Chronic Exposure to Normobaric Hypoxia Increases Testosterone Levels and Testosterone/Cortisol Ratio in Cyclists
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Experimental Design and Training Program
2.3. Measurements during the Experiment
2.4. Statistical Analysis
3. Results
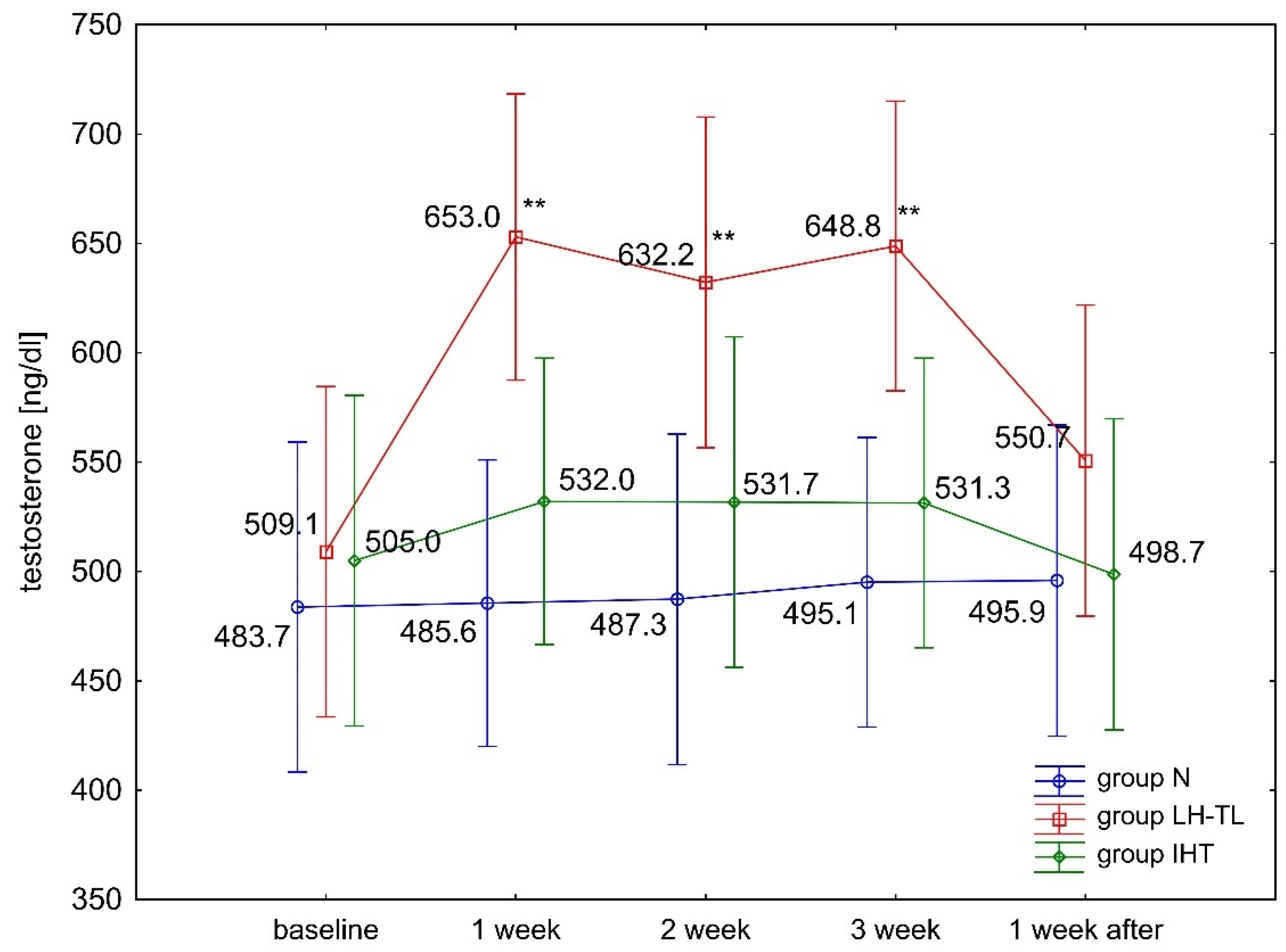
3.1. Changes in Testosterone and Cortisol Levels
3.2. Changes in Training Load and Biochemical Variables
3.3. Changes in Selected Hematological Variables in LH–TL Group
4. Discussion
4.1. Testosterone
4.2. Cortisol
4.3. T/C Ratio
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995, 20, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in sports and exercise: Tracking health, performance, and recovery in athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Viru, A.; Viru, M. Cortisol-essential adaptation hormone in exercise. Int. J. Sports Med. 2004, 25, 461–464. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Harkonen, M.; Kuoppasalmi, K.; Huhtaniemmi, I.; Tikkanen, H.; Remes, K.; Dessypris, A.; Karvonen, J. Effect of training on plasma anabolic and catabolic steroid hormones and their response during physical exercise. Int. J. Sports Med. 1986, 7, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Gastmann, U.; Petersen, K.G.; Bachl, N.; Seidel, A.; Khalaf, A.N.; Fischer, S.; Keul, J. Training-overtraining: Performance, and hormone levels, after a defined increase in training volume versus intensity in experienced middle- and long-distance runners. Br. J. Sports Med. 1992, 26, 233–242. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J. Resistance Exercise Overtraining and Overreaching. Neuroendocrine Responses. Sports Med. 1997, 23, 106–129. [Google Scholar] [CrossRef]
- Chicharro, J.L.; Lucia, A.; Perez, M.; Vaquero, A.F.; Urena, R. Saliva composition and exercise. Sports Med. 1998, 26, 17–27. [Google Scholar] [CrossRef]
- Crewther, B.T.; Cook, C.; Cardinale, M.; Weatherby, R.P.; Lowe, T. Two emerging concepts for elite athletes. The short-term effects of testosterone and cortisol on the neuromuscular system and the dose-response training role of these endogenous hormones. Sports Med. 2011, 41, 103. [Google Scholar] [CrossRef]
- Flynn, M.; Pizza, F.; Brolinson, P. Hormonal Responses to Excessive Training: Influence of Cross Training. In. J. Sports Med. 1997, 18, 191–196. [Google Scholar] [CrossRef]
- Filaire, E.; Legrand, B.; Lac, G.; Pequignot, J.M. Training of elite cyclists: Effects on mood state and selected hormonal responses. J. Sports Sci. 2004, 22, 1025–1033. [Google Scholar] [CrossRef]
- Vervoorn, C.; Vermulst, L.J.; Boelens-Quist, A.M.; Koppeschaar, H.P.; Erich, W.B.; Thijssen, J.H.; de Vries, W.R. Seasonal changes in performance and free testosterone: Cortisol ratio of elite female rowers. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 14–21. [Google Scholar] [CrossRef]
- Hoogeveen, A.R.; Zonderland, M.L. Relationships between testosterone, cortisol and performance in professional cyclists. Int. J. Sports Med. 1996, 17, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Lucía, A.; Chicharro, J.L.; Pérez, M.; Serratosa, L.; Bandrés, F.; Legido, J.C. Reproductive function in male endurance athletes: Sperm analysis and hormonal profile. J. Appl. Physiol. 1996, 81, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Slivka, D.R.; Hailes, W.S.; Cuddy, J.S.; Ruby, B.C. Effects of 21 days of intensified training on markers of overtraining. J. Strength Cond. Res. 2010, 24, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Sivaprakasam, S. Effects of training load on some hormonal, hematological and biochemical profile of male cyclists. Ann. Appl. Sport Sci. 2014, 2, 1–12. [Google Scholar] [CrossRef][Green Version]
- Ndon, J.A.; Snyder, A.C.; Foster, C.; Wehrenberg, W.B. Effects of chronic intense exercise training on the leukocyte response to acute exercise. Int. J. Sports Med. 1992, 13, 176–182. [Google Scholar] [CrossRef]
- Chapman, R.F.; Stray-Gundersen, J.; Levine, B.D. Individual variation in response to altitude training. J. Appl. Physiol. 1998, 85, 1448–1456. [Google Scholar] [CrossRef]
- Rusko, H.; Tikkanen, H.; Hamalainen, I.; Kalliokoski, K.; Puranen, A. Effect of living in hypoxia and training in normoxia on sea level VO2max and red cell mass. Med. Sci. Sports Exerc. 1999, 31, 86. [Google Scholar] [CrossRef]
- Faiss, R.; Willis, S.; Born, D.P.; Sperlich, B.; Vesin, J.M.; Holmberg, H.C.; Millet, G.P. Repeated double-poling sprint training in hypoxia by competitive cross-country skiers. Med. Sci. Sports Exerc. 2015, 47, 809–817. [Google Scholar] [CrossRef]
- Brocherie, F.; Millet, G.P.; Hauser, A.; Steiner, T.; Rysman, J.; Wehrlin, J.P.; Girard, O. “Live high-train low and high” hypoxic training improves team-sport performance. Med. Sci. Sports Exerc. 2015, 47, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Wilk, R.; Karpiński, J.; Chalimoniuk, M.; Zajac, A.; Langfort, J. Intermittent hypoxic training improves anaerobic performance in competitive swimmers when implemented into a direct competition mesocycle. PLoS ONE 2017, 12, e0180380. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Fidos-Czuba, O.; Płoszczyca, K.; Zając, A.; Langfort, J. Comparison of the effect of intermittent hypoxic training vs. the live high, train low strategy on aerobic capacity and sports performance in cyclists in normoxia. Biol. Sport 2018, 35, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Bril, G.; Płoszczyca, K.; Piotrowicz, Z.; Chalimoniuk, M.; Roczniok, R.; Zembroń-Łacny, A.; Gerasimuk, D.; Langfort, J. Intermittent hypoxic training at lactate threshold intensity improves aiming performance in well-trained biathletes with little change of cardiovascular variables. BioMed Res. Int. 2019, 25, 1287506. [Google Scholar] [CrossRef] [PubMed]
- Savourey, G.; Launay, J.C.; Besnard, Y.; Guinet, A.; Travers, S. Normo- and hypobaric hypoxia: Are there any physiological differences? Eur. J. Appl. Physiol. 2003, 89, 122–126. [Google Scholar] [CrossRef]
- Płoszczyca, K.; Langfort, J.; Czuba, M. The effects of altitude training on erythropoietic response and hematological variables in adult athletes: A narrative review. Front. Physiol. 2018, 9, 375. [Google Scholar] [CrossRef]
- Strüder, H.K.; Hollmann, W.; Donike, M.; Platen, P.; Weber, K. Effect of O2 availability on neuroendocrine variables at rest and during exercise: O2 breathing increases plasma prolactin. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 443–449. [Google Scholar] [CrossRef]
- Svendsen, I.S.; Hem, E.; Gleeson, M. Effect of acute exercise and hypoxia on markers of systemic and mucosal immunity. Eur. J. Appl. Physiol. 2016, 116, 1219–1229. [Google Scholar] [CrossRef]
- Woods, D.R.; O’Hara, J.P.; Boos, C.J.; Hodkinson, P.D.; Tsakirides, C.; Hill, N.E.; Jose, D.; Hawkins, A.; Phillipson, K.; Hazlerigg, A.; et al. Markers of physiological stress during exercise under conditions of normoxia, normobaric hypoxia, hypobaric hypoxia, and genuine high altitude. Eur. J. Appl. Physiol. 2017, 117, 893–900. [Google Scholar] [CrossRef]
- Richalet, J.P.; Rutgers, V.; Bouchet, P.; Rymer, J.C.; Kéromès, A.; Duval-Arnould, G.; Rathat, C. Diurnal variations of acute mountain sickness, colour vision, and plasma cortisol and ACTH at high altitude. Aviat. Space Environ. Med. 1989, 60, 105–111. [Google Scholar]
- Basu, M.; Pal, K.; Prasad, R.; Malhotra, A.S.; Rao, K.S.; Sawhney, R.C. Pituitary, gonadal and adrenal hormones after prolonged residence at extreme altitude in man. Int. J. Androl. 1997, 20, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Benso, A.; Broglio, F.; Aimaretti, G.; Lucatello, B.; Lanfranco, F.; Ghigo, E.; Grottoli, S. Endocrine and metabolic responses to extreme altitude and physical exercise in climbers. Eur. J. Endocrinol. 2007, 157, 733–740. [Google Scholar] [CrossRef]
- Ermolao, A.; Travain, G.; Facco, M.; Zilli, C.; Agostini, C.; Zaccaria, M. Relationship between stress hormones and immune response during high-altitude exposure in women. J. Endocrinol. Investig. 2009, 32, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.; Pousette, A.; Carlstrom, K.; Askenberger, M.; Johansson, C. Anabolic and catabolic hormonal response of elite runners to training at high altitude. Scand. J. Med. Sci. Sports 1992, 2, 10–15. [Google Scholar] [CrossRef]
- Banfi, G.; Marinelli, M.; Roi, G.S.; Agape, V. Usefulness of free testosterone/cortisol ratio during a season of elite speed skating athletes. Int. J. Sports Med. 1993, 14, 373–379. [Google Scholar] [CrossRef]
- Vasankari, T.J.; Rusko, H.; Kujala, U.M.; Huhtaniemi, I.T. The effect of ski training at altitude and racing on pituitary, adrenal and testicular function in men. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 221–225. [Google Scholar] [CrossRef]
- Uchakin, P.; Gotovtseva, E.; Levine, B.D.; Stray-Gundersen, J. Neuroimmune Humoral changes associated with altitude training. Med. Sci. Sports Exerc. 1995, 27, 174. [Google Scholar] [CrossRef]
- Rusko, H.; Kirvesniemi, H.; Paavonlainen, L.; Vähäsöyrinki, P.; Kyrö, K.P. Effect of altitude training on sea level aerobic and anaerobic power in elite athletes. Med. Sci. Sports Exerc. 1996, 28, 124. [Google Scholar] [CrossRef]
- Gore, C.J.; Hahn, A.G.; Rice, A.; Bourdon, P.; Lawrence, S.; Walsh, C.; Stanef, T.; Barnes, P.; Parisotto, R.; Martin, D.; et al. Altitude training at 2690 m does not increase total haemoglobin mass or sea level VO2max in world champion track cyclists. J. Sci. Med. Sport 1998, 1, 156–170. [Google Scholar] [CrossRef]
- Wilber, R.L.; Drake, S.D.; Hesson, J.L.; Nelson, J.A.; Kearney, J.T.; Dallam, G.M.; Williams, L.L. Effect of altitude training on serum creatine kinase activity and serum cortisol concentration in triathletes. Eur. J. Appl. Physiol. 2000, 81, 140–147. [Google Scholar] [CrossRef]
- Tiollier, E.; Schmitt, L.; Burnat, P.; Fouillot, J.P.; Robach, P.; Filaire, E.; Guezennec, C.; Richalet, J.P. Living high-training low altitude training: Effects on mucosal immunity. Eur. J. Appl. Physiol. 2005, 94, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Kuipers, H.; Snyder, A.C.; Keizer, H.A.; Jeukendrup, A.; Hesselink, M. A New Approach for the determination of ventilatory and lactate thresholds. Int. J. Sports Med. 1992, 13, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Zajac, A.; Cholewa, J.; Poprzęcki, S.; Waśkiewicz, Z.; Mikołajec, K. Lactate threshold (D-Max Method) and maximal lactate steady state in cyclists. J. Hum. Kinet. 2009, 21, 49–56. [Google Scholar] [CrossRef]
- Płoszczyca, K.; Jazic, D.; Piotrowicz, Z.; Chalimoniuk, M.; Langfort, J.; Czuba, M. Comparison of maximal lactate steady state with anaerobic threshold determined by various methods based on graded exercise test with 3-min stages in elite cyclists. BMC Sports Sci. Med. Rehabil. 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Maszczyk, A.; Gerasimuk, D.; Roczniok, R.; Fidos-Czuba, O.; Zając, A.; Gołaś, A.; Mostowik, A.; Langfort, J. The effects of hypobaric hypoxia on erythropoiesis, maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes. J. Sports Sci. Med. 2014, 13, 912–920. [Google Scholar] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Wiśniewska, A.; Płoszczyca, K.; Czuba, M. Changes in erythropoietin and vascular endothelial growth factor following the use of different altitude training concepts. J. Sports Med. Phys. Fitness 2020, 60, 677–684. [Google Scholar] [CrossRef]
- Sawhney, R.C.; Chhabra, P.C.; Malhotra, A.S.; Singh, T.; Riar, S.S.; Rai, R.M. Hormone profiles at high altitude in man. Andrologia 1985, 17, 178–184. [Google Scholar] [CrossRef]
- Barnholt, K.E.; Hoffman, A.R.; Rock, P.B.; Muza, S.R.; Fulco, C.S.; Braun, B.; Holloway, L.; Mazzeo, R.S.; Cymerman, A.; Friedlander, A.L. Endocrine responses to acute and chronic high-altitude exposure (4300 meters): Modulating effects of caloric restriction. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 1078–1088. [Google Scholar] [CrossRef]
- Humpeler, E.; Skrabal, F.; Bartsch, G. Influence of exposure to moderate altitude on the plasma concentration of cortisol, aldosterone, renin, testosterone, and gonadotropins. Eur. J. Appl. Physiol. 1980, 45, 167–176. [Google Scholar] [CrossRef]
- Feng, L.S.; Hong, P.; Zong, P.F.; Guo, J.; Li, F.T. Effects of altitude training on serum hormone of male middle and long distance runners. Sports Sci. 2000, 20, 49–52. [Google Scholar]
- Shahani, S.; Braga-Basaria, M.; Maggio, M.; Basaria, S. Androgens and erythropoiesis: Past and present. J. Endocrinol. Investig. 2009, 32, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F. Serum testosterone levels and excessive erythrocytosis during the process of adaptation to high altitudes. Asian J. Androl. 2013, 15, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Chaupis, D. Higher androgen bioactivity is associated with excessive erythrocytosis and chronic mountain sickness in Andean Highlanders: A review. Andrologia 2015, 47, 729–743. [Google Scholar] [CrossRef]
- Colice, G.L.; Ramirez, G. Effect of hypoxemia on the renin-angiotensin-aldosterone system in humans. J. Appl. Physiol. 1995, 58, 724–730. [Google Scholar] [CrossRef]
- Morishima, T.; Goto, K. Successive exposure to moderate hypoxia does not affect glucose metabolism and substrate oxidation in young healthy men. SpringerPlus 2014, 3, 370. [Google Scholar] [CrossRef]
- Woods, D.R.; Davison, A.; Stacey, M.; Smith, C.; Hooper, T.; Neely, D.; Turner, S.; Peaston, R.; Mellor, A. The cortisol response to hypobaric hypoxia at rest and post-exercise. Horm. Metab. Res. 2012, 44, 302–305. [Google Scholar] [CrossRef]
- Berglund, B. High-altitude training: Aspects of hematological adaptation. Sports Med. 1992, 14, 289–303. [Google Scholar] [CrossRef]
- Millet, G.; Faiss, R.; Pialoux, V. Point: Hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J. Appl. Physiol. 2012, 112, 1783–1784. [Google Scholar] [CrossRef]
- Mounier, R.; Brugniaux, J.V. Counterpoint: Hypobaric hypoxia does not induce different responses from normobaric hypoxia. J. Appl. Physiol. 2012, 112, 1784–1786. [Google Scholar] [CrossRef]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extrem. Physiol. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Saugy, J.; Schmitt, L.; Hauser, A.; Constantin, G.; Cejuela, R.; Faiss, R.; Wehrlin, J.P.; Rosset, J.; Robinson, N.; Millet, G.P. Same performance changes after Live High-Train Low in normobaric vs. hypobaric hypoxia. Front. Physiol. 2016, 7, 138. [Google Scholar] [CrossRef]
- Timon, R.; Olcina, G.; Padial, P.; Bonitch-Góngora, J.; Martínez-Guardado, I.; Benavente, C.; de la Fuente, B.; Feriche, B. Effects of resistance training in hypobaric vs. normobaric hypoxia on circulating ions and hormones. Int. J. Environ. Res. Public Health 2022, 19, 3436. [Google Scholar] [CrossRef] [PubMed]




| LH–TL n = 10 | IHT n = 10 | N n = 10 | Results of One-Way ANOVA | |
|---|---|---|---|---|
| Age (y) | 20.5 ± 2.9 | 20.7 ± 3.1 | 21.8 ± 4.0 | F = 0.230 p = 0.977 |
| Height (cm) | 181 ± 4.3 | 178.0 ± 5.3 | 178.2 ± 3.4 | F = 1.750 p = 0.193 |
| Weight (kg) | 69.6 ± 3.9 | 67.5 ± 5.3 | 68.1 ± 4.8 | F = 0.442 p = 0.647 |
| FAT (%) | 8.4 ± 2.6 | 10.6 ± 2.0 | 8.4 ± 2.4 | F = 2.903 p = 0.082 |
| VO2max (mL/kg/min) | 66.0 ± 4.1 | 67.6 ± 2.7 | 67.0 ± 2.9 | F = 0.541 p = 0.844 |
| WRLT (W) | 292 ± 21.4 | 286.0 ± 25.0 | 280 ± 21.1 | F = 2.154 p = 0.255 |
| Day | Microcycle 1 | Microcycle 2 | Microcycle 3 | Microcycle 4 |
|---|---|---|---|---|
| 1 | T1 + 2 h endurance training (60–75% of WRLT) | T2 + 2 h endurance training (60–75% of WRLT) | T3 + 2 h endurance training (60–75% of WRLT) | Day off |
| 2 | 3–4 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) | 3–4 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) | 3–4 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) | 1 h active recovery ride < 55% WRLT |
| 3 | T1 + 2 h endurance training (60–75% of WRLT) | T2 + 2 h endurance training (60–75% of WRLT) | T3 + 2 h endurance training (60–75% of WRLT) | 2 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) |
| 4 | Strength endurance (gym) Upper body | Strength endurance (gym) Upper body | Strength endurance (gym) Upper body | Strength endurance (gym) Upper body |
| 5 | T1 + 2 h endurance training (60–75% of WRLT) | T2 + 2 h endurance training (60–75% of WRLT) | T3 + 2 h endurance training (60–75% of WRLT) | T1 + 1 h endurance training (60–75% of WRLT) |
| 6 | 3–4 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) | 3–4 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) | 3–4 h of endurance training 60–75% of WRLT with high-speed intervals (2 × 6 × 10 s-max) | 2 h of endurance training 60–75% of WRLT |
| 7 | Day off | Day off | Day off | Day off |
| Variables | Group | Measurement | ||||
|---|---|---|---|---|---|---|
| Baseline (x ± SD) | 1 Week (x ± SD) | 2 Weeks (x ± SD) | 3 Weeks (x ± SD) | 4 Weeks (x ± SD) | ||
| Training load (TSS) | LH–TL | 462 ± 35 | 1094 * ± 63 | 1147 * ± 73 | 1283 * ± 69 | 412 ± 27 |
| IHT | 451 ± 25 | 1128 * ± 49 | 1164 * ± 69 | 1276 * ± 76 | 387 ± 22 | |
| N | 434 ± 29 | 1152 * ± 51 | 1194 * ± 76 | 1308 * ± 86 | 426 ± 31 | |
| CK (U/I) | LH–TL | 90.1 ± 34.3 | 139.3 ± 48.9 | 159.7 * ± 58.3 | 161.7 * ± 62.1 | 98.7 ± 27.7 |
| IHT | 110.9 ± 32.1 | 151.8 ± 52.6 | 168.1 * ± 64.1 | 175.1 * ± 52.7 | 115.8 ± 34.1 | |
| N | 89.3 ± 28.4 | 147.2 * ± 42.6 | 158.8* ± 49.4 | 161.7 * ± 55.7 | 108.4 ± 32.2 | |
| URIC (mg/dL) | LH–TL | 4.75 ± 0.34 | 4.98 ± 0.24 | 5.65 * ± 0.26 | 5.81 * ± 0.31 | 4.79 ± 0.34 |
| IHT | 4.83 ± 0.29 | 5.12 ± 0.34 | 5.74 * ± 0.38 | 5.97 * ± 0.42 | 4.89 ± 0.29 | |
| N | 4.91 ± 0.38 | 5.21 ± 0.41 | 5.84 * ± 0.46 | 6.01 * ± 0.52 | 4.87 ± 0.31 | |
| Variable | Baseline (x ± SD) | After 4 Weeks (x ± SD) | ∆ (x ± SD) |
|---|---|---|---|
| RBC (million/μL) | 5.01 ± 0.2 | 5.33 ** ± 0.23 | 0.32 ± 0.19 |
| HGB (g/dL) | 15.3 ± 0.67 | 16.3 ** ± 0.76 | 1.0 ± 0.27 |
| HCT (%) | 44.5 ± 2.3 | 46.5 ** ± 2.5 | 2.0 ± 1.08 |
| Ret (%) | 1.00 ± 0.19 | 1.38 * ± 0.13 | 0.38 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czuba, M.; Płoszczyca, K.; Kaczmarczyk, K.; Langfort, J.; Gajda, R. Chronic Exposure to Normobaric Hypoxia Increases Testosterone Levels and Testosterone/Cortisol Ratio in Cyclists. Int. J. Environ. Res. Public Health 2022, 19, 5246. https://doi.org/10.3390/ijerph19095246
Czuba M, Płoszczyca K, Kaczmarczyk K, Langfort J, Gajda R. Chronic Exposure to Normobaric Hypoxia Increases Testosterone Levels and Testosterone/Cortisol Ratio in Cyclists. International Journal of Environmental Research and Public Health. 2022; 19(9):5246. https://doi.org/10.3390/ijerph19095246
Chicago/Turabian StyleCzuba, Miłosz, Kamila Płoszczyca, Katarzyna Kaczmarczyk, Józef Langfort, and Robert Gajda. 2022. "Chronic Exposure to Normobaric Hypoxia Increases Testosterone Levels and Testosterone/Cortisol Ratio in Cyclists" International Journal of Environmental Research and Public Health 19, no. 9: 5246. https://doi.org/10.3390/ijerph19095246
APA StyleCzuba, M., Płoszczyca, K., Kaczmarczyk, K., Langfort, J., & Gajda, R. (2022). Chronic Exposure to Normobaric Hypoxia Increases Testosterone Levels and Testosterone/Cortisol Ratio in Cyclists. International Journal of Environmental Research and Public Health, 19(9), 5246. https://doi.org/10.3390/ijerph19095246







