Abstract
The efficient, stable, and selective photocatalytic conversion of nitric oxide (NO) into harmless products such as nitrate (NO3−) is greatly desired but remains an enormous challenge. In this work, a series of BiOI/SnO2 heterojunctions (denoted as X%B-S, where X% is the mass portion of BiOI compared with the mass of SnO2) were synthesized for the efficient transformation of NO into harmless NO3−. The best performance was achieved by the 30%B-S catalyst, whose NO removal efficiency was 96.3% and 47.2% higher than that of 15%B-S and 75%B-S, respectively. Moreover, 30%B-S also exhibited good stability and recyclability. This enhanced performance was mainly caused by the heterojunction structure, which facilitated charge transport and electron-hole separation. Under visible light irradiation, the electrons gathered in SnO2 transformed O2 to ·O2− and ·OH, while the holes generated in BiOI oxidized H2O to produce ·OH. The abundantly generated ·OH, ·O2−, and 1O2 species effectively converted NO to NO− and NO2−, thus promoting the oxidation of NO to NO3−. Overall, the heterojunction formation between p-type BiOI and n-type SnO2 significantly reduced the recombination of photo-induced electron-hole pairs and promoted the photocatalytic activity. This work reveals the critical role of heterojunctions during photocatalytic degradation and provides some insight into NO removal.
1. Introduction
The majority of the world’s population lives in areas where the air quality level exceeds the World Health Organization (WHO) criterion [1,2]. Although air pollution is not visible, it causes severe damage to human health and the environment [3]. Nitrogen oxides (NOx) are typical air pollutants that are not only harmful at concentrations as low as 21 ppb (40 μg m−3) [4] and are also deemed responsible for multiple atmospheric environmental issues, including photochemical smog, acid rain, and haze [5,6,7]. The efficient reduction of ambient NOx concentrations is therefore urgent in developing countries. In its 14th Five-Year Plan, the Chinese government set a target to lower NOx concentrations by over 10% in 2025 compared with 2020. About 90–95% of total NOx emissions consist of nitric oxide (NO). Therefore, developing effective NO elimination technologies would be of great significance [8].
The removal of NO via photocatalytic technology shows good promise because photocatalytic methods are environmentally friendly and cost-effective compared with conventional NO removal approaches such as selective catalytic reduction (SCR). Currently, the photocatalytic conversion of NO to harmless nitrate (NO3−) is under investigation, but this strategy is hampered by the low conversion efficiency and toxic/undesired by-product formation [9,10]. NO is inevitably transformed into nitrogen dioxide (NO2), which is more harmful than NO. Therefore, promoting the reaction selectivity to enhance the generation of NO3− as the main product is an important problem that still remains to be solved for the successful photocatalytic removal of NO [11,12].
Metal oxide semiconductors such as TiO2, ZnO, and SnO2 have received extensive attention due to their simple preparation, intensive sources, low cost, and high stability [13]. However, the application of single photocatalysts is restricted by low visible light absorption and detrimental electron-hole recombination [14,15]. The combination of materials is a valuable strategy for improving the utilization of sunlight and the separation of photogenerated charge carriers, thus boosting the photocatalytic activities [16]. It is found that the TiO2/Nb2O5 heterostructure can improve the NO photodegradation by 7.0-folds and 3.8-folds higher removal efficiency, compared with pure Nb2O5 and TiO2, respectively [17]. Moreover, the ZnO/rGO composite is confirmed to possess remarkable performance in gaseous acetaldehyde degradation, which is 1.6 times superior to that of pure ZnO [18]. SnO2 is regarded as one of the best n-type direct band gap semiconductor photocatalysts. It possesses good stability, outstanding optical properties, and excellent electronic properties [19]. The proportion of visible light (50%) in the solar spectrum is far greater than that of ultraviolet light (4%). However, SnO2 can only be excited by ultraviolet light because of its wide band gap (3.5–3.7 eV) [20,21]. Hence, broadening the spectrum of light able to be absorbed by SnO2 would help realize its practical application. Among the various modification methods, photosensitization presents an exciting strategy for efficiently promoting visible light photocatalysis. In this method, a photosensitizer that matches the band structure of the semiconductor with a wide band gap is selected [22,23]. Bismuth oxyhalides BiOX (X = Cl, Br, and I) can act as photosensitizers with narrow band gaps and superior characteristics under visible light [24]. Therefore, the light absorption spectrum of SnO2 should be broadened into the visible light range by designing heterostructured photocatalysts combined with BiOX.
In this work, p-n heterojunction photocatalysts were synthesized by grinding SnO2 and BiOI mixtures at different ratios (denoted as BiOI/SnO2) for NO removal. The facile synthesis method enabled the environmental application of photocatalysts. The photocatalytic performance of the BiOI/SnO2 catalysts was evaluated. The structure, morphology, chemical state, and optical properties of BiOI/SnO2 were characterized. In addition, the photocatalytic NO degradation mechanism was illustrated based on in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analysis. This work provides a strategy for exploiting highly efficient visible-light-driven heterojunction photocatalysts to control air pollution.
2. Materials and Methods
2.1. Materials
Tin chloride pentahydrate (SnCl4·5H2O) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium hydroxide (NaOH) and potassium iodide (KI) were obtained from Chongqing Chuandong Chemical Co., Ltd. (Chongqing, China). Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ethanol was purchased from Chengdu Chron Chemicals Co., Ltd. (Chengdu, China). NO (100 ppm) and dry air consisting of N2 and O2 with a 4:1 volume ratio were supplied by Chongqing Rising Gas (Chongqing, China). All the chemicals were of analytical grade and were used without further purification.
2.2. Synthesis of p-BiOI/n-SnO2 Heterojunction
The synthesis procedure for preparing the p-BiOI/n-SnO2 heterojunction is displayed in Figure 1. SnO2 was prepared by hydrothermal method. Briefly, 10 mmol SnCl4·5H2O and 5 mmol NaOH were separately dissolved in 50 mL deionized water and stirred for 30 min. Next, these two solutions were mixed together. The resulting mixture was stirred for another 30 min, and 50 mL of ethanol was then added to the mixed solution under stirring. This solution was transferred into a 200 mL stainless steel Telfon-lined autoclave, which was sealed, heated to 120 °C, and held at this temperature for 12 h. After cooling down to room temperature, the precipitates were collected by vacuum filtration and washed with deionized water and absolute ethanol. The obtained samples were dried at 70 °C for 12 h and then ground into SnO2 powder for further usage. BiOI was obtained through a facile precipitation method. First, 2 mmol Bi(NO3)3·5H2O and 2 mmol KI were separately dissolved in 60 mL deionized water, denoted as A solution and B solution, respectively. After dissolution was complete, solution A was added dropwise into solution B. The resulting mixture was stirred for 30 min. Precipitates were collected after 2 h of precipitation, and the precipitates were then washed three times with deionized water and absolute ethanol. The samples were dried at 60 °C for 24 h and then ground into SnO2 powder for further usage. A series of p-BiOI/n-SnO2 heterojunctions were synthesized by varying the BiOI mass to 15%, 30%, and 75% of the SnO2 mass, and these samples were denoted as X%B-S (X = 15, 30, 75). For comparison, pristine SnO2 and BiOI were also prepared.
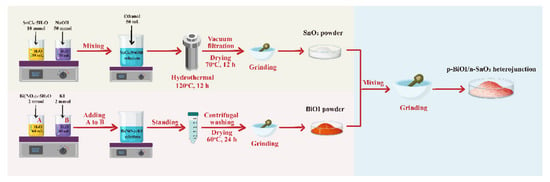
Figure 1.
Fabrication procedure of p-BiOI/n-SnO2 heterojunction.
2.3. Characterization
X-ray diffraction (XRD) patterns of the samples were measured to characterize their structure and crystallinity using a Rigaku Miniflex II diffractometer (Tokyo, Japan) with a Cu Kα radiation source (λ = 1.5406 Å) and 2θ scanning speed of 10° min−1. The elemental composition and corresponding valence states on the sample surfaces were determined by X-ray photoelectron spectroscopy (XPS) using a Kratos Axis Ultra DLD spectrometer (Manchester, UK). Field emission scanning electron microscopy (SEM, JEOL model JSM-6335 F, Tokyo, Japan) was used to analyze the morphology of the samples. UV-vis diffuse reflectance spectra (UV-vis DRS) was performed with a Shimadzu UV-1800 spectrometer (Kyoto, Japan), and photoluminescence spectra (PL) were obtained using a JASCO Spectrofluorometer FP-8200 (Tokyo, Japan) to characterize the optical properties of the samples. Electron spin resonance (ESR) signals were recorded by a JESFA200 spectrometer (Tokyo, Japan), and 5,5-Mimethyl-1-pyrroline N-oxide (DMPO), 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), and 4-oxo-2,2,6,6-tetramethylpiperidine (4-oxo-TEMP) served as spin-trap reagents to trap photogenerated hydroxyl radicals (·OH) as well as superoxide anion radicals (·O2−), electrons, and singlet oxygen (1O2), respectively.
2.4. Photocatalytic NO Oxidation
The photocatalytic activities of the synthesized catalysts for NO oxidation were evaluated by measuring their gas-phase NO removal efficiency in a continuous flow reactor with dimensions of 30 cm × 15 cm × 10 cm. In detail, 0.1 g of the catalyst was ultrasonically dispersed and uniformly coated onto two glass culture dishes with diameters of 12 cm, followed by vacuum drying at 60 °C for 30 min. NO from a compressed gas cylinder (15 mL min−1) was diluted to a concentration of 500 ppb by an air stream (2.4 L min−1), which was continuously bubbled into the reactor. After adsorption–desorption equilibrium was achieved, a 150 W commercial tungsten halogen lamp (UV cutoff filter, λ ≥ 420 nm) was turned on to initiate the photocatalytic NO oxidation. The concentrations of NO and NO2 were measured by a NOx analyzer (Thermo Scientific, 42i-TL, Waltham, MA, USA) and sampled every minute for 30 min. The removal efficiency () of NO was calculated with the equation , where and represent the concentrations of NO in the inlet and outlet, respectively. In addition, the catalyst stability was evaluated by performing NO oxidation five consecutive times following the same procedure. The photocatalyst did not undergo any treatment at the end of each cycle, and photocatalytic oxidation was initiated by visible light irradiation after adsorption–desorption equilibrium was achieved under dark conditions.
2.5. In Situ DRIFTS Analysis
In situ diffuse reflectance Fourier transform infrared spectroscopy (DRIFTS) measurements were performed in an in situ diffuse-reflectance cell (Harrick, New York, NY, USA) in a TENSOR II FT-IR spectrometer (Bruker, Ettlingen, Germany) to investigate the adsorption and reaction behavior of NO over the catalysts. Before measurements were performed, the obtained samples were pretreated for 20 min at 110 °C in a high-temperature reaction chamber to remove water, carbon dioxide, and carbohydrates from the catalyst surface. The baseline was recorded before injecting the reaction gas into the reaction chamber. The flow rate of the gas mixture (He, O2, and NO) was 100 mL min−1, and the concentration of NO was 50 ppm diluted by O2. After adsorption equilibrium was achieved, each catalyst was illuminated by visible light to initiate the photocatalytic reaction. Data were collected every 2 min for 30 min.
3. Results and Discussion
3.1. Photocatalytic Activity
The photocatalytic performance of the prepared pristine BiOI, SnO2, and BiOI/SnO2 composites was evaluated under visible-light irradiation. Figure 2a shows the variation in NO concentration (expressed as a normalized concentration) in the presence of the prepared photocatalysts. The pristine uncombined SnO2 and BiOI exhibited weak photocatalytic activity, only oxidizing 3.1% and 5.1% of the NO, respectively. X%B-S exhibited enhanced photoactivity compared to pristine SnO2 and BiOI, indicating that heterojunction formation was beneficial for improving the photocatalytic activity [25]. The NO removal efficiency of the BiOI/SnO2 composites dramatically increased in the first 6 min and reached a maximum at 6–8 min. Remarkably, the visible-light driven-driven oxidation performance of X%B-S appeared to slightly decrease after 6–8 min, possibly due to the accumulation of reaction intermediates. The generation of intermediates could block the adsorption and active sites [26]. Optimal results were obtained using 30%B-S as the photocatalyst to achieve NO removal efficiency of up to 47.1%. The removal efficiency of 30%B-S was 96.3% and 47.2% higher than that of 15%B-S and 75%B-S, respectively. This was also better than the performance of other reported p-n heterojunction photocatalysts shown in Table 1, such as 20%BiOI/ZnWO4 (32.32%) [27], Bi2O3/Bi2O2CO3 (35%) [28], Bi2O2CO3/ZnFe2O4 (35%) [16], and 4%β-Bi2O3/CeO2-δ (42.9%) [29]. In addition, the NO removal efficiency of 30%B-S did not significantly change after five consecutive cycles (Figure 2b), indicating the excellent stability and repeatability of its photocatalytic performance. The decrease in photocatalytic activity of 30%B-S (2.3%) was much lower than that of B-S-4 (15.5%) after four cycles [30]. XRD pattern further demonstrated that no obvious change occurred in the structure of 30%B-S during the cyclic test (Figure 2c). Therefore, the synthesized 30%B-S was an efficient and stable photocatalyst for long-term NO purification.
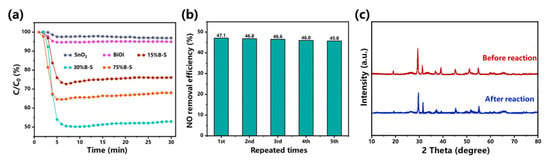
Figure 2.
(a) Photocatalytic activity of as-synthesized catalysts for NO removal, (b) stability test of 30%B-S, and (c) XRD patterns of 30%B-S before and after cyclic test.

Table 1.
NO removal performance of reported p-n heterojunction photocatalysts.
3.2. Microstructure and Chemical Composition
The crystallinity of pristine BiOI, SnO2, and the BiOI/SnO2 composites was identified by X-ray diffraction (XRD). As shown in Figure 3, all the characteristic diffraction peaks of SnO2 and BiOI were in good agreement with those pertaining to the SnO2 tetragonal phase (PDF#41-1445) and BiOI tetragonal phase (PDF#10-0445), respectively. The composites possessed all the characteristic peaks of pristine BiOI and SnO2 without any peak shift, implying that the crystal structures of the constituent monomers were not affected by the preparation of X%B-S. Moreover, with increasing BiOI content in X%B-S, the characteristic peaks pertained to BiOI became sharper and similar to those of pure BiOI, while the diffraction peaks corresponding to the (110) crystal plane of SnO2 gradually weakened. This indicated that the composite contained both BiOI and SnO2.
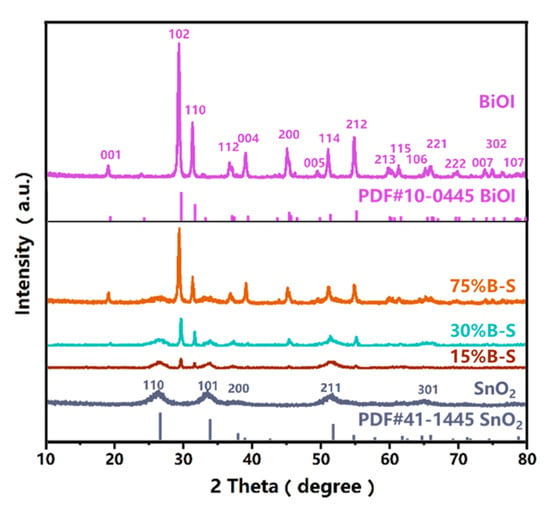
Figure 3.
XRD patterns of BiOI, SnO2, and X%B-S (where X = 15, 30, and 75).
X-ray photoelectron spectroscopy (XPS) measurements were performed to further characterize the surface elemental compositions of pristine BiOI, SnO2, and 30%B-S. Figure 4a demonstrates the coexistence of Bi, Sn, O, and I elements in 30%B-S. The high-resolution Sn 3d spectra of SnO2 and 30%B-S (Figure 4b) depict one pair of strong peaks located at 486.44 and 494.96 eV. These peaks were attributed to Sn 3d3/2 and Sn 3d5/2, respectively, suggesting that the valence state of Sn was positive tetravalent [31]. Figure 4c shows that the Bi 4f spectra had two peaks at 159.02 and 164.32 eV, which were associated with Bi 4f7/2 and Bi 4f5/2, respectively. These peaks were ascribed to the Bi3+ in neat BiOI [25]. In addition, the Bi3+ peaks slightly shifted to lower binding energies in the 30%B-S spectrum, which was possibly attributed to the electron transfer between BiOI and SnO2 [32]. A similar shift in binding energy was also observed in the I 3d spectra. As shown in Figure 4d, the I 3d peaks of BiOI at 618.94 and 630.42 eV were assigned to I 3d5/2 and I 3d3/2, respectively [33]. The O 1s spectra were resolved as three peaks at 530.42, 530.22, and 532.04 eV, which corresponded to the Sn-O bonds of SnO2 (530.38 eV) [34], Bi-O bonds of BiOI (530.31 eV) [25], and H-O bonds [35], respectively (Figure 4e). In general, the binding energies of the Bi 4f, O 1s, and I 3d peaks of 30%B-S shifted to lower values in comparison with neat BiOI. Meanwhile, the binding energies of the Sn 3d and O 1s peaks of 30%B-S shifted to higher values compared to those in neat SnO2. This demonstrated the existence of electron transfer from BiOI to SnO2 and the formation of a p-n heterojunction [36].
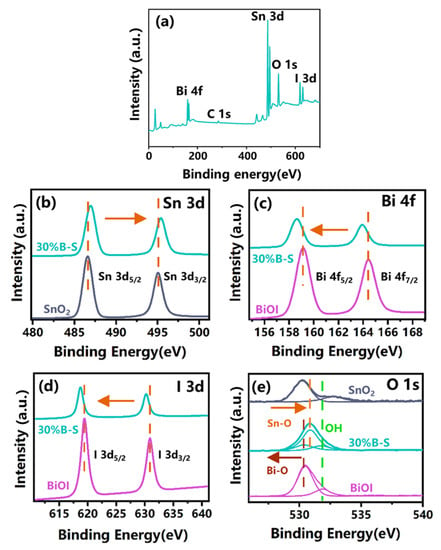
Figure 4.
XPS survey spectrum of (a) 30%B-S. High-resolution XPS spectra of (b) Sn 3d, (c) Bi 4f, (d) I 3d, and (e) O 1s for BiOI, SnO2, and 30%B-S.
The morphological features of pristine BiOI, SnO2, and 30%B-S were observed by scanning electron microscopy (SEM). Figure 5a shows that BiOI possessed thin regular nanosheets (thickness: 50–60 nm) with smooth surfaces, indicating its good crystallinity and uniformity. Figure 5b shows that SnO2 consisted of nanoparticle aggregates, with average nanoparticle sizes ranging from 20 to 30 nm. The BiOI/SnO2 composite shown in Figure 5c retained the same nanoparticle aggregate morphology.
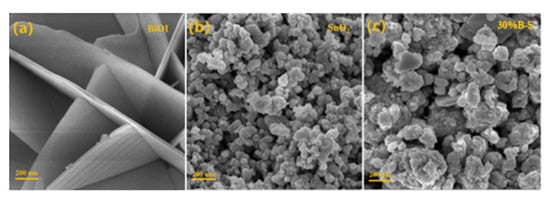
Figure 5.
SEM images of (a) BiOI, (b) SnO2, and (c) 30%B-S.
3.3. Optical and Photoelectrochemical Properties
According to UV-vis diffuse reflectance spectra (UV-vis DRS) analysis (Figure 6a), the pristine SnO2 only responded to ultraviolet light, with its absorption edge located at around 380 nm. However, the pristine BiOI responded in both the ultraviolet and visible light regions, with an absorption edge of 720 nm. Compared with neat SnO2, the optical absorption edge of X%B-S (X = 15, 30, 75) exhibited a redshift. Moreover, X%B-S (especially 30%B-S) possessed the better light harvesting performance compared with pristine BiOI and SnO2. These results can be ascribed to the formation of BiOI-SnO2 heterojunctions [37]. The optical band edges of BiOI and SnO2 were calculated using the Tauc equation , where , , , and are the absorption coefficient, Planck’s constant, light frequency, and band gap energy, respectively. is a constant, while is defined by the optical transition of the semiconductor (namely, for direct transition and for indirect transition) [38]. Typically, indirect transition occurs in BiOI and SnO2, so should equal 4 [39,40]. The band gap energy can thus be estimated by the intercept of the tangents in a plot of versus light energy (), as shown in Figure 6b. The band gap energies of BiOI and SnO2 were 1.74 and 3.15 eV, respectively. The band edge energies of the conduction band (CB) and valence band (VB) of BiOI and SnO2 were further estimated by the following empirical formulas:
where represents the energy of free electrons on the hydrogen scale (~4.5 eV), is the semiconductor band gap, and is the absolute electronegativity of the semiconductor, expressed as the geometric mean of the electronegativity of the constituent atoms. The values of for BiOI and SnO2 were calculated to be 5.99 eV and 6.25 eV [40,41], respectively. Hence, the and of BiOI were calculated to be ca. 2.36 eV and 0.62 eV, while those of SnO2 were about 3.325 eV and 0.175 eV, respectively.
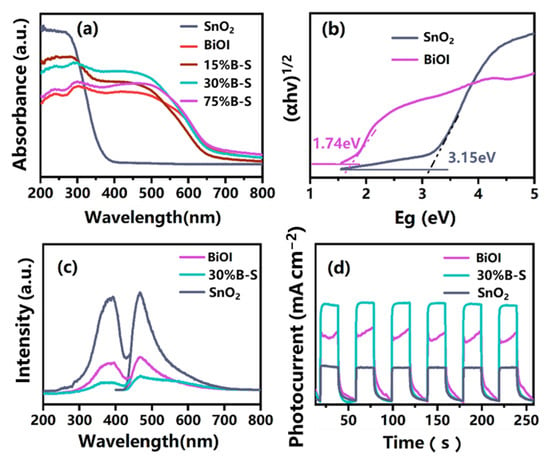
Figure 6.
(a) UV-vis diffuse reflectance spectra (UV-vis DRS) of prepared samples, (b) plot of versus light energy () for BiOI and SnO2, (c) photoluminescence spectra (PL), and (d) photocurrent density of BiOI, SnO2, and 30%B-S.
Figure 6c shows the photoluminescence spectra (PL) curves of the prepared samples, which were used to evaluate the separation and recombination efficiencies of photoexcited electron-hole pairs. The peak intensities of 30%B-S were significantly lower than those of neat BiOI and SnO2, demonstrating that BiOI doping significantly inhibited the recombination of photogenerated charge carriers. Photocurrent response tests revealed the relatively low photocurrent response of individual BiOI and SnO2 (Figure 6d). The photocurrent density of 30%B-S was enhanced by 1.5 and 0.5 times compared to neat SnO2 and BiOI, respectively, suggesting the effective separation efficiency of electron-hole pairs in the BiOI-SnO2 heterojunction [42]. This was in accordance with PL analysis.
3.4. Enhanced Photocatalytic Oxidation Mechanism
ESR analysis was used to detect the generation of active species with strong oxidant capacity, including e−, ·OH, ·O2−, and 1O2 on the catalyst surfaces [43]. Unlike neat BiOI and SnO2, the trapped e− signal of 30%B-S sharply declined after illumination (Figure 7a). This was potentially because the electrons were consumed to generate the reactive oxygen species [44]. As illustrated in Figure 7b–d, the ESR signals of the radicals were not detected after illuminating neat SnO2 with visible light. This was because the large band gap of neat SnO2 could not be adequately excited to generate charge carriers. The ·OH, 1O2, and ·O2− signal densities of 30%B-S were significantly stronger than those of pristine BiOI. In the dark, almost no distinct characteristic peaks were observed, suggesting that few or no ·OH, 1O2, and ·O2− species were produced. Notably, after illumination for 2 min, four similar characteristic peaks appeared in the 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-·OH spectrum of 30%B-S, with a peak intensity ratio of 1:2:2:1 (Figure 7b). The DMPO-·OH signals increased with increasing visible light illumination time, indicating the rapid generation of ·OH during the photocatalytic process. This was possibly because the BiOI doped in SnO2 promoted the activation of H2O molecules and the separation of spatial charges, leading to the transformation of H2O and the generation of ·OH species. Typical peaks of the DMPO superoxide adduct, such as DMPO-·O2−, and the 4-oxo-2,2,6,6-tetramethylpiperidine (4-oxo-TEMP) singlet oxygen adduct, such as 4-oxo-TEMP-1O2, were detected in both neat BiOI and 30%B-S. A strong response was achieved by 30%B-S (Figure 7c,d). Thus, the production of ·O2− and 1O2 was significantly enhanced by BiOI doping, indicating the important roles of ·O2− and 1O2 in the photocatalytic oxidation of NO.
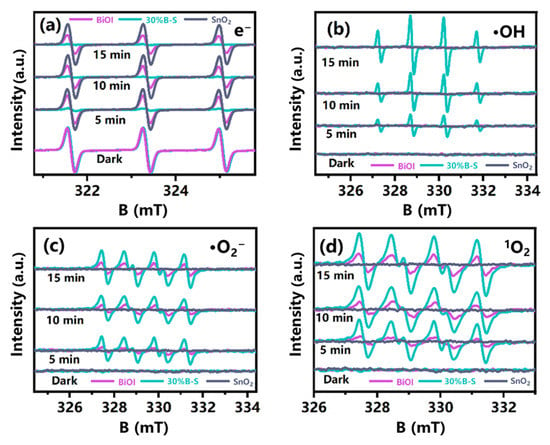
Figure 7.
Spin-trapping electron spin resonance (ESR) spectra of (a) e−, (b) ·OH, (c) ·O2−, and (d) 1O2 for the BiOI, SnO2, and 30%B-S samples in the dark and under visible light illumination.
In situ DRIFTS was used to explore the NO photocatalytic oxidation pathway of the BiOI-SnO2 heterojunction. Figure 8a,c,e show the adsorption bands of species related to NO under dark ambient conditions for BiOI, SnO2, and 30%B-S, respectively. NO can interact with the nitrogen or oxygen atoms on the SnO2 surface to produce cationic dimer, two forms of nitrosyls bound to surface tin atoms and a nitrite Sn-O-NO-species [45]. The layered structure of BiOI nanoplate can provide the sufficient surface area for NO adsorption. The combination of SnO2 and BiOI contributed to the formation of more reactive sites for the NO adsorption [46], leading to the generation of more species on 30%B-S compared with BiOI and SnO2.The peaks located at 1633 cm−1 and 844 cm−1 in Figure 8e were respectively ascribed to the adsorbed NO and NO2− generated on the surface of 30%B-S [47,48]. Moreover, the typical absorption peak of NO3− (1340 cm−1) was detected in 30%B-S [8], which was attributed to the oxidation of NO by the active radicals adsorbed on the surface of the BiOI-SnO2 heterojunction. The NO3− peak remained stable over the adsorption period, indicating that the oxidation reaction only occurred at the moment NO was absorbed. Then adsorption equilibrium was reached. The adsorbed NO preferentially attacked surface ·OH groups, generating NO− and NOH through a reaction (i.e., 3NO + OH− = NO2 + NO− + NOH). The N-O-H was detected at 1154 cm−1 [49], similar to the absorption band of NO3−. After achieving adsorption–desorption equilibrium and under light illumination, bands at 1544 cm−1 and 1359 cm−1 corresponding to NO2 were observed in the BiOI and SnO2 spectra [50,51], respectively, while the NO3− signal was undetectable (Figure 8b,d). More reactions were excited on the surface of 30%B-S under light irradiation, as indicated by appearance of increased absorption peaks (Figure 8f). This suggested that the formation of the heterojunction strengthened the visible light absorption capacity of the photocatalyst. The distinct absorption bands of NO2− and NO3− (861 cm−1 and 1252 cm−1) were observed [8,52], and the signal response of NO3− rapidly increased with increasing light irradiation time. No NO2 peak was detected, revealing that 30%B-S heterojunction can efficiently transform NO into harmless NO3− products.
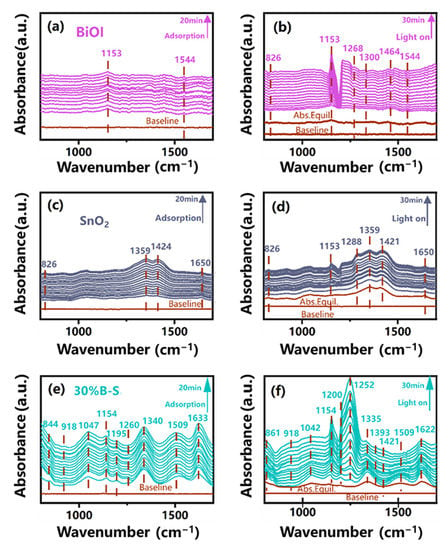
Figure 8.
In situ DRIFTS spectra of NO adsorption and visible-light reaction processes over (a,b) BiOI, (c,d) SnO2, and (e,f) 30%B-S.
Considering these experimental results and theoretical analysis, a possible improved photocatalytic mechanism of NO oxidation over the p-BiOI/n-SnO2 heterojunction was proposed, as depicted in Figure 9. BiOI is a p-type semiconductor with a Fermi level (Ef) similar to the VB, and SnO2 is a typical n-type with the Ef located near the CB. The coupling of BiOI with SnO2 enables the energy bands of BiOI to increase, while the energy bands of SnO2 decrease. Once the Ef of BiOI and SnO2 shift to the same level and reach equilibrium, a p-n heterojunction is formed. Ultimately, the CB of BiOI shifts to an energy level higher than that of SnO2, leading to the migration of photoexcited electrons from the CB of BiOI to SnO2. The driving force of this migration is the energy difference between the CB of BiOI and SnO2 under visible light irradiation. The electrons gathered on the CB of SnO2 convert O2 into ·O2− and ·OH. 1O2 is generated by the reaction of ·O2− with photogenerated holes. Moreover, the residual holes in the VB of BiOI oxidize H2O to produce ·OH. The generation of an internal electric field and construction of the p-n heterojunction changes the transmission pathway of the photo-induced charge carriers and strengthens the separation of electron-hole pairs, significantly promoting visible light photocatalytic NO oxidation.
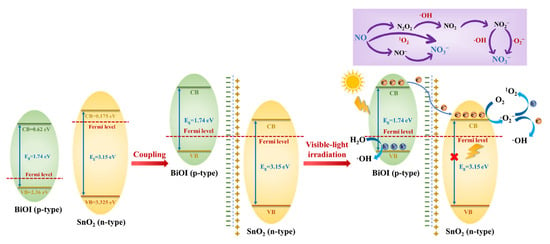
Figure 9.
The proposed photocatalytic mechanism of NO oxidation by the BiOI/SnO2 heterojunction.
4. Conclusions
A p-BiOI/n-SnO2 heterojunction with excellent activity, stability, and selectivity was successfully synthesized in this work. Among the prepared heterojunctions, the 30%B-S photocatalyst exhibited the most enhanced photoreactivity under visible light illumination, with a NO removal efficiency that was 96.3% and 47.2% greater than that of 15%B-S and 75%B-S, respectively. In addition, the NO removal efficiency of 30%B-S did not noticeably change after five cycles. The experimental results and theoretical analysis confirmed that NO was transformed into harmless NO3− as the main final product over the p-BiOI/n-SnO2 heterojunction. The heterojunction changed the transmission pathway of photogenerated carriers and promoted the separation of electron-hole pairs, resulting in remarkable photocatalytic performance for NO purification. Overall, the proposed p-BiOI/n-SnO2 heterojunction photocatalyst shows good promise for the efficient oxidation of NO to NO3−.
Author Contributions
Writing—original draft preparation, investigation, H.C.; investigation, data curation, Y.H. and Z.Y.; conceptualization, supervision, validation, J.Y.; formal analysis, Y.X. and J.Z.; writing—review and editing, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21906151) and the Scientific Research Foundation of Zhejiang University of Water Resources and Electric Power (XKY2022005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Q.W.; Xiao, R.; Lei, X.; Yu, T.; Mo, J.H. Experimental and modeling investigations on the adsorption behaviors of indoor volatile organic compounds in an in-situ thermally regenerated adsorption-board module. Build. Environ. 2021, 203, 108065. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P.; Nguyen, V.-H. Tailoring cadmium sulfide-based photocatalytic nanomaterials for water decontamination: A review. Environ. Chem. Lett. 2021, 19, 271–306. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.-H.; Thakur, S.; Nguyen, C.C.; Kim, S.Y.; Le, Q.V.; et al. An overview on recent progress in photocatalytic air purification: Metal-based and metal-free photocatalysis. Environ. Res. 2022, 214, 113995. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. Air pollution is greatest environmental threat to health. JAMA 2018, 319, 1085. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.F.; Ma, X.F.; Lu, K.D.; Jiang, M.Q.; Zou, Q.; Wang, H.C.; Zeng, L.M.; Zhang, Y.H. Direct evidence of local photochemical production driven ozone episode in Beijing: A case study. Sci. Total Environ. 2021, 800, 148868. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Zhu, L.S.; Wang, X.P.; Qiu, X.X.; Qian, W.R.; Wang, L.L. Assessing environmental impact of NOX and SO2 emissions in textiles production with chemical footprint. Sci. Total Environ. 2022, 831, 154961. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, R.L.; Chen, Y.T.; Yan, Y.; Hu, G.R.; Wang, S.S. Quantifying the source and formation of nitrate in PM2.5 using dual isotopes combined with Bayesian mixing model: A case study in an inland city of southeast China. Chemosphere 2022, 308, 136097. [Google Scholar] [CrossRef]
- Hu, L.Z.; Wang, T.; Nie, Q.Q.; Liu, J.Y.; Cui, Y.P.; Zhang, K.F.; Tan, Z.C.; Yu, H.S. Single Pd atoms anchored graphitic carbon nitride for highly selective and stable photocatalysis of nitric oxide. Carbon 2022, 200, 187–198. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.Y.; Sun, Y.J.; Wang, H.; Jiang, G.M.; Lee, S.C.; Dong, F. Enhancing ROS generation and suppressing toxic intermediate production in photocatalytic NO oxidation on O/Ba co-functionalized amorphous carbon nitride. Appl. Catal. B Environ. 2018, 237, 938–946. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, W.D.; Li, J.Y.; Jiang, G.M.; Zhou, Y.; Lee, S.C.; Dong, F. Transformation pathway and toxic intermediates inhibition of photocatalytic NO removal on designed Bi metal@defective Bi2O2SiO3. Appl. Catal. B Environ. 2019, 241, 187–195. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, J.C.; Saianand, G.; Lee, K.P.; Sonar, P.; Dharmarajan, R.; Hou, Y.L.; Ann, K.Y.; Kannan, W.; Kim, W.J. Recent progress in the abatement of hazardous pollutants using photocatalytic TiO2-based building materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Heterogeneous photocatalysis and prospects of TiO2-based photocatalytic deNOxing the atmospheric environment. Catalysts 2018, 8, 553. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.H.; Sanaye, R.; Amani, A.M. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, W.D.; Cui, W.; Li, J.Y.; Sun, Y.J.; Jiang, G.M.; Huang, H.W.; Zhang, Y.X.; Dong, F. Reactant activation and photocatalysis mechanisms on Bi-metal@Bi2GeO5 with oxygen vacancies: A combined experimental and theoretical investigation. Chem. Eng. J. 2019, 370, 1366–1375. [Google Scholar] [CrossRef]
- Liu, H.M.; Liu, M.N.; Nakamura, R.; Tachibana, Y. Primary photocatalytic water reduction and oxidation at an anatase TiO2 and Pt-TiO2 nanocrystalline electrode revealed by quantitative transient absorption studies. Appl. Catal. B Environ. 2021, 296, 120226. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, D.D.; Zhang, Q.; Zhang, Y.F.; Cao, J.J.; Shen, Z.X.; Ho, W.K.; Lee, S.C. Synthesis of a Bi2O2CO3/ZnFe2O4 heterojunction with enhanced photocatalytic activity for visible light irradiation-induced NO removal. Appl. Catal. B Environ. 2018, 234, 70–78. [Google Scholar] [CrossRef]
- Bi, X.; Du, G.H.; Kalam, A.; Sun, D.F.; Zhao, W.Q.; Yu, Y.; Su, Q.M.; Xu, B.S.; Al-Sehemi, A.G. Constructing anatase TiO2/Amorphous Nb2O5 heterostructures to enhance photocatalytic degradation of acetaminophen and nitrogen oxide. J. Colloid Interface Sci. 2021, 601, 346–354. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.Q.; Liu, J.; Pareek, V.K.; Wang, S.B. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Khan, A.; Ullah, I.; Khan, A.U.; Ahmad, B.; Katubi, K.M.; Alsaiari, N.S.; Saleem, M.; Abdullah, M.Z.; Liu, J.J. Photocatalytic degradation and electrochemical energy storage properties of CuO/SnO2 nanocomposites via the wet-chemical method. Chemosphere 2023, 313, 137482. [Google Scholar] [CrossRef]
- Li, D.G.; Huang, J.X.; Li, R.B.; Chen, P.; Chen, D.N.; Cai, M.X.; Liu, H.J.; Feng, Y.P.; Lv, W.Y.; Liu, G.G. Synthesis of a carbon dots modified g-C3N4/SnO2 Z-scheme photocatalyst with superior photocatalytic activity for PPCPs degradation under visible light irradiation. J. Hazard. Mater. 2021, 401, 123257. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yang, S.C.; Wang, Z.; Qin, H.J.; Lyu, G.J.; Chen, J.C.; Yang, G.H. High selective conversion of fructose to lactic acid by photocatalytic reforming of BiOBr/Znx@SnO2-n in alkaline condition. J. Catal. 2022, 413, 843–857. [Google Scholar] [CrossRef]
- Wang, C.L.; Guo, G.Y.; Zhu, C.J.; Li, Y.Q.; Jin, Y.B.; Zou, B.S.; He, H.; Wang, A.L. Facile synthesis, characterization, and photocatalytic evaluation of In2O3/SnO2 microsphere photocatalyst for efficient degradation of Rhodamine B. Nanomaterials 2022, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; He, Y.; Du, X.; Chu, P.K.; Zhang, Y.H. A general and facile approach to heterostructured core/shell BiVO4/BiOI p-n junction: Room-temperature in situ assembly and highly boosted visible-light photocatalysis. ACS Sustain. Chem. Eng. 2015, 3, 3262–3273. [Google Scholar] [CrossRef]
- Reddy, K.H.; Martha, S.; Parida, K.M. Fabrication of novel p-BiOI/n-ZnTiO3 heterojunction for degradation of rhodamine 6G under visible light irradiation. Inorg. Chem. 2013, 52, 6390–6401. [Google Scholar] [CrossRef]
- Liu, H.J.; Liu, C.W.; Liu, M.; Zhang, S.S.; Zhang, H.K.; Yang, L.; Zhang, S.Q. One-pot hydrothermal synthesis of SnO2/BiOBr heterojunction photocatalysts for the efficient degradation of organic pollutants under visible light. ACS Appl. Mater. Interfaces 2018, 10, 28686–28694. [Google Scholar] [CrossRef]
- Yi, J.J.; Liao, J.Z.; Xia, K.X.; Song, Y.H.; Lian, J.B.; She, X.J.; Liu, Y.X.; Yuan, S.Q.; Dong, F.; Xu, H.; et al. Integrating the merits of two-dimensional structure and heteroatom modification into semiconductor photocatalyst to boost NO removal. Chem. Eng. J. 2019, 370, 944–951. [Google Scholar] [CrossRef]
- Gong, S.W.; Zhu, G.Q.; Bello, I.A.; Rao, F.; Li, S.P.; Gao, J.Z.; Zubairu, S.M.; Peng, J.H.; Hojamberdiev, M. Construction of 1D/2D BiOI/ZnWO4 p-n heterojunction photocatalyst with enhanced photocatalytic removal of NO. J. Chem. Technol. Biotechnol. 2020, 95, 1705–1716. [Google Scholar] [CrossRef]
- Lu, Y.F.; Huang, Y.; Zhang, Y.F.; Cao, J.J.; Li, H.W.; Bian, C.; Lee, S.C. Oxygen vacancy engineering of Bi2O3/Bi2O2CO3 heterojunctions: Implications of the interfacial charge transfer, NO adsorption and removal. Appl. Catal. B Environ. 2018, 231, 357–367. [Google Scholar] [CrossRef]
- Nie, J.L.; Zhu, G.Q.; Zhang, W.B.; Gao, J.Z.; Zhong, P.; Xie, X.T.; Huang, Y.; Hojamberdiev, M. Oxygen vacancy defects-boosted deep oxidation of NO by β-Bi2O3/CeO2-δ p-n heterojunction photocatalyst in situ synthesized from Bi/Ce(CO3)(OH) precursor. Chem. Eng. J. 2021, 424, 130327. [Google Scholar] [CrossRef]
- Wu, H.Z.; Yuan, C.W.; Chen, R.M.; Wang, J.D.; Dong, F.; Li, J.Y.; Sun, Y.J. Mechanisms of interfacial charge transfer and photocatalytic NO oxidation on BiOBr/SnO2 p-n heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 43741–43749. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. A simple large-scale method for preparation of g-C3N4/SnO2 nanocomposite as visible-light-driven photocatalyst for degradation of an organic pollutant. Mater. Express 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Xu, S.M.; Jin, B.Y.; Ge, X.; Qian, X.; Xu, L.X.; Chen, F.Q.; Zhan, X.L.; Yang, Y.R.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Li, Y.P.; Shu, S.X.; Huang, L.Y.; Liu, J.W.; Liu, J.; Yao, J.; Liu, S.; Zhu, M.H.; Huang, L.J. Construction of a novel double S-scheme structure WO3/g-C3N4/BiOI: Enhanced photocatalytic performance for antibacterial activity. J. Colloid Interf. Sci. 2023, 633, 60–71. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.H.; Yang, S.L.; Wu, H.S.; Wu, Y.X.; Wu, L.D.; Pan, J.; Xiong, X. In situ construction of an SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, Y.P.; Huang, L.Y.; Yang, L.; Wang, H.; Liu, J.; Liu, J.W.; Song, Z.W.; Huang, L.J. Enhanced photocatalytic antibacterial and degradation performance by n-p type 0D/2D SnO2−x/BiOI photocatalyst under LED light. Chem. Eng. J. 2021, 411, 128505. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.Y.; Zhou, N.N.; Jiang, L.; Liu, H.C.; He, Q.R.; Deng, C.H.; Chu, D.L.; Zhang, M.; Sun, Z.Q. Construction of p-n heterostructured BiOI/TiO2 nanosheets arrays for improved photoelectrochemical water splitting performance. Appl. Surf. Sci. 2022, 601, 154277. [Google Scholar] [CrossRef]
- Long, D.; Tu, Y.P.; Chai, Y.Q.; Yuan, R. Photoelectrochemical assay based on SnO2/BiOBr p-n heterojunction for ultrasensitive DNA detection. Anal. Chem. 2021, 93, 12995–13000. [Google Scholar] [CrossRef]
- Nawaz, A.N.; Khan, A.; Ali, N.; Mao, P.; Gao, X.Y.; Ali, N.; Bilal, M.; Khan, H. Synthesis of ternary-based visible light nano-photocatalyst for decontamination of organic dyes-loaded wastewater. Chemosphere 2022, 289, 133121. [Google Scholar] [CrossRef]
- Sharma, N.; Pap, Z.; Székely, I.; Focsan, M.; Karacs, G.; Nemeth, Z.; Garg, S.; Hernadi, K. Combination of iodine-deficient BiOI phases in the presence of CNT to enhance photocatalytic activity towards phenol decomposition under visible light. Appl. Surf. Sci. 2021, 565, 150605. [Google Scholar] [CrossRef]
- Selvarajan, S.; Malathy, P.; Suganthi, A.; Rajarajan, M. Fabrication of mesoporous BaTiO3/SnO2 nanorods with highly enhanced photocatalytic degradation of organic pollutants. J. Ind. Eng. Chem. 2017, 53, 201–212. [Google Scholar] [CrossRef]
- Sun, Y.J.; Xiao, X.; Dong, X.A.; Dong, F.; Zhang, W. Heterostructured BiOI@La(OH)3 nanorods with enhanced visible light photocatalytic NO removal. Chin. J. Catal. 2017, 38, 217–226. [Google Scholar] [CrossRef]
- Yang, W.P.; Ren, Q.; Zhong, F.Y.; Wang, Y.X.; Wang, J.L.; Chen, R.M.; Li, J.Y.; Dong, F. Promotion mechanism of -OH group intercalation for NOx purification on BiOI photocatalyst. Nanoscale 2021, 13, 20601–20608. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, L.B.; Li, Y.; Shang, D.H.; Zheng, W.W.; Zhan, S.H. Unraveling reactive oxygen species formation mechanism of Fenton catalyst and its application in environmental treatment. Chin. J. Rare Met. 2022, 46, 428–437. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Bredow, T.; Pacchioni, G. NO adsorption on the stoichiometric and reduced SnO2 (110) surface. Theor. Chem. Acc. 2005, 114, 52–59. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Zeng, G.M. Fabrication of SnO2 nanopaticles/BiOI n-p heterostructure for wider spectrum visible-light photocatalytic degradation of antibiotic oxytetracycline hydrochloride. ACS Sustain. Chem. Eng. 2017, 5, 5134–5147. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.Y.; Dong, F.; Sun, Y.J.; Jiang, G.M.; Cen, W.L.; Lee, S.C.; Wu, Z.B. Highly efficient performance and conversion pathway of photocatalytic NO oxidation on SrO-clusters@amorphous carbon nitride. Environ. Sci. Technol. 2017, 51, 10682–10690. [Google Scholar] [CrossRef]
- Chen, P.; Sun, Y.J.; Liu, H.J.; Zhou, Y.; Jiang, G.M.; Lee, S.C.; Zhang, Y.X.; Dong, F. Facet-dependent photocatalytic NO conversion pathways predetermined by adsorption activation patterns. Nanoscale 2019, 11, 2366–2373. [Google Scholar] [CrossRef]
- Xin, Y.; Zhu, Q.H.; Gao, T.; Li, X.M.; Zhang, W.; Wang, H.; Ji, D.H.; Huang, Y.; Padervand, M.; Yu, F.; et al. Photocatalytic NO removal over defective Bi/BiOBr nanoflowers: The inhibition of toxic NO2 intermediate via high humidity. Appl. Catal. B Environ. 2023, 324, 122238. [Google Scholar] [CrossRef]
- Wen, X.L.; Jiang, X.L.; Jin, T.X.; Chen, H.; Zhang, X.H.; Wang, S.Y. Constructing oxygen vacancies on Bi2MoO6 nanosheets by aqueous ammonia etching with enhanced photocatalytic NO oxidation performance. Energy Fuels 2022, 36, 11485–11494. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.Y.; Shi, X.J.; Huang, T.T.; Lee, S.C.; Huang, Y.; Cao, J.J. Constructing Pd/ferroelectric Bi4Ti3O12 nanoflake interfaces for O2 activation and boosting NO photo-oxidation. Appl. Catal. B Environ. 2022, 302, 120876. [Google Scholar] [CrossRef]
- Zou, Y.Z.; Xie, Y.; Yu, S.; Chen, L.C.; Cui, W.; Dong, F.; Zhou, Y. SnO2 quantum dots anchored on g-C3N4 for enhanced visible-light photocatalytic removal of NO and toxic NO2 inhibition. Appl. Surf. Sci. 2019, 496, 143630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).