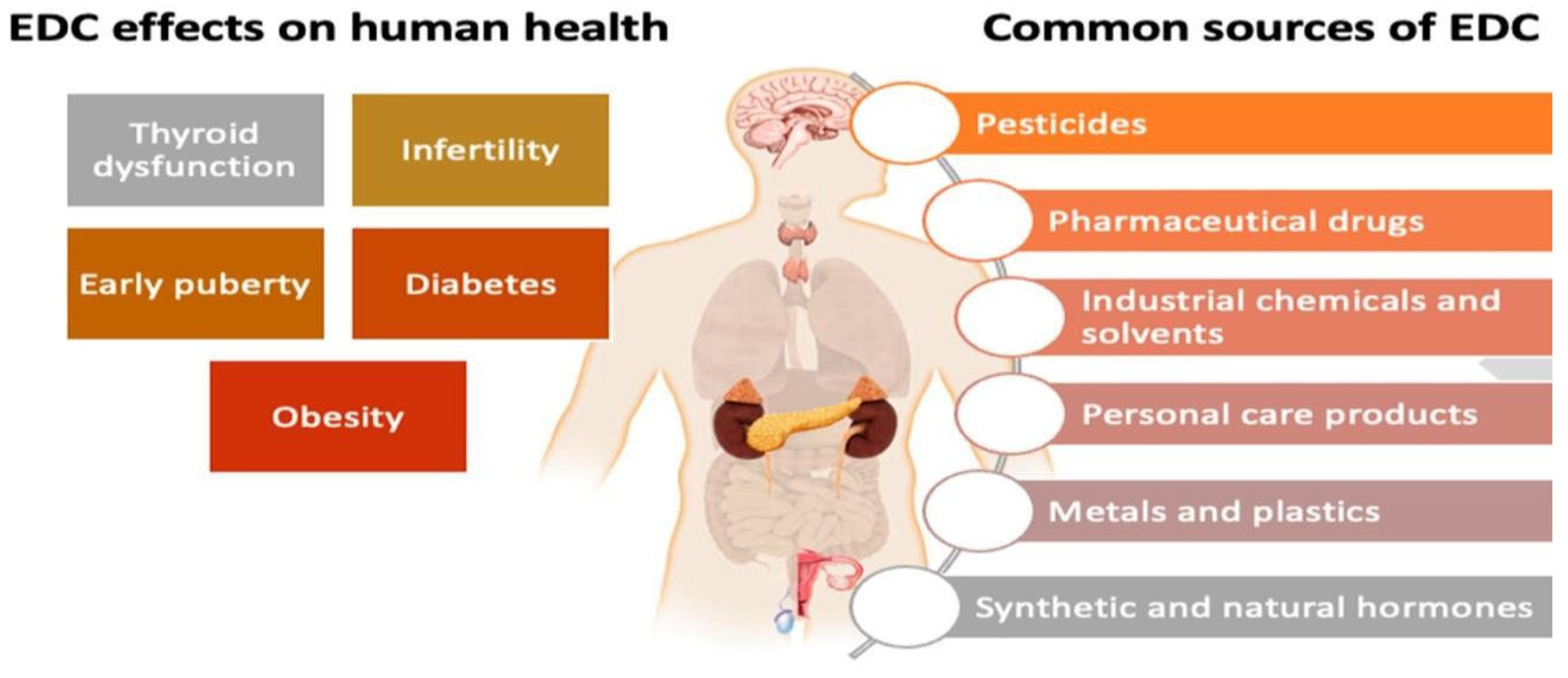
4.2.2. Endocrine Disruptors in Grey Wastewater
4.2.3. Nanoparticles and Microplastics in Grey Water
4.3. Microbial Communities and Their Pathogenesis in Grey Wastewater
4.3.1. Bacterial Communities and Their Pathogenesis
4.3.2. Viral Communities and Their Pathogenesis
4.3.3. Fungal Communities and Their Pathogenesis
4.3.4. Microbial Parasites and Their Pathogenesis
5. Benefits, Challenges, and Sustainable Use of Grey Water
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abed, S.N.; Almuktar, S.A.; Scholz, M. Impact of Storage Time on Characteristics of Synthetic Greywater for Two Different Pollutant Strengths to Be Treated or Recycled. Water Air Soil Pollut. 2020, 231, 211. [Google Scholar] [CrossRef]
- Radingoana, M.P.; Dube, T.; Mazvimavi, D. Progress in greywater reuse for home gardening: Opportunities, perceptions and challenges. Phys. Chem. Earth Parts A/B/C 2020, 116, 102853. [Google Scholar] [CrossRef]
- Van de Walle, A.; Kim, M.; Alam, M.K.; Wang, X.; Wu, D.; Dash, S.R.; Rabaey, K.; Kim, J. Greywater reuse as a key enabler for improving urban wastewater management. Environ. Sci. Ecotechnol. 2023, 16, 100277. [Google Scholar] [CrossRef] [PubMed]
- Noman, E.A.; Al-Gheethi, A.A.S.; Radin Mohamed, R.M.S.; Talip, B.A.; Nagao, H.; Mohd Kassim, A.H.; Bakar, S.A. Consequences of the improper disposal of greywater. In Management of Greywater in Developing Countries: Alternative Practices, Treatment and Potential for Reuse and Recycling; Springer: Berlin/Heidelberg, Germany, 2019; pp. 33–50. [Google Scholar]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Leonard, M.; Gilpin, B.; Robson, B.; Wall, K. Field study of the composition of greywater and comparison of microbiological indicators of water quality in on-site systems. Environ. Monit. Assess. 2016, 188, 475. [Google Scholar] [CrossRef]
- Boamah, T. Solutions for Decentralized Greywater Treatment. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2020. [Google Scholar]
- Khajvand, M.; Mostafazadeh, A.K.; Drogui Tyagi, R.D. Management of greywater: Environmental impact, treatment, resource recovery, water recycling, and decentralization. Water Sci. Technol. 2022, 86, 909–937. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.J.; Badkubi, M. Application of horizontal series filtration in greywater treatment: A semi-industrial study. Australas. J. Water Resour. 2020, 24, 236–247. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Merayo, N.; Prinsen, P.; Luque, R.; Blanco, A.; Zhao, M. A review on greywater reuse: Quality, risks, barriers and global scenarios. Rev. Environ. Sci. Bio Technol. 2019, 18, 77–99. [Google Scholar] [CrossRef]
- Filali, H.; Barsan, N.; Souguir, D.; Nedeff, V.; Tomozei, C.; Hachicha, M. Greywater as an alternative solution for a sustainable management of water resources—A review. Sustainability 2022, 14, 665. [Google Scholar] [CrossRef]
- Khapra, R.; Singh, N. Physical, chemical, and biological evaluation of domestic laundry greywater discharges to attract reclamation strategies and reuse applications in urban settings. Environ. Qual. Manag. 2023, 33, 209–221. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-based remediation as an effective approach for removal of environmental pollutants—A review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Khajvand, M.; Mostafazadeh, A.K.; Drogui, P.; Tyagi, R.D.; Brien, E. Greywater characteristics, impacts, treatment, and reclamation using adsorption processes towards the circular economy. Environ. Sci. Pollut. Res. 2022, 29, 10966–11003. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.; Gibbons, D.; O’Dwyer, M.; Curran, T.P. International evolution of fat, oil and grease (FOG) waste management–A review. J. Environ. Manag. 2017, 187, 424–435. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S.; Trivedi, S. Ecological floating bed (EFB) for decontamination of polluted water bodies: Design, mechanism and performance. J. Environ. Manag. 2019, 251, 109550. [Google Scholar] [CrossRef]
- Rakesh, S.; Ramesh, D.P.; Murugaragavan, R.; Avudainayagam, S.; Karthikeyan, S. Characterization and treatment of grey water: A review. Int. J. Commun. Syst. 2020, 8, 34–40. [Google Scholar] [CrossRef]
- Mahlobo, M. Reduction and monitoring of fat, oil and grease in the Hillcrest area and the greater Ethekwini Municipality. In Proceedings of the Water Institute of South Africa–Biennial Conference, Sun City, South Africa, 18–22 May 2008. [Google Scholar]
- Shokri, A.; Fard, M.S. A critical review in electrocoagulation technology applied for oil removal in industrial wastewater. Chemosphere 2022, 288, 132355. [Google Scholar] [CrossRef]
- Tetteh, E.K. Optimisation of Dissolved Air Flotation (DAF) for Separating Industrial Mineral Oil From Water. Master’s Thesis, Durban University of Technology, Durban, South Africa, 2018. [Google Scholar]
- Romero-Güiza, M.; Asiain-Mira, R.; Alves, M.; Palatsi, J. Induced air flotation for fat, oil, and grease recovery in urban wastewater: A proposed methodology for system optimization and case study. J. Water Process Eng. 2022, 50, 103201. [Google Scholar] [CrossRef]
- Khalil, M.; Liu, Y. Greywater Biodegradability and Biological Treatment Technologies: A Critical Review. Int. Biodeterior. Biodegrad. 2021, 161, 105211. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Gupta, D.K. Constructed wetland, an eco-technology for wastewater treatment: A review on various aspects of microbial fuel cell integration, low temperature strategies and life cycle impact of the technology. Renew. Sustain. Energy Rev. 2021, 148, 111261. [Google Scholar] [CrossRef]
- Shaikh, I.N.; Ahammed, M.M. Quantity and Quality Characteristics of Greywater: A Review. J. Environ. Manag. 2020, 261, 110266. [Google Scholar] [CrossRef]
- Eriksson, E.; Donner, E. Metals in greywater: Sources, presence and removal efficiencies. Desalination 2009, 248, 271–278. [Google Scholar] [CrossRef]
- Shaikh, I.N.; Ahammed, M.M. Effect of Operating Mode on the Performance of Sand Filters Treating Greywater. Environ. Sci. Pollut. Res. 2021, 28, 38209–38223. [Google Scholar] [CrossRef] [PubMed]
- Huelgas, A.; Nakajima, M.; Nagata, H.; Funamizu, N. Comparison between Treatment of Kitchen-sink Wastewater and a Mixture of Kitchen-sink and Washing-machine Wastewaters. Environ. Technol. 2009, 30, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ghaitidak, D.M.; Yadav, K.D. Reuse of Greywater: Effect of Coagulant. Desalin. Water Treat. 2015, 54, 2410–2421. [Google Scholar] [CrossRef]
- Li, W.; Raoult, D.; Fournier, P. Bacterial Strain Typing in the Genomic Era. FEMS Microbiol. Rev. 2009, 33, 892–916. [Google Scholar] [CrossRef]
- Pidou, M.; Avery, L.; Stephenson, T.; Jeffrey, P.; Parsons, S.A.; Liu, S.; Memon, F.A.; Jefferson, B. Chemical Solutions for Greywater Recycling. Chemosphere 2008, 71, 147–155. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Abirami, M.; Yuvaraja, N. Photodegradation of Contaminants in Bathroom Wastewater under UV Illumination Source. Mater. Today Proc. 2020, 33, 2697–2700. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Keitt, T.H. Resilience vs. historical contingency in microbial responses to environmental change. Ecol. Lett. 2015, 18, 612–625. [Google Scholar] [CrossRef]
- Ekwanzala, M.D.; Dewar, J.B.; Kamika, I.; Momba, M.N.B. Tracking the environmental dissemination of carbapenem-resistant Klebsiella pneumoniae using whole genome sequencing. Sci. Total Environ. 2019, 691, 80–92. [Google Scholar] [CrossRef]
- Corvalan, C.; Villalobos Prats, E.; Sena, A.; Campbell-Lendrum, D.; Karliner, J.; Risso, A.; Wilburn, S.; Slotterback, S.; Rathi, M.; Stringer, R.; et al. Towards climate resilient and environmentally sustainable health care facilities. Int. J. Environ. Res. Public Health 2020, 17, 8849. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.S.; Akinbola, G.; Shah, N. A model-based optimization study on greywater reuse as an alternative urban water resource. Sustain. Prod. Consum. 2020, 22, 186–194. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, B.; Jiang, M.; Jin, Y.; Chang, J. Performance and microbial community features of tidal-flow biochar-amended constructed wetlands treating sodium dodecyl sulfate (SDS)-containing greywater. J. Clean. Prod. 2023, 396, 136545. [Google Scholar] [CrossRef]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Parmar, S.; Daki, S.; Bhattacharya, S.; Shrivastav, A. Microorganism: An ecofriendly tool for waste management and environmental safety. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–193. [Google Scholar]
- Zeng, Q.; Li, Y.; Gu, G. Nitrate-dependent Degradation of 17α-ethinylestradiol by Acclimated Activated Sludge under Anaerobic Conditions. J. Chem. Technol. Biotechnol. 2009, 84, 1841–1847. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Mousavi, S.A.; Darvishi, P. Greywater as a sustainable source for development of green roofs: Characteristics, treatment technologies, reuse, case studies and future developments. J. Environ. Manag. 2021, 295, 112991. [Google Scholar] [CrossRef]
- Samayamanthula, D.R.; Sabarathinam, C.; Bhandary, H. Treatment and effective utilization of greywater. Appl. Water Sci. 2019, 9, 90. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.S.; Noman, E.A.; Radin Mohamed, R.M.S.; Talip, B.A.; Mohd Kassim, A.H.; Ismail, N. Disinfection Technologies for Household Greywater. In Management of Greywater in Developing Countries. Alternative Practices, Treatment and Potential for Reuse and Recycling; Springer: Berlin/Heidelberg, Germany, 2019; pp. 185–203. [Google Scholar]
- Oteng-Peprah, M.; Acheampong, M.A.; DeVries, N.K. Greywater characteristics, treatment systems, reuse strategies and user perception—A review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Noman, E.A.; Al-Gheethi, A.A.S.; Bakar, S.A.; Radin Mohamed, R.M.S.; Talip, B.A.; Mohd Kassim, A.H. Treatment Technologies of Household Greywater. In Management of Greywater in Developing Countries: Alternative Practices, Treatment and Potential for Reuse and Recycling; Springer: Berlin/Heidelberg, Germany, 2019; pp. 125–147. [Google Scholar]
- Bader, A.C.; Hussein, H.J.; Jabar, M.T. BOD: COD ratio as Indicator for wastewater and industrial water pollution. Int. J. Spec. Educ. 2022, 37, 2164–2171. [Google Scholar]
- Katukiza, A.Y.; Ronteltap, M.; Niwagaba, C.B.; Kansiime, F.; Lens, P.N.L. Grey water characterisation and pollutant loads in an urban slum. Int. J. Environ. Sci. Technol. 2015, 12, 423–436. [Google Scholar] [CrossRef]
- Khanam, K.; Patidar, S.K. Greywater Characteristics in Developed and Developing Countries. Mater. Today Proc. 2022, 57, 1494–1499. [Google Scholar]
- Vigiak, O.; Grizzetti, B.; Udias-Moinelo, A.; Zanni, M.; Dorati, C.; Bouraoui, F.; Pistocchi, A. Predicting biochemical oxygen demand in European freshwater bodies. Sci. Total Environ. 2019, 666, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.N.; Ahammed, M.M. Quantity and quality characteristics of greywater from an Indian household. Environ. Monit. Assess. 2022, 194, 191. [Google Scholar] [CrossRef]
- Seenirajan, M.; Sasikumar, S.; Antony, E. Design of Grey Water Treatment Units. Int. Res. J. Eng. Technol. 2018, 5, 4243–4250. [Google Scholar]
- Samer, M. Biological and chemical wastewater treatment processes. In Wastewater Treatment Engineering; IntechOpen: London, UK, 2015; p. 150. [Google Scholar]
- Yin, H.; Wang, Y.; Huang, J. Photodegradation-induced biological degradation of treated wastewater effluent organic matter in receiving waters. Water Res. 2021, 204, 117567. [Google Scholar] [CrossRef]
- Arifin, S.N.H.; Mohamed, R.M.S.R.; Ismail, N.R.; Al-Gheethi, A.; Bakar, S.A. Effects of direct discharge of domestic greywater to nearby water body. Mater. Today Proc. 2020, 31, A126–A136. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Ruegg, P.L. Practical Food Safety Interventions for Dairy Production. J. Dairy Sci. 2003, 86, E1–E9. [Google Scholar] [CrossRef]
- Ekwanzala, M.D.; Budeli Momba, M. Sustainable solutions to the impact of industrial water pollution on the environment and community health. In Sustainable Industrial Water Use: Perspectives, Incentives, and Tools; IWA Publishing: London, UK, 2021; p. 127. [Google Scholar]
- Huang, Y.; Han, R.; Qi, J.; Duan, H.; Chen, C.; Lu, X.; Li, N. Health risks of industrial wastewater heavy metals based on improved grey water footprint model. J. Clean. Prod. 2022, 377, 134472. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–125. [Google Scholar]
- Raj, D.; Maiti, S.K. Sources, bioaccumulation, health risks and remediation of potentially toxic metal (loid) s (As, Cd, Cr, Pb and Hg): An epitomised review. Environ. Monit. Assess. 2020, 192, 108. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Teow, Y.H.; Sum, J.Y.; Ng, Z.J.; Mohammad, A.W. Water pathways through the ages: Integrated laundry wastewater treatment for pollution prevention. Sci. Total Environ. 2021, 760, 143966. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Manna, K. Sources and toxicological effects of lead on human health. Indian J. Med. Spec. 2019, 10, 66–71. [Google Scholar]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog; IntechOpen: London, UK, 2019; Volume 10, pp. 70–90. [Google Scholar]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.S.; Anusha, L.; Choudhary, R.; Lvov, V.; Tovar, G.I. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; MMS, C.P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial Wastewater and Its Toxic Effects; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L.; et al. Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the United States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.C.; Negra, M.D.; Kaiser, A.; Pinelo, M. Enzyme immobilization on inorganic surfaces for membrane reactor applications: Mass transfer challenges, enzyme leakage and reuse of materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Jyothi, N.R.; Farook, N.A.M. Mercury toxicity in public health. In Heavy Metal Toxicity in Public Health; IntechOpen: London, UK, 2020; pp. 1–12. [Google Scholar]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Dou, S.; Huang, W. Biomagnification of methylmercury in a marine food web in Laizhou Bay (North China) and associated potential risks to public health. Mar. Pollut. Bull. 2020, 150, 110762. [Google Scholar] [CrossRef] [PubMed]
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- Govil, K.; Krishna, A.K. Soil and water contamination by potentially hazardous elements: A case history from India. In Environmental Geochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 567–597. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Hilal, E.Y.; Elkhairey, M.A.E.; Osman, A.O.A. The role of zinc, manganse and copper in rumen metabolism and immune function: A review article. Open J. Anim. Sci. 2016, 6, 304. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ravindran, V.; Nidheesh, P.V.; Rayalu, S. Optical sensing of copper and its removal by different environmental technologies. ChemistrySelect 2020, 5, 10432–10474. [Google Scholar] [CrossRef]
- Manasa, R.L.; Mehta, A. Wastewater: Sources of pollutants and its remediation. Environ. Biotechnol. 2020, 2, 197–219. [Google Scholar]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y. Recent Developments in Physical, Biological, Chemical, and Hybrid Treatment Techniques for Removing Emerging Contaminants from Wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Ali, A.; Phull, A.-R.; Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Tracy, J.W.; Guo, A.; Liang, K.; Bartram, J.; Fisher, M. Sources of and solutions to toxic metal and metalloid contamination in small rural drinking water systems: A rapid review. Int. J. Environ. Res. Public Health 2020, 17, 7076. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Kour, A.; Sharma, P. Ecological impact of heavy metals on aquatic environment with reference to fish and human health. J. Appl. Nat. Sci. 2022, 14, 1471–1484. [Google Scholar]
- Malhotra, N.; Ger, T.R.; Uapipatanakul, B.; Huang, J.C.; Chen, K.H.C.; Hsiao, C.D. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Mahboob, S. Environmental pollution of heavy metals as a cause of oxidative stress in fish: A review. Life Sci. J. 2013, 10, 336–347. [Google Scholar]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhaop, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- Karim, N. Copper and human health-a review. J. Bahria Univ. Med. Dent. Coll. 2018, 8, 117–122. [Google Scholar] [CrossRef]
- Anyanwu, B.O.; Ezejiofor, A.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy metal mixture exposure and effects in developing nations: An update. Toxics 2018, 6, 65. [Google Scholar] [CrossRef]
- Sharma, N.; Sodhi, K.K.; Kumar, M.; Singh, D.K. Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100388. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. A review on accessible techniques for removal of hexavalent Chromium and divalent Nickel from industrial wastewater: Recent research and future outlook. J. Clean. Prod. 2021, 295, 126229. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An overview of chromium removal techniques from tannery effluent. Appl. Water Sci. 2020, 10, 205. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Thomas, M.; Białecka, B.; Zdebik, D. Removal of copper, nickel and tin from model and real industrial wastewater using sodium trithiocarbonate: The negative impact of complexing compounds. Arch. Environ. Prot. 2018, 44, 33–47. [Google Scholar]
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6509–6518. [Google Scholar] [CrossRef]
- Sharma, R.; Verma, N.; Lugani, Y.; Kumar, S.; Asadnia, M. Conventional and Advanced Techniques of Wastewater Monitoring and Treatment. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–48. [Google Scholar]
- Sadr, S.M.K. Application of Membrane Assisted Technologies in Water Reuse Scenarios. Ph.D. Thesis, University of Surrey, Guildford, UK, 2014. [Google Scholar]
- Singh, A.; Singh, D.; Yadav, H. Impact and assessment of heavy metal toxicity on water quality, edible fishes and sediments in lakes: A review. Trends Biosci. 2017, 10, 1551–1560. [Google Scholar]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Dwumfour-Asare, B.; Adantey, P.; Nyarko, K.B.; Appiah-Effah, E. Greywater Characterization and Handling Practices among Urban Households in Ghana: The Case of Three Communities in Kumasi Metropolis. Water Sci. Technol. 2017, 76, 813–822. [Google Scholar] [CrossRef]
- Edwin, G.A.; Gopalsamy, P.; Muthu, N. Characterization of Domestic Gray Water from Point Source to Determine the Potential for Urban Residential Reuse: A Short Review. Appl. Water Sci. 2014, 4, 39–49. [Google Scholar] [CrossRef]
- Leong, J.Y.C.; Chong, M.N.; Poh, P.E. Assessment of Greywater Quality and Performance of a Pilot-Scale Decentralised Hybrid Rainwater-Greywater System. J. Clean. Prod. 2018, 172, 81–91. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, A.K.; Bhunia, P.; Dash, R.R. Removal of surfactants in greywater using low-cost natural adsorbents: A review. Surf. Interfaces 2021, 27, 101532. [Google Scholar]
- Long, D.C. Greening of consumer cleaning products. In Green Techniques for Organic Synthesis and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 91–115. [Google Scholar]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Jena, G.; Dutta, K.; Daverey, A. Surfactants in water and wastewater (greywater): Environmental toxicity and treatment options. Chemosphere 2023, 341, 140082. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Sobrino-Figueroa, A. Toxic effect of commercial detergents on organisms from different trophic levels. Environ. Sci. Pollut. Res. 2018, 25, 13283–13291. [Google Scholar] [CrossRef]
- Podder, A.; Sadmani, A.A.; Reinhart, D.; Chang, N.B.; Goel, R. Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Hamza, R.A.; Iorhemen, O.T.; Tay, J.H. Occurrence, impacts and removal of emerging substances of concern from wastewater. Environ. Technol. Innov. 2016, 5, 161–175. [Google Scholar] [CrossRef]
- Mariana, M.; Hps, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Kulkarni, D.; Jaspal, D. Surfactants in waste water: Development, current status and associated challenges. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B.l. Cationic surfactants: Self-assembly, structure-activity correlation and their biological applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-based materials for water treatment: Adsorption, photocatalytic degradation, disinfection, antifouling, and nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Slabov, V. Bioreagents in Froth Flotation and Flocculation of Copper, Iron and cerium Oxides. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2024. [Google Scholar]
- Aleid, G.M.; Alshammari, A.S.; Tripathy, D.B.; Gupta, A.; Ahmad, S. Polymeric Surfactants: Recent Advancement in Their Synthesis, Properties, and Industrial Applications. Macromol. Chem. Phys. 2023, 224, 2300107. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Bhatt, J.; Rai, A.K.; Gupta, M.; Vyas, S. Surfactants: An Emerging Face of Pollution. In Micropollutants and Challenges: Emerging in the Aquatic Environments and Treatment Processes; Elsevier: Amsterdam, The Netherlands, 2020; p. 145. [Google Scholar]
- Freeling, F.; Alygizakis, N.A.; von der Ohe, P.C.; Slobodnik, J.; Oswald, P.; Aalizadeh, R.; Cirka, L.; Thomaidis, N.S.; Scheurer, M. Occurrence and potential environmental risk of surfactants and their transformation products discharged by wastewater treatment plants. Sci. Total Environ. 2019, 681, 475–487. [Google Scholar] [CrossRef]
- Krishnan, S.; Chandran, K.; Sinnathambi, C.M. Wastewater treatment technologies used for the removal of different surfactants: A comparative. Int. J. Appl. Chem. 2016, 12, 727–739. [Google Scholar]
- da Silva, S.W.; Klauck, C.R.; Siqueira, M.A.; Bernardes, A.M. Degradation of the commercial surfactant nonylphenol ethoxylate by advanced oxidation processes. J. Hazard. Mater. 2015, 282, 241–248. [Google Scholar] [CrossRef]
- Mokashe, N.; Chaudhari, B.; Patil, U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int. J. Biol. Macromol. 2018, 117, 493–522. [Google Scholar] [CrossRef]
- Arora, U.; Khuntia, H.K.; Chanakya, H.N.; Kapley, A. Surfactants: Combating the fate, impact, and aftermath of their release in the environment. Int. J. Environ. Sci. Technol. 2023, 20, 11551–11574. [Google Scholar] [CrossRef]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment trends and combined methods in removing pharmaceuticals and personal care products from wastewater—A review. Membranes 2023, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Najmi, M.; Mehrnia, M.R.; Tashauoei, H.R.; Iranpoury, A.; Alivand, M.S. Removal of personal care products (PCPs) from greywater using a submerged membrane bioreactor (SMBR): The effect of hydraulic retention time. J. Environ. Chem. Eng. 2020, 8, 104432. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Antonellis, C. Pharmaceuticals and Personal Care Products in New Hampshire: Prevalence and the Influence of Microbial Communities on Removal During Wastewater Treatment. Master’s Thesis, University of New Hampshire: Durham, NH, USA, 2021. [Google Scholar]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential environmental and human health risks caused by antibiotic-resistant bacteria (ARB), antibiotic resistance genes (ARGs) and emerging contaminants (ECs) from municipal solid waste (MSW) landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Saifur, S.; Gardner, C.M. Loading, transport, and treatment of emerging chemical and biological contaminants of concern in stormwater. Water Sci. Technol. 2021, 83, 2863–2885. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen—Toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.; Meyer, A.S.; Raj, A. Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci. Total Environ. 2021, 777, 145988. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 256920. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation–a Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; El-Sheekh, M.; Ma, Y.; Pugazhendhi, A.; Natarajan, D.; Kandasamy, G.; Raja, R.; Kumar, R.M.S.; Kumarasamy, S.; Sathiyan, G.; et al. Current status of microbes involved in the degradation of pharmaceutical and personal care products (PPCPs) pollutants in the aquatic ecosystem. Environ. Pollut. 2022, 300, 118922. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-Disruptive Chemicals as Contaminants of Emerging Concern in Wastewater and Surface Water: A Review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Sangeetha, S.; Vimalkumar, K.; Loganathan, B.G. Environmental contamination and human exposure to select endocrine-disrupting chemicals: A review. Sustain. Chem. 2021, 2, 343–380. [Google Scholar] [CrossRef]
- Ghosh, A.; Tripathy, A.; Ghosh, D. Impact of endocrine disrupting chemicals (EDCs) on reproductive health of human. Proc. Zool. Soc. 2022, 75, 16–30. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-disrupting compounds: An overview on their occurrence in the aquatic environment and human exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Yao, B.; Zhi, D.; Luo, L.; Zhou, Y. Endocrine disrupting chemicals in the environment: Environmental sources, biological effects, remediation techniques, and perspective. Environ. Pollut. 2022, 310, 119918. [Google Scholar] [CrossRef]
- Badamasi, I.; Odong, R.; Masembe, C. Threats posed by xenoestrogenic chemicals to the aquatic ecosystem, fish reproduction and humans: A review. Afr. J. Aquat. Sci. 2020, 45, 243–258. [Google Scholar] [CrossRef]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef]
- Encarnação, T.; Pais, A.A.; Campos, M.G.; Burrows, H.D. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar] [CrossRef]
- Goralczyk, K. A review of the impact of selected anthropogenic chemicals from the group of endocrine disruptors on human health. Toxics 2021, 9, 146. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.-B. Endocrine-disrupting chemicals and disease endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Esfandiari, A.; Mowla, D. Investigation of microplastic removal from greywater by coagulation and dissolved air flotation. Process Saf. Environ. Prot. 2021, 151, 341–354. [Google Scholar] [CrossRef]
- Galvão, A.; Aleixo, M.; De Pablo, H.; Lopes, C.; Raimundo, J. Microplastics in wastewater: Microfiber emissions from common household laundry. Environ. Sci. Pollut. Res. 2020, 27, 26643–26649. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal oxides nanoparticles: General structural description, chemical, physical, and biological synthesis methods, role in pesticides and heavy metal removal through wastewater treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Prakash, A.; Some, S.; Singh, C.; Bezner Kerr, R.; Caretta, M.A.; Conde, C.; Ferre, M.R.; Schuster-Wallace, C.; Maria Pahlen, M.C.; et al. Synergies and Trade-Offs between Climate Change Adaptation Options and Gender Equality: A Review of the Global Literature. Humanit. Soc. Sci. Commun. 2022, 9, 251. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free. Radic. Res. 2021, 55, 331. [Google Scholar] [CrossRef] [PubMed]
- Roy, K. Chemometrics and Cheminformatics in Aquatic Toxicology; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Parkins, M.D.; Lee, B.E.; Acosta, N.; Bautista, M.; Hubert, C.R.; Hrudey, S.E.; Frankowski, K.; Pang, X.L. Wastewater-based surveillance as a tool for public health action: SARS-CoV-2 and beyond. Clin. Microbiol. Rev. 2024, 37, e00103-22. [Google Scholar] [CrossRef]
- Awasthi, A.; Gandhi, K.; Rayalu, S. Greywater treatment technologies: A comprehensive review. Int. J. Environ. Sci. Technol. 2024, 21, 1053–1082. [Google Scholar] [CrossRef]
- Cui, Q.; Huang, Y.; Wang, H.; Fang, T. Diversity and abundance of bacterial pathogens in urban rivers impacted by domestic sewage. Environ. Pollut. 2019, 249, 24–35. [Google Scholar] [CrossRef]
- Nagarkar, M.; Keely, S.P.; Brinkman, N.E.; Garland, J.L. Human-and infrastructure-associated bacteria in greywater. J. Appl. Microbiol. 2021, 131, 2178–2192. [Google Scholar] [CrossRef]
- Cho, S.; Jackson, C.R.; Frye, J.G. The prevalence and antimicrobial resistance phenotypes of Salmonella, Escherichia coli and Enterococcus sin surface water. Lett. Appl. Microbiol. 2020, 71, 3–25. [Google Scholar] [CrossRef]
- Duijster, J.W.; Franz, E.; Neefjes, J.; Mughini-Gras, L. Bacterial and parasitic pathogens as risk factors for cancers in the gastrointestinal tract: A review of current epidemiological knowledge. Front. Microbiol. 2021, 12, 790256. [Google Scholar] [CrossRef]
- Benami, M.; Gillor, O.; Gross, A. Potential health and environmental risks associated with onsite greywater reuse: A review. Built Environ. 2016, 42, 212–229. [Google Scholar] [CrossRef]
- Guarin, T.C.; Pagilla, K.R. Microbial community in biofilters for water reuse applications: A critical review. Sci. Total Environ. 2021, 773, 145655. [Google Scholar] [CrossRef] [PubMed]
- Shingare, R.P.; Thawale, P.R.; Raghunathan, K.; Mishra, A.; Kumar, S. Constructed wetland for wastewater reuse: Role and efficiency in removing enteric pathogens. J. Environ. Manag. 2019, 246, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Mbanga, J.; Abia, A.L.K.; Amoako, D.G.; Essack, S.Y. Quantitative microbial risk assessment for waterborne pathogens in a wastewater treatment plant and its receiving surface water body. BMC Microbiol. 2020, 20, 346. [Google Scholar] [CrossRef] [PubMed]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. Water Qual. 2017, 401–416. [Google Scholar]
- Khalid, S.; Shahid, M.; Natasha; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public health 2018, 15, 895. [Google Scholar] [CrossRef]
- Bej, S.; Swain, S.; Bishoyi, A.K.; Mandhata, C.P.; Sahoo, C.R.; Padhy, R.N. Wastewater-associated infections: A public health concern. Water Air Soil Pollut. 2023, 234, 444. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Bhatt, A.; Arora, P.; Prajapati, S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: A review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020, 8, 104429. [Google Scholar] [CrossRef]
- Boussettine, R.; Hassou, N.; Bessi, H.; Ennaji, M.M. Waterborne transmission of enteric viruses and their impact on public health. In Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 907–932. [Google Scholar]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pierre Contreras, G.; Conei Valencia, D.; Lizama, L.; Vargas Zuñiga, D.; Avendaño Carvajal, L.F.; Ampuero Llanos, S. An old acquaintance: Could adenoviruses be our next pandemic threat? Viruses 2023, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Wartecki, A.; Rzymski, P. On the coronaviruses and their associations with the aquatic environment and wastewater. Water 2020, 12, 1598. [Google Scholar] [CrossRef]
- Mahgoub, S.A. Microbial hazards in treated wastewater: Challenges and opportunities for their reusing in Egypt. In Unconventional Water Resources and Agriculture in Egypt; Springer: Berlin/Heidelberg, Germany, 2019; pp. 313–336. [Google Scholar]
- Walker, D.I.; Adriaenssens, E.M.; McDonald, J.E.; Hillary, L.S.; Malham, S.K.; Jones, D.L. Viral indicators for tracking domestic wastewater contamination in the aquatic environment. Water Res. 2020, 181, 115926. [Google Scholar]
- Ibrahim, Y.; Ouda, M.; Kadadou, D.; Banat, F.; Naddeo, V.; Alsafar, H.; Yousef, A.F.; Barceló, D.; Hasan, S.W. Detection and removal of waterborne enteric viruses from wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 105613. [Google Scholar] [CrossRef]
- Jalali Milani, S.; Nabi Bidhendi, G. A review on the potential of common disinfection processes for the removal of virus from wastewater. Int. J. Environ. Res. 2022, 16, 9. [Google Scholar] [CrossRef]
- Lim, K.-Y.; Hamilton, A.J.; Jiang, S.C. Assessment of public health risk associated with viral contamination in harvested urban stormwater for domestic applications. Sci. Total Environ. 2015, 523, 95–108. [Google Scholar] [CrossRef]
- Sims, N.; Kasprzyk-Hordern, B. Future perspectives of wastewater-based epidemiology: Monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020, 139, 105689. [Google Scholar] [CrossRef]
- Ram, N.; Gable, L.; Ram, J.L. The future of wastewater monitoring for the public health. Univ. Richmond Law Rev. 2021, 56, 911. [Google Scholar] [CrossRef]
- Divya, K.S.; Murthy, S.M.; Jogaiah, S. Ecological studies of fungal biodiversity in freshwater and their broad-spectrum applications. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 631–648. [Google Scholar]
- Mir-Tutusaus, J.A.; Baccar, R.; Caminal, G.; Sarrà, M. Can white-rot fungi be a real wastewater treatment alternative for organic micropollutants removal? A review. Water Res. 2018, 138, 137–151. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, H.; Feng, Q.; Wu, X.; Zhang, Y.; McCarthy, A.J.; Sekar, R. Changes in fungal community structure in freshwater canals across a gradient of urbanization. Water 2020, 12, 1917. [Google Scholar] [CrossRef]
- Yang, W.; Cai, C.; Guo, Y.; Wu, H.; Guo, Y.; Dai, X. Diversity and Fate of Human Pathogenic Bacteria, Fungi, Protozoa, and Viruses in Full-Scale Sludge Treatment Plants. J. Clean. Prod. 2022, 380, 134990. [Google Scholar] [CrossRef]
- González-Abradelo, D.; Pérez-Llano, Y.; Peidro-Guzmán, H.; del Rayo Sánchez-Carbente, M.; Folch-Mallol, J.L.; Aranda, E.; Vaidyanathan, V.K.; Cabana, H.; Gunde-Cimerman, N.; Batista-García, R.A. First demonstration that ascomycetous halophilic fungi (Aspergillus sydowii and Aspergillus destruens) are useful in xenobiotic mycoremediation under high salinity conditions. Bioresour. Technol. 2019, 279, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Kou, Y. Ectomycorrhizal fungi: Participation in nutrient turnover and community assembly pattern in forest ecosystems. Forests 2020, 11, 453. [Google Scholar] [CrossRef]
- Baker, P.; Tiroumalechetty, A.; Mohan, R. Fungal enzymes for bioremediation of xenobiotic compounds. In Recent Advancement in White Biotechnology Through Fungi: Volume 3: Perspective for Sustainable Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 463–489. [Google Scholar]
- Mhlongo, T.N.; Ogola, H.J.O.; Selvarajan, R.; Sibanda, T.; Kamika, I.; Tekere, M. Occurrence and diversity of waterborne fungi and associated mycotoxins in treated drinking water distribution system in South Africa: Implications on water quality and public health. Environ. Monit. Assess. 2020, 192, 519. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Vilchez-Vargas, R.; Kerckhof, F.M.; Aranda, E.; González-López, J.; Rodelas, B. Community structure, population dynamics and diversity of fungi in a full-scale membrane bioreactor (MBR) for urban wastewater treatment. Water Res. 2016, 105, 507–519. [Google Scholar] [CrossRef]
- Lavanya, V.; Kannan, D. Research Article Grey Water Treatment Using Effective Micro-organisms and its Impact on Water Qualities. J. Appl. Sci. 2019, 19, 188–198. [Google Scholar] [CrossRef]
- Yadav, A.N. Recent Trends in Mycological Research; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Isaza-Pérez, F.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Potential of residual fungal biomass: A review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031. [Google Scholar]
- da Silva, A.F.; Banat, I.M.; Giachini, A.J.; Robl, D. Fungal biosurfactants, from nature to biotechnological product: Bioprospection, production and potential applications. Bioprocess Biosyst. Eng. 2021, 44, 2003–2034. [Google Scholar]
- Goutam, J.; Sharma, J.; Singh, R.; Sharma, D. Fungal-mediated bioremediation of heavy metal–polluted environment. In Microbial Rejuvenation of Polluted Environment: Volume 2; Springer: Berlin/Heidelberg, Germany, 2021; pp. 51–76. [Google Scholar]
- Verma, P.P.; Shelake, R.M.; Das, S.; Sharma, P.; Kim, J.Y. Plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF): Potential biological control agents of diseases and pests. In Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Springer: Berlin/Heidelberg, Germany, 2019; pp. 281–311. [Google Scholar]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Public health risks associated with food-borne parasites. EFSA J. 2018, 16, e05495. [Google Scholar] [PubMed]
- Siwila, J.; Mwaba, F.; Chidumayo, N.; Mubanga, C. Food and waterborne protozoan parasites: The African perspective. Food Waterborne Parasitol. 2020, 20, e00088. [Google Scholar] [CrossRef] [PubMed]
- Candela, E.; Goizueta, C.; Periago, M.V.; Muñoz-Antoli, C. Prevalence of intestinal parasites and molecular characterization of Giardia intestinalis, Blastocystis sand Entamoeba histolytica in the village of Fortín Mbororé (Puerto Iguazú, Misiones, Argentina). Parasites Vectors 2021, 14, 510. [Google Scholar] [CrossRef]
- Benami, M.; Gillor, O.; Gross, A. Potential Microbial Hazards from Graywater Reuse and Associated Matrices: A Review. Water Res. 2016, 106, 183–195. [Google Scholar] [CrossRef]
- Moropeng, R.C.; Budeli, P.; Mpenyana-Monyatsi, L.; Momba, M.N.B. Dramatic reduction in diarrhoeal diseases through implementation of cost-effective household drinking water treatment systems in Makwane village, Limpopo province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 410. [Google Scholar] [CrossRef]
- Nampijja, M. The Impact of Helminth Infections on Developmental and Educational Outcomes. In Handbook of Applied Developmental Science in Sub-Saharan Africa; Springer: Berlin/Heidelberg, Germany, 2017; pp. 133–156. [Google Scholar]
- Krumrie, S.; Capewell, P.; Smith-Palmer, A.; Mellor, D.; Weir, W.; Alexander, C.L. A scoping review of risk factors and transmission routes associated with human giardiasis outbreaks in high-income settings. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100084. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, J.; Mao, X. Removal of Pathogens in Onsite Wastewater Treatment Systems: A Review of Design Considerations and Influencing Factors. Water 2021, 13, 1190. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability 2017, 10, 86. [Google Scholar] [CrossRef]
- Hazra, M.; Durso, L.M. Performance efficiency of conventional treatment plants and constructed wetlands towards reduction of antibiotic resistance. Antibiotics 2022, 11, 114. [Google Scholar] [CrossRef]
- Bui, S.; Oppedal, F.; Sievers, M.; Dempster, T. Behaviour in the toolbox to outsmart parasites and improve fish welfare in aquaculture. Rev. Aquac. 2019, 11, 168–186. [Google Scholar] [CrossRef]
- Guichard, M.; Dietemann, V.; Neuditschko, M.; Dainat, B. Advances and perspectives in selecting resistance traits against the parasitic mite Varroa destructor in honey bees. Genet. Sel. Evol. 2020, 52, 71. [Google Scholar] [CrossRef] [PubMed]
- Chaoua, S.; Boussaa, S.; Khadra, A.; Boumezzough, A. Efficiency of two sewage treatment systems (activated sludge and natural lagoons) for helminth egg removal in Morocco. J. Infect. Public Health 2018, 11, 197–202. [Google Scholar] [CrossRef]
- Buchmann, K. Control of parasitic diseases in aquaculture. Parasitology 2022, 149, 1985–1997. [Google Scholar] [CrossRef]
- Olanrewaju, O.O.; Ilemobade, A.A. Greywater Reuse Review and Framework for Assessing Greywater Treatment Technologies for Toilet Flushing. Adv. Res. 2015, 5, 1–25. [Google Scholar] [CrossRef]
- Oh, K.S.; Leong, J.Y.C.; Poh, P.E.; Chong, M.N.; Von Lau, E. A review of greywater recycling related issues: Challenges and future prospects in Malaysia. J. Clean. Prod. 2018, 171, 17–29. [Google Scholar] [CrossRef]
- Ammar, E.; Maury, H.; Morin, L.; Sghir, A. Environmental, economic, and ethical assessment of the treated wastewater and sewage sludge valorization in agriculture. In Interaction and Fate of Pharmaceuticals in Soil-Crop Systems: The Impact of Reclaimed Wastewater; Springer: Berlin/Heidelberg, Germany, 2021; pp. 49–78. [Google Scholar]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Boano, F.; Caruso, A.; Costamagna, E.; Ridolfi, L.; Fiore, S.; Demichelis, F.; Galvão, A.; Pisoeiro, J.; Rizzo, A.; Masi, F. A review of nature-based solutions for greywater treatment: Applications, hydraulic design, and environmental benefits. Sci. Total Environ. 2020, 711, 134731. [Google Scholar] [CrossRef]
- Rieckmann, M. Education for Sustainable Development Goals: Learning Objectives; UNESCO Publishing: Paris, France, 2017; ISBN 9231002090. [Google Scholar]
- Zhang, D.; Mishra, S.; Brynjolfsson, E.; Etchemendy, J.; Ganguli, D.; Grosz, B.; Lyons, T.; Manyika, J.; Niebles, J.C.; Sellitto, M.; et al. The AI index 2021 annual report. arXiv 2021, arXiv:2103.06312. [Google Scholar]
| Parameters | Kitchen Wastewater | Bathroom Wastewater | |||||||
|---|---|---|---|---|---|---|---|---|---|
| [27] | [28] | [24] | [29] | [30,31] | [28] | [24] | |||
| Temperature (°C) | 24.4–30.9 | 25.8–29.0 | |||||||
| Total solids (mg/L) | 679–1272 | 777 | |||||||
| Total suspended solids (mg/L) | 11–3934 | 134–1300 | 58–78 | 19–793 | |||||
| Total dissolved solids (mg/L) | 312–903 | 810.6 | |||||||
| pH | 5.5 ± 0.5 | 6.5–7.7 | 5.58–10 | 5.9–7.4 | 7.3–7.8 | 8.1 | 7.1–7.6 | 5.98–8.40 | |
| BOD (mg/L) | 40.8–890 | 185–2460 | 536–1460 | 166 ± 37 | 7.2 | 129–173 | 20–673 | ||
| COD (mg/L) | 770–2050 | 110 ± 100 | 58–1340 | 411–8071 | 26–2050 | 575 ± 98 | 48 | 230–367 | 64–903 |
| Heavy Metals | Concentrations Quantified Mg/L | Source of Greywater | Country | Citations |
|---|---|---|---|---|
| Fe | 0–0.233 | Bathroom | Ghana | [105] |
| 0–0.15 | Shower | India | [106] | |
| 0–0.17 | Wash basin | India | [106] | |
| 0–0.81 | Kitchen | India | [106] | |
| 0–0.095 | Kitchen | Ghana | [105] | |
| 0–1.34 | Laundry | India | [106] | |
| 0–0.367 | Salon | Ghana | [105] | |
| 0–0.129 | Laundry | Ghana | [105] | |
| 0–0.005 | Rainwater | India | [107] | |
| Zn | 0–0.04–0.10 0–2.59 0–2.03 0–0.11 0–0.055 0–0.018 0–0.044 | Domestic Shower India Kitchen Kitchen Laundry Laundry Salon | Ghana India India India Ghana India Ghana India | [105] [106] [106] [106] [105] [106] [105] [107] |
| Pb | 0–0.087 | Bathroom | Ghana | [105] |
| N.D | Shower | India | [106] | |
| N.D | Wash basin | India | [106] | |
| 0–0.04 | Kitchen | Ghana | [106] | |
| 0–0.095 | Kitchen | Ghana | [105] | |
| <0.03 | Laundry | India | [106] | |
| 0–0.099 | Laundry | Ghana | [105] | |
| 0.064 | Salon | Ghana | [105] | |
| Cd | 0–0.001 | Bathroom | Ghana | [105] |
| 0–0.005 | Shower | India | [106] | |
| <0.001 | Wash Basin | India | [106] | |
| N.D | Kitchen | India | [106] | |
| N.D | Kitchen | Ghana | [105] | |
| N.D 0.001 | Laundry | India | [106] | |
| 0.001 | Laundry | Ghana | [105] | |
| 0.001 | Salon | Ghana | [105] | |
| Cu | 0–0.004 | Shower | India | [106] |
| <0.005 | Wash Basin | India | [106] | |
| 0–0.02 | Kitchen | India | [106] | |
| 0–0.10 | Laundry | India | [106] | |
| Cr | N.D | Shower | India | [106] |
| N.D | Wash Basin | India | ||
| 0–0.39 | Kitchen | India | ||
| <0.004 | Laundry | India |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budeli, P.; Sibali, L.L. Greywater Reuse: Contaminant Profile, Health Implications, and Sustainable Solutions. Int. J. Environ. Res. Public Health 2025, 22, 740. https://doi.org/10.3390/ijerph22050740
Budeli P, Sibali LL. Greywater Reuse: Contaminant Profile, Health Implications, and Sustainable Solutions. International Journal of Environmental Research and Public Health. 2025; 22(5):740. https://doi.org/10.3390/ijerph22050740
Chicago/Turabian StyleBudeli, Phumudzo, and Linda Lunga Sibali. 2025. "Greywater Reuse: Contaminant Profile, Health Implications, and Sustainable Solutions" International Journal of Environmental Research and Public Health 22, no. 5: 740. https://doi.org/10.3390/ijerph22050740
APA StyleBudeli, P., & Sibali, L. L. (2025). Greywater Reuse: Contaminant Profile, Health Implications, and Sustainable Solutions. International Journal of Environmental Research and Public Health, 22(5), 740. https://doi.org/10.3390/ijerph22050740






