Abstract
Background: The occupational demands of professional airline pilots such as shift work, work schedule irregularities, sleep disruption, fatigue, physical inactivity, and psychological stress may promote adverse outcomes to cardiometabolic health. This review investigates the prevalence of cardiometabolic health risk factors for airline pilots. Methods: An electronic search was conducted utilizing PubMed, MEDLINE (via OvidSP), CINAHL, PsycINFO, SPORTDiscus, CENTRAL, and Web of Science for publications between 1990 and February 2022. The methodological quality of included studies was assessed using two quality assessment tools for cross-sectional and clinical trial studies. The prevalence of physiological, behavioral, and psychological risk factors was reported using descriptive analysis. Results: A total of 48 studies derived from 20 different countries, reviewing a total pooled sample of 36,958 airline pilots. Compared with general population estimates, pilots had a similar prevalence for health risk factors, yet higher sleep duration, lower smoking and obesity rates, less physical activity, and a higher overall rate of body mass index >25. Conclusions: The research reported substantial prevalence >50% for overweight and obesity, insufficient physical activity, elevated fatigue, and regular alcohol intake among pilots. However, the heterogeneity in methodology and the lack of quality and quantity in the current literature limit the strength of conclusions that can be established. Enhanced monitoring and future research are essential to inform aviation health practices and policies (Systematic Review Registration: PROSPERO CRD42022308287).
1. Introduction
Cardiometabolic noncommunicable diseases (NCDs) such as cardiovascular disease (CVD), stroke, type 2 diabetes (T2D), and their primary risk factors are a leading public health concern that produce significant and growing economic costs globally [1]. The leading cause of mortality worldwide is CVD [2], which has been reported as the most frequent cause of permanent groundings among Korean airline pilots [3]. Cardiovascular and cerebrovascular incidents have also been reported among the most prevalent causes of flight incapacitation in the United Kingdom [4].
Airline pilots experience unique occupational demands which may promote adverse outcomes to cardiometabolic health, including shift work, work schedule irregularities, sleep disruption, fatigue, the sedentary nature of the job, and stress demands associated with flight safety [3,5,6,7,8]. Cardiometabolic diseases are associated with numerous modifiable risk factors across physiological, behavioral, and psychological domains [1]. Central and systemic obesity, hypertension, dyslipidemia, hyperglycemia, insulin resistance, and adipose dysfunction are among prevalent physical risk factors associated with increased risk of CVD and T2D [1,9]. Modifiable behavioral risk factors [10] such as unhealthy diet, physical inactivity, excessive alcohol consumption, and tobacco smoking, along with psychological risk factors including high fatigue [11] and depression [12], are each independently established as risk factors for cardiometabolic diseases.
To date, no systematic reviews have been published pertaining to the evaluation of modifiable health risk factor prevalence among airline pilots. Estimations of health risk prevalence are important for monitoring of trends and to inform risk reduction interventions; hence, the aim of the current review was to critically analyze the global literature to quantify the prevalence of modifiable cardiometabolic health risk factors among commercial airline pilots. The findings from this review may be valuable to inform aviation health practices and policies for supporting pilot health, enhancing flight operation safety, and identifying deficiencies within the literature base to inform future research.
2. Materials and Methods
2.1. Protocol
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) [13]. The protocol of this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42022308287).
2.2. Literature Search
An electronic search was conducted utilizing PubMed, MEDLINE (via OvidSP), CINAHL, PsycINFO, SPORTDiscus, CENTRAL, and Web of Science. A broad search strategy was implemented to gather literature published between 1 January 1990 and 28 February 2022. Key terms incorporated in the search string were relating to airline pilots and cardiometabolic health risk prevalence (see Table 1). The search was limited to peer-reviewed publications in English. Eligible publications were extracted, and their reference lists were manually checked for potentially relevant studies. The reference lists of existing review articles pertaining to aviation medicine were also cross-checked for relevant articles.

Table 1.
Search terms blocks were combined for text and word search in PubMed and adapted to the remaining databases: 1 and 2; 1 and 3; 1, 2, and 3.
2.3. Eligibility Criteria
Publications were identified for inclusion on the basis of population, literature type, publication date, and cardiometabolic health risk eligibility. The population criteria for inclusion were fixed-wing pilots (airline, commercial, civilian), and no restrictions were placed on fleet type (short-haul, long-haul, mixed-fleet). Articles were excluded if they included pilots with <1 year experience of being a pilot, as well as those who worked part-time, were a helicopter pilot, or worked in noncivil aviation roles (Air Force, military, army, or private), and if they were published before 1990. Literature sources that met inclusion were peer-reviewed original articles (retrospective, prospective, cross-sectional, case–control, cohort, and experimental), and other sources were excluded, e.g., literature reviews, commentaries, and editorials. To be eligible for inclusion, publications had to report on at least one of the following cardiometabolic health risk markers: blood pressure (BP), body composition (body mass, body mass index [BMI], waist circumference, waist-to-hip ratio, body fat percentage, lean mass percentage, visceral adiposity), glycemic control (fasting or postprandial glucose, HbA1c), insulin (fasting, insulin sensitivity, or insulin resistance), inflammation (C-reactive protein, inflammatory markers), blood lipid panel (total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides (TG)), microalbuminuria, endothelial or microvascular dysfunction, alcohol consumption, smoking, dietary behaviors (fruit and vegetable intake, high-energy-dense intake, high-saturated-fat intake, high sugar intake, or low-fiber), physical activity (sedentary behavior, moderate-to-vigorous physical activity (MVPA), or daily steps), cardiorespiratory fitness (submaximal or maximal oxygen consumption [VO2]), sleep (hours per night, sleep quality), psychosocial stress (stress, depression, anxiety, and fatigue), and self-rated health.
To avoid including studies involving work duty-induced inflation of cardiometabolic risk prevalence, studies pertaining to outcome measures recorded preceding (<24 h), during, or acutely following (<48 h) long-haul flights were excluded. Where available, nonflight duty baseline data from these studies were utilized. Studies reporting data exclusively on pilot subpopulations (e.g., diabetic or obese pilots) were excluded. A hand search of recent issues of prominent aviation journals was conducted to screen for any recently published articles that were not yet indexed and apparent on the systematic search.
2.4. Screening Process
The lead author conducted the initial literature search, and results were downloaded and imported into Endnote citation software (Endnote x9, Clarivate Analytics, Philadelphia, PA, USA) for collation and duplicate removal. Thereafter, articles were exported to Microsoft Excel (Microsoft® Excel version 16.54) for further removal of duplicates and subsequent eligibility screening. Initial title and abstract screening was conducted by one author and cross-checked by a second reviewer. Subsequently, potentially eligible articles from the initial screening progressed to full-text evaluation of eligibility for inclusion. Discrepancies in outcomes between the reviewers were resolved via discussion and consultation with a third reviewer.
2.5. Methodological Quality Assessment
The methodological quality of publications included for this review was independently assessed by two reviewers. The risk-of-bias quality assessment checklist (adapted from Hoy and colleagues [14]) was utilized for evaluation of cross-sectional studies, which consisted of four external validity items and six internal validity items. Clinical trials were evaluated utilizing the risk-of-bias tool from Cochrane [15]. The summative quality assessment for each publication was expressed as being of low quality (high risk of bias), moderate quality (high risk of bias), or high quality (low risk of bias). Consistent with the Grades of Recommendation, Assessment, Development, and Evaluation and Cochrane approaches, total scores for the cross-sectional study assessment were grouped as the following thresholds: very high risk of bias (0–4 points), high risk of bias (5–6 points), or low risk of bias (7–10 points). Clinical trials were rated as ‘high’, ‘low’, or ‘unclear’ for seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and outcome-specific evaluations of risk of bias.
2.6. Data Extraction
The study country, aim, design, participant characteristics, outcomes of interest, and instruments for included publications were extracted (Appendix A). If necessary, additional publication information was sought from trial registries, article supplementary materials, or direct contact with article authors. Study data were independently extracted and coded by one reviewer, and a second reviewer independently extracted and coded 20% of the included studies for process cross-evaluation. Any discrepancies between reviewers were resolved via discussion and consultation with a third reviewer if necessary. For clinical trials included in our analysis, descriptive data were extracted from their reported baseline data, and post-intervention data were not included. For between-group studies that only reported subgroup descriptive statistics (e.g., interventional and control), we computed the combined population mean using Cochran’s formula [16].
2.7. Analysis of Data
The prevalence of cardiometabolic health risks was reported using descriptive analysis. Available data were sought and extracted from included publications for descriptive analysis, including one or multiple of the following available statistical metrics: mean descriptive statistics, prevalence proportions, incidence rates, standardized incidence ratios, prevalence ratios, odds ratios, risk ratios, or scoring outcomes derived from relevant self-report instruments. The meta-analysis estimates for proportions and descriptive statistics for cardiometabolic health risk factors were calculated by weighing the studies according to their sample size within pooled samples. A 95% confidence interval was presented alongside pooled prevalence statistics. Meta-analyses were not conducted for some cardiometabolic risk factors due to a low number of studies reporting the parameter of interest (n < 4) or due to methodological heterogeneity. Data were entered into an Excel spreadsheet (Microsoft, Seattle, WA, USA) and then imported into statistical software SPSS v28 for Windows (IBM, New York, NY, USA), where meta-analysis interpretation was performed.
3. Results
3.1. Study Selection
The search strategy produced 6138 unique results, 107 of which were deemed potentially eligible at primary screening. After full-text reviews, 48 passed eligibility evaluation for inclusion. A PRISMA flowchart depicting stages of the selection process is illustrated in Figure 1.
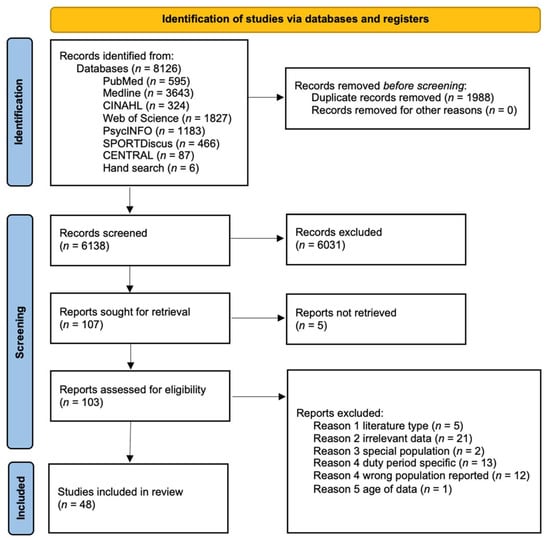
Figure 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2. Study Characteristics
The 48 studies involved a total of 36,958 participants, included in 46 cross-sectional studies and three clinical trials (Figure 2). The characteristics of the included studies are summarized in Appendix A. Across all studies, males represented 96% of participants. The mean age of participants was 40 ± 11 years according to 35/48 studies which reported the mean age. The most prevalent age range reported in the remaining studies was 35–45 years. Twenty-five studies reported self-report subjective data, 14 utilized a combination of self-report subjective and objective data, and five reported only objective data. The included studies were conducted in 20 different countries or regions, including Brazil (five), China (five), New Zealand (four), Finland (three), Indonesia (three), Sweden (three), the United Kingdom (three), the United States (three), Korea (two), the Netherlands (two), Portugal (two), and one study each from Arab states, Australia, Europe, Germany, India, Oceania, Saudi Arabia, Spain, and Thailand. Four studies involved participants from numerous countries.
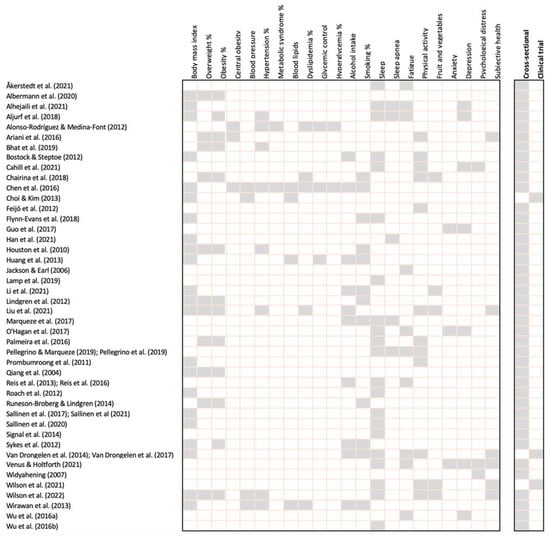
Figure 2.
Cardiometabolic risk markers and airline pilot outcome summary for each study.
3.3. Quality of Reviewed Articles
The results of the risk-of-bias assessment are displayed in Table 2 and Table 3. Of the 48 publications included in the review, four were considered of low methodological quality with a high risk of bias and 13 were considered of high methodological quality with a low risk of bias. Weak external validity was apparent for most cross-sectional studies, with a paucity of random sampling (n = 39) and high nonresponse bias (n = 33) as leading factors. Lacking reliability and validity of outcome measures (n = 17) and inappropriate observed prevalence period (n = 14) were prominent factors of poor internal validity among cross-sectional studies. The three clinical trials reviewed ranged from low to moderate quality, all exhibiting high risk of bias for allocation concealment and blinding of participants.

Table 2.
Methodological quality scores of cross-sectional studies.

Table 3.
Risk-of-bias assessment of clinical trials.
3.4. Physiological Cardiometabolic Risk Factors among Pilots
Twenty-eight studies investigated physiological cardiometabolic risk factors. From the 22 studies reporting BMI, 12 were objectively measured and 10 were based on self-report data. The overall objectively measured BMI (n = 20,279) pooled mean was 26.1 ± 3.0 kg/m2 and the overall subjective BMI (n = 3710) pooled mean was 24.7 ± 3.1 kg/m2. For females, one study (n = 661) reported an objectively measured BMI of 23.9 kg/m2 (20.0–27.7), and another (n = 32) reported a subjective BMI as 22.7 kg/m2.
Eleven studies investigated the prevalence of overweight and obesity; five (n = 19,171) were objectively measured and six (n = 3309) were based on self-reporting from participants. The pooled mean for objective measures of overweight and obesity were 47.5% (47.4–47.5%) and 11.6% (11.6–11.7%), respectively. One study reported obesity only, revealing a prevalence of 20% [6]. The pooled mean for subjective measures of overweight and obesity was 43.6% (43.3–43.9%) and 12.4% (11.9–12.9%), respectively. The overall pooled prevalence of overweight plus obesity was 59.1% (59.0–59.2%) for objective measures and 56.0% (55.5–56.5%) for subjective measures. The combined pooled prevalence from subjective and objective measures for overweight, obesity, and overweight plus obesity was 46.8% (46.7–46.9), 11.7% (11.6–11.8%), and 58.6% (58.5–58.7%), respectively. One study [32] (n = 661) reported the prevalence of overweight and obesity for females as 28% and 6%, respectively. The prevalence of metabolic syndrome was reported by two studies, ranging from 15% [21] to 38% [27]. Furthermore, these studies reported objectively measured central obesity (>102 cm) prevalence as 18% [21] and 64% [27]. Only one study investigated C-reactive protein levels, reporting a mean hs-CRP serum level of 1.68 ± 1.79 (mg/L) [21].
Four studies (n = 16,327) reported the prevalence of hypertension (BP ≥ 140/90 mmHg) from objective measurement as 29% [32], 28% [27], 26% [56], and 11% [23], with a pooled prevalence of 27.6% (27.5–27.7%). Furthermore, one study (n = 303) reported the prevalence of elevated BP (≥130/85 mmHg) as 38% [21]. Derived from four studies [3,27,56,57], the objectively measured pooled mean systolic blood pressure (SBP) was 126 ± 14 mmHg, and the objectively measured pooled mean diastolic blood pressure (DBP) was 79 ± 9 mmHg. The prevalence of self-reported known hypertension of participants in three studies was 13% [20], 7% [38], and 6% [57]. One study reported the prevalence of objective hypertension for females as 14% [32].
HDL cholesterol and triglycerides were reported in four studies [3,27,33,57] (n = 1640), revealing pooled means of 1.3 ± 0.9 mmol/L and 19 ± 1.6 mmol/L, respectively. Additionally, three studies reported the prevalence of low HDL as 8% [21], 46% [27], and 57% [26] and of elevated triglycerides as 24% [21], 28% [27], and 29% [26]. The pooled mean of three studies [3,33,57] (n = 1337) reporting TC was 5.3 ± 1.0 mmol/L, and an LDL cholesterol mean of 3.3 ± 0.9 mmol/L was derived from two studies [3,33] (n = 742). The prevalence of self-reported known dyslipidemia of participants in two studies was 10% [57] and 19% [38]. Only two studies investigated hyperglycemia, reporting the prevalence as 31% [21] (≥100 mg/dL) and 30% [27] (≥5.6 mmol/L).
3.5. Behavioral Cardiometabolic Risk Factors among Pilots
Thirty-one studies included the evaluation of behavioral cardiometabolic risk factors. Alcohol intake was investigated in 10 samples of airline pilots [6,24,27,33,36,38,39,46,47,57,60,61]; one study utilized a validated questionnaire [36], and five studies [27,33,36,38,39] (n = 2538) ascertained “regular alcohol intake” on the basis of a participant self-recall question, producing a pooled prevalence of 52% (51.3–53.1). Twelve studies [6,26,27,29,32,33,36,37,39,49,57,60] (n = 19,116) reported smoking prevalence, yet no studies evaluated quantity or frequency of smoking. The pooled prevalence was 9.4% (9.3–9.5%). One study reported the prevalence of smoking for females as 6% [32].
From the 20 studies evaluating sleep, seven studies objectively measured sleep hours with actigraphy (n = 1764) [29,35,48,50,51,52,53,59], and six used self-recall methods (n = 2224) [17,24,39,56,59,62]. The pooled means for objective and self-recall sleep hours per night were 7.2 ± 1.1 and 7.0 ± 0.6, respectively. Three studies reported the prevalence of <6 h of sleep per night as 23% [43], 20% [61], and 22% [20]. Furthermore, other studies reported that <6 h of sleep per night was associated with obesity [41] and poor sleep quality [42] within participants. The prevalence of excessive sleepiness assessed by the Epworth Sleepiness Scale (score ≥ 10) was reported by five studies [19,20,39,42,46], exhibiting a pooled prevalence of 44.5% (44.1–44.8%). Among four studies reporting high obstructive sleep apnea (OSA) risk ascertained from the Berlin Questionnaire, the prevalence was 5% [19], 20% [39], 21% [42], and 29% [20], providing a pooled mean of 21.4% (21.3–21.5%).
The prevalence of self-reported insufficient physical activity (<150 min MVPA per week) was reported in five studies (n = 2233) providing a pooled prevalence of 51.5% (51.3–51.7%) [22,26,42,56,62]. Additionally, <150 min MVPA per week was found to be associated with obesity in one study which reported a prevalence ratio of 1.08 (0.98–1.19) [41]. One study reported the mean days per week of moderate physical activity and strenuous physical activity as 3.3 ± 1.9 and 2.0 ± 1.4, respectively. Another study reported the mean walking minutes and MVPA minutes per week as 110 ± 117 and 145 ± 72, respectively.
Three studies (n = 955) reported the prevalence of subjective insufficient daily fruit intake as 33% (<200 g/day) [38], 60% [56], and 65% (<2 servings/day) [62] and of insufficient daily vegetable intake as 19% (<300 g/day) [38], 47% [62], and 48% [56] (<3 servings/day). From these studies, two reported the prevalence of combined insufficient fruit and vegetable intake as 68% [56] and 84% [62]. One study reported the mean number of snacks per duty as 4 ± 3 [60].
3.6. Psychological Cardiometabolic Risk Factors among Pilots
Sixteen studies included an evaluation of psychological cardiometabolic risk factors. Among 10 studies investigating the prevalence of psychological fatigue, four studies (n = 2987) utilized the Fatigue Severity Scale (FSS), two of which reported a psychological fatigue prevalence (FSS ≥ 4 mean score) of 77% [54] and 89% [46,47]. Another two studies reported the severe psychological fatigue prevalence (FSS ≥ 36 total score) as 33% [19] and 68% [20]. The prevalence of elevated psychological fatigue in the remaining studies (n = 2719) was reported as 5% [58], 27% [42,43], 30% [60,61], and 75% [34], each produced with different methodology.
Seven studies subjectively measured the prevalence of depression, with a pooled mean of 21% (20.8–21.6) for mild depression derived from five studies [19,25,30,54,58] (n = 3411) utilizing the Patient Health Questionnaire (PHQ-9; score ≥ 10). One study reported a depression prevalence of 35% [20] according to the Hospital Anxiety and Depression Scale (score > 8), whereas another study reported depression or anxiety within the last 12 months as 54.4% [40]. One study reported mild depression (PHQ-9 score ≥ 10) prevalence in females as 11% [58]. Two studies (n = 2527) reported the prevalence of mild anxiety derived from the Generalized Anxiety Disorder-7 (GAD-7; score > 10) scale, noting 4% [30] and 7% [54]. The prevalence of below-average or poor subjective self-rated health was reported in three studies (n = 1282) as 8% [38], 25% [56], and 39% [22], each derived from different methodology.
4. Discussion
To our knowledge, this is the first comprehensive synthesis of published research pertaining to physiological, behavioral, and psychological cardiometabolic health risk factors among this unique occupational group. Our findings provide stakeholders including aviation medical professionals, policymakers, researchers, clinicians, and occupational health authorities with a scientific synthesis of the magnitude of prevalence of cardiometabolic health risk factors among commercial pilots. These findings may be beneficial to inform developments in aviation health practices and policies to support pilot health and wellness, to mitigate risks of occupational morbidity, medical conditions causing loss of license, and medical incapacity, and to support flight safety [5].
Findings from the review suggest similar health risk factor prevalence to the general population, yet higher sleep duration, less physical activity, lower smoking rates, higher regular alcohol consumption, less obesity, and a higher overall rate of body mass index >25 among pilots. We discovered, within the literature reviewed, a dominance of self-reported data, with most studies exhibiting moderate to high risk of methodological bias. Indeed, there are limited high-quality studies within the field, warranting the need for future research to address the gaps within and strengthen the body of knowledge.
4.1. Prevalence of Physiological Cardiometabolic Risk Factors among Pilots
As described by the International Civil Aviation Organization (ICAO) aviation medical regulations, cardiometabolic health risk data are acquired routinely during aviation medical examinations for pilots >35 years old for CVD risk assessment, which include BMI, BP, resting heart rate, blood lipids, and HbA1c [5]. In 2015, the global prevalence of overweight, obesity, and overweight plus obesity in the general population was reported as 38.7%, 16.4%, and 55.1%, respectively [63]. This general population estimate is relative to the country, age, and sex characteristics represented in the present review of studies conducted among pilots. Past research has reported a lower prevalence of overweight and obesity in pilots compared to the general population [6,64,65]. Indeed, the present review found that pilots had a 4.7% lower prevalence of obesity than the general population [63]. As obesity is a major risk factor for diseases such as CVD and T2D [66], the lower rate of obesity within pilots may promote a lower pilot population cardiometabolic disease relative risk compared to the general population.
Interestingly, with overweight and obesity pooled together, we discovered that pilots had an overall 3.5% higher rate of overweight plus obesity compared to the general population (58.6% and 55.1%, respectively). This finding suggests that past reports of lower rates of overweight and obesity within pilots [6,64,65] compared to the general population may be archaic, and future research should investigate the underlying causal mechanisms that contribute to overweight and obesity rates among pilots. A noteworthy consideration for interpretation of this information is the lack of random sampling and potential response bias within studies on pilots compared to the general population, which adds a notable limitation to the validity of prevalence comparisons between populations.
Most countries represented within the present review were high-income countries. In 2010, the global hypertension prevalence was estimated as 31.1% (30.0–32.2%), while that among high-income countries was estimated as 28.5% (27.3–29.7%) [67]. We found four studies reporting the prevalence of hypertension within pilots, ranging from 11% to 29% [23,27,32,56], with a pooled prevalence slightly lower than the general population at 27.6%. Airline pilots undergo regular medical examinations evaluating BP [5], and this regular active monitoring may promote the observed lower hypertension prevalence. However, one study reported a 38% [21] prevalence of pilots exhibiting elevated BP (≥130/85 mmHg); thus, it would be valuable for future epidemiological research to report the prevalence of elevated BP, accompanying hypertension rates in order to better inform researchers on the distribution of BP ranges across the pilot population.
Prospective epidemiological studies have consistently reported that unfavorable blood lipid profiles are associated with increased incidence of metabolic syndrome (MS) and NCDs such as CVD [68]. We found three studies reporting the prevalence of markers of dyslipidemia in pilots, with prevalence of low HDL ranging from 8% to 57% and of elevated TG ranging from 24% to 29% [21,26,27], whereas MS prevalence ranged from 15% [21] to 38% [27]. These evident variances in prevalence rates based on the small sample of studies (n = 3) [21,26,27] illustrate the need for further research to be conducted regarding quantification of these risk factors in pilots to reach valid inferences of their prevalence.
The global prevalence of diabetes is rising, and it has been estimated that 49.7% of people living with diabetes are undiagnosed [69]. Investigation of impaired glucose tolerance and hyperglycemia is scarce in the airline pilot literature, with only two studies reporting the prevalence of hyperglycemia (30.4–31.3%) [21,27] and scant research reporting on the prevalence of elevated HbA1c, which is the leading diagnostic criteria for T2D [69]. This dearth of information may be attributable to past barriers for diabetic pilots to operate commercials flights due to the risk of incapacitation from hypoglycemia while flying, yet recent advances in insulin therapies, monitoring techniques, and modes of administration have given rise to policy developments reducing barriers for diabetic pilots to operate commercial flights [70]. Seemingly, there is a need for more research attention on glycemic control and identifying the prevalence of elevated risk markers for T2D among airline pilots.
4.2. Prevalence of Behavioral Cardiometabolic Risk Factors among Pilots
From the 31 studies we found reporting on behavioral risk factors, sleep was the most frequently reported risk factor (n = 21). Sleep disruption is an inherent risk for pilots as occupational characteristics such as extended duty periods, work schedules, crossing time zones, and sleep restrictions cause perturbance of sleep routine consistency [71]. Recent research has indicated that sleep difficulty is frequently expressed as a primary source of work induced stress among pilots [25]. The present review found the mean pooled sleep hours per night to range from 7.0 h to 7.1 h, indicating that the population mean falls within the lower range of sleep guidelines for health in adults, which has been reported as the attainment of 7–9 h [72]. We found three studies reporting the prevalence of short sleep (<6 h) ranging from 20% to 23% [20,59,61], which is comparably lower than a USA-based study noting a prevalence of ≤6 h sleep as 29% among the general population in 2012 [73]. Indeed, due to the influence of fatigue on flight safety, pilots are often subject to fatigue management training via aviation medical management [71,74], facilitating the implementation of adaptive coping strategies to mitigate fatigue which may support the attainment of sleep guidelines within the population.
Past research has reported lower sex- and age-adjusted prevalence for smoking and higher levels of physical exercise among aircrews compared to the general population [65]. Indeed, we found a pooled smoking prevalence of 9% among pilots, which is considerably lower than a 2015 prevalence estimate of 25% for smoking among the global male general population [75]. Interestingly, for physical activity, we found a pooled prevalence of 51.5% for insufficient physical activity among pilots, which is markedly higher than a recent global prevalence estimate of 32% (30–33%) in 2016 among the general population within high-income countries [76]. However, our findings were only derived from four studies using self-recall data and small samples, making comparisons with the general population of scant validity. Future research utilizing objective outcome measures is important to further evaluate the accuracy of current findings.
Alcohol use is a leading risk factor for global disease burden, with the global prevalence of current drinking estimated as 47% in 2017 [77]. According to five studies we found, the pooled mean for regular alcohol intake among pilots was 52%. However, the lack of quantity and the low-quality methodology among studies minimize the validity of prevalence estimation. Furthermore, pilots may be inherently biased to misrepresent their true alcohol intake to aviation medical professionals or researchers due to aviation regulations prohibiting alcohol consumption within 8 h of acting as a crew member and existing alcohol testing mandates [78].
Dietary behaviors are a leading risk factor for obesity and cardiometabolic diseases such as CVD and T2D [79]. Previous studies have conveyed occupational factors such as inconsistent mealtimes, physical inactivity on duty, suboptimal airport and airline catering options, and shift work as factors that may be detrimental to healthy dietary patterns among pilots [6,7,21]. There is a dearth of literature pertaining to the quantification of dietary behaviors among pilots, with only two studies identified in this review reporting the prevalence of pilots who were not achieving daily fruit and vegetable intake guidelines of ≥5 servings ranging from 68–84% [56,62]. Although lacking validity, this estimate is comparable to the estimated global prevalence estimate of 79% derived from the World Health Survey 2002–2004 [80], relative to the country and sex characteristics represented in the present review.
4.3. Prevalence of Psychological Cardiometabolic Risk Factors among Pilots
High levels of psychological fatigue are associated with elevated risk of CVD and excess mortality within the general population [11] and are detrimental to a pilot’s ability to safely operate the aircraft or perform safety-related duties [5]. We found the prevalence of elevated psychological fatigue to range from 5% to 77% [19,42,43,46,47,54,58,60,61] And of severe psychological fatigue to range from 33% to 68% [19,20]. The heterogeneity of methodology among studies within pilots inhibits valid comparisons with the general population. Nonetheless, with numerous studies reporting noteworthy rates of elevated psychological fatigue during nonduty periods, this warrants further research regarding the development of innovative interventions to better facilitate fatigue mitigation in this occupational group.
Major depressive disorder is associated with elevated cardiometabolic risk factors and poor health outcomes [12]. Depression and mental health issues among pilots have been proposed as contributing factors in numerous flight incidents resulting in mass casualties [40]. Thus, psychological risk factors are pertinent to pilot health and wellness and, in turn, flight operation safety. We discovered a pooled mean of 21% for mild depression [19,25,30,54,58] among pilots. Comparatively, a prevalence of 21% for mild depression was reported within a general population sample using congruent methodology, delineating a similar prevalence between populations.
4.4. Study Strengths
To the authors’ knowledge there is no published scientific synthesis of cardiometabolic health risk factor data for airline pilots. The studies included in the systematic review were derived from 20 different countries from around the globe. Therefore, the findings are not localized to a certain region and are relevant data pertaining to the global airline pilot population. This review revealed insights that diverge from previous assumptions regarding cardiometabolic health among airline pilots, thus providing useful data which may inform public health practice and the development of targeted initiatives to support occupational health and safety.
4.5. Study Limitations
As this review sought to identify baseline nonwork duty-related prevalence of cardiometabolic health risk factors, we did not examine risk factor quantification during or immediately following flight duty periods, as these work characteristics often elicit acute inflated risk prevalence for factors such as psychological fatigue, sleep disruption, and other psychological distress-related parameters [39,52]. Thus, the present review did not capture the magnitude of work duty-induced perturbations to behavioral and psychological cardiometabolic health risks.
Furthermore, as we sought to identify the prevalence of cardiometabolic health risks among the overall airline pilot population, we did not stratify outcomes by fleet division, such as short-haul, long-haul, or mixed-fleet. The comparison of health risk prevalence between fleet divisions may be an appropriate scope for a future systematic review, which would add to the literature for understanding the magnitude of health risk difference between pilot rosters.
Due to the heterogeneity of publication dates among the literature featured in our review, the global general population prevalence comparison studies utilized may not optimally align with timepoints from studies among pilots and should be considered by readers with our presented population comparisons. Additionally, the heterogeneity of measurements of cardiometabolic parameters among the airline pilot studies reviewed and the general population estimates should be considered in the interpretation of our findings.
Lastly, the low quantity of robust studies limits the generalizability of the current findings reported within the literature. Future high-quality epidemiological research utilizing validated measurements will be valuable to increase the probability of attaining reliable conclusions pertaining to the health risk prevalence within the pilot population. To provide further meaningful insight into pilot cardiometabolic health risk and to address gaps in the literature, research attention pertaining to the assessment of glycemic control (i.e., HbA1c) and blood lipids, objectively measured health behaviors (dietary behaviors, physical activity, alcohol intake), and wider assessment of depressive symptoms among the airline pilot population would provide valuable contributions to advance the body of knowledge.
5. Conclusions
The findings of this review provide synthesis on the prevalence and magnitude of cardiometabolic health risk factors among airline pilots. A wide range of prevalence rates were reported for many investigated health risk parameters in the literature, with pervasiveness of overweight and obesity, insufficient physical activity, elevated psychological fatigue, insufficient fruit and vegetable intake, and regular alcohol consumption among pilots. The inherent bias, dominance of self-report data, and heterogeneity of methodology mean that it was not possible to establish strong conclusions. Future research utilizing objective measures and robust random sampling strategies are advocated to strengthen the validity of prevalence estimates and enhance the generalizability of findings.
Author Contributions
D.W. and N.G. participated in the conceptualization and design of the work, data collection and analysis, interpretation of the results, and manuscript writing; M.D. and B.J. contributed to the data analysis, interpretation of the results, and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests.
Appendix A

Table A1.
Summary of characteristics of studies that investigated cardiometabolic health risk parameters among airline pilots.
Table A1.
Summary of characteristics of studies that investigated cardiometabolic health risk parameters among airline pilots.
| Study (Year) | Aim of Study | Design; Data; Country | Sample; % Male; Age | Key Findings | Instruments |
|---|---|---|---|---|---|
| Åkerstedt et al. (2021) [17] | Investigate associations among schedule, fatigue, and sleep | Cross-sectional; subjective; Sweden | n = 89; 76%; 38 ± 9 years | Fatigue (KSS) = 4.2 ± 1.8; sleep = 6.8 ± 1.4 h; sleep, duty time, and early starts are important predictors of fatigue in the 24 h window and that the number of very early starts and short sleep have cumulative effects on fatigue across a 7 day work period | Karolinska Sleepiness Scale; sleep duration self-report |
| Albermann et al. (2020) [18] | Evaluate the prevalence of lower-back pain compared with the general population | Cross-sectional; subjective; Germany | n = 698; 92%; 40 ± 9 years | BMI = 24.4 ± 2.7; overweight = 35% and obesity = 3.2%; chronic lower-back pain = 83%; time spent sitting during work = 90% | BMI (self-report); Oswestry Lower-Back Pain Disability Index |
| Alhejaili et al. (2021) [19] | Evaluate the presence of obstructive sleep apnea in pilots | Cross-sectional; subjective and objective; Saudi Arabia | n = 39; 100%; 43 ± 10 years | BMI = 24.5 ± 2.4; insomnia prevalence (AIS ≥ 6) = 31%; high risk of obstructive apnea = 5%; abnormal sleepiness = 23%; mild depression = 26%; moderate severity depression = 10%; suboptimal sleep quality = 39%; severe fatigue = 33%; VAFS abnormal fatigue = 23% | BMI; Athens insomnia scale (AIS); Berlin Questionnaire; Epworth Sleepiness Scale (ESS); Pittsburgh Sleep Quality Index (PSQI); Fatigue Severity Scale (FSS); Visual Analog Fatigue Scale (VAFS); Patient Health Questionnaire (PHQ-9) |
| Aljurf et al. (2018) [20] | Evaluate the prevalence of fatigue, depression, sleepiness, and the risk of obstructive sleep apnea | Cross-sectional; subjective; Arab states | n = 328; 99%; 41 ± 10 years | BMI = 27.6 ± 5.0; BMI ≥ 30 = 24%; Sleep <6 h = 22%; known hypertension = 13%; severe fatigue (FSS ≥ 36) = 68%; reported mistakes being made in the cockpit because of fatigue = 67%; ESS excessive sleepiness = 34%; high risk of OSA = 29%; depression (HADS ≥ 8) = 35% | Fatigue Severity Scale (FSS); Berlin Questionnaire; Epworth Sleepiness Scale (ESS); Hospital Anxiety and Depression Scale (HADS) |
| Alonso-Rodríguez and Medina-Font (2012) [21] | Evaluate C-reactive protein levels and the prevalence of metabolic syndrome | Cross-sectional; objective; Spain | n = 1009; 100%; 42 ± 11 years | elevated BP = 38%; hyperglycemia = 31%; elevated serum triglycerides = 24%; abdominal obesity = 18%; low HDL cholesterol = 8%; hs-CRP serum levels = 1.7 ± 1.8 mg/L; high hs-CRP incidence increased with age; metabolic syndrome (MS) prevalence = 15%; MS in pilots <35 years old = 4%; MS in pilots 35–50 years old = 14%; MS in pilots >50 years old = 29%; hs-CRP was significantly higher in pilots with MS than those without MS | Venous blood test; waist circumference; blood pressure; not all instruments specified |
| Ariani et al. (2016) [22] | Evaluate physical exercise habits and associated factors | Cross-sectional; subjective; Indonesia | n = 332; 100%; 20–29 years = 39%, 30–39 years = 23%, 40–49 years = 21%, 50–65 years = 16% | <150 MVPA min per week = 56%; overweight = 28% and obesity = 53%; central obesity = 46%; low or average SLS score (≤24) = 39% | BMI (self-report); Satisfaction with Life Scale (SLS); not all instruments specified |
| Bhat et al. (2019) [23] | Examine the prevalence of hypertension and obesity and their relationship | Cross-sectional; objective; India | n =1185; 89%; 35 ± 14 years | Overweight = 39% and obesity = 7%; hypertension = 11% | BMI; blood pressure |
| Bostock and Steptoe (2012) [24] | Investigate work schedule influence on diurnal cortisol rhythm | Cross-sectional; subjective; the United Kingdom | n = 30; 100%; 20–29 years = 15%, 30–39 years = 41%, 40–49 years = 30%, 50–65 years = 15% | BMI = 25.6 ± 2.5; sleep = 8.2 ± 1 h; consumed alcohol on nonwork days = 52%; exercised >10 min on nonwork days = 28% | BMI (self-report); not all instruments specified |
| Cahill et al. (2021) [25] | Investigate the relationship among work-related stress, wellbeing, and coping mechanisms | Cross-sectional; subjective; international | n = 821; 88%; <25 years = 5%, 25–35 years = 27%, 36–45 years = 31%, 46–55 years = 26%, 56–65 years = 12% | Mild depression = 40%; moderate-severity depression = 4%; severe depression = 2%; regular exercise (≥3 times per week) = 25%; perceived regular sleep difficulties = 81%; regular work stress digestive symptoms = 59%; regular work stress induced psychosocial distress = 37% | Patient Health Questionnaire-9 (PHQ-9); Oldenburg Burnout |
| Chairina et al. (2018) [26] | Identify the risk factors associated with dyslipidemia | Cross-sectional; subjective and objective; Indonesia | n = 128; 100%; not reported | Overweight = 20% and obesity = 65%; <150 MVPA min per week = 71%; inappropriate or excessive food intake = 66%; smoking = 45%; dyslipidemia = 62%; elevated TG = 29%; elevated LDL = 47%; low HDL = 57% | Instruments not specified |
| Chen et al. (2016) [27] | Evaluate metabolic syndrome and periodontal disease status | Cross-sectional; objective; China | n = 303; 100%; 35 ± 8 years | BMI = 23.6 ± 2.6; smoking = 33%; regular alcohol drinker = 20%; metabolic syndrome =3 8%; elevated waist circumference = 64%, 87.6 ± 8.5 (cm); low HDL-C levels = 46%, 1.2 ± 1.9 (mmol/L); elevated fasting plasma glucose = 30%, 5.4 ± 0.6 (mmol/L); high systolic BP = 28%, 124 ± 11 (mmHg); elevated TG levels = 28%, 1.5 ± 0.8 (mmol/L); high diastolic pressure = 17%, 79 ± 7 (mmHg) | Venous blood test; saliva test; periodontal examination; blood pressure; waist circumference; BMI; Community Periodontal Index |
| Choi and Kim (2013) [3] | Evaluate the effects of physical examination and diet consultation on risk factors for CVD | Clinical trial; subjective and objective; Korea | n = 326; 100%; 30–39 years = 47%, 40–49 years = 33%, 50–59 years = 20% | TC > 220 mg/dL = 18%; TC (mg/dL) = 236 ± 13; HDL (mg/dL) = 51 ± 11; LDL (mg/dL) = 155 ± 16; TG (mg/dL) = 154 ± 81; BMI = 24.5 ± 2.1; weight (kg) = 73 ± 8; SBP (mmHg) = 118 ± 12; DBP (mmHg) = 76 ± 9 | BMI; venous blood test; blood pressure |
| Feijó et al. (2012) [28] | Evaluate the prevalence of common mental disorders and related factors | Cross-sectional; subjective; Brazil | n = 807; 92%; 46 years | Regular physical activity practice = 61%; common mental disorders = 7% | Self-Reporting Questionnaire—20 items |
| Flynn-Evans et al. (2018) [29] | Investigate work schedule effects on neurobehavioral performance and sleep | Cross-sectional; subjective and objective; USA | n = 44; 91%; 31 ± 7 years | BMI = 24.2 ± 2.6; sleep = 6.8 ± 0.9 h; sleep latency 18%; sleep efficiency = 83%; smoking habit = 5% | Sleep diary; Psychomotor Vigilance Task; Samn–Perelli fatigue scale; actigraphy |
| Guo et al. (2017) [30] | Investigate the effects of emotional intelligence on depression and anxiety | Cross-sectional; subjective; China | n = 319; 100%; 31 ± 6 years | Mild depression = 24%; moderate depression = 1%; mild anxiety = 4%; moderate anxiety = 0.3% | Trait Meta Mood Scale; Proactive Coping Scale; The Patient Health Questionnaire (PHQ-9); Generalized Anxiety Disorder-7 (GAD-7) |
| Han et al. (2021) [31] | Investigate the occurrence of obstructive sleep apnea | Cross-sectional; subjective and objective; Korea | n = 103; 100%; 44 ± 8 years | BMI = 24.6 ± 2.1; neck circumference = 38 ± 2 (cm); OSA high risk = 32% | BMI; Epworth Sleepiness Scale (ESS); Berlin questionnaire; neck circumference; polysomnography; apnea–hypopnea index; oxygen desaturation index; respiratory disturbance index |
| Houston et al. (2010) [32] | Identify the 10 year absolute CVD risk of pilots using a cardiovascular disease risk prediction model | Cross-sectional; subjective and objective; the United Kingdom | n = 14,379; 95%; not reported | BMI = 26.0 (male) and 23.9 (female); overweight = 47% (male) and 28% (female); obesity = 12% (male) and 6% (female); smoking = 8% (male) and 6% (female); hypertension = 29% (male) and 14% (female); population 10 year absolute CVD risk = 8% ± 7%; 10 year absolute CVD risk >20% (high risk) was 9% for males and 0% for females | BMI; blood pressure; not all instruments specified |
| Huang et al. (2013) [33] | Evaluate distribution of APOE gene polymorphism, dyslipidemia, and overweight | Cross-sectional; subjective and objective; China | n = 416; 100%; 39 ± 11 years | BMI = 24.2 ± 2.5; fasting glucose = 5.2 ± 0.6 (mmol/L); smoking = 54%; regular alcohol intake = 32%; total cholesterol = 4.6 ± 0.9 (mmol/L); LDL (mmol/L) = 2.8 ± 0.8; HDL = 1.3 ± 0.3 (mmol/L); TG = 1.6 ± 0.9 (mmol/L) | BMI; venous blood test; not all instruments specified |
| Jackson and Earl (2006) [34] | Evaluate fatigue prevalence | Cross-sectional; subjective; the United Kingdom | n = 162; 94%; 38 ± 9 years, range 21–59 years | Global CFS fatigue score = 18 ± 5; severe fatigue on the CFS = 75%; “fatigue worse than 2 years ago” = 81%; “feel tired with impaired judgement while flying?” = 80%; “concerned with the level of fatigue you experience?” = 78% | Chronic Fatigue Scale (CFS) |
| Lamp et al. (2019) [35] | Evaluate sleep timing and duration | Cross-sectional; subjective and objective; USA | n = 92; 84%; 51 ± 9 years | Sleep = 8.2 ± 1.7h | Actigraphy |
| Li et al. (2021) [36] | Investigate the prevalence of functional gastrointestinal disorders and associated triggers | Cross-sectional; subjective; China | n = 212; 100%; 34 ± 7 years | BMI = 23.8 ± 2.4, range 19–29; regular alcohol = 31%; smoking = 49%; functional gastrointestinal disorder prevalence = 39% | BMI (self-report); semi-quantitative food frequency questionnaire (SQFFQ) |
| Lindgren et al. (2012) [37] | Investigate associations among digestive symptoms and diet, insomnia, and lifestyle factors | Cross-sectional; subjective; Sweden | n = 354; 91%; 49 ± 6 years | Male BMI = 25.2; female BMI = 22.7; overall overweight = 41% and obesity = 4%; smoking = 5% | BMI (self-report); not all instruments specified |
| Liu et al. (2021) [38] | Investigate health-related quality of life and its related factors | Cross-sectional; subjective; China | n = 373; 100%; 35 ± 8 years | BMI = 23.8 ± 2.2; hypertension = 7%; dyslipidemia = 19%; overweight = 46% and obesity = 3%; smoking = 39%; regular alcohol intake = 38%; physical activity days per week = 2 (range 1–3); vegetable intake ≤300 g per day = 19%; fruit intake ≤200 g per day = 33%; self-rated health (very poor or poor) = 13%; self-rated quality of life (very poor or poor) = 8%; self-rated energy and fatigue (very poor or poor) = 6% | BMI (self-report); WHOQOL-BREF; not all instruments specified |
| Marqueze et al. (2017) [39] | Evaluate factors associated with unintentional sleep at work of airline pilots | Cross-sectional; subjective; Brazil | n = 1234; 97%; 39 ± 10 years | Smoking = 7%; regular alcohol = 75%; moderate alcohol intake = 24%; harmful use of alcohol = 1%; sleep 6.9 ± 1.2 h; unintentional sleep while on duty = 58%; sleep quality “fairly or very bad” = 11%; OSA high risk = 20%; excessive sleepiness = 42% | Alcohol Use Disorders Identification Test; Karolinska Sleep Questionnaire; Berlin questionnaire; Epworth Sleepiness Scale; Work Ability Index |
| O’Hagan et al. (2017) [40] | Investigate the differences in self-reported depression or anxiety | Cross-sectional; subjective; Europe | n = 701; 95%; not reported | Depression or anxiety in the past 12 months prevalence = 54%; working >41 h per week, sleep disruption, elevated fatigue, and being female were factors associated with higher probability of reporting feeling depressed or anxious in the last 12 months | Internally validated questionnaire |
| Palmeira et al. (2016) [41] | Identify the prevalence and associated factors of overweight and obesity | Cross-sectional; subjective; Brazil | n = 1198; 100%; 39 ± 10 years, range 21–67 years | Overweight = 54% and obesity = 15%; factors associated with obesity included ≤150 min of weekly physical activity, ≤6 h of sleep during days off, sleepiness, and time of being a pilot were associated with obesity | BMI (self-report); Karolinska Sleep Questionnaire |
| Pellegrino and Marqueze (2019) [42]; Pellegrino et al. (2019) [43] | Investigate the association of work organization and sleep aspects with work ability | Cross-sectional; subjective; Brazil | n =1234; 97%; 39 ± 10 years | <150 MVPA min per week = 50%; perceived insufficient sleep = 32%; excessive sleepiness = 43%; perceived of high fatigue = 27%; OSA high risk = 21%; poor sleep quality = 48%; poor sleep quality was associated with shift characteristics, being insufficiently physically active, and sleeping <6 h on days off. | Karolinska Sleepiness Scale; Berlin questionnaire; Epworth Sleepiness Scale; Yoshitake questionnaire; Work Ability Index; Job Stress Scale; Need for Recovery Scale |
| Prombumroong et al. (2011) [44] | Evaluate the prevalence of lower-back pain and associated factors | Cross-sectional; subjective; Thailand | n = 684; 100%; 40 ± 10 years | BMI = 24.3 ± 2.8; no regular exercise = 64%; lower-back pain in the last 12 months = 56% | BMI (self-report); Job Content Questionnaire Thai version (JCQ Thai version); Nordic questionnaire for lower-back pain |
| Qiang et al. (2004) [45] | Evaluate the association of body mass index with cardiovascular disease | Cross-sectional and prospective; subjective and objective; USA | n =3019; 100%; range 45–54 years | BMI = 27.2 ± 3.4; overweight = 55% and obesity = 7%; pilots who were overweight and obese had 6% and 22% higher CVD risk, respectively | BMI; blood pressure |
| Reis et al. (2013) [46]; Reis et al. (2016) [47] | Evaluate the prevalence of fatigue and compare the differences among fatigue, sleep, and labor specificities | Cross-sectional; subjective; Portugal | n = 456; 97%; 39 ± 8 years | Total fatigue prevalence (FSS ≥ 4) = 89%; JSS ≥ 4 = 35.0%; excessive sleepiness = 59%; alcohol intake >3 times per week = 1% | Internally validated questionnaire; Fatigue Severity Scale (FSS); Epworth Sleepiness Scale (ESS); Jenkins Sleep Scale (JSS) |
| Roach et al. (2012) [48] | Evaluate the impact of work schedule on the sleep and fatigue | Cross-sectional; subjective and objective; Australia | n = 19; 100%; 54 ± 2 years | BMI = 25.0 ± 2.4; sleep hours = 7.2 h | Samn–Perelli Fatigue Checklist; actigraphy; not all instruments specified |
| Runeson-Broberg and Lindgren (2014) [49] | Assess the prevalence of musculoskeletal symptoms | Cross-sectional; subjective; Sweden | n = 354; 91%; 31–40 years = 9%, 41–50 years = 61%, 51–60 years = 29%, 61+ years = 2% | Overweight = 41% and obesity = 4%; smokers = 5% | BMI (self-report); Nordic questionnaire for analyzing musculoskeletal symptoms |
| Sallinen et al. (2017) [50]; Sallinen et al. (2021) [52] | Evaluate and compare sleep patterns, sleepiness, and management strategies | Cross-sectional; subjective and objective; Finland | n = 477; 100%; 43 ± 7 years | BMI = 25.1 ± 2.9; sleep = 7 h 27 min ± 51 min | Actigraphy; Karolinska Sleepiness Scale; BMI (self-report) |
| Sallinen et al. (2020) [51] | Compare sleepiness ratings of airline pilot and truck drivers | Cross-sectional; subjective and objective; Finland | n = 33; 100%; 44 ± 7 years | Sleep = 7 h 48 min ± 56 min; BMI = 25.6 ± 3.6; KSS = 4.0 | Actigraphy; Karolinska Sleepiness Scale; BMI (self-report); not all instruments specified |
| Signal et al. (2014) [53] | Evaluate the uptake and effectiveness of fatigue mitigation guidance material | Cross-sectional; objective; New Zealand | n = 52; 100%; 55 years | Sleep hours = 7.0 ± 1.2 h; sleep efficiency = 88 ± 5% | Actigraphy |
| Sykes et al. (2012) [6] | Compare the prevalence of medical conditions and risk factors with the general population | Cross-sectional; subjective and objective; New Zealand | n = 595; 97%; not reported | BMI = 27.1; obesity prevalence = 20%; smoking = 2%; alcoholic drink per week = 5.4 | Instruments not specified |
| Van Drongelen et al. (2014) [60]; Van Drongelen et al. (2017) [61] | Investigate the effects of an mHealth intervention to mitigate fatigue and determine risk factors for fatigue | Clinical trial; subjective; the Netherlands | n = 502; 93%; 41 ± 8 years | BMI = 24.1 ± 2.3; alcohol intake several days per week = 67%; smoking = 11.2%; CIS = 62 ± 22; moderate physical activity (days p/w) = 3.3 ± 1.9; strenuous physical activity (days p/w) = 2.0 ± 1.4; number of snacks per duty = 4.6 ± 3.6; sleep quality (1–20 scale) = 7.5 ± 3.9; sleep duration <6 h = 20%; health perception (1–5 scale, higher value denotes better health) = 3.4 ± 0.8; CIS fatigue prevalence = 30% | BMI (self-report); Checklist Individual Strength (CIS); Need for Recovery scale; Dutch Questionnaire on the Experience and Evaluation of Work; Jenkins Sleep Scale; Pittsburgh Sleep Quality Index; Short Form 36-item (SF-36) Health Survey |
| Venus and Holtforth (2021) [54] | Evaluate work schedule effects on fatigue risks on flight duty, stress, sleep problems, fatigue severity, wellbeing, and mental health | Cross-sectional; subjective; International | n = 406; 92%; 41 ± 11 years | PHQ stress = 5.0 ± 3.5; WHO5 PR (wellbeing) = 55 ± 20; PHQ-8 = 5.7 ± 4.4; SRQ-20 (common mental disorders) = 3.9 ± 4.0; Fatigue Severity Scale = 4.5 ± 1.0; Jenkins Sleep Scale = 2.0 ± 1.1; high fatigue = 33% and severe fatigue = 42%; PHQ8 ≥ 10 = 19%; GAD-7 = 3.9 ± 3.8; GAD7 ≥ 10 = 7.2% | Fatigue Severity Scale; Jenkins Sleep Scale; WHO5; Self-Reporting Questionnaire-20 (SRQ20); Patient Health Questionnaire (PHQ-8); Generalized Anxiety Disorder-7 (GAD-7) |
| Widyahening (2007) [55] | Identify the effect of work stressors and other factors on mental–emotional disturbances among airline pilots | Cross-sectional; subjective; Indonesia | n = 109; 100%; <40 years = 65%, >40 years = 56% | Mental–emotional disturbance = 39%; poor physical conditions, high work stressors, and household tension were associated with mental–emotional disturbance | Symptom Checklist 90 (SCL90) questionnaire |
| Wilson et al. (2021) [62] | Evaluate the efficacy of an intervention for enhancing health behaviors | Clinical trial; subjective; New Zealand | n = 79; 82%; 42 ± 12 years | Sleep = 7.2 ± 0.5 h; PSQI global score = 5.4 ± 2.7; weekly walking min = 110 ± 117; weekly MVPA min = 145 ± 72; <150 MVPA min per week = 49%; fruit and vegetable intake (servings/day) = 3.6 ± 0.9; <2 fruit (servings/day) = 65%; <3 vegetables (servings/day) = 47%; <5 fruit and vegetables (servings/day) = 84%; physical health score (SF-12v2) = 48 ± 7; mental health score (SF-12v2) = 51 ± 5 | Pittsburgh Sleep Quality Index (PSQI); International Physical Activity Questionnaire (IPAQ) Short Form; Short Health Form 12v2 (SF-12v2); dietary recall |
| Wilson et al. (2022) [56] | Explore the prevalence and distribution of health risk factors in airline pilots and compare these with the general population | Cross-sectional; subjective and objective; New Zealand | n = 504; 91%; 46 ± 10 years | BMI = 26.6; overweight = 51%; obesity = 16%; SBP = 131 ± 13; DBP = 81 ± 9; hypertension = 27%; sleep <7 h = 34%; sleep = 7 h 11 min; weekly MVPA = 141 ± 87; insufficient physical activity = 48%; physical health score (SF-12v2) = 47 ± 6; mental health score (SF-12v2) = 49 ± 8; fruit and vegetable intake (servings/day) = 3.7 ± 1.7; <2 fruit (servings/day) = 60%; <3 vegetables (servings/day) = 48%; <5 fruit and vegetables (servings/day) = 68%; poor or fair self-rated health = 25% | International Physical Activity Questionnaire (IPAQ) Short Form; Short Health Form 12v2 (SF-12v2); dietary recall |
| Wirawan et al. (2013) [57] | Investigate the prevalence of excessive CVD risk score | Cross-sectional; subjective and objective; Oceania | n = 595; 100%; 46 ± 12 years | BMI = 26.5 ± 4.0; smoking = 2%; alcohol consumption 5 ± 6 u/week; known hypertension = 6%; SBP = 128 ± 15; DBP = 78 ± 10; hyperlipidemia history = 10%; TC = 5.3 ± 1.1; HDL = 1.3 ± 0.5; TG = 1.1 ± 0.8; cholesterol–HDL ratio = 3.9 ± 1.4; pilots who were found to have 5 year CVD risk score of 10–15% or higher = 3.5% | Instruments not specified |
| Wu et al. (2016a) [58] | Investigate the prevalence of depression | Cross-sectional; subjective; international | n = 1826; 86%; not reported | 13% of males and 11% of females met depression threshold; 4.1% reported suicidal thoughts within the past two weeks; 5% reported experiencing fatigue daily | Patient Health Questionnaire 9 (PHQ-9) |
| Wu et al. (2016b) [59] | Characterize sleep behaviors | Cross-sectional; objective; international | n = 332; 100%; 52 years, range 23–64 years | Sleep = 7.6 h (self-report) and 6.8 h (objective); sleep ≤ 6 h = 23%; sleep > 9 h = 1% | Actigraphy and self-report |
Note: AIS = Athens Insomnia Scale; BMI = body mass index; BP = blood pressure; CIS = Checklist Individual Strength; CRP = C-reactive protein; CSF = Chronic Fatigue Scale; CVD = cardiovascular disease; DBP = diastolic blood pressure; ESS = Epworth Sleepiness Scale; FSS = Fatigue Severity Scale; GAD-7 = Generalized Anxiety Disorder-7; HADS = Hospital Anxiety and Depression Scale; HDL = high-density lipoprotein; IPAQ = International Physical Activity Questionnaire; JSS = Jenkins Sleep Scale; KSS = Karolinska Sleepiness Scale; LDL = low-density lipoprotein; mmHg = millimeters of mercury; MS = metabolic syndrome; MVPA = moderate-to-vigorous physical activity; OSA = obstructive sleep apnea; PHQ-8 = Patient Health Questionnaire 8; PHQ-9 = Patient Health Questionnaire 9; PSQI = Pittsburgh Sleep Quality Index; SBP = systolic blood pressure; SCL90 = Symptom Checklist 90; SF-36 = Short Form 36-item Health Survey; SLS = Satisfaction with Life Scale; SRQ20 = Self-Reporting Questionnaire-20; TG = triglycerides; VAFS = Visual Analog Fatigue Scale; WHOQOL-BREF = World Health Organization Quality of Life Brief Form.
References
- Miranda, J.J.; Barrientos-Gutiérrez, T.; Corvalan, C.; Hyder, A.A.; Lazo-Porras, M.; Oni, T.; Wells, J.C.K. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat. Med. 2019, 25, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, K.Y. Effects of physical examination and diet consultation on serum cholesterol and health-behavior in the Korean pilots employed in commercial airline. Ind. Health 2013, 51, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Radcliffe, S.A. The annual incapacitation rate of commercial pilots. Aviat. Space Environ. Med. 2012, 83, 42–49. [Google Scholar] [CrossRef] [PubMed]
- International Civial Aviation Authority. Manual of Civil Aviation Medicine, 3rd ed.; International Civial Aviation Authority: Montreal, QC, Canada, 2012; p. 580. [Google Scholar]
- Sykes, A.J.; Larsen, P.D.; Griffiths, R.F.; Aldington, S. A Study of Airline Pilot Morbidity. Aviat. Space Environ. Med. 2012, 83, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Driller, M.; Winwood, P.; Johnston, B.; Gill, N. The Effects of a Brief Lifestyle Intervention on the Health of Overweight Airline Pilots during COVID-19: A 12-Month Follow-Up Study. Nutrients 2021, 13, 4288. [Google Scholar] [CrossRef] [PubMed]
- Coombes, C.; Whale, A.; Hunter, R.; Christie, N. Sleepiness on the flight deck: Reported rates of occurrence and predicted fatigue risk exposure associated with UK airline pilot work schedules. Saf. Sci. 2020, 129, 104833. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Marmot, M.; Bell, R. Social determinants and non-communicable diseases: Time for integrated action. BMJ 2019, 364, l251. [Google Scholar] [CrossRef]
- Basu, N.; Yang, X.; Luben, R.N.; Whibley, D.; Macfarlane, G.J.; Wareham, N.J.; Khaw, K.-T.; Myint, P.K. Fatigue is associated with excess mortality in the general population: Results from the EPIC-Norfolk study. BMC Med. 2016, 14, 122. [Google Scholar] [CrossRef]
- Lasserre, A.M.; Strippoli, M.P.F.; Glaus, J.; Gholam-Rezaee, M.; Vandeleur, C.L.; Castelao, E.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Preisig, M. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Mol. Psychiatry 2017, 22, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Akerstedt, T.; Klemets, T.; Karlsson, D.; Habel, H.; Widman, L.; Sallinen, M. Acute and cumulative effects of scheduling on aircrew fatigue in ultra-short-haul operations. J. Sleep Res. 2021, 30, e13305. [Google Scholar] [CrossRef]
- Albermann, M.; Lehmann, M.; Eiche, C.; Schmidt, J.; Prottengeier, J. Low Back Pain in Commercial Airline Pilots. Aerosp. Med. Hum. Perform. 2020, 91, 940–947. [Google Scholar] [CrossRef]
- Alhejaili, F.; Hafez, A.; Wali, S.; Alshumrani, R.; Alzehairi, A.M.; Balkhyour, M.; Pandi-Perumal, S.R. Prevalence of Obstructive Sleep Apnea Among Saudi Pilots. Nat. Sci. Sleep 2021, 13, 537–545. [Google Scholar] [CrossRef]
- Aljurf, T.M.; Olaish, A.H.; BaHammam, A.S. Assessment of sleepiness, fatigue, and depression among Gulf Cooperation Council commercial airline pilots. Sleep Breath. 2018, 22, 411–419. [Google Scholar] [CrossRef]
- Alonso-Rodriguez, C.; Medina-Font, J. High Sensitivity C-Reactive Protein in Airline Pilots with Metabolic Syndrome. Aviat. Space Environ. Med. 2012, 83, 504–508. [Google Scholar] [CrossRef]
- Ariani, C.; Soemarko, D.S.; Yuliawati, I.; Basuki, B. Flight hours of unplanned flight and other risk factors affecting exercise habit among commercial pilots in Indonesia. Health Sci. J. Indones. 2016, 8, 36–42. [Google Scholar] [CrossRef]
- Bhat, K.G.; Verma, N.; Pant, P.; Marwaha, M.P.S. Hypertension and obesity among civil aviation pilots. Aerosp. Med. Hum. Perform. 2019, 90, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Bostock, S.; Steptoe, A. Influences of early shift work on the diurnal cortisol rhythm, mood and sleep: Within-subject variation in male airline pilots. Psychoneuroendocrinology 2013, 38, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.; Cullen, P.; Anwer, S.; Wilson, S.; Gaynor, K. Pilot Work Related Stress (WRS), Effects on Wellbeing and Mental Health, and Coping Methods. Int. J. Aerosp. Psychol. 2021, 31, 87–109. [Google Scholar] [CrossRef]
- Chairina, N.; Werdhani, R.A.; Gathmyr, D. Association of total flight hours with lipid blood profiles among civilian pilots in Indonesia. J. Phys. Conf. Ser. 2018, 1073, 042012. [Google Scholar] [CrossRef]
- Chen, X.; Xie, L.; Liu, Y.; Chen, D.J.; Yu, Q.; Gan, X.Q.; Yu, H.Y. Metabolic Syndrome and Periodontal Disease Among Civilian Pilots. Aerosp. Med. Hum. Perform. 2016, 87, 1016–1020. [Google Scholar] [CrossRef]
- Feijo, D.; Luiz, R.R.; Camara, V.M. Common Mental Disorders Among Civil Aviation Pilots. Aviat. Space Environ. Med. 2012, 83, 509–513. [Google Scholar] [CrossRef]
- Flynn-Evans, E.E.; Arsintescu, L.; Gregory, K.; Mulligan, J.; Nowinski, J.; Feary, M. Sleep and neurobehavioral performance vary by work start time during non-traditional day shifts. Sleep Health 2018, 4, 476–484. [Google Scholar] [CrossRef]
- Guo, Y.N.; Ji, M.; You, X.Q.; Huang, J. Protective Effects of Emotional Intelligence and Proactive Coping on Civil Pilots’ Mental Health. Aerosp. Med. Hum. Perform. 2017, 88, 858–865. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, G.Y.; Hyun, W.; Kim, Y.; Jang, J.S. Obstructive sleep apnea in airline pilots during daytime sleep following overnight flights. J. Sleep Res. 2021, 30, e13375. [Google Scholar] [CrossRef]
- Houston, S.; Mitchell, S.; Evans, S. Application of a Cardiovascular Disease Risk Prediction Model Among Commercial Pilots. Aviat. Space Environ. Med. 2010, 81, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.; Feng, Y.J.; Chen, W.R. The distribution of apolipoprotein E gene polymorphism in Chinese civil aircrews, and a possible risk factor to their overweight and dyslipidemia is cumulative flight time. Clin. Chim. Acta 2013, 416, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.A.; Earl, L. Prevalence of fatigue among commercial pilots. Occup. Med.-Oxf. 2006, 56, 263–268. [Google Scholar] [CrossRef]
- Lamp, A.; McCullough, D.; Chen, J.M.C.; Brown, R.E.; Belenky, G. Pilot Sleep in Long-Range and Ultra-Long-Range Commercial Flights. Aerosp. Med. Hum. Perform. 2019, 90, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, J.R.; Yin, D.W.; Zhang, Y.H.; Shan, D.Z.; Jiang, X.; Shang, L. Prevalence and trigger factors of functional gastrointestinal disorders among male civil pilots in China. Sci. Rep. 2021, 11, 2021. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, T.; Runeson, R.; Wahlstedt, K.; Wleslander, G.; Dammstrom, B.G.; Norback, D. Digestive Functional Symptoms Among Commercial Pilots in Relation to Diet, Insomnia, and lifestyle Factors. Aviat. Space Environ. Med. 2012, 83, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.B.; Qiu, B.; Zhang, C.Y.; Deng, M.Z.; Liang, Z.H.; Qi, Y.M. Health-related quality of life in pilots of a Chinese commercial airline. Arch. Environ. Occup. Health 2021, 76, 511–517. [Google Scholar] [CrossRef]
- Marqueze, E.C.; Nicola, A.C.B.; Diniz, D.; Fischer, F.M. Working hours associated with unintentional sleep at work among airline pilots. Rev. De Saude Publica 2017, 51, 61. [Google Scholar] [CrossRef]
- O’Hagan, A.D.; Issartel, J.; Nevill, A.; Warrington, G. Flying Into Depression: Pilot’s Sleep and Fatigue Experiences Can Explain Differences in Perceived Depression and Anxiety Associated With Duty Hours. Workplace Health Saf. 2017, 65, 109–117. [Google Scholar] [CrossRef]
- Palmeira, M.L.D.; Marqueze, E.C. Excess weight in regular aviation pilots associated with work and sleep characteristics. Sleep Sci. 2016, 9, 266–271. [Google Scholar] [CrossRef]
- Pellegrino, P.; Marqueze, E.C. Aspects of work and sleep associated with work ability in regular aviation pilots. Rev. De Saude Publica 2019, 53, 31. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, P.; Moreno, C.R.D.; Marqueze, E.C. Aspects of work organization and reduced sleep quality of airline pilots. Sleep Sci. 2019, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Prombumroong, J.; Janwantanakul, P.; Pensri, P. Prevalence of and Biopsychosocial Factors Associated with Low Back Pain in Commercial Airline Pilots. Aviat. Space Environ. Med. 2011, 82, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Li, G.; Rebok, G.W.; Baker, S.P. Body mass index and cardiovascular disease in a birth cohort of commuter air carrier and air taxi pilots. Ann. Epidemiol. 2005, 15, 247–252. [Google Scholar] [CrossRef]
- Reis, C.; Mestre, C.; Canhao, H. Prevalence of Fatigue in a Group of Airline Pilots. Aviat. Space Environ. Med. 2013, 84, 828–833. [Google Scholar] [CrossRef]
- Reis, C.; Mestre, C.; Canhao, H.; Gradwell, D.; Paiva, T. Sleep and Fatigue Differences in the Two Most Common Types of Commercial Flight Operations. Aerosp. Med. Hum. Perform. 2016, 87, 811–815. [Google Scholar] [CrossRef]
- Roach, G.D.; Petrilli, R.M.A.; Dawson, D.; Lamond, N. Impact of Layover Length on Sleep, Subjective Fatigue Levels, and Sustained Attention of Long-Haul Airline Pilots. Chronobiol. Int. 2012, 29, 580–586. [Google Scholar] [CrossRef]
- Runeson-Broberg, R.; Lindgren, T.; Norback, D. Musculoskeletal symptoms and psychosocial work environment, among Swedish commercial pilots. Int. Arch. Occup. Environ. Health 2014, 87, 685–693. [Google Scholar] [CrossRef]
- Sallinen, M.; Sihvola, M.; Puttonen, S.; Ketola, K.; Tuori, A.; Harma, M.; Kecklund, G.; Akerstedt, T. Sleep, alertness and alertness management among commercial airline pilots on short-haul and long-haul flights. Accid. Anal. Prev. 2017, 98, 320–329. [Google Scholar] [CrossRef]
- Sallinen, M.; Pylkkonen, M.; Puttonen, S.; Sihvola, M.; Akerstedt, T. Are long-haul truck drivers unusually alert? A comparison with long-haul airline pilots. Accid. Anal. Prev. 2020, 137, 105442. [Google Scholar] [CrossRef]
- Sallinen, M.; Onninen, J.; Ketola, K.; Puttonen, S.; Tuori, A.; Virkkala, J.; Akerstedt, T. Self-reported reasons for on-duty sleepiness among commercial airline pilots. Chronobiol. Int. 2021, 38, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Signal, T.L.; Mulrine, H.M.; van den Berg, M.J.; Smith, A.A.; Gander, P.H.; Serfontein, W. Mitigating and monitoring flight crew fatigue on a westward ultra-long-range flight. Aviat. Space Environ. Med. 2014, 85, 1199–1208. [Google Scholar] [CrossRef]
- Venus, M.; Holtforth, M.G. Short and Long Haul Pilots Rosters, Stress, Sleep Problems, Fatigue, Mental Health, and Well-Being. Aerosp. Med. Hum. Perform. 2021, 92, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Widyahening, I.S. High level of work stressors increase the risk of mental-emotional disturbances among airline pilots. Med. J. Indones. 2007, 16, 117–121. [Google Scholar] [CrossRef][Green Version]
- Wilson, D.; Driller, M.; Johnston, B.; Gill, N. The prevalence and distribution of health risk factors in airline pilots: A cross-sectional comparison with the general population. Aust. N. Z. J. Public Health 2022. [Google Scholar] [CrossRef]
- Wirawan, I.M.A.; Aldington, S.; Griffiths, R.F.; Ellis, C.J.; Larsen, P.D. Cardiovascular Investigations of Airline Pilots with Excessive Cardiovascular Risk. Aviat. Space Environ. Med. 2013, 84, 608–612. [Google Scholar] [CrossRef]
- Wu, A.C.; Donnelly-McLay, D.; Weisskopf, M.G.; McNeely, E.; Betancourt, T.S.; Allen, J.G. Airplane pilot mental health and suicidal thoughts: A cross-sectional descriptive study via anonymous web-based survey. Environ. Health A Glob. Access Sci. Source 2016, 15, 121. [Google Scholar] [CrossRef]
- Wu, L.J.; Gander, P.H.; van den Berg, M.J.; Signal, T.L. Estimating long-haul airline pilots’ at-home baseline sleep duration. Sleep Health 2016, 2, 143–145. [Google Scholar] [CrossRef]
- van Drongelen, A.; Boot, C.R.L.; Hlobil, H.; Twisk, J.W.R.; Smid, T.; van der Beek, A.J. Evaluation of an mHealth intervention aiming to improve health-related behavior and sleep and reduce fatigue among airline pilots. Scand. J. Work Environ. Health 2014, 40, 557–568. [Google Scholar] [CrossRef]
- van Drongelen, A.; Boot, C.R.L.; Hlobil, H.; Smid, T.; van der Beek, A.J. Risk factors for fatigue among airline pilots. Int. Arch. Occup. Environ. Health 2017, 90, 39–47. [Google Scholar] [CrossRef]
- Wilson, D.; Driller, M.; Ben, J.; Gill, N. The effectiveness of a 17-week lifestyle intervention on health behaviors among airline pilots during COVID-19. J. Sport Health Sci. 2021, 10, 333–340. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- De Stavola, B.L.; Pizzi, C.; Clemens, F.; Evans, S.A.; Evans, A.D.; Silva, I.D. Cause-specific mortality in professional flight crew and air traffic control officers: Findings from two UK population-based cohorts of over 20,000 subjects. Int. Arch. Occup. Environ. Health 2012, 85, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, C.; Evans, S.A.; De Stavola, B.L.; Evans, A.; Clemens, F.; Silva, I.D. Lifestyle of UK commercial aircrews relative to air traffic controllers and the general population. Aviat. Space Environ. Med. 2008, 79, 964–974. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health guidelines. Arch. Intern. Med. 2002, 162, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Russell-Jones, D.L.; Hutchison, E.J.; Roberts, G.A. Pilots flying with insulin-treated diabetes. Diabetes Obes. Metab. 2021, 23, 1439–1444. [Google Scholar] [CrossRef]
- Wingelaar-Jagt, Y.Q.; Wingelaar, T.T.; Riedel, W.J.; Ramaekers, J.G. Fatigue in Aviation: Safety Risks, Preventive Strategies and Pharmacological Interventions. Front. Physiol. 2021, 12, 712628. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cunningham, T.J.; Croft, J.B. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep 2015, 38, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.J.; Powell, D.; Broadbent, E. Fatigue self-management strategies and reported fatigue in international pilots. Ergonomics 2004, 47, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y.; et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef]
- Kraus, C.K.; Li, G. Pilot alcohol violations reported in U.S. newspapers, 1990-2006. Aviat. Space Environ. Med. 2006, 77, 1288–1290. [Google Scholar]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Murphy, M.M.; Barraj, L.M.; Spungen, J.H.; Herman, D.R.; Randolph, R.K. Global assessment of select phytonutrient intakes by level of fruit and vegetable consumption. Br. J. Nutr. 2014, 112, 1004–1018. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).