Abstract
This review of the literature examines diseases and pathogen characteristics on military vessels, in order to improve the success of missions on a boat. Our aim is to understand the spread of disease, aiming to maximize biological resilience and hopefully eliminate outbreaks. Keyword research was conducted from various sources of information, including scientific publications, theses, public health organization websites, and clinical trials. A synthesis of bacterial, viral, fungal, and parasitosis characteristics was established, and a risk prioritization index was defined, based on contagiousness (basic reproduction number (R0)) and clinical severity. For instance, COVID-19 was assessed as moderately contagious, with critical severity, and Influenza A H1N1 as having a minor level of contagiousness with critical severity, resulting in a level two out of three risk prioritization index. This approach demonstrates that while diseases have numerous characteristics, a method for classifying them by isolating specific criteria and prioritizing them could be proposed. In conclusion, further work is needed to analyze onboard operator activities and develop simulation models related to pathogen characteristics.
1. Introduction
In the past, several outbreaks of infectious diseases have been reported on ships, emphasizing the challenges posed by enclosed, densely populated environments [1,2,3,4]. Ships, whether military or civilian, present unique conditions that can facilitate pathogen transmission due to limited space, shared facilities, and prolonged close-contact interactions. Among the epidemics identified were gastroenteritis on the cruise ships Aurora (2003) and Oasis of the Seas (2019) [5], pneumonia on the USS Boxer helicopter carrier (2007), the H1N1 influenza pandemic on the USS Theodore Roosevelt aircraft carrier (military) (2009) [6] and that on the Ocean Dream cruise ship (2009). The COVID-19 pandemic, which emerged in late 2019, further demonstrated the vulnerability of maritime settings. Large-scale outbreaks occurred on cruise ships such as the Diamond Princess and Grand Princess, and on Nile River cruises [7,8], as well as in the naval forces of several countries, including the French aircraft carrier Charles de Gaulle [9], the American aircraft carrier USS Theodore Roosevelt [10,11], and the destroyer USS Kidd [7]. The study by De Laval et al. [9] showed that a large proportion of the crew of the Charles de Gaulle was infected, highlighting the rapid spread of SARS-CoV-2 within a confined environment. Crowded conditions, shared ventilation, and frequent interactions among sailors facilitated the transmission of the virus. The majority of cases were symptomatic, but most cases were mild to moderate, with only a limited number of individuals requiring hospitalization. Nevertheless, despite attempts at isolation and social distancing on board, the virus spread before strict measures could be implemented, leading to the mission being cut short. On the USS Theodore Roosevelt, more than 1200 sailors were infected, representing approximately 25% of the crew. A portion of the crew was quarantined in Guam, and this had a major impact on the ship’s operational readiness [10]. This pandemic showed that an epidemiological crisis can have major effects on the availability of forces and their capacity to carry out missions. Indeed, in the confined environment of a ship with enclosed areas, there is inevitably close contact between crew members over an extended period of time, which may exacerbate disease spread. Consequently, ships can represent an ideal environment for the transmission of infections among crew members, and naval operations must integrate robust prevention and mitigation strategies to minimize the operational disruption caused by infectious diseases.
By detecting pathogens as early as possible, a ship’s crew can develop strong biological resilience, which will help control the impact of a potential epidemic threat. Biological resilience can be defined as “the ability to prevent, limit the effects of, and bounce back from biological disturbance linked to pathogenic elements of natural or terrorist origin. The aim is to enable military ships to recover function and operational efficiency as effectively as possible to a state equivalent to the prior disturbance” [12,13,14]. This biological resilience is based on a systemic approach to natural or terrorist-caused biological risks, establishing hypotheses to ensure the operational effectiveness of ships. This study is a scientific literature review aiming to define relevant diseases and the characteristics of associated pathogens. Indeed, there are numerous pathogens that vary in danger level, with several modes of transmission. It is necessary to identify those characteristics that favor transmission, along with detectability thresholds, clinical evaluation criteria, and acceptability thresholds.
The aim of this study was threefold: (1) to understand the intrinsic characteristics (standard values and severity thresholds) that favor the spread of pathogens, (2) to describe those diseases with a probability of occurrence in naval military settings and classify them according to a risk prioritization index that takes into account clinical severity and contagiousness, in order to target the most high-risk pathogens, and (3) to establish a roadmap for future studies to simulate pathogen propagation in naval forces. By integrating epidemiological modeling, risk assessment, and operational constraints, this research aims to enhance the resilience of military fleets and ensure their sustained operational effectiveness in the face of biological threats.
2. Literature Review
2.1. Search Strategy for the Characterization of Pathogens
The characteristics of the pathogens responsible for the various identified diseases were investigated. Pathogens can be classified into four categories: viruses, bacteria, fungi, and parasites.
In order to identify relevant studies on pathogen characteristics that influence the propagation of diseases, a keyword search was conducted across various knowledge bases, including PubMed, Google Scholar, Web of Knowledge, and ScienceDirect.
Different sources of information were used, such as scientific publications, theses, official public health organization websites, and clinical trials. The selection of articles was based on their relevance and the number of citations.
The research strategy was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines. The PRISMA methodology was chosen for this study to ensure a rigorous and reproducible research process. This methodology provides a structured approach to systematically identifying, selecting, and analyzing relevant literature, which is essential for synthesizing high-quality evidence on pathogen transmission in naval environments. The use of PRISMA offers several advantages.
- It minimizes selection bias by following a predefined inclusion and exclusion criteria framework, ensuring that only relevant and high-quality studies are considered.
- It enhances transparency by providing a detailed flow diagram of the study selection process, allowing for better reproducibility of the research.
- PRISMA improves the reliability of findings by promoting a comprehensive and structured literature review process, facilitating the identification of key trends and gaps in the existing knowledge.
The search used the following keywords: [pathogens] OR [bacteria OR viruses OR fungi OR parasitosis] AND [characteristics OR contagiousness OR spread OR transmission OR emission OR survival time]. The study selection was conducted based on several key criteria. Studies were selected within a defined publication timeframe (2010 to the present) to ensure the relevance of the findings. Only studies available in accessible languages, with a preference for English-language publications, were considered. Priority was given to studies focusing on pathogen transmission, epidemics, and biological resilience specifically in maritime contexts. Duplicate records and studies with limited applicability to the research scope were removed. Finally, to ensure scientific rigor, priority was given to peer-reviewed articles and high-quality studies. The flow chart is presented in Figure 1.
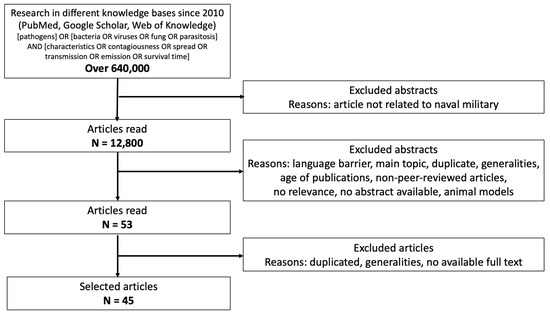
Figure 1.
Flow chart illustrating how research into the identified articles was performed, following the PRISMA guidelines.
2.2. Characterization of Pathogens
2.2.1. Pathogen Transmission
There are several modes of pathogen transmission, including airborne, direct contact, and vehicle transmission. On military ships, the greatest risk of contagiousness remains airborne transmission. Viruses are responsible for the majority of respiratory tract infections. Respiratory viruses can be transmitted via two primary modes: indirect contact (e.g., with a fomite, any object or clothing can carry infection), and air transmission (inhalation and direct deposition) [15,16]. Small droplets can quickly evaporate during the transition from the high relative humidity of the respiratory tract to the ambient air’s relative humidity. Azimi et al. (2020) [17] demonstrated that aerosol inhalation was probably the dominant contributor to COVID-19 transmission among the passengers of the Diamond Princess cruise, even under the conservative assumption of high ventilation rates and no air recirculation conditions.
2.2.2. Contagiousness
Contagiousness can predict the spread of infectious diseases. Several factors influence contagiousness, for which the basic reproduction number (R0) acts as a means of quantifying contagiousness. R0 is defined as the average number of secondary infections produced by an infected individual in a susceptible host population [18]. Although R0 may vary, depending on ship-specific environmental factors (crew density, confined spaces, and ventilation systems that affect pathogen spread), control measures (vaccination and the presence of strict protocols), and contextual variabilities (population size, pre-existing immunity, crew fatigue, and limited access to medical care), it remains a factor to consider because R0 helps to evaluate the potential spread of an infectious disease within a specific environment, such as on a military vessel. Moreover, when identifying pathogens with a high R0, it is possible to prioritize infection prevention and control measures on board. Finally, R0 is a parameter used in epidemiological models to estimate the effectiveness of containment, ventilation, or vaccination strategies in enclosed environments. When the R0 value is less than 1, each infected individual, on average, transmits the pathogen to fewer than one other individual, leading to the pathogen’s eventual disappearance from the population. Conversely, when the R0 value is greater than 1, the number of cases increases on average over time, potentially leading to an epidemic [19]. Huang et al. (2021) [20] demonstrated in their study that a higher asymptomatic ratio leads to more infectious contacts.
R0 is a central parameter in the mathematical theory of epidemics. Assuming “panmixia” (i.e., randomness in a population), where the population is considered homogeneously susceptible, the basic reproduction number is the product of three parameters: the probability (β) of transmission of the virus during contact with the risk, the number of contacts (c) at risk, and the duration (d) of the generation interval between two infections (which are often equated with the length of the contagious period) [21].
R0 = β × c × d
R0 is not constant but is instead a random variable. It is not measured from epidemic curves but instead requires a back-calculation process based on the theory of epidemics and observation of the speed of propagation, particularly the time required for the number of cases to double. In the simple model mentioned above, the formula for estimating R0 is based on the estimation of doubling time (Td) and generation interval (d) [21].
R0 = (d × ln(2) + Td)/Td
For example, in the case of an epidemic with an observed doubling duration, where Td = 3 days and the generation interval d = 6 days, the R0 is 2.4.
Diseases transmitted through the air exhibit a range of R0 values that are not represented by a single average value but instead by thresholds that depend on many factors. For instance, tuberculosis has an R0 value ranging from 0.26 to 4.3, making it less transmissible than COVID-19, which has an R0 ranging from 1.4 to 8.9. Factors influencing airborne transmission include the viral load in respiratory particles of different sizes, the stability of the virus in aerosols, and the dose–response relationship for each virus.
Through experimental approaches, it is possible to estimate the number of infectious virus particles in contaminated fluids with the infectious dose 50 (ID50) criteria, i.e., the dose of virus required to infect 50% of exposed animals in animal studies. From dose–response studies in animals, we can establish a relationship between a dose of virus particles administered in units of PFU and the AID50 value, as was modeled for SARS-CoV in mice [22].
The unit of infectious dose used in infection risk prediction models is the quantum. A quantum is defined as the dose of airborne droplet nuclei required to cause infection in 63% of susceptible persons [23]. The quantum was originally a parameter calculated from the percentage of infected subjects in scenarios or aerosol challenge tests.
The quanta emission rate of the contaminated person (quanta.h−1) is estimated by considering certain physiological parameters (e.g., exhalation rate according to the level of vocal activity, speaking, singing, shouting, or physical activity) and virus-specific parameters (e.g., viral load in the exhaled air and number of infectious viral particles). The infectious dose can also be expressed as quanta concentration per unit volume of air (quanta/m3), taking into account cumulative exposure time and the dilution volume (e.g., the volume of a room). Infection risk estimation models use two approaches to estimate the quanta emission rate.
In the first approach, it can be modeled from a retrospective analysis of outbreaks [24,25,26,27,28].
In the second approach, the quanta emission rate is modeled from the predictive estimate of the infectious particle load expelled by patient zero [23,28,29,30].
Despite numerous scientific articles on the subject, it remains difficult to draw definitive conclusions and be quantitatively accurate about the transmissibility of epidemics. Indeed, the numerical values vary according to the contexts and studies. In particular, the lack of consensus on the complexity of a pathogen’s transmission and the multiplicity of influencing parameters leaves many questions open in this field [15].
2.2.3. Environmental Conditions
Environmental conditions can also influence the spread of pathogens [31]. Enveloped viruses, such as COVID-19, are more sensitive to heat and ultraviolet light, making them less stable at high temperatures. Other viruses, such as influenza, are transmitted more efficiently at 5 °C than at 20 °C [32]. At 20 °C, the transmission efficiency of an influenza isolate exhibits a bimodal dependence on relative humidity (RH), with airborne (i.e., droplet or aerosol) transmission being maximal at 20–35% RH, poor at 50% RH, moderate at 65% RH, and absent at 80% RH [32]. Viruses also require a certain moisture level to survive and may become dehydrated and lose their ability to infect hosts in dry environments.
Bacteria can grow rapidly in hot, humid environments, such as in unrefrigerated food, but can also survive in cold, dry environments, such as stainless-steel surfaces. Temperature and humidity can also affect the ability of bacteria to produce toxins and infect hosts.
Fungi also have specific temperature and humidity requirements for their survival and growth. They can thrive in hot, humid environments, such as poorly ventilated bathrooms, but can also survive in dry, cool environments, such as cellars. Fungi can also produce spores that can be carried through the air and inhaled, causing allergies and fungal disease.
In summary, viruses, bacteria, and fungi have specific temperature and humidity requirements for their survival and ability to infect hosts. Hot, humid environments can promote the growth of bacteria and fungi, while cold, dry environments can enhance virus survival.
2.2.4. Survival Time Outside the Host
The survival time of pathogens outside the host refers to the duration during which a pathogen can survive in the environment, i.e., on surfaces, in the air, or in water, without the presence of a host. Survival time varies with the type of pathogen, temperature, humidity, and type of surface [32,33,34]. Methods used to measure pathogen survival may include cell culture, staining assays, immunofluorescence assays, and quantitative PCR. The survival time of pathogens is usually expressed in hours or in days. For instance, the SARS-CoV-2 virus, responsible for the COVID-19 pandemic, can survive on stainless-steel surfaces for up to 72 h, on cardboard for up to 24 h, and on copper for up to 3 h.
2.2.5. Emission
The last relevant characteristic is emission, which is defined as the speed at which an individual loads the air with pathogens. The emission factor is measured in quanta per hour (quanta·h−1). A quantum corresponds to the dose of aerosol sufficient to infect 63% of susceptible individuals. Emission factors depend on the symptomatic nature of the infection, physical activity, and vocalization. For SARS-CoV-2, the emission rate was assessed as varying between 1 quanta·h−1 and 100 quanta·h−1 by measuring the viral concentration in saliva [23], or between 0.37 quanta·h−1 and 32 quanta·h−1 using Monte-Carlo simulations [29]. In general, symptomatic people have a higher ability to spread pathogens through the air, mainly through coughing and sneezing. Studies have shown that coughing and sneezing can spread droplets at a speed of up to 50 m per second, while speech can spread smaller droplets at a speed of up to 10 m per second. However, it is important to note that airborne pathogen transmission also depends on the precautions taken to prevent spread, such as wearing masks, adequate ventilation of enclosed spaces, physical distancing, and hand hygiene [35,36].
2.2.6. Severity of Disease
The severity of an emerging infectious disease is generally measured by three criteria: the proportion of hospitalized cases, the proportion of hospitalized cases in intensive care, and deaths. Data on hospital admissions and intensive care are not always available in real time. The calculation of mortality rates is subject to reporting biases, both for the number of deaths and for the number of confirmed or unconfirmed cases. For example, when only symptomatic cases, or even hospitalized cases, are reported, a much higher rate is obtained than if the entire population is screened, including paucisymptomatic (only mild symptoms) or even asymptomatic cases. More reliable estimates can be obtained from more localized epidemics, which allow for an almost systematic screening of cases and the exhaustive recording of deaths. This was the case on cruise ships (the Diamond Princess being the first) or military ships (such as the Charles de Gaulle aircraft carrier) during the COVID-19 pandemic [21].
The study of excess mortality helps to overcome these potential biases. Excess mortality is a statistical concept based on comparing observed mortality rates during an epidemic with mortality rates recorded in previous years [21].
3. Methodology
A list of diseases was compiled to better understand each disease (symptoms, incubation period, contagiousness, etc.) and to provide details about the mode of contamination. The selection of specific pathogens for analysis was guided by several key criteria to ensure their relevance to naval environments. Priority was given to pathogens known for their ability to spread in confined settings such as ships (e.g., COVID-19, influenza, and tuberculosis). The selection criteria also considered pathogens that were historically involved in outbreaks aboard military and civilian vessels, in order to reflect real-world risks. Infectious agents demonstrating resilience against standard prevention measures, such as norovirus, were also included due to their high transmissibility and containment challenges. To determine the probability of occurrence, a multi-faceted approach was employed, integrating epidemiological data, studies from analogous environments (e.g., hospitals and military bases), and expert assessments from specialized medical professionals. Diseases that would not be encountered in naval military environments due to their mode of transmission were excluded from the study (e.g., Bilharzia). In addition, the French Military Health Service (SSA) vaccination schedule was also considered [37]. Naval military ships are differentiated from civilian ships by their construction and purpose. Military ships are designed to be highly efficient, reliable, and scalable, incorporating the latest technologies in restricted spaces to enable marines to protect their country’s sovereignty across the globe. The military ships currently used by France include aircraft carriers, surface combatant vessels, patrol vessels, amphibious helicopter carriers, and submarines. To fulfill their missions, these military ships may need to make stopovers in various parts of the world, making them vulnerable to epidemics, hence the importance of ensuring their resilience to such threats. The diseases included in the analysis were classified according to a risk prioritization index that considers both clinical severity and contagiousness.
Both clinical severity and contagiousness were classified into five categories (Table 1 and Table 2), based on a literature review. The relevance and application of the rating grids were then validated by professionals in the medical field.

Table 1.
Classifications for clinical severity.

Table 2.
Ratings for the contagiousness index (R0).
In our study, despite non-constant but fluctuating values, and in order not to complicate the analysis, we have chosen to use R0 as a measure of contagiousness.
By combining the contagiousness level and severity of a disease, we can classify epidemics by their level of risk. This approach is similar to the one used by the Institute for Disease Modeling at Harvard University. The risk prioritization index (RPI) was also calculated by multiplying clinical severity by contagiousness (Table 3). Since French sailors were vaccinated against a number of diseases, according to the SSA vaccination schedule [37], a coefficient of less than 1 can be applied to the RPI to account for vaccination. This coefficient is based on the assumption that sailors are vaccinated before the deployment, ensuring maximum vaccination coverage:

Table 3.
Risk prioritization index. Clinical severity: 5—catastrophic; 4—critical; 3—major; 2—moderate; 1—minor. Contagiousness: 5—extreme; 4—very high; 3—significant; 2—moderate; and 1—minor.
- A coefficient of 0.2 was applied to diseases with a vaccine effectiveness rate between 66% and 100% [38,39,40,41,42,43,44,45,46];
- A coefficient of 0.5 was applied to diseases with a vaccine effectiveness rate between 35% and 65% [47]; and
- A coefficient of 0.8 was applied to diseases with a vaccine effectiveness rate between 0 and 34%.
Risks are classified into three categories, based on the importance of the military ship’s mission:
- Category 1 (10 ≤ RPI ≤ 25): used for diseases with a significant impact on the military ship’s mission;
- Category 2 (5 ≤ RPI ≤ 9): used for diseases with a moderate impact on the military ship’s mission; and
- Category 3 (1 ≤ RPI ≤ 4): used for diseases with a low impact on the military ship’s mission.
Several agents can be used to intentionally infect people, leading to a significant number of illnesses and deaths. This act is known as bioterrorism. Bioterrorism agents can be classified into three categories:
- Category A: high-priority agents, which include organisms that pose a significant risk to national security due to several factors:
- They are easily disseminated or transmitted from person to person;
- They have high mortality rates, with the potential for a major public health impact;
- They have the ability to cause public panic and social disruption; and
- They require specific enhancements in disease surveillance.
- Category B: second-highest priority agents, which include pathogens that:
- Are moderately easy to disseminate;
- Cause moderate morbidity rates and low mortality rates; and
- Require specific enhancements in disease surveillance.
- Category C: third-highest priority agents, which include emerging pathogens (such as Nipah virus and Hantavirus) that could potentially be engineered for mass dissemination in the future, due to:
- Their availability;
- Their ease of production and dissemination; and
- Their potential for high morbidity and mortality rates, and their significant health impact.
Some infections considered to be bioterrorism threats are classified and have limited data available in the literature. As a result, only a few examples of such infections have been presented in Table 4.

Table 4.
(a): Diseases related to viruses, with their descriptions and risk prioritization calculations. Diseases that are not encountered in the military naval environment are listed, but they have not been classified according to the risk prioritization index (the boxes have been grayed out). (b): Diseases related to bacteria, with their descriptions and risk prioritization calculations. Diseases that are not encountered in the military naval environment are listed, but they have not been classified according to the risk prioritization index (the boxes have been grayed out). (c): Diseases related to parasites, with their descriptions and risk prioritization calculations. Diseases that are not encountered in the military naval environment are listed, but they have not been classified according to the risk prioritization index (the boxes have been grayed out). (d): Diseases related to fungi, with their descriptions and risk prioritization calculations. Diseases that are not encountered in the military naval environment are listed, but they have not been classified according to the risk prioritization index (the boxes have been grayed out).
4. Results
A total of fifty-eight infectious diseases were listed and described. Table 4a presents diseases related to viruses, Table 4b shows those related to bacteria, Table 4c shows those related to parasites, and finally, Table 4d shows those related to fungi.
Only diseases with a probable occurrence onboard were assessed in terms of clinical severity and contagiousness. The evaluation of contagiousness and clinical severity, which was reviewed by three medical doctors (A.G., F.L., and P.P.), including an infectious disease specialist (A.G.), remains a subjective assessment based on the scientific literature.
The highest-risk diseases in a military naval environment share several characteristics in terms of contagiousness and severity. They can be categorized into three main modes of transmission: direct contact with bodily fluids (Ebola, rabies, smallpox, and hepatitis E), fecal–oral transmission through contaminated water or food (cholera, Shiga toxin-producing bacteria, shigellosis, and hepatitis E), and airborne or droplet transmission (meningitis, pneumonic plague, tuberculosis, and smallpox). Among them, smallpox, pneumonic plague, and tuberculosis are particularly dangerous due to their airborne transmission in confined spaces, facilitating rapid contagion aboard a ship. Similarly, fecal–oral diseases such as cholera and shigellosis pose a major risk in an environment where potable water and food supplies are centralized, increasing the potential for outbreaks. Some diseases, such as Ebola and rabies, are less contagious but extremely lethal. Finally, tuberculosis and hepatitis E can develop into chronic infections, posing a persistent risk to crews. In a military naval setting, where close quarters and limited resources make it difficult to isolate infected individuals and implement rapid countermeasures, these diseases represent a significant threat to operational health and readiness.
5. Discussion
This literature review describes various diseases that can spread aboard military ships, and it emphasizes the characteristics of those pathogens that contribute to their transmission. Key among these characteristics are the reproduction number (R0) and the contagiousness, which together enable the definition of a risk prioritization index (RPI). The ultimate goal of this study is to minimize the operational impact on military boat missions by identifying those pathogens that pose the greatest risk. Based on the analysis conducted, pathogens can be classified according to four main groups: emerging diseases (e.g., COVID-19), serious diseases with low contagiousness (e.g., hepatitis), highly contagious diseases with low severity (e.g., varicella), and bioterrorism threats (e.g., smallpox).
Other similar studies on infectious diseases in confined or enclosed environments, particularly on military ships, cruise ships, and certain facilities like hospitals and detention centers, have already been conducted. These environments share common characteristics, such as restricted spaces, limited ventilation conditions, and a high number of close interactions, which facilitate the spread of pathogens. Previous research has examined infectious disease outbreaks on ships, particularly of respiratory viruses like SARS-CoV-2 and influenza, showing that airborne transmission is a major mode of spread in these contexts [48]. For example, the study by Rocklöv et al. (2020) [49] on COVID-19 aboard the Diamond Princess cruise ship demonstrates that, despite high ventilation rates and the absence of air recirculation, aerosol transmission remained dominant. This study highlights the difficulty of containing these pathogens in confined environments and underscores the limitations of traditional ventilation measures in these spaces. Our review corroborates these findings by identifying the key characteristics of pathogens that promote contagion in naval environments, such as the stability of airborne particles and the basic reproduction number (R0). Similarly, a study by Whittaker et al. (2004) [50] on viral gastroenteritis outbreaks aboard military ships showed that strict control measures, like enhanced ventilation and surface disinfection, are crucial, but are sometimes insufficient against interpersonal transmission in restricted spaces.
A crucial aspect requiring further exploration is how specific naval environmental conditions influence pathogen transmission dynamics. Unlike other enclosed settings such as hospitals or office buildings, military ships present unique challenges. For example, while ships are equipped with advanced ventilation systems, the efficiency of these systems in filtering airborne pathogens varies. Several studies [51,52] have indicated that even with high ventilation rates, aerosolized pathogens can persist and spread across compartments, necessitating additional control measures such as localized air filtration and UV-based disinfection systems. In addition, the high-density living conditions on military ships, coupled with frequent social and operational interactions, create ideal conditions for rapid disease transmission. Close-quarters sleeping arrangements, shared dining spaces, and communal hygiene facilities all contribute to the increased risk of outbreaks. Furthermore, the size of naval vessels varies depending on the type of ship, and the number of crew members on board also depends on the specific vessel. As a result, the available space per sailor in square meters will differ according to ship type. For instance, the level of proximity among crew members is much higher on a submarine than on an aircraft carrier. Submarines are designed to operate in confined spaces, meaning that sailors have very limited personal space. In contrast, aircraft carriers, being significantly larger, offer a larger volume per person, thereby reducing the risk of contamination. In addition, military ships often operate in isolated environments for extended periods, unlike land-based facilities where infected individuals can be quickly isolated or evacuated. This limited access to external medical resources requires a proactive approach, including onboard diagnostic capabilities and contingency planning for medical evacuations.
Some pathogens also present a greater challenge in naval environments due to their ability to spread through multiple transmission routes, making mitigation strategies more complex. For example, COVID-19 spreads via airborne aerosols, droplets, contaminated surfaces, and, potentially, fecal–oral transmission, requiring multiple layers of prevention. Norovirus, a common cause of viral gastroenteritis, is transmitted through direct contact, contaminated food/water, and airborne particles from vomiting, making control measures such as strict hygiene and disinfection essential. Influenza primarily spreads through aerosols but can also be transmitted via contaminated surfaces; therefore, it requires combined strategies of vaccination, ventilation, and surface cleaning. These examples highlight the complexity of disease control aboard military ships, where the confined nature of the environment amplifies the difficulty of limiting transmission.
In military contexts, several studies have focused on managing biological threats and emerging pathogens. For instance, the risk prioritization model in this study, which combines clinical severity and the reproduction potential index (RPI), is similar to methodologies used by the Harvard University Institute for Disease Modeling [53] to rank biological agents based on their spread potential in military settings. These approaches underscore the importance of prioritizing pathogens with high reproduction potential and those representing significant threats to military missions due to their rapid spread.
Airborne transmission has also been observed during outbreaks of influenza and other respiratory viruses on planes, which share similar confined, high-density environments with ships. These findings emphasize the challenges in containing pathogens in such environments, reinforcing the importance of understanding how pathogens propagate through the air in confined spaces [48]. Research conducted in hospitals also indicates that infection spread is often controlled through monitoring and rapid response, as well as measures such as isolating infected patients [54]. However, naval environments, due to their mobility and lack of equivalent medical facilities, require additional approaches, including advanced detection technologies and the integration of containment measures during ship design.
Recent research has criticized the use of the R0 index as a standard measure of contagiousness, especially in closed environments where factors such as population density, close interactions, and ventilation can influence epidemiological outcomes [55]. This study could benefit from models that incorporate additional parameters, such as crew movement analysis and quantum emissions in shared spaces. Previous work by Hsu et al. (2023) [56] shows that models based on these factors produce more accurate predictions for outbreaks in confined environments, such as cruise ships and military bases.
This approach underscores the complexity of managing disease risks on military ships and highlights several limitations. One limitation is the use of R0, which is often criticized due to the lack of consensus within the scientific community regarding its estimation [19]. The subjectivity of the evaluation of clinical severity and contagiousness, although based on scientific publications, is also a limitation of this study. Furthermore, while the criteria discussed in this study provide a foundation for simulating pathogen spread, understanding crew activities will be crucial for accurate transmission modeling. This activity analysis must be integrated as a parameter of these simulations. Indeed, several studies have shown that the majority of infections are transmitted between passengers [57]. Therefore, an analysis of crew movements—who performs which tasks, as well as where and when—will need to be integrated into these simulations.
The next steps of this work include 1) reducing the list of biological risk agents based on their common characteristics, and 2) developing methods and tools to assess, simulate, and improve the resilience of military ships [58].
Better identification and understanding of pathogens will improve detection (e.g., PCR tests and air quality measurements), prevention (e.g., an awareness of barrier gestures and isolation), and elimination (e.g., air or water decontamination). These measures can be taken into account in the design of ship architecture, with technological monitoring and cost-benefit analysis guiding decisions. Currently, several advanced solutions are being studied for implementation on military vessels, with all the integration constraints that this entails. For example, high-efficiency particulate air (HEPA) filters and ultraviolet germicidal irradiation (UVGI) are being tested to reduce the airborne transmission of pathogens. These systems can be incorporated into ventilation networks to provide continuous air purification. Among other technologies, the development of onboard biosensors capable of detecting airborne or waterborne pathogens in real time is a key area of research. These sensors use molecular detection techniques such as CRISPR-based assays or rapid PCR diagnostics. Self-disinfecting surfaces, antimicrobial coatings, and automated UV-C robots can also be used to reduce contamination risks in high-contact areas. Finally, improvements in ballast water management and onboard water purification technologies, such as ozone- and plasma-based disinfection, are being evaluated to prevent the spread of waterborne pathogens. Regarding the crew, future ship designs may include dedicated isolation spaces equipped with negative pressure systems to contain infectious diseases more effectively. Health monitoring systems could also analyze crew health data, detect early signs of disease outbreaks, and optimize response strategies through predictive modeling.
Rooms identified through simulation models and activity analysis could benefit from dedicated approaches such as air filtration, systematic surface disinfection, non-ionizing electric fields [59], or the use of far-UVC lamps to significantly reduce the risk of pathogen spread.
This study provides a foundation for further assessing the spread of pathogens aboard military ships. The results can be refined to target those pathogens that have the greatest impact on operational missions and that require appropriate countermeasures. Additional studies are needed to identify common characteristics among the pathogens of interest. These characteristics will then help to determine effective control strategies, which can be evaluated through:
- Experimental trials in a controlled environment to test the effectiveness of detection, identification, containment, and elimination solutions adapted to the constraints of the maritime environment (humidity, salinity, and vibrations); and
- Numerical simulations (particularly computational fluid dynamics) to model the spread of pathogens and assess preventive and detection measures.
This study highlights the high risk of airborne transmission and emphasizes the importance of studying this mode of transmission in future research. In addition, an analysis of sailors’ activities on board would help identify practices and times of day that promote disease transmission. However, since access to ships is restricted due to military confidentiality, the use of agent-based simulation models would be helpful.
6. Conclusions
In light of the results from similar studies, this review emphasizes that managing infectious diseases in military naval settings requires specific solutions, such as modeling crew interactions and integrating containment measures into the design of the ships themselves. The adoption of measures inspired by studies of other closed environments could also strengthen the biological resilience of military ships, helping to better anticipate and contain outbreaks. By developing early detection strategies and tailoring interventions to the characteristics of military ships, this study could also inspire more robust epidemic prevention policies, thereby minimizing operational risks.
Author Contributions
All authors listed have made substantial, direct, and intellectual contributions to the work and approved it for publication: conceptualization, L.P.-C.; methodology, L.P.-C.; analysis, L.P.-C., A.G. F.L., P.P.; validation, A.G., F.L., P.P.; writing-original draft preparation, L.P.-C.; writing-review and editing, L.P.-C., A.G., F.L., C.I., A.D., P.P.; supervision, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this article are available on PubMed, Web of Knowledge, and Google Scholar, and are referenced at the end of this article.
Acknowledgments
The authors would like to acknowledge: Baptiste Roussine, Charlie Yams, and Charlotte Roger for their assistance in analyzing disease risk, Art Mallinson (Vancouver, BC, Canada) for his helpful advice in the final read-through of the manuscript, and Clément Beghin for his contribution to the simulation aspect of this study.
Conflicts of Interest
Laetitia Peultier-Celli, Franck Letourneur, Clara Inghels, and Audrey Duclos are employed by the Naval Group company, and Philippe Perrin and Alain Gérard are employed by the University of Lorraine and the University Hospital of Nancy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fernandes, E.G.; de Souza, P.B.; de Oliveira, M.E.B.; Lima, G.D.; Pellini, A.C.G.; Ribeiro, M.C.S.; Sato, H.K.; Ribeiro, A.F.; Yu, A.L.F. Influenza B outbreak on a cruise ship off the São Paulo Coast, Brazil. J. Travel Med. 2014, 21, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, R.; Keenan, A.; Sopwith, W.; Smith, K.; Quigley, C.; Mutton, K.; Dardamissis, E.; Nichols, G.; Harris, J.; Gallimore, C.; et al. Norovirus outbreak in a cruise ship sailing around the British Isles: Investigation and multi-agency management of an international outbreak. J. Infect. 2010, 60, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xiang, X.; Xiong, Y.; Ling, H.; Shen, H.; Deng, W.; Tang, W.; Shen, T.; Li, Q. Outbreak of acute gastroenteritis caused by norovirus genogroup II attributed to contaminated cold dishes on a cruise ship in Chongqing, China, 2017. Int. J. Environ. Res. Public Health 2018, 15, 2823. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, M.E.; Cortes, J.; Hall, A.J.; Vaughan, G.; Howard, C.; Gregoricus, N.; Cramer, E.H. Disease transmission and passenger behaviors during a high morbidity norovirus outbreak on a cruise ship, January 2009. Clin. Infect. Dis. 2011, 52, 1116–1122. [Google Scholar] [CrossRef]
- Moira, P.; Mylonopoulos, D.; Terzoglou, E. Health Issues and Cruising in COVID-19 Era. Int. J. Res. Tour. Hosp. 2020, 6, 12–22. [Google Scholar]
- Brewster, R.K.; Chan, K.; Allen, H.; Sundermann, A.; Keane, S.; Boles, C. Future directions of infection control and risk management on military vessels: A narrative review. J. Public Health Emerg. 2022, 6, 33. [Google Scholar] [CrossRef]
- Kordsmeyer, A.-C.; Mojtahedzadeh, N.; Heidrich, J.; Militzer, K.; von Münster, T.; Belz, L.; Jensen, H.-J.; Bakir, S.; Henning, E.; Heuser, J.; et al. Systematic review on outbreaks of SARS-CoV-2 on cruise, navy and cargo ships. Int. J. Environ. Res. Public Health 2021, 18, 5195. [Google Scholar] [CrossRef]
- Sekizuka, T.; Itokawa, K.; Kageyama, T.; Saito, S.; Takayama, I.; Asanuma, H.; Nao, N.; Tanaka, R.; Hashino, M.; Takahashi, T.; et al. Haplotype networks of SARS-CoV-2 infections in the Diamond Princess cruise ship outbreak. Proc. Natl. Acad. Sci. USA 2020, 117, 20198–20201. [Google Scholar] [CrossRef]
- de Laval, F.; Chaudet, H.; Gorgé, O.; Marchi, J.; Lacrosse, C.; Dia, A.; Marbac, V.; Mrenda, B.M.; Texier, G.; Letois, F.; et al. Investigation of a COVID-19 outbreak on the Charles de Gaulle aircraft carrier, March to April 2020: A retrospective cohort study. Eurosurveillance 2022, 27, 2100612. [Google Scholar] [CrossRef]
- Kasper, M.R.; Geibe, J.R.; Sears, C.L.; Riegodedios, A.J.; Luse, T.; Von Thun, A.M.; McGinnis, M.B.; Olson, N.; Houskamp, D.; Fenequito, R.; et al. An outbreak of COVID-19 on an aircraft carrier. N. Engl. J. Med. 2020, 383, 2417–2426. [Google Scholar] [CrossRef]
- Malone, J.D. USS Theodore Roosevelt, COVID-19, and ships: Lessons learned. JAMA Netw. Open 2020, 3, e2022095. [Google Scholar] [CrossRef]
- Pourabdollahian, B.; Eslami, Y.; Chenouard, R.; da Cunha, C. (Chemnitz, Germany). A correlated redefinition of the concept of resilience in a production system. Advances in Production Management Systems, APMS2024. Personal communication, 2024. [Google Scholar]
- Patry, E.; Bourgeois, Q.; Bernaud, V.; de Wykerslooth, C. Résilience des Armées. DIA-3.4.1_Résilience 2022, N°23/ARM/CICDE/NP. Available online: https://www.defense.gouv.fr/sites/default/files/cicde/20220208-NP-DIA-3.4.1_RESILIENCE2022-VF.pdf (accessed on 29 March 2025).
- Bhamra, R.; Dani, S.; Burnard, K. Resilience: The concept, a literature review and future directions. Int. J. Prod. Res. 2011, 49, 5375–5393. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.; Conly, J.; Silva, C.P.; Malik, M.; Eremin, S. Infection prevention and control measures for acute respiratory infections in healthcare settings: An update. East. Mediterr. Health J. 2013, 19, S39–S47. [Google Scholar] [CrossRef]
- Azimi, P.; Keshavarz, Z.; Laurent, J.G.C.; Stephens, B.; Allen, J.G. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2015482118. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Huang, L.-S.; Li, L.; Dunn, L.; He, M. Taking account of asymptomatic infections: A modeling study of the COVID-19 outbreak on the Diamond Princess cruise ship. PLoS ONE 2021, 16, e0248273. [Google Scholar] [CrossRef]
- Flahaut, A.; Slama, R.; Spira, A. Que dit la science à propos de l’épidémiologie des maladies infectieuses émergentes ? Inserm, From science to health, 2020, 12p. Available online: https://www.inserm.fr/wp-content/uploads/2021-05/inserm-miseaupointepidemiomalinfectieuses-majmai2021.pdf (accessed on 29 March 2025).
- Watanabe, T.; Bartrand, T.A.; Weir, M.H.; Omura, T.; Haas, C.N. Development of a Dose-Response Model for SARS Coronavirus. Risk Anal. 2010, 30, 1129–1138. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef]
- Prentiss, M.; Chu, A.; Berggren, K. Superspreading events without superspreaders: Using high attack rate events to estimate Nº for airborne transmission of COVID-19. MedRxiv 2020, 28p. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef]
- Miller, S.L.; Nazaroff, W.W.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Marr, L.C.; Morawska, L.; Noakes, C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2020, 31, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Rojas, A.P.; Kropff, E.; Bahnfleth, W.; Buonanno, G.; Dancer, S.; Kurnitski, J.; Li, Y.; Loomans, M.; Marr, L.; et al. Practical indicators for Risk of Airborne Transmission in Shared Indoor Environments and their application to COVID-19 Outbreaks. Environ. Sci. Technol. 2022, 56, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Poydenot, F.; Abdourahamane, I.; Caplain, E.; Der, S.; Haiech, J.; Jallon, A.; Khoutami, I.; Loucif, A.; Marinov, E.; Andreotti, B. Risk assessment for long- and short-range airborne transmission of SARS-CoV-2, indoors and outdoors. PNAS Nexus 2022, 1, pgac223. [Google Scholar] [CrossRef]
- Buonanno, G.; Morawska, L.; Stabile, L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: Prospective and retrospective applications. Environ. Int. 2020, 145, 106112. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K. Modeling airborne pathogen transport and transmission risks of SARS-CoV-2. Appl. Math. Model. 2021, 95, 297–319. [Google Scholar] [CrossRef]
- McMichael, A.J.; Campbell-Lendrum, D.H.; Corvalan, C.F.; Ebi, K.L.; Githeko, A.K.; Scheraga, J.D.; Woodward, A. International consensus on the science of climate and health: The IPCC Third Assessment Report. Clim. Chang. Hum. Health Risks Responses 2003, 2, 103–132. [Google Scholar]
- Pica, N.; Bouvier, N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012, 2, 90–95. [Google Scholar] [CrossRef]
- Fuster-Valls, N.; Hernández-Herrero, M.; Marín-De-Mateo, M.; Rodríguez-Jerez, J.J. Effect of different environmental conditions on the bacteria survival on stainless steel surfaces. Food Control. 2008, 19, 308–314. [Google Scholar] [CrossRef]
- Hanczvikkel, A.; Tóth, Á. Quantitative study about the role of environmental conditions in the survival capability of multidrug-resistant bacteria. J. Infect. Public Health 2018, 11, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Service de Santé des Armées (SSA). Calendrier Vaccinal à L’incorporation. Available online: https://professionnels.vaccination-info-service.fr/var/vis/storage/original/application/download/20221222_NP_DCSSA-SDD-OS_annexes1_6_fiches_techniques_calendrier-vaccinal-2023%20(4).pdf (accessed on 29 March 2025).
- Boogaard, J.v.D.; de Gier, B.; Lima, P.d.O.B.; Desai, S.; de Melker, H.E.; Hahné, S.J.; Veldhuijzen, I.K. Immunogenicity, duration of protection, effectiveness and safety of rubella containing vaccines: A systematic literature review and meta-analysis. Vaccine 2001, 39, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Chit, A.; Zivaripiran, H.; Shin, T.; Lee, J.K.H.; Tomovici, A.; Macina, D.; Johnson, D.R.; Decker, M.D.; Wu, J. Acellular pertussis vaccines effectiveness over time: A systematic review, meta-analysis and modeling study. PLoS ONE 2018, 13, e0197970. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Langan, R.C.; Goodbred, A.J. Hepatitis A. Am. Fam. Physician 2021, 104, 368–374. [Google Scholar]
- Pillsbury, A.; Quinn, H. An assessment of measles vaccine effectiveness, Australia, 2006–2012. West. Pac. Surveill. Response 2015, 6, 43–50. [Google Scholar] [CrossRef]
- Deeks, S.L.; Lim, G.H.; Simpson, M.A.; Gagné, L.; Gubbay, J.; Kristjanson, E.; Fung, C.; Crowcroft, N.S. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. Can. Med. Assoc. J. 2011, 183, 1014–1020. [Google Scholar] [CrossRef]
- A Truelove, S.; Keegan, L.T.; Moss, W.J.; Chaisson, L.H.; Macher, E.; Azman, A.S.; Lessler, J. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin. Infect. Dis. 2019, 71, 89–97. [Google Scholar] [CrossRef]
- Brisson, M.; Edmunds, W.; Gay, N. Varicella vaccination: Impact of vaccine efficacy on the epidemiology of VZV. J. Med. Virol. 2003, 70, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Quiambao, B.; Peyrani, P.; Li, P.; Cutler, M.W.; Van Der Wielen, M.; Perez, J.L.; Webber, C. Efficacy and safety of a booster dose of the meningococcal A, C, W, Y-tetanus toxoid conjugate vaccine administered 10 years after primary vaccination and long-term persistence of tetanus toxoid conjugate or polysaccharide vaccine. Hum. Vaccines Immunother. 2020, 16, 1272–1279. [Google Scholar] [CrossRef]
- Milligan, R.; Paul, M.; Richardson, M.; Neuberger, A. Vaccines for preventing typhoid fever. Cochrane Database Syst. Rev. 2018, 2018, CD001261. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Xiao, S.; Lei, H. Comparative analysis of inflight transmission of SARS-CoV-2, influenza, and SARS-CoV-1. Epidemiol. Infect. 2023, 151, 1–22. [Google Scholar] [CrossRef]
- Rocklöv, J.; Sjödin, H.; Wilder-Smith, A. COVID-19 outbreak on the Diamond Princess cruise ship: Estimating the epidemic potential and effectiveness of public health countermeasures. J. Travel Med. 2020, 27, taaa030. [Google Scholar] [CrossRef]
- Whittaker, D.R.; Callan, J.E.; Campbell, J.T.; McCarten, M.D. Viral Gastroenteritis: The USS THEODORE ROOSEVELT Experience. Mil. Med. 2004, 169, 747–750. [Google Scholar] [CrossRef]
- Salmonsmith, J.; Ducci, A.; Guo, L.; Torii, R.; Balachandran, R.; Houlihan, C.; Epstein, R.; Rubin, J.; Tiwari, M.K.; Lovat, L.B. The influence of mechanical ventilation and portable air cleaners upon aerosol spread in a hospital outpatients clinic. Aerosol Sci. Technol. 2024, 1–12. [Google Scholar] [CrossRef]
- Berry, G.; Parsons, A.; Morgan, M.; Rickert, J.; Cho, H. A review of methods to reduce the probability of the airborne spread of COVID-19 in ventilation systems and enclosed spaces. Environ. Res. 2021, 203, 111765. [Google Scholar] [CrossRef] [PubMed]
- Harvard University Institute fo Disease Modeling. Available online: https://hsph.harvard.edu/research/communicable-disease-ccdd/ (accessed on 29 March 2025).
- Abbas, M.; Nunes, T.R.; Martischang, R.; Zingg, W.; Iten, A.; Pittet, D.; Harbarth, S. Nosocomial transmission and outbreaks of coronavirus disease 2019: The need to protect both patients and healthcare workers. Antimicrob. Resist. Infect. Control. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Sy, K.T.L.; White, L.F.; Nichols, B.E. Population density and basic reproductive number of COVID-19 across United States counties. PLoS ONE 2021, 16, e0249271. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chen, J.-K.; Wikramaratna, P.S.; Yen, A.M.-F.; Chen, S.L.-S.; Chen, H.-H.; Lai, C.-C. Can ship travel contain COVID-19 outbreak after re-opening: A Bayesian meta-analysis. Epidemiology Infect. 2023, 151, e99. [Google Scholar] [CrossRef]
- Xi, Z.; Meng, D.; Zhao, J. The Analysis of COVID-19 Transmission on Diamond Princess Cruise Ship. In Proceedings of the 2021 IEEE 10th Data Driven Control and Learning Systems Conference (DDCLS), Suzhou, China, 14–16 May 2021; pp. 1224–1229. [Google Scholar]
- Inghels, C.; Da Cunha, C.; Billon-Denis, E.; Duclos, A.; Beghin, C. (Strasbourg, France). Methods and tools for biological risk assessment of naval vessels. International conference CBRNE Research & Innovation. Personal communication, 2024. [Google Scholar]
- Narayan, R.; Kundu, D.; Ghatak, A.; Tripathi, S.; Datta, S. Efficient elimination of airborne pathogens: A study on aerosolized Mycobacterium tuberculosis and SARS-CoV-2 using ZeBox technology. J. Hosp. Infect. 2022, 129, 17–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).