Abstract
Previous studies reported an increased risk of benign paroxysmal positional vertigo (BPPV) in patients with migraine. Hence, we aimed to assess the risk of migraine in patients with BPPV. This cohort study was conducted using the Taiwan National Health Insurance Research Database. The BPPV cohort consisted of patients aged <45 years with a diagnosis of BPPV between 2000 and 2009. An age- and sex-matched comparison group free from a history of BPPV or migraine was selected. All cases were followed up from 1 January 2000 to 31 December 2010 or until death or a diagnosis of migraine. The baseline demographic characteristics in both groups were compared using Student’s t-test and the chi-square test. Cox proportional hazards regression analysis was used to estimate the hazard ratio for migraine in the BPPV cohort compared with the comparison group after adjustment for age, sex, and comorbidities. Notably, 117 of the 1386 participants with BPPV and 146 of the 5544 participants without BPPV developed migraine. After adjustment for age, sex, and comorbidities, BPPV showed an adjusted hazard ratio indicating a 2.96-fold increased risk of migraine (95% confidence interval: 2.30–3.80, p < 0.001). We found that BPPV is associated with an increased risk of a migraine diagnosis.
1. Introduction
Benign paroxysmal positional vertigo (BPPV) is the most common cause of vertigo. A study reported that the lifetime prevalence rate of BPPV is 2.4% [1]. Idiopathic BPPV is more prevalent in older adults than in younger adults, with a peak onset between 50 and 60 years. It is also more prevalent in females than males, with a male-to-female ratio of 1:2–1:3. The typical symptom of BPPV is recurrent episodes of vertigo lasting for ≤1 min provoked by head movement. While each episode is brief, vertigo typically recurs in weeks without therapy [2] and is thought to be caused by the displacement of otoliths from otolithic organs into the semicircular canals [3,4]. However, the cause of the otolith displacement remains unknown. The Dix–Hallpike maneuver and head-roll test induce nystagmus in patients with suspected BPPV. After a clinical diagnosis of BPPV, Epley’s canalith-repositioning maneuver is used to treat patients immediately.
Migraine is the second most prevalent neurologic disorder, with a male-to-female ratio of 1:3 [5]. The 1-year prevalence rate of migraine was reported to be 9.1% in Taiwan [6]. The prevalence of migraine in males is highest in the age range of 25–29 years (8.3%), whereas it is highest in females in the age range of 30–34 years (21.1%). Most migraine diagnoses are made clinically according to the International Classification of Headache Disorders, 2nd Edition, 2004 [7]. With core symptoms and adequate follow-up time, a clinical diagnosis of migraine is primarily made in an outpatient setting. However, physicians should consider secondary headache disorder if the onset of migraine is noted after the age of 50 years [5,7]. Migraine is now a manageable disease entity with much evidence supporting preventive and abortive treatments [8]. Among the various subtypes of migraine, vestibular migraine introduced in the International Classification of Headache Disorders appendix, 3rd Edition, stands out with its presentation of vestibular symptoms lasting between 5 min and 72 h [9]. The duration of vestibular migraine symptoms is longer than that of BPPV [10,11]. Characteristic features of migrainous positional vertigo include short duration, frequent recurrence, onset in early life, migrainous symptoms during episodes of positional vertigo, and atypical positional nystagmus [11,12,13,14,15]. However, the vestibular migraine criteria are for research purposes, and better scientific evidence is warranted before vestibular migraine can be formally accepted.
As per Kayan et al., patients with migraine presented with vertigo three times more often than patients with tension-type headaches (26.5% vs. 7.8%) [16]. Dizziness and vertigo occur in 20–30% and 25–26% of patients with a primary complaint of migraine, respectively [17]. Hence, some clinical questions, such as “Do patients suffer BPPV before migraine onset?” and “If a patient had been diagnosed with BPPV, will the exposure to BPPV increase the risk of migraine in the patient?” arose. Even with multiple cross-sectional studies showing the association between migraine and BPPV, the main conundrum is that both disease entities are very frequent in the general population. However, there seems to be no cohort study investigating the incidence of migraine in adults with BPPV. Among the various types of vertigo and relatively nonspecific dizziness, we chose BPPV as the exposure in this cohort study because BPPV is the most common type of vertigo. This study aimed to investigate the risk of migraine in patients with BPPV using a retrospective cohort study design and a population-based research database.
2. Materials and Methods
2.1. National Health Insurance Research Database
The National Health Insurance Research Database (NHIRD) is a comprehensive database covering approximately 100% of the population in Taiwan. All information that would expose a person’s identity had been de-identified. The regulations from the Bureau of National Health Insurance (NHI) and the National Health Research Institute maintain the confidentiality of data. The 2000 Longitudinal Health Insurance Database (LHID) is a data subset randomly sampling 1 million people from the population between 1996 and 2010. The LHID used the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) to record disease entities. It is openly provided to researchers in Taiwan for scientific and epidemiological purposes. It includes the medical claims data for outpatient and inpatient services. The full ethical privacy of this study was approved by the Research Ethics Committee of China Medical University, and it was exempt from full review. This study used the outpatient records in the NHIRD as the data source.
2.2. Study Population
Given the background knowledge of the peak age of migraine onset being 25–35 years of age in Taiwan and the high possibility of misclassification bias in migraine diagnosis in patients >50 years, we collected data on newly diagnosed BPPV (ICD-9-CM code 386.11) cases between 2000 and 2009 from the LHID, with the baseline age being <45 years. First, patients with a previous diagnosis of migraine were excluded to avoid reverse causality. Then, we defined the index date of the BPPV case as the date of the initial BPPV diagnosis. Next, the date, month, and year of the index date of the selected case were assigned to the comparison cohort. Afterward, we adopted an individual matching method using age and sex at a ratio of 4:1 to randomly select individuals without a history of BPPV or migraine before the index date.
2.3. Baseline Characteristics
We included comorbidities before the index date as the baseline comorbidities, including hypertension (ICD-9-CM codes 401–405), diabetes mellitus (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), anxiety (ICD-9-CM codes 300.0, 300.2, 300.3, 308.3, and 309.81), and depression (ICD-9-CM codes 296.2–296.3, 300.4 and 311), to further adjust for possible confounding factors. The comorbidities were selected because hypertension, diabetes mellitus, and hyperlipidemia are known risk factors for BPPV and BPPV recurrence [18,19], and anxiety and depression are known comorbidities in migraine [20].
2.4. Outcome
The primary outcome was the occurrence of migraine, defined as the first ambulatory visit associated with an ICD-9-CM code of 346, during the follow-up period. Both cohorts were followed up until 31 December 2010, or until death or the diagnosis of migraine.
2.5. Statistical Analysis
For descriptive statistical analysis, we used the chi-squared test and Student’s t-test to compare baseline demographic characteristics between the BPPV and matched control groups. We calculated the cumulative incidence of migraine for each group by dividing the total number of migraine events by the total sum of the follow-up person-years (per 10,000 person-years). We used the Cox proportional hazards regression model to analyze the overall, age-specific, sex-specific, and each comorbidity-specific risks of developing migraine associated with BPPV. Furthermore, we calculated the adjusted hazard ratio (aHR) of migraine presenting with a 95% confidence interval (CI) for the BPPV group after adjusting for age, sex, and comorbidities.
The statistical program SAS 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. The Kaplan-Meier estimate for measuring the cumulative incidence in both groups was calculated using R software (R Foundation for Statistical Computing, Vienna, Austria). Then, we compared the differences in the cumulative incidence curves between the two groups using the log-rank test. Statistical significance was set at p < 0.05 in a two-tailed test.
3. Results
3.1. Baseline Demographic Characteristics in the Study Groups
Within the 11-year study period, 1386 patients without previous a BPPV or migraine history who had a baseline age <45 years were diagnosed with BPPV. Based on individual matching using age and sex at a 4:1 ratio, 5544 patients were included in the control group after excluding those with a history of migraine or BPPV before the index date. The distribution of age and sex was similar between the two groups. In both cohorts, the mean age was 33.2 years. In addition, both cohorts had a female predominance (68.0%).
The baseline demographic status and comorbidities between the BPPV and control groups are shown in Table 1. The mean follow-up duration was 6.23 and 6.46 years in the BPPV and control groups, respectively. The prevalence rate of all comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, anxiety, and depression, was considerably higher in the BPPV group than in the control group.

Table 1.
Baseline demographic status of patients within the BPPV and control groups.
3.2. Comparison of the Incidence of Migraine Diagnosis between the BPPV and Control Groups
Table 2 shows the comparison of migraine incidence between BPPV and matched control patients. Within the 11-year follow-up period, 117 (1.35%) and 146 (0.41%) patients had migraine in the BPPV and control groups, respectively. The overall cumulative incidence of migraine was 3.31-fold higher in the BPPV group than in the matched control group (135.4 vs. 40.8 per 10,000 person-years). After adjusting for demographic characteristics, the BPPV cohort had a 2.96-fold higher risk of migraine than the matched control cohort (aHR: 2.96, 95% CI: 2.30–3.80).

Table 2.
Incidence of migraine in the BPPV and comparison groups using multivariate Cox proportional hazards regression analysis.
As shown in Figure 1, the BPPV group had a higher cumulative incidence of migraine than the matched control group, with the log-rank test showing the difference (p < 0.001).
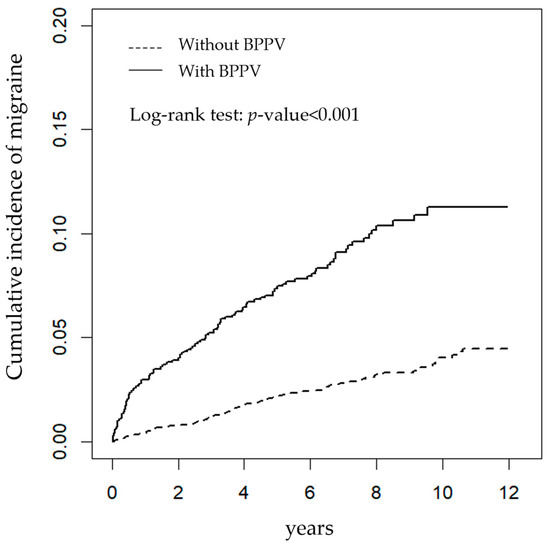
Figure 1.
The cumulative incidence of newly diagnosed migraine using Kaplan-Meier survival analysis in patients with (solid line) and without (dashed line) benign paroxysmal positional vertigo (BPPV).
3.3. Risk Factors for Migraine in Patients with BPPV
Using Cox proportional hazards regression analysis, a markedly higher hazard ratio of having a migraine in this specific BPPV sample and matched control cohorts was observed in females (aHR: 2.91; 95% CI: 2.30–3.80), those with hyperlipidemia (aHR:1.77; 95% CI: 1.16–2.70), and those with anxiety (aHR: 1.49; 95% CI: 1.03–2.14) (Table 3).

Table 3.
Incidence of migraine and multivariate Cox proportional hazards regression analysis measures of hazard ratio for the study cohort.
3.4. Subgroup Analysis of Migraine Risk
The subgroup analysis showed that an increased migraine risk was associated with age, sex, hypertension, diabetes mellitus, hyperlipidemia, anxiety, and depression (Table 4). However, sex may be associated with migraine occurrence in patients with BPPV. Male BPPV patients may have a more increased hazard ratio for developing migraine than female patients with BPPV. The migraine incidence in control males was 10.5 per 10,000 person-years, approximately one-fourth of the mean incidence (40.8 per 10,000 person-years) in the whole control group. This may amplify the increased aHR in the subgroup analysis of males compared with females.

Table 4.
Subgroup analysis of migraine in the BPPV and age- and sex-matched control cohorts.
3.5. Sensitivity Test
Further analysis of migraine risk stratified by different specialties diagnosing BPPV is presented in Table 5; this demonstrated a consistently higher migraine risk in the BPPV group stratified by different specialties making the BPPV diagnosis.

Table 5.
Migraine risk stratified by different specialties diagnosing BPPV.
4. Discussion
This study mainly demonstrated that the risk of migraine was 2.96 times higher in the BPPV cohort than in the age- and sex-matched control cohort after adjusting for age, sex, and comorbidities. The incidence of migraine diagnosis was 135.4 per 10,000 person-years in patients with BPPV, whereas it was 40.8 in the age- and sex-matched control group. Furthermore, a higher risk of migraine was associated with three factors in patients aged <45 years and with a BPPV history: female sex (aHR: 2.91), hyperlipidemia (aHR: 1.77), and anxiety (aHR: 1.49). To our knowledge, this study is the first population-based cohort study to show that BPPV is associated with an increased risk of migraine.
Our study has various clinical implications. The atypical earlier onset age of BPPV among patients in clinical settings should prompt physicians to note migraine-related history, especially in female patients, those with a history of hyperlipidemia, or those with a history of anxiety. This is because these factors were independently associated with a higher migraine risk in patients with BPPV.
We limited the inclusion baseline age of patients to <45 years for three reasons. First, it avoids misclassification bias in the diagnosis of migraine in patients >50 years [10]. Second, the age specification limits the effect of baseline comorbidities and other unmeasured confounding factors leading to unmeasured selection bias and confounding regarding the risk of migraine between the two groups. Third, based on the 11-year timeframe of the database research and the peak age of migraine prevalence in males and females being 25–29 and 30–34 years, respectively, in Taiwan [6], it is reasonable to establish an age specification of <45 years to evaluate the age range associated with the highest risk as that between 25 and 34 years for the diagnosis of migraine in the included BPPV and age- and sex-matched control groups.
The mean baseline age of the BPPV and matched control cohorts were 33.2 and 33.1 years, respectively. However, this is not the typical prevalence age of patients with BPPV. Therefore, the number of patients in our BPPV group was relatively low. However, the mean baseline age is in the age range (25–34 years) of migraine peak prevalence in Taiwan. Furthermore, the male: female ratio was approximately 1:2, which is relatively consistent with the general epidemiological sex ratio of patients with BPPV. Comorbidities such as hypertension, diabetes mellitus, hyperlipidemia, anxiety, and depression were notably more common in the BPPV group than in the control group. While other methods, such as propensity score matching, may adjust the possible baseline demographic differences at the beginning of sample selection to reduce the selection bias and impact of confounding factors, the representative control group will also be changed from an age- and sex-matched general population sample to a more comorbidity-bound population sample. In this study, we aimed to compare the risk of migraine between patients with BPPV and a more general population to increase the external validity of the result.
Because our study showed a lower prevalence rate of migraine than the epidemiological study, we changed our paper title from “migraine” to “migraine diagnosis.” A previous study in Taiwan showed a 1-year migraine prevalence rate of 9.1% [6]. In our study, the incidence of migraine was 135.4 per 10,000 person-years in the BPPV group, whereas it was 40.8 in the age- and sex-matched control cohort. The 1-year prevalence rate of migraine in both cohorts was 1.35% and 0.41%, respectively, during the study period. This is far below the expected prevalence rate of migraine.
There are several epidemiological reports associating migraine with BPPV. However, to the best of our knowledge, there are neither cohort nor case-control studies investigating migraine risk in patients with BPPV. Nevertheless, a few cross-sectional studies have reported on the associations between migraine and BPPV. In a cross-sectional study by Lee et al., migraine is associated with BPPV with an odds ratio of 2.05 compared with the control group (p < 0.001) [21]. Another cross-sectional study showed that patients with BPPV had a three times higher historical rate of migraine than the general population and a higher family history of migraine (58.4% vs. 12.6%) and episodic vertigo (44.9% vs. 18%) in patients with BPPV than in patients with other dizziness and vertigo [22]. However, a cross-sectional study showed no significant association between migraine and BPPV in 508 patients in balance clinics [23]. Two population-based cohort studies investigated BPPV occurrence in patients with migraine. The first study, in Taiwan, showed a 2.03-fold higher risk of having BPPV in patients with migraine than in the age-, sex-, and comorbidities-matched controls (incidence rate ratio: 2.03; 95% CI: 1.41–2.97; p < 0.001) [24]. In the study with the same database as ours, no age specification was given due to the natural course of migraine peak age (25–34 years), followed by BPPV peak age (50–60 years). The second study, which had a larger sample size and a longer follow-up duration and was conducted in Korea, showed a 2.54-fold higher incidence of BPPV in migraine patients (aHR, 2.54; 95% CI: 2.41–2.68) [25]. The longer duration may more likely expose the patients in the migraine group between 50–60 years to BPPV. This may increase the likelihood of BPPV occurrence in the Korean study. Table 6 provides a general overview of the findings on the associations between BPPV and migraine in previous cohort studies.

Table 6.
Associations between BPPV and migraine in cohort studies.
Many observational surveys, including case series, have reported the association between BPPV and migraine [26,27,28,29,30,31]. The association between BPPV and migraine is not always consistent due to different study populations. Generally, these studies presented what is observed in the vertigo clinic, that patients with BPPV have a higher migraine prevalence. Patients with BPPV and a history of migraine tended to be younger with a female predominance. As presented by Faralli et al., the mean age was younger in patients with BPPV and migraine, and the recurrence rate of >4 documented episodes of BPPV was higher than in patients with BPPV without headache or migraine (39 vs. 53 years and 19.4% vs. 7.3%). No significant difference was observed in the number of maneuvers needed to achieve recovery between the two groups [27]. In headache clinics, neurologists seldom find patients with migraine with a history of BPPV [16,32]. Instead, in interviewing the patients, complaints of dizziness and vertigo existed. Therefore, observational reports suggested a higher prevalence of vertigo in patients with classical migraine than in patients with common migraine, tension-type headaches, and cluster headaches (42%, 12%, 17%, and 14%, respectively) [32]. Another study reported a higher vertigo prevalence in migraine than in tension-type headache (26.5% vs. 7.8% p < 0.001) [16]. Interestingly, there was no difference in neurotologic abnormality in patients with migraine presenting with or without vestibular symptoms [33]. As noticed in the literature, neurologists found a higher prevalence of vertigo in patients with migraine than in patients with tension-type headaches. Moreover, otolaryngologists found a higher migraine history in patients with BPPV than other dizziness and vertigo. The lack of time sequence between migraine and BPPV in cross-sectional studies and the lack of control groups in case series studies are the main limitations of the study design. A cohort study is the better observational study design in this situation. Another summary of published cross-sectional studies and case series studies investigating associations between BPPV and migraine with the exclusion of vestibular migraine is presented in Table 7.

Table 7.
Associations between BPPV and migraine in cross-sectional studies and case series studies.
The pathophysiological association between migraine and BPPV remains unclear. Three mechanisms associating migraine with BPPV have been previously proposed. First, vasospasm caused by migraine may be responsible for vertigo or cochlear symptoms. Baloh postulated that ontological symptoms in patients with migraine might occur from vasospasm or a certain iron channel disorder [34]. Ishiyama et al. proposed that repeated vasospasm can influence the microvasculature of the inner ear [35]. Repeated vasospasm may stress and damage the vestibular cells, thus resulting in the dislodgement of otoconia from the macula. The suppression of inner-ear microvasculature further leads to cochlear symptoms, such as hearing loss and vestibular symptoms. This association mechanism was supported by a previous study that showed an increased incidence rate of sudden sensorineural hearing loss in patients with migraine [36]. Second, the effect of neuropeptides, such as substance P, neurokinin A, nitrous oxide, and calcitonin gene-related peptide (CGRP), may cause sudden sensorineural hearing loss in patients with BPPV. CGRP is a neuropeptide involved in the efferent synapses of hair cell organs, such as the cochlea, semicircular canal, and lateral line [37]. Deletion of CGRP in transgenic mice was associated with reduced suprathreshold cochlear nerve activity and decreased vestibulo–ocular reflex gain. The recent success of many CGRP-related medications for migraine implies that the vestibular system plays an essential role in the pathogenesis of migraine [8]. Third, the CAMERA study demonstrated that a combination of hypoperfusion and embolism is the most likely mechanism for posterior circulation infarction in migraine [38]. The relative hypoperfusion may result in vertigo, as noted in vertebra–basilar insufficiency [38,39]. A cross-sectional study demonstrated the possible association between vertebral artery abnormality and BPPV [40].
Benign paroxysmal vertigo of childhood is one of the periodic childhood syndromes commonly regarded as a precursor of migraine, as described in the International Classification of Headache Disorders, 3rd Edition [9]. In this study, the annual conversion rate from BPPV to migraine was low (1.35%), with an 11-year accumulation incidence of 8.4%. In benign paroxysmal vertigo of childhood, the accumulation incidence rate of migraine with an average follow-up duration of 18 years and 15.7 years was 33.3% and 21%, respectively [41,42]. Hence, we were not able to infer further whether BPPV is a precursor to migraine. Rather, we postulated that a shared pathophysiological relationship might exist. This study’s result is also consistent with the previous notion that patients with acute migrainous vertigo develop central and peripheral vestibular dysfunctions [33].
This study has three major strengths. First, combined with the nationwide population-based cohort, the large sample size of one million citizens covered under the national health insurance in Taiwan and the 11-year adequate follow-up period for migraine occurrence provide enough power for differences between the two groups. Second, with the age specification of <45 years as the inclusion criterion, we tried to avoid a higher probability of migraine misclassification bias in the older population. Although some of the patients with migraine may present to the clinic after years of migraine suffering at age ≥50 years, we set the age specification not for definition purposes but rather for the need for validity. Moreover, with an age specification of <45 years in our study, the mean baseline age of both groups was 32 years. This was also good for migraine occurrence because migraine is a relatively early-onset disease entity with a peak onset age of 25–34 years in Taiwan. The 11-year follow-up period for migraine occurrence was adequate.
This study had some limitations. First, selection bias is prominent in our study, as shown in our baseline demographic state (Table 1), even with the baseline age specification of <45 years. Second, although known confounding factors, such as female sex, were controlled using a matching method, confounding is still a major source of bias in cohort studies because possible unknown confounders may exist. Third, misclassification of BPPV and migraine is still possible in this cohort study, even with the age specification to control the bias of inappropriate diagnosis of migraine in patients >50 years. However, it is worth noting that the differentiation of peripheral and central vertigo may occasionally be difficult in a clinical setting. In the case of BPPV, the difficulty may be ameliorated but not completely overcome. However, the lack of validation studies of BPPV in the database made this a serious limitation of our study. Finally, the severity, duration, and frequency of BPPV and migraine were not recorded in our database, and further exploration of their association was impossible.
5. Conclusions
Our study indicated a 2.96-fold higher risk of a migraine diagnosis in patients with BPPV than in age- and sex-matched controls in Taiwan. In addition, three other factors, including female sex, hyperlipidemia, and anxiety, were associated with an increased migraine diagnosis in this study.
Author Contributions
Conceptualization, I.-A.S., S.-J.W. and T.-C.L.; methodology, T.-C.L. and C.-Y.H.; software, C.-Y.H.; validation, I.-A.S. and S.-J.W.; formal analysis, I.-A.S.; investigation, I.-A.S.; resources, C.-Y.H.; data curation, C.-Y.H.; writing—original draft preparation, I.-A.S.; writing—review and editing, T.-C.L. and S.-J.W.; visualization, C.-Y.H.; supervision, S.-J.W. and T.-C.L.; project administration, S.-J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of China Medical University and Hospital (protocol code: CMUH104-REC2-115(CR-7), 27 May 2022).
Informed Consent Statement
Patient consent was waived as the data used in this study were deidentified and encrypted.
Data Availability Statement
The National Health Insurance Record Database is publicly available from the Taiwan National Health Institute. With academic or commercial requests, Taiwan citizens can have access to the research database.
Acknowledgments
We are grateful to the Health Data Science Center, China Medical University Hospital for providing administrative and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, J.-S.; Zee, D.S. Clinical Practice. Benign paroxysmal positional vertigo. N. Engl. J. Med. 2014, 370, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- von Brevern, M.; Bertholon, P.; Brandt, T.; Fife, T.; Imai, T.; Nuti, D.; Newman-Toker, D. Benign paroxysmal positional vertigo: Diagnostic criteria consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. Acta Otorrinolaringol. 2017, 68, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Welling, D.B.; Parnes, L.S.; O’Brien, B.; Bakaletz, L.O.; Brackmann, D.E.; Hinojosa, R. Particulate matter in the posterior semicircular canal. Laryngoscope 1997, 107, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Parnes, L.S.; McClure, J.A. Free-floating endolymph particles: A new operative finding during posterior semicircular canal occlusion. Laryngoscope 1992, 102, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Woldeamanuel, Y.W.; Cowan, R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017, 372, 307–315. [Google Scholar] [CrossRef]
- Wang, S.J.; Fuh, J.L.; Young, Y.H.; Lu, S.R.; Shia, B.C. Prevalence of migraine in Taipei, Taiwan: A population-based survey. Cephalalgia 2000, 20, 566–572. [Google Scholar] [CrossRef]
- Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd Edition. Cephalalgia 2004, 24 (Suppl. S1), 9–160. [CrossRef]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
- GBD 2016 Headache Collaborators Global, Regional, and National Burden of Migraine and Tension-Type Headache, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [CrossRef]
- Lempert, T.; Neuhauser, H. Epidemiology of vertigo, migraine and vestibular migraine. J. Neurol. 2009, 256, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Holle, D.; Naegel, S.; Diener, H.-C.; Obermann, M. Vestibular migraine. Cephalalgia 2015, 35, 262–270. [Google Scholar] [CrossRef] [PubMed]
- von Brevern, M.; Neuhauser, H. Epidemiological evidence for a link between vertigo and migraine. J. Vestib. Res. 2011, 21, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Kim, S.K.; Park, C.H.; Lee, J.H. Vestibular-evoked myogenic potentials in migrainous vertigo. Otolaryngol. Head Neck. Surg. 2011, 144, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, V.; Piazza, F.; Pisani, F.; Marciano, E. Neuro-otological features of benign paroxysmal vertigo and benign paroxysmal positioning vertigo in children: A follow-up study. Brain Dev. 2006, 28, 80–84. [Google Scholar] [CrossRef]
- Kayan, A.; Hood, J.D. Neuro-otological manifestations of migraine. Brain 1984, 107, 1123–1142. [Google Scholar] [CrossRef]
- Bayazit, Y.; Yilmaz, M.; Mumbuç, S.; Kanlikama, M. Assessment of migraine-related cochleovestibular symptoms. Rev. Laryngol. Otol. Rhinol. 2001, 122, 85–88. [Google Scholar]
- Chen, J.; Zhao, W.; Yue, X.; Zhang, P. Risk factors for the occurrence of benign paroxysmal positional vertigo: A systematic review and meta-analysis. Front. Neurol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Cui, K.; Liu, C. Risk factors for benign paroxysmal positional vertigo recurrence: A systematic review and meta-analysis. J. Neurol. 2021, 268, 4117–4127. [Google Scholar] [CrossRef]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and Systems of Care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.H.; Kwon, Y.S.; Lee, J.J.; Sohn, J.H. Risk of vestibulocochlear disorders in patients with migraine or non-migraine headache. J. Pers. Med. 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Uneri, A. Migraine and benign paroxysmal positional vertigo: An outcome study of 476 patients. Ear Nose Throat J. 2004, 83, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Teixido, M.; Baker, A.; Isildak, H. Migraine and benign paroxysmal positional vertigo: A single-institution review. J. Laryngol. Otol. 2017, 131, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Liu, C.-J.; Lin, L.-Y.; Chen, T.-J.; Wang, S.-J. Migraine is associated with an increased risk for benign paroxysmal positional vertigo: A nationwide population-based study. J. Headache Pain 2015, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hong, S.M.; Park, I.-S.; Choi, H.G. Association between migraine and benign paroxysmal positional vertigo among adults in South Korea. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Pollak, L.; Pollak, E. Headache during a cluster of benign paroxysmal positional vertigo attacks. Ann. Otol. Rhinol. Laryngol. 2014, 123, 875–880. [Google Scholar] [CrossRef]
- Faralli, M.; Cipriani, L.; Del Zompo, M.R.; Panichi, R.; Calzolaro, L.; Ricci, G. Benign paroxysmal positional vertigo and migraine: Analysis of 186 cases. B-ENT 2014, 10, 133–139. [Google Scholar]
- Yetiser, S.; Gokmen, M.H.A. Clinical aspects of benign paroxysmal positional vertigo associated with migraine. Int. Tinnitus J. 2015, 19, 64–68. [Google Scholar] [CrossRef]
- Hilton, D.B.; Luryi, A.L.; Bojrab, D.I.; Babu, S.C.; Hong, R.S.; Bojrab, D.I., 2nd; Santiago Rivera, O.J.; Schutt, C.A. Comparison of associated comorbid conditions in patients with benign paroxysmal positional vertigo with or without migraine history: A large single institution study. Am. J. Otolaryngol. 2020, 41, 102650. [Google Scholar] [CrossRef]
- Gupta, A.; Kushwaha, A.K.; Sen, K.; Bajaj, B.K. Association between benign positional vertigo and migraine in Indian population. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 420–423. [Google Scholar] [CrossRef]
- Kim, E.K.; Pasquesi, L.; Sharon, J.D. Examining Migraine as a predictor of benign paroxysmal positional vertigo onset, severity, recurrence, and associated falls. Cureus 2022, 14, e28278. [Google Scholar] [CrossRef] [PubMed]
- Kuritzky, A.; Ziegler, D.K.; Hassanein, R. Vertigo, motion sickness and migraine. Headache 1981, 21, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Casani, A.P.; Sellari-Franceschini, S.; Napolitano, A.; Muscatello, L.; Dallan, I. Otoneurologic dysfunctions in migraine patients with or without vertigo. Otol. Neurotol. 2009, 30, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.W. Neurotology of migraine. Headache 1997, 37, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, A.; Jacobson, K.M.; Baloh, R.W. Migraine and benign positional vertigo. Ann. Otol. Rhinol. Laryngol. 2000, 109, 377–380. [Google Scholar] [CrossRef] [PubMed]
- El-Saied, S.; Joshua, B.-Z.; Segal, N.; Kraus, M.; Kaplan, D.M. Sudden hearing loss with simultaneous posterior semicircular canal BPPV: Possible etiology and clinical implications. Am. J. Otolaryngol. 2014, 35, 180–185. [Google Scholar] [CrossRef]
- Jones, S.M.; Vijayakumar, S.; Dow, S.A.; Holt, J.C.; Jordan, P.M.; Luebke, A.E. Loss of α-calcitonin gene-related peptide (ACGRP) reduces otolith activation timing dynamics and impairs balance. Front. Mol. Neurosci. 2018, 11, 289. [Google Scholar] [CrossRef]
- Kruit, M.C.; Launer, L.J.; Ferrari, M.D.; van Buchem, M.A. Infarcts in the posterior circulation territory in migraine. the population-based MRI CAMERA study. Brain 2005, 128, 2068–2077. [Google Scholar] [CrossRef]
- Bruzzone, M.G.; Grisoli, M.; De Simone, T.; Regna-Gladin, C. Neuroradiological features of vertigo. Neurol. Sci. 2004, 25 (Suppl. S1), S20–S23. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Zhang, H.; Xu, Y.; Fu, S.; Yu, M.; Ji, P. Evaluation of vertebrobasilar artery changes in patients with benign paroxysmal positional vertigo. Neuroreport 2013, 24, 741–745. [Google Scholar] [CrossRef]
- Lindskog, U.; Odkvist, L.; Noaksson, L.; Wallquist, J. Benign paroxysmal vertigo in childhood: A long-term follow-up. Headache 1999, 39, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Batuecas-Caletrío, A.; Martín-Sánchez, V.; Cordero-Civantos, C.; Guardado-Sánchez, L.; Marcos, M.R.; Fabián, A.H.; Benito González, J.J.; Santa Cruz-Ruiz, S. Is benign paroxysmal vertigo of childhood a migraine precursor? Eur. J. Paediatr. Neurol. 2013, 17, 397–400. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).