Abstract
Adolescent COVID-19 vaccination has stalled at 53% in the United States. Vaccinating adolescents remains critical to preventing the continued transmission of COVID-19, the emergence of variants, and rare but serious disease in children, and it is the best preventive measure available to return to in-person schooling. We investigated parent–adolescent COVID-19 vaccine decision-making. Between 24 February and 15 March 2021, we conducted surveys and 12 focus groups with 46 parent–adolescent dyads in Southern California. Parents and adolescents completed a survey prior to participation in a focus group discussion, which focused on exploring COVID-19 vaccine acceptance or uncertainty and was guided by the 5C vaccine hesitancy model. Parents uncertain about vaccinating adolescents expressed low vaccine confidence and high COVID-19 disease risk complacency. Parents who accepted COVID-19 vaccination for adolescents expressed high confidence in health authority vaccine recommendations, high perceived COVID-19 risk, and collective responsibility to vaccinate children. Additionally, unique pandemic-related factors of vaccine acceptance included vaccinating for emotional health, resuming social activities, and vaccine mandates. Among parents, 46% were willing to vaccinate their adolescent, 11% were not, and 43% were unsure. Among adolescents, 63% were willing to vaccinate. Despite vaccine availability, 47% of adolescents remain unvaccinated against COVID-19. Factors associated with vaccine uncertainty and acceptability inform health care practitioner, school, community, and public health messaging to reach parents and adolescents.
1. Introduction
An estimated 72.8 million children live in the United States, comprising 22.2% of the population. Adolescents aged 12–17 comprise 7.7% of that group, or 25.1 million children [1,2]. Reaching community immunity against COVID-19 and preventing the continued spread of COVID-19 and emergence of new variants requires pediatric vaccination [3]. Adolescents are vectors of SARS-CoV-2 transmission [4,5] and therefore, each adolescent vaccinated reduces the risk of viral infection in the community [6,7]. These are indirect benefits of vaccination for adolescents. Nevertheless, these indirect benefits are justified, given vaccination’s role in reducing the continued spread of emerging variants such as the delta or omicron variant, offering greater protection for children attending in-person school and social activities, and preventing disruptions to daily life [4].
However, vaccinating adolescents against COVID-19 also has direct benefits. Although severe illness in adolescents is rare, vaccination is warranted, given that it can effectively prevent severe illness, hospitalization, and long COVID-19 [8,9]. Since the beginning of the pandemic, more than 7.5 million children including adolescents have been confirmed positive for COVID-19, representing 1 in 10 children or an estimated 20.8% of cumulative COVID-19 cases, as of December 2021 [10]. Only 24 states report on hospitalization among children, which appears to be uncommon [10,11,12]. However, more than 8000 children have experienced severe illness, and among adolescent COVID-19-related hospitalizations, there have been 721 reported deaths, many of which occurred among Black and Hispanic adolescents [13]. Vaccines are available and provide protection against severe COVID-19, chronic post-COVID-19 sequelae (i.e., long COVID-19), and rare albeit serious complications, such as multisystem inflammatory disease in children [14,15]. Hospitalization among unvaccinated adolescents is 10 times higher than vaccinated adolescents [16].
Southern California, and Orange County in particular, features diverse communities in terms of racial and ethnic makeup, political ideologies, and a history of communities of color being disproportionately affected by COVID-19 during the pandemic [17,18,19]. Since the 2000s, Orange County has witnessed dramatic growth, with more than 3 million residents, of which 35% are Hispanic or Latino and 26% of those 35% who speak Spanish at home; 21% of its population is Asian; 46% of households speak another language at home; an estimated 35% lives in multigenerational households; and 30% was born in another country [18]. As part of California’s COVID-19 policy, Orange County families also experienced being part of the first state in the country to close in-person schools and transition to virtual learning early in the pandemic. By early 2021, many families were eager for their children to return to school, and families, pediatricians, epidemiologists, and scholars were anticipating the emergency approval and recommendation for the COVID-19 vaccine to become available to adolescents [20,21].
On 10 May 2021, the Pfizer BioNTech COVID-19 vaccine became available to adolescents aged 12–17 in the United States after the U.S. Food and Drug Administration issued an emergency use authorization [22]. Although there was an initial peak in vaccine uptake in June 2021, 7 months later, at the end of 2021, only 53% of adolescents had completed their vaccination series [13]. Parental willingness to vaccinate adolescents against COVID-19 varies widely [21,23,24]. Research exploring parental decision-making is needed to inform the design of effective communication that promotes vaccination. Rare incidents of vaccine-induced myocarditis [25,26] also affected parent decision-making, especially among parents of adolescent sons; however, the Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention (CDC) deemed that the vaccine benefits outweighed the rare myocarditis risk for vaccinating adolescents, especially adolescents with comorbidities [26].
The 5C vaccine hesitancy model gives insight into factors that contribute to vaccine uncertainty or acceptance. The 5C model consists of five constructs: (a) vaccine confidence (trust in the safety and effectiveness of vaccines, the development process, the system that delivers them, and the competence of health care providers); (b) risk complacency (perceptions regarding disease risk and vaccine utility); (c) vaccine constraints (practical barriers to vaccinating such as availability, affordability, and health literacy); (d) vaccine calculation (the need for extensive information searching and weighing vaccine benefits and risks); and (e) collective responsibility to vaccinate for others [27]. The following study was guided by the 5C vaccine hesitancy model and aimed to qualitatively explore parental COVID-19 vaccine decision-making for their adolescent children.
2. Materials and Methods
2.1. Study Design, Participants, and Setting
The study consisted of recruiting parent–adolescent dyads from Orange County, California, to explore factors that contribute to COVID-19 vaccination uncertainty or acceptance. People of color became a majority minority in the county in the early 2000s. Orange County has outpaced the nation in its dramatic population growth and demographic transformation, driven by growing Latino and Asian American populations. The 35% Hispanic minority, with 26.8% speaking Spanish at home and many of whom live in Santa Ana and Anaheim, have been disproportionately affected by COVID-19 [17]. Another sizeable minority in Orange County is Asians at 21% of the population. A sizable subgroup and enclave of Vietnamese people reside in the county and are characterized by diverse political ideologies. More than 700,000 people in California live in multigenerational households, with a sizeable portion in Orange County, and 30% of county residents were born in another country [18]. Our recruitment sampling methods mirrored the county’s demographics, sampling based on the diverse race and ethnic makeup, Spanish-speaking residents, multigenerational households, diverse occupations, educational attainment, and income.
Parents and adolescents completed a one-time electronic survey prior to participation in a one-time virtual focus group discussion. The 5C model guided development of the survey and the focus group discussion guide. Analysis of parent COVID-19 vaccine decision-making for their adolescent children is presented in this article, as are the adolescent survey results. Dyadic analysis of adolescent–parent discussion is reported separately.
2.2. Data Collection
Between 24 February and 15 March 2021 (prior to the emergency authorization of the Pfizer BioNTech vaccine for adolescents aged 12–17), we conducted 12 virtual focus groups with parent–adolescent dyads in Orange County. Interested parents contacted the study coordinator, with whom eligibility was confirmed per telephone. Families, i.e., parent–adolescent dyads, provided verbal informed consent by telephone before they agreed to participate in the study. The study purpose and involvement was explained (one-time survey and one-time virtual focus group discussion), as was the voluntary nature of participation and the USD $50 compensation. Participants received a study information sheet (written summary of what was reviewed) by email, and parents also received a link for each parent and adolescent to an electronic survey to complete prior to the virtual focus group. In two instances, the email was sent to the adolescent when the parent did not have an email address. A 24-h period was given prior to scheduling focus groups. Of the 12 focus groups, nine were held in English and three in Spanish. Each focus group was moderated by one of five trained moderators. After logging into the Zoom meeting together, parents and adolescents introduced themselves. Parents were then asked to step away while adolescents had a 30-min discussion with the moderator. Parents were then asked to return (and adolescents to step away) to participate in a 60-min discussion. Focus groups were video recorded for accuracy, and participants were asked to keep their web cameras on. The size of the focus groups ranged from three to six participants. Six focus groups were composed of three parents, three groups had four parents, two groups had five parents, and one group had six parents. Households received a USD $50 electronic gift card as compensation.
2.3. Recruitment
We virtually recruited with the assistance of community partners who distributed electronic flyers (in English and Spanish) through local schools, listservs, and Children’s Hospital of Orange County. Parents were recruited from communities known to have higher COVID-19 morbidity and mortality risk attributed to race and ethnicity (e.g., Santa Ana), live in multigenerational households, have children with chronic conditions, and reflect a range of parental occupations [17]. Parents were screened by telephone for eligibility: (a) parent of a middle or high school student aged 11–18 years old; (b) English or Spanish speaker; and (c) Orange County resident. Parents with multiple eligible adolescents were asked to consider one for enrollment in the study. The study coordinator verbally reviewed the study purpose and participant involvement, as previously described, then the parent and the adolescent agreed to participate verbally by telephone prior to scheduling the focus group (24 h were given prior to scheduling). Families received the study information by email to the parent. The study was approved by the university’s institutional review board to comply with human protections (HS#2021-6423). Signed informed consent was waived.
2.4. Parent Survey and Focus Group Discussion Guide
The electronic 54-item survey and semistructured focus group guide were developed for parents and adolescents (see Supplemental Material). This article reports predominantly on the parents’ responses, focusing on the discovery of factors contributing to vaccine uncertainty and confidence. Both study instruments (survey and focus group discussion guide) were informed by the 5C vaccine hesitancy model to investigate aspects of vaccine confidence, COVID-19 perceived risk for adolescents, constraints to vaccinating, perceptions of collective responsibility to vaccinate, and calculation (extent of deliberation about vaccine benefits and risks). Survey domains included questions regarding parent–child vaccine communication, ranking of COVID-19 vaccine concerns and motivators to vaccinate, parenting style, positive expectancies regarding the pending approval of adolescent COVID-19 vaccination, decision-making under conditions of uncertainty, vaccine information sources, and sociodemographics, including adolescents’ receipt of the influenza vaccine in the last year. See Table 1 for parent survey question domains. The adolescent survey and focus group discussion guide were similar but adjusted for an adolescent perspective and language.

Table 1.
Overview of Survey Question Domains for Parents.
The focus group discussion guide consisted of five parts: (a) parental decision-making for vaccinating their children in general, (b) parental decision-making for COVID-19 vaccination, (c) desired COVID-19 vaccine information related to adolescents, (d) vaccine information sources, and (e) thoughts on vaccinating children 11 years old or younger (see Table 2).

Table 2.
Focus Group Discussion Guide.
2.5. Data Analysis
For the survey, the data analysis consisted of calculating descriptive statistics (i.e., frequencies, percentages, and means) for survey responses related to the COVID-19 vaccination status of parents, vaccine intent for children, adolescent influenza vaccination status, ranking of COVID-19 vaccine concerns and motivators, and family (parent and adolescent) sociodemographic information.
Qualitative data analysis was conducted of the transcribed audio-recordings of the parent and adolescent focus group discussions. Data were transcribed verbatim for accuracy and personal identifiers were replaced with pseudonyms. The Spanish recorded data were transcribed into Spanish first, then back translated into English to ensure accuracy. We took a phronetic iterative approach [28,29,30] to analyze the data and discover emergent themes relevant to parent COVID-19 vaccine decision-making. Phronetic refers to the Greek word phronesis, which prioritizes contextual knowledge. An iterative approach to data analysis involved first inductively analyzing the data line-by-line and tagging segments with descriptive codes (describing what was said and developing labels), and then using the 5C vaccine hesitancy model to deductively analyze the extent to which parents expressed aspects of the five Cs of vaccine acceptability: confidence, COVID-19 risk complacency, constraints (practical barriers), calculation (weighing benefits and risks to vaccinating), and collective responsibility to vaccinate children against COVID-19 once the emergency authorization for adolescents is approved [28,30]. All coders initially independently read the transcript data (i.e., data immersion). Segments of data were then tagged and labeled with descriptive codes (primary coding). A codebook was then developed, containing transcript quotes exemplifying aspects of research questions (e.g., what influences decision-making regarding vaccinating, with 32 descriptive codes for contributors to vaccine uncertainty and 30 descriptive codes for contributors to vaccine acceptability). For data analysis of the Spanish language data, two coders fluent in Spanish analyzed the data, whereas another coder analyzed the English version of the Spanish data. Coders met to discuss emergent codes from the data and organize codes into themes. Secondary data analysis involved organizing the descriptive codes into higher-order themes as they related to answering research questions regarding what factors contribute to parent acceptance or uncertainty to vaccinate their children against COVID-19 when the vaccine is approved for adolescents. Data and supporting quotes were organized into parent COVID-19 vaccine decision-making themes, which expressed vaccine uncertainty and acceptance, corresponding to the 5C model constructs [27,31]. Vaccine decision-making factors not recognized by the 5C model were identified and characterized as additional, newly discovered unique pandemic-related factors motivating vaccine acceptance or uncertainty.
3. Results
The 12 focus groups featured 46 parents and 46 adolescents. The mean parent age was 45 and the mean adolescent age was 14. All parents except one were mothers, most parents (74%) had a bachelor’s degree education or higher, nearly half of parents (48%) reported being Latino, 22% of parents reported having an adolescent with a chronic medical condition, and 30% of parents lived in multigenerational households. More than half of adolescents identified as male, most (93%) attended online distance learning for school at the time of the study, and many (67%) had been vaccinated against influenza in the last year. Nearly half of parents (46%) were willing to vaccinate their adolescent against COVID-19, 11% were not, and 43% were unsure. Many adolescents (63%) were willing to be vaccinated, 20% neither agreed nor disagreed, and 17% said they were not willing to be vaccinated. Among parents, 28% were vaccinated against COVID-19 at the time of the study. Sociodemographics are reported in Table 3.

Table 3.
Parent and Adolescent Dyad Demographics (N = 46).
Survey results of parents’ and adolescents’ ranking of COVID-19 vaccination concerns and motivators are shown in Figure 1, Figure 2, Figure 3 and Figure 4. Their concerns centered on vaccine confidence, with top concerns for parents focusing on long-term vaccine side effects. One of the adolescents’ top concerns in addition to confidence was fear of needles. Parents’ and adolescents’ motivators to vaccinate showed that confidence in the vaccine ranked at the top. Additionally, unique pandemic-related factors that motivated willingness to vaccinate included vaccinating for social benefit and collective responsibility.
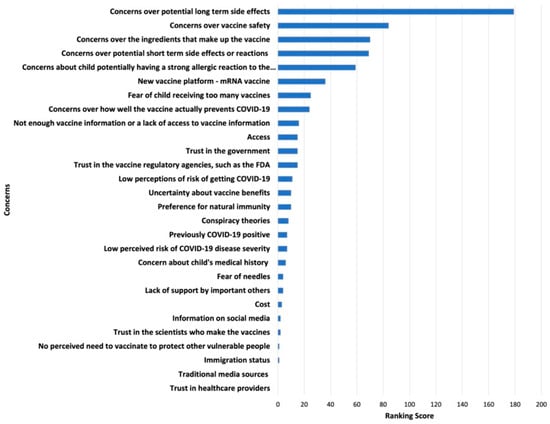
Figure 1.
Top Parental Concerns for Vaccinating Adolescents against COVID-19.
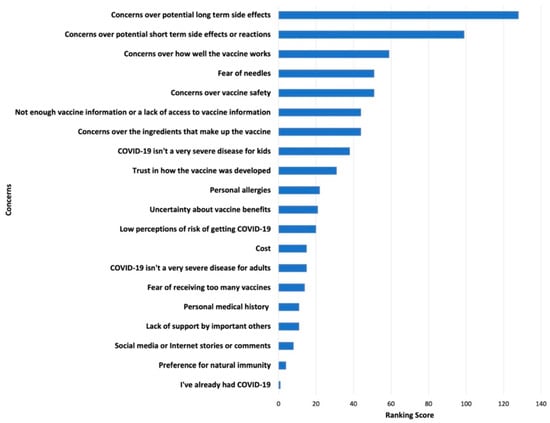
Figure 2.
Top Adolescent Concerns for Vaccinating against COVID-19.
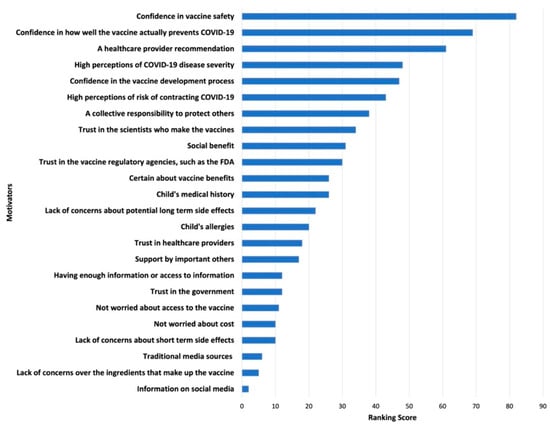
Figure 3.
Top Parental Motivators for Vaccinating Adolescents against COVID-19.
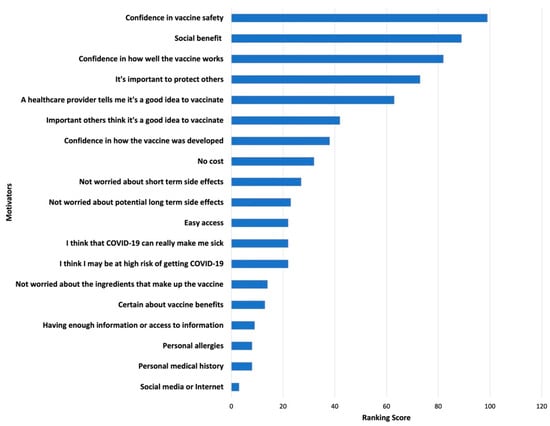
Figure 4.
Top Adolescent Motivators for Vaccinating against COVID-19.
For the qualitative analysis, results are reported as constructs from the 5C model that contributed to COVID-19 vaccine uncertainty or acceptance among parents (see Figure 5).
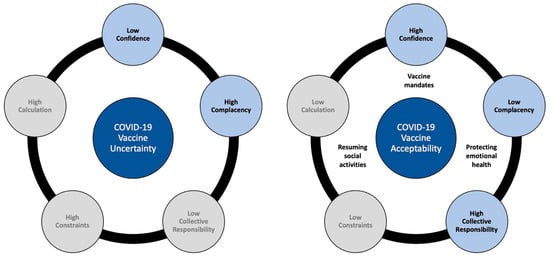
Figure 5.
Graphical Representation of Qualitative Results.
3.1. COVID-19 Vaccine Uncertainty
From analysis of parents’ discussions expressing vaccine uncertainty, two of the five Cs emerged as relevant: low confidence in the COVID-19 vaccine and high complacency about disease risk for adolescents. Constraints, calculation, and collective responsibility constructs did not emerge among parent expressing vaccine uncertainty.
3.1.1. Low Confidence in the COVID-19 Vaccine
A key theme regarding parental vaccine uncertainty was expressions of low confidence in the COVID-19 vaccine. Many parents (n = 32 of 46) expressed low vaccine confidence in 20 distinct ways. Parents raised concerns about the vaccine’s safety and effectiveness and described mistrust in pharmaceutical companies, regulatory agencies, public health authorities, and health care providers. For vaccine safety, parents expressed concerns over unknown long-term side effects on their adolescent’s health and development, an unpredictable immune response to the vaccine, the possibility of the vaccine exacerbating pre-existing medical condition such as asthma, and uncertainty about vaccine ingredients. Parents who expressed greater worry about vaccine effects expressed concerns for younger (i.e., preteen) and smaller children being able to handle the immune response induced by the vaccine. When discussing vaccine effectiveness, parents expressed concerns over short-lived immunity and a lack of protection against emerging variants.
Parents’ mistrust in pharmaceutical companies and regulatory agencies was expressed in their comments about the COVID-19 vaccine development process being rushed and there being “corners cut”. Parents questioned whether pharmaceutical companies valued and prioritized the health of children. Additionally, some parents expressed that vaccine companies lacked transparency about the vaccine’s ingredients and technologies (i.e., mRNA platform), which led to skepticism and hesitation. Parents’ vaccine uncertainty was expressed in their discussion about the political and public health pressure to approve and roll out a vaccine quickly.
Parents also reported mistrust in public health authorities and health care providers. A few participants doubted public health agencies such as the CDC, given their inconsistent mask-wearing recommendations. Some parents noted a preference for discussing the COVID-19 vaccine with family or friends working in health care, rather than their own provider or their adolescent’s pediatrician. These parents perceived some health care providers as having a vested position in not questioning the credibility of the vaccine. A few parents also expressed low confidence in providers knowing much about the vaccine or its ingredients.
Ultimately, for some parents, vaccine concerns and mistrust undermined the confidence needed to view the vaccination as a protective measure for adolescents. Parents described waiting months to years to vaccinate their adolescent, to observe the vaccine’s effect on other children. Uncertain parents emphasized needing more long-term clinical data and time to consider vaccinating their adolescent.
3.1.2. High Complacency regarding COVID-19 Disease Risk
Slightly more than one third of parents (32.6%, n = 15), did not perceive COVID-19 as a significant health threat to their adolescent children or perceived COVID-19 risk as manageable. High disease complacency was expressed in 12 ways. Parents reported that their adolescent either was not at risk of contracting SARS-CoV-2 or had already contracted the virus and the risk was manageable. Parents cited sheltering in place, social distancing, and online distance learning as measures that safeguarded children. Managing risk of infection was also facilitated by a parent’s ability to control their adolescent’s social activity. Parents who reported less compliance with preventive measures often acknowledged their adolescent’s risk of exposure but normalized the risk. One parent discussed not feeling at risk because the virus seemed impossible to contract despite them attending several social functions during the pandemic. Some parents attributed this lack of susceptibility to the adolescent’s immune system.
Low risk perceptions related to disease severity were expressed among parents of healthy children. Low risk perceptions persisted even among parents who had personal experiences with COVID-19 or who knew someone who had contracted the disease. For example, one mother whose son previously had COVID-19 described the illness as “manageable” and said that her son was “resilient”. She described being more worried about the stigma and inconvenience of having an infected child than the disease. Parents of young adolescents did not perceive the vaccine as necessary and reported an intent to delay vaccination due to low risk perceptions. Participant quotes highlighting these findings are presented in Table 4.

Table 4.
Uncertainty in Vaccine Decision-Making.
3.2. COVID-19 Vaccine Acceptability
From analysis of parent discussions expressing vaccine acceptance, three of the five Cs emerged as relevant: high confidence in the COVID-19 vaccine, low complacency regarding disease risk, and a collective responsibility to vaccinate. Three additional unique pandemic-related factors emerged as relevant in shaping parents’ acceptance of vaccinating adolescents: vaccinating for emotional health, resuming social activities, and vaccine mandates as motivators.
3.2.1. High Confidence in the COVID-19 Vaccine
Close to 40% (39.1%, n = 18) of parents expressed high vaccine confidence, and they expressed it in seven ways. They discussed trusting their doctor, expressing trust in science, personal observations that others were vaccinated without complications, family norms to vaccinate, perceiving the vaccine as safe due to vaccine trial information, messages from public health authorities encouraging vaccination, and perceiving their older adolescent children as able to handle the immune response like adults. An explicit, strong health care provider recommendation to vaccinate adolescents certainly carried weight in deciding whether to vaccinate adolescents against COVID-19. One parent expressed that she needed to be “talked off the ledge” by her children’s pediatrician to vaccinate her children against COVID-19 when it becomes available. Parents often described health care providers as educated and positioned to increase vaccine literacy. Participants expressed interest in wanting to understand the science behind the vaccine before deciding whether to vaccinate. Parents of adolescents with underlying health conditions valued recommendations by their child’s health care specialist. Parents also stated they would consider the recommendations of family and friends in the health care field. Overall, parents cited trust in science and providers as a reason for being more accepting of a COVID-19 vaccine for adolescents.
Greater confidence in the COVID-19 vaccine was ascribed by parents when they articulated being more confident about vaccinating, because they personally knew individuals who had been vaccinated without side effects or complications. Additionally, parents described having confidence in vaccinating because of growing up in a family or household where vaccinating was perceived as protective and normative.
3.2.2. Low Complacency regarding COVID-19 Disease Risk
Parents who intended to vaccinate their adolescents were more concerned about the risk of disease than vaccine side effects. Low complacency was expressed in eight ways by 37% of parents (n = 17). Parents voiced concerns about severe disease and death from COVID-19. Some parents were worried that contracting SARS-CoV-2 would exacerbate their adolescent’s pre-existing health conditions. Parents with high risk perceptions shared stories about prior illnesses in the household and stated that these experiences set the context for why they intended to vaccinate their adolescent.
3.2.3. High Collective Responsibility to Vaccinate
Collective responsibility was expressed in eight ways by 43.5% of parents (n = 20). Parents reported wanting to vaccinate their adolescent to protect their social circle: older grandparents living in the household, the adolescent’s teachers, neighbors, local grocery workers, and other individuals with whom their adolescent interacts (e.g., friends, friends’ grandparents, and teammates). Although parents did not perceive children to be at risk of severe disease, many worried about their adolescent being a source of viral transmission. Fear of infecting others was associated with feelings of guilt. Participant quotes highlighting vaccine acceptance are presented in Table 5.

Table 5.
Vaccine Acceptability.
3.2.4. Vaccinating for Emotional Health
Unique to the pandemic, 23.9% of parents (n = 11) discussed vaccinating their adolescent for emotional health. Parents discussed how socially isolated their children had been in the past year and expressed the desire for their adolescent to attend school in person. Parents worried about the long-term impact of distance learning on their child’s development. Parents also described the COVID-19 vaccine as a way to end the pandemic and return to normal life, citing vaccine intentions for their own emotional health and describing fatigue from living in fear during the pandemic. Parents perceived vaccination as offering a sense of security and emotional relief.
3.2.5. Vaccinating to Resume Social Activities
Related to emotional health, 21.7% of parents (n = 10) discussed vaccinating to resume social activities, including in-person schooling, afterschool activities, sports events, school graduation (from middle or high school), birthday parties, sleepovers, and socializing with family and friends. Vaccinating adolescents would allow them to safely resume social activities.
3.2.6. Vaccinating Due to Mandates
A smaller percentage of parents, 17.4% (n = 8), reported that they would vaccinate their adolescents only if vaccines were mandated. Vaccine mandates included those issued by the adolescent’s middle or high school and the state, but parents also discussed of mandates for college enrollment, travel, and work. A few parents expressed concerns over schools preemptively mandating vaccination. Participant quotes highlighting pandemic-related factors are presented in Table 6.

Table 6.
Pandemic-Related Factors in COVID-19 Vaccine Decision-Making.
4. Discussion
This study identified how parents expressed COVID-19 vaccine acceptance or uncertainty for their adolescent children early in the pandemic, when vaccination for adults had just recently been approved by emergency authorization and approval for adolescent COVID-19 vaccination was still pending (the study was conducted February and March 2021, and the vaccine for adolescents was approved in May 2021). Despite the COVID-19 vaccine being available for adolescents in the United States since May 2021, adolescent vaccination stalled at 53% at the end of 2021 [2], with vaccination rates varying significantly across states and communities. An estimated 9.5 million 12- to 17-year-olds still need to be vaccinated [32]. Even in Orange County, California, adolescent vaccination rates vary widely by city from 49% to 95% as of February 2022 [33]—a seeming microcosm of the nation, whose adolescent vaccination rates also vary widely by state and community [33]. The success of any vaccine program to reduce the burden of COVID-19 depends on high vaccine acceptance and uptake, including adolescents but most importantly, their parents [34]. Vaccinating adolescents plays a critical role in limiting the spread of COVID-19 [6,9] and is the best preventive measure available to offer safe in-person schooling. In addition to the direct benefit of minimizing rare yet possibly severe COVID-19 disease, the indirect benefits of vaccinating adolescents, i.e., for preventing community transmission, prevail and are critical. The CDC released a statement highlighting the need to urgently increase COVID-19 vaccination coverage, particularly in light of the spread of new variants [35].
Understanding parent vaccine decision-making for adolescents is important for delivering tailored vaccine messaging to subgroups of parents who may turn to health care practitioners, public health, community advocates, and school officials for vaccine recommendations or cues about its importance. In our study, parents’ uncertainty about vaccinating their adolescent against COVID-19 reflects vaccine attitudes among parents in Orange County, California, during the early pandemic—a period of high uncertainty. During this time, parents’ vaccine attitudes reflected low vaccine confidence and high complacency about COVID-19 risk in children among parents who were uncertain. Parent vaccine attitudes may be changing among some, given the rising proportion of COVID-19 infections among children (18.6%) and that for subgroups of children, especially unvaccinated children, COVID-19 disease risk may result in hospitalization and in rare cases, multisystem inflammatory disease or death [10].
Among parents confident in vaccinating their adolescents at a time when the vaccine had not received emergency authorization, high vaccine confidence, low risk complacency, and a high collective responsibility to vaccinate characterized vaccine acceptance attitudes. Three additional vaccine acceptance factors were discovered, which have not been recognized in vaccine-specific theories: vaccinating for emotional health, to resume social activities, and in response to mandates. Parents uncertain about vaccinating their children expressed low confidence in the vaccine and mistrust in government regulatory agencies, pharmaceutical companies, and public health officials. Parents’ mistrust exhibited a spillover effect from early pandemic blunders, when public health authorities gave inconsistent messaging on masks [36,37].
Vaccine hesitancy factors recognized by the 5C model that were not raised by parents at the time of the study (February–March 2021) included constraints (not being able to access vaccination) and calculation (extensively searching for COVID-19 information). Similar to a national study [21], low vaccine confidence because of unknown long-term outcomes was expressed by parents, as were questions about vaccine ingredients, vaccine efficacy given variants, and wanting more safety information about clinical trials involving adolescents. The vaccination status of the parent did not necessarily align with the parent’s vaccine attitude to vaccinating their child, nor did the parent’s vaccine attitude always align with their child’s vaccine attitude. That is, some parents were vaccinated but did not want or deem it necessary to vaccinate their children. Additionally, in some cases, parents expressed not intending to vaccinate their adolescent children, whereas the adolescent child expressed the intent or desire to vaccinate. Although preteen children (5th and 6th graders) tended to mirror their parent’s vaccine attitudes, older adolescent children (high school age) in some cases had greater access to independent vaccine information through school or social media and as a result, expressed greater confidence in wanting to vaccinate once the vaccine became available. Among the 28% of vaccinated parents at the time of the study in February–March 2021, one parent did not want her children vaccinated because she had experienced adverse vaccine reactions, whereas another expressed not perceiving the need for her sons to be vaccinated because they had already contracted COVID-19 and she felt the risk was manageable. Achieving community immunity did not factor into this parent’s vaccine attitude regarding her sons.
Parents whose adolescent child had comorbidities were both uncertain about and accepting of vaccination. These parents expressed turning to their physician for guidance. Similar to findings from a national survey [38], our findings suggest the continued importance of having individuals with medical credibility, e.g., pediatricians, public health officials, and school nurses, answer parents’ questions to increase vaccine confidence. Additionally, findings suggest the continued importance of having localized community efforts to increase vaccine education, opportunities to answer parents’ questions, and access to vaccination in the community. In light of the increasing spread of variants in the United States and the rapidly changing risk environment for adolescents [35], COVID-19 vaccine discussions with parents and adolescents continue to be critical. Our findings provide guidance on key areas to emphasize in discussions with parents and their adolescents who have yet to be vaccinated.
Factors associated with vaccine acceptance and uncertainty may inform effective messaging. With the continued spread of COVID-19, parents uncertain about vaccinating need to be reminded by trusted pediatricians of the importance and priority of vaccinating adolescents. We now know that risk to adolescents, especially those with comorbidities, may be higher, especially for potentially severe COVID-19 or long COVID-19. Receiving explicit vaccine recommendations from a personal pediatrician and positive vaccine norms from the community (especially family members) may signal to parents who are uncertain that prioritizing vaccination is important. Provider recommendation was explicitly mentioned as important by parents, in contrast to a national survey study of parents, who indicated that a health care provider vaccine recommendation would not change their vaccine intentions [21]. Lessons from other adolescent vaccine attitude studies (e.g., HPV vaccination) suggest that parents receiving a strong explicit vaccine recommendation from the family pediatrician is a necessary condition to increase vaccination [39,40]. In addition to pediatrician recommendation, community and school vaccine norms may have an influence on parents’ decisions, because some families visit their pediatrician less during the pandemic and may be in more regular communication with schools for COVID-19 guidance. Nationally, adolescent immunizations have dropped during the pandemic [38,41].
Trusted pediatric practitioners and school and community nurses who engage families in vaccine conversations may benefit in shifting the conversation to social benefits or the collective responsibility of vaccinating to improve vaccine acceptance. Our study findings revealed that unique pandemic-related factors play an important role in COVID-19 vaccine acceptance, including vaccinating for emotional health, resuming social activities, and vaccine mandates. Parents and adolescents expressed social reasons for wanting to vaccinate, such as resuming in-person activities after having experienced social isolation during the pandemic.
As with other adolescent vaccines such as HPV vaccination, having health care practitioners take a nonconfrontational announcement approach to explicitly recommend COVID-19 vaccination (as the default and recommended action rather than a question to parents) may be needed to more effectively reach parents who either mistrust the vaccine or view COVID-19 risk as manageable [39,42]. Parents may benefit from being reminded of the rapidly changing risk environment, including the relaxation of restrictions, delta and omicron variants, elimination of mask mandates in many states, and inconsistent protective behaviors of others. Vaccination can prevent severe COVID-19 disease, given that adolescents no longer live in protected “bubbles”.
Parents also expressed vaccinating their children in response to policy cues to prioritize vaccination through mandates, whether for school, travel, state, or work mandates. Mandating vaccination signals prioritizing the recommended behavior in response to societal risk [21]. Social, emotional, and policy options are important to consider for pediatric, school nurse, and public health official messaging. Listening to parents’ concerns and questions and answering their questions continue to be important as a point-of-care educational opportunity to allay parents’ concerns and build trust [43].
Study limitations include the fact that findings reflect parental perspectives prior to the emergency authorization of COVID-19 vaccination for adolescents, during a rapidly evolving risk environment. At the time of this study, no vaccines had been approved for adolescents, and this discussion was one of possibility. Adults who were health care workers and more recently, teachers, had received the opportunity to be vaccinated, but there was no timeline for when adolescents would have approval and access to vaccines. The identified factors continue to be critical for effective messaging to vaccinate the remaining 47% of unvaccinated adolescents in the United States, who are at 10-fold higher risk for potentially more severe outcomes should they contract COVID-19. In contrast to the United States, countries such as the United Kingdom have not prioritized vaccinating adolescents as a policy until recently [8,9,44].
This study explored perspectives of parents and adolescents in Orange County, California, and reflects a time when most adolescents there were being schooled online from home. Orange County’s demographics reflect a diverse racial and ethnic makeup of predominantly White, 35% Hispanic (Mexican and El Salvadorean), and 22% Asian (e.g., Vietnamese, Korean, Chinese, and Indian) [18], distinct from not only the rest of the country but also neighboring rural counties, such as Imperial County, in Southern California. Parents’ vaccine attitudes and access may vary significantly in neighboring rural Imperial or urban Los Angeles counties. Interviews with parents from different regions of the country such as in the Southeast or who have low educational attainment may reveal additional factors such as vaccine access (finding time to take off work to vaccinate adolescents) related to vaccine attitudes. School-based vaccine clinics may benefit families in such cases. Our recruitment strategy sampled parents who mirrored the community in which the study was conducted, with diversity in race and ethnicity, multigenerational households, children with chronic conditions, and children who were schooled online. A notable limitation is that the education level of parent participants was relatively high, with almost three quarters having a college education. This may have obscured other concerns among families with lower levels of educational attainment. We know from other studies that low-income communities have expressed COVID-19 vaccine uncertainty [21,45]. A Kaiser Family Foundation study revealed that low-income parents are more concerned about taking time off work and traveling to a vaccine site to vaccinate their children [46]. Thus, vaccine constraints—one of the 5Cs not found in this study—could play a role for some parents not sampled in this study. Another limitation is that we did not collect data on religiosity, which the literature has shown can influence vaccine attitudes in some cases. Finally, the limitation of conducting virtual (Zoom) parent discussions must be recognized. The disadvantages of virtual discussion groups include not being able to read nonverbal cues as accurately, technology glitches, not being able to control for others present or listening on the call (i.e., lack of privacy possibly affecting what a person discloses), and the sample being limited to those who have Zoom technology. On the other hand, advantages of virtual discussion groups include cost and time savings, effective access and convenience, expanding the geographic range of inclusion, and improving the inclusion of parents who might otherwise not be able to attend.
5. Conclusions
With the Pfizer BioNTech vaccine authorized for use in children aged 5 or older in the United States, public health messaging, especially by pediatricians, school administrators, nurses, pharmacists, local community leaders, and public health officials, will continue to be paramount in addressing the educational needs of parents and adolescents to bolster vaccine confidence and the understanding of how vaccines protect and benefit children’s health [38,47,48]. Continued parent education about vaccines is needed, including eliciting and addressing questions from parents and adolescents. Educational opportunities for parents and adolescents may be more accessible, either through schools or local community leaders, to answer parents’ questions as part of trust building [45,48,49]. In discussions with families, pediatricians, community, and school clinic workers should walk the tightrope of being transparent about possible rare vaccine side effects such as myocarditis [26] or anxiety-induced syncope [50] while allaying parents’ and adolescents’ vaccine concerns. Parent vaccine messaging may benefit from emphasizing that through vaccination, their children will be more protected and can resume social activities, sports, travel, and in-person school or summer camps safely, with less likelihood of acquiring and transmitting COVID-19 and less disruption to their adolescents’ lives. Messaging to reach hesitant parents may also include messaging via adolescents, who in some cases have greater and independent access to COVID-19 vaccine information and a greater willingness to vaccinate than their parents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19074212/s1. Survey instrument and focus group discussion guide.
Author Contributions
Conceptualization, S.H., D.H.S., E.J.F. and D.M.C.; methodology, E.J.F., S.H., D.H.S. and H.C.H.; validation, S.H., E.J.F., M.R. and S.N.L.; formal analysis, E.J.F., M.R. and S.N.L.; investigation, S.H.; resources, D.M.C., D.M.C. and B.B.-A.; data curation, E.J.F., M.R., S.N.L. and S.H.; writing—original draft preparation, E.J.F. and S.H.; writing—review and editing, H.C.H., D.H.S. and A.G.; project administration, A.G.; funding acquisition, D.M.C. and B.B.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Institute for Clinical and Translational Science (ICTS) at the University of California, Irvine, and the National Institutes of Health (NIH) Community Engagement Alliance (CEAL) against COVID-19 Disparities. This research was, in part, funded by the National Institutes of Health (NIH) Agreement OT2HL158287. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of California, Irvine (protocol code UCI IRB HS# 2021-6423, approved 5 February 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. IRB was approved for this study and included a waiver of signed consent. All participants consented verbally, received a study information sheet, and agreed to participate.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the families for their time and participation in the study. We thank Robynn Zender for her organization, study design, and recruitment support, which made this project happen.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- US Census Quick Facts Percent Children under the Age of 18. 2020. Available online: https://www.census.gov/quickfacts/fact/table/US# (accessed on 3 February 2022).
- American Academy of Pediatrics (AAP). Children and COVID-19 Vaccination Trends: AAP Analysis of Data Posted by the Centers for Disease Control and Prevention (CDC) on All States Except Idaho as of December 29, 2021; AAP: Itasca, IL, USA, 2021. [Google Scholar]
- Aschwanden, C. Five reasons why COVID-19 herd immunity is probably impossible. Nature 2021, 591, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.; Yousaf, A.R.; Chang, K.; Schwartz, N.G.; McDaniel, C.J.; Lee, S.H.; Szablewski, C.M.; Brown, M.; Drenzek, C.L.; Dirlikov, E.; et al. Household transmission of SARS-CoV-2 from children and adolescents. N. Engl. J. Med. 2021, 385, 954–956. [Google Scholar] [CrossRef]
- Yonker, L.; Boucau, J.; Regan, J.; Choudhary, M.C.; Burns, M.D.; Young, N.; Farkas, E.J.; Davis, J.P.; Moschovis, P.P.; Kinane, T.B.; et al. Virologic features of SARS-CoV-2 infection in children. J. Infect. Dis. 2021, 224, 1821–1829. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Didierlaurent, A.; Tilmanne, A.; Smeesters, P.; Marchant, A. Pediatric COVID-19: Immunopathogenesis, transmission and prevention. Vaccines 2021, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.M.; Afghani, B.; Byington, C.L.; Cunningham, C.K.; Golub, S.; Lu, K.D.; Radom-Aizik, S.; Ross, L.F.; Singh, J.; Smoyer, W.E.; et al. SARS-CoV-2 vaccine testing and trials in the pediatric population: Biologic, ethical, research, and implementation challenges. Pediatric Res. 2021, 90, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S. Crossing the rubicon: A fine line between waiting and vaccinating adolescents against COVID-19. J. Infect. 2021, 83, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Shiri, T.; Evans, M.; Talarico, C.A.; Morgan, A.R.; Mussad, M.; Buck, P.O.; McEwan, P.; Strain, W.D. Vaccinating adolescents and children significantly reduces COVID-19 morbidity and mortality across all ages: A population-based modeling study using the UK as an example. Vaccines 2021, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- AAP. Children and COVID-19: State Data Report; AAP and Children’s Hospital Association: Itasca, IL, USA, 2021. [Google Scholar]
- Delahoy, M.; Ujamaa, D.; Whitaker, M.; O’Halloran, A.; Anglin, O.; Burns, E.; Cummings, C.; Holstein, R.; Kambhampati, A.K.; Milucky, J.; et al. Hospitalizations associated with COVID-19 among children and adolescents—COVID-NET, 14 states, March 1, 2020–August 14, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1255–1260. [Google Scholar] [CrossRef]
- Tsabouri, S.; Makis, A.; Kosmeri, C.; Siomou, E. Risk factors for severity in children with Coronavirus disease 2019: A comprehensive literature review. Pedatric Clin. N. Am. 2021, 68, 321–338. [Google Scholar] [CrossRef]
- AAP. Children and COVID-19 State Data Report; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- McCormick, D.; Richardson, L.C.; Young, P.R.; Viens, L.J.; Gould, C.V.; Kimball, A.; Pindyck, T.; Rosenblum, H.G.; Siegel, D.A.; Vu, Q.M.; et al. Deaths in children and adolescents associated with COVID-19 and MIS-C in the United States. Pediatrics 2021, 148, e2021052273. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.; Gilani, Z.; Godfred-Cato, S.; Belay, E.D.; Feldstein, L.R.; Patel, M.M.; Randolph, A.G.; Newhams, M.; Thomas, D.; Magleby, R.; et al. Incidence of Multisystem Inflammatory Syndrome in Children among US Persons infected with SARS-CoV-2. JAMA Netw. Open 2021, 4, e2116420. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Gee, J.; Baggs, J.; Abara, W.E.; Marquez, P.; Thompson, D.; Su, J.R.; Licata, C.; Rosenblum, H.G.; Myers, T.R.; et al. COVID-19 vaccine safety in adolescents aged 12–17 years—United States, December 14, 2020–July 16, 2021. In Morbidity and Mortality Weekly Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; pp. 1053–1058. [Google Scholar]
- Parker, D.M.; Bruckner, T.; Vieira, V.M.; Medina, C.; Minin, V.N.; Felgner, P.L.; Dratch, A.; Zahn, M.; Bartell, S.M.; Boden-Albala, B. Predictors of test positivity, mortality, and seropositivity during the early Coronavirus disease epidemic, Orange County, CA, USA. Emerg. Infect. Dis. 2021, 27, 2604–2618. [Google Scholar] [CrossRef] [PubMed]
- Together, O.H. Population by Language Spoken at Home; OC Healthier Together Dashboard: Orange County, CA, USA, 2022. [Google Scholar]
- William, D. Recall, Special Elections: Looking Back on the Year in Orange County Politics. 2022. Available online: https://spectrumnews1.com/ca/orange-county/politics/2022/01/04/orange-county-politics--2021-in-review (accessed on 6 February 2022).
- Pfizer. Pfizer-Biontech Announces Positive Toplinei Results of Pivotal COVID-19 Vaccine Study in Adolescents; Pfizer: Mainz, Germany, 2021. [Google Scholar]
- Scherer, A.; Gedlinske, A.M.; Parker, A.M.; Gidengil, C.A.; Askelson, N.M.; Petersen, C.A.; Woodworth, K.R.; Lindley, M.C. Acceptability of Adolescent COVID-19 Vaccination among Adolescents and Parents of Adolescents—United States, April 15–23, 2021. In Morbidity and Morality Weekly Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; pp. 997–1003. [Google Scholar]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight against Pandemic; US Food & Drug Administration: Silver Spring, MD, USA, 2021.
- Montalti, M.; Rallo, F.; Guaraldi, F.; Bartoli, L.; Po, G.; Stillo, M.; Perrone, P.; Squillace, L.; Dallolio, L.; Pandolfi, P.; et al. Would parents get their children vaccinated against SARS-CoV-2? Rate and predictors of vaccine hesitancy according to a survey over 5000 families from Bologna, Italy. Vaccines 2021, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.; Ozkaya-Parlakay, A.; Senel, E. Evaluation of COVID-19 vaccine refusal in parents. Pediatric Infect. Dis. J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Steele, J.M.; Fonseca, B.; Huang, S.; Shah, S.; Maskatia, S.A.; Buddhe, S.; Misra, N.; Ramachandran, P.; Gaur, L.; et al. COVID-19 vaccination—Associated myocarditis in adolescents. Pediatrics 2021, 148, e2021053427. [Google Scholar] [CrossRef] [PubMed]
- CDC. Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines among Adolescents and Young Adults; Department of Immunization and Vaccines, CDC: Atlanta, Georgia, 2021. [Google Scholar]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.J. Data analysis basics: A phronetic iterative approach. In Qualitative Research Methods: Collecting Evidence, Crafting Analysis, Communicating Impact; Tracy, S.J., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 208–235. [Google Scholar]
- Tracy, S.J. A phronetic iterative approach to data analysis in qualitative research. J. Qual. Res. 2018, 19, 61–76. [Google Scholar]
- Tracy, S.J. Advanced data analysis: The art and magic of interpretation. In Qualitative Research Methods: Collecting Evidence, Crafting Analysis, Communicating Impact; Tracy, S.J., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 236–264. [Google Scholar]
- Hennink, M.M.; Kaiser, B.N.; Weber, M.B. What influences saturation? Estimating sample sizes in focus group research. Qual. Health Res. 2019, 29, 1483–1496. [Google Scholar] [CrossRef]
- Murthy, B.; Zell, E.; Saelee, R.; Murthy, N.; Meng, L.; Meador, S.; Reed, K.; Shaw, L.; Gibbs-Scharf, L.; Gibbs-Scharf, L.; et al. COVID-19 Vaccination Coverage among Adolescents Aged 12–17 Years—United States, December 14, 2020–July 31, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1206–1213. [Google Scholar] [CrossRef]
- OC Health Care Agency. COVID-19 Vaccination Status of Youths. COVID-19 Vaccination Status of Youths Age 12–17. 2022. Available online: https://ochca.maps.arcgis.com/apps/dashboards/8202b85d0e944b88af5a1140c9206031 (accessed on 30 January 2022).
- Plotkin, S.A.; Levy, O. Considering mandatory vaccination of children for COVID-19. Pediatrics 2021, 147, e2021050531. [Google Scholar] [CrossRef] [PubMed]
- CDC. Delta Variant: What We Know about the Science; Division of Viral Diseases, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://stacks.cdc.gov/view/cdc/108671 (accessed on 3 February 2022).
- Latkin, C.; Dayton, L.; Yi, G.; Konstantopoulos, A.; Boodram, B. Trust in a COVID-19 vaccine in the U.S.: A social-ecological perspective. Soc. Sci. Med. 2021, 270, 113684. [Google Scholar] [CrossRef]
- Hopfer, S.; Fields, E.J.; Lu, Y.; Ramakrishnan, G.; Grover, T.; Bai, Q.; Huang, Y.; Li, C.; Mark, G. The social amplification and attenuation of COVID-19 risk perception shaping mask wearing behavior: A longitudinal Twitter analysis. PLoS ONE 2021, 16, e0257428. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.J.; Rosenberg, J.; Waselewski, M.E.; Amaro, X.; Wasag, J.; Chang, T. National study of youth opinions on vaccination for COVID-19 in the U.S. J. Adolesc. Health 2021, 68, 869–872. [Google Scholar] [CrossRef]
- Brewer, N.T.; Hall, M.E.; Malo, T.L.; Gilkey, M.B.; Quinn, B.; Lathren, C. Announcements versus conversations to improve HPV vaccination coverage: A randomized trial. Pediatrics 2017, 139, e20161764. [Google Scholar] [CrossRef] [PubMed]
- Hopfer, S.; Wright, M.; Pellman, H.; Wasserman, R.; Fiks, A.G. HPV vaccine recommendation profiles among a national network of pediatric practitioners: Understanding contributors to parental vaccine hesitancy and acceptance. Hum. Vaccine Immunother. 2019, 15, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- NCI-Designated Cancer Centers Call for Urgenet Action to Get HPV Vaccination Back on Track. 2021. Available online: https://www.asco.org/sites/new-www.asco.org/files/NCI-CC-Statement-HPV-2021.pdf (accessed on 2 November 2021).
- Malo, T.L.; Hall, M.E.; Brewer, N.T.; Lathren, C.R.; Gilkey, M.B. Why is announcement training more effective than conversation training for introducing HPV vaccination? A theory-based investigation. Implement. Sci. 2018, 13, 57–68. [Google Scholar] [CrossRef]
- Dempsey, A.; O’Leary, S. Human Papillomavirus Vaccination: Narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad. Pediatrics 2018, 18, S23–S27. [Google Scholar] [CrossRef]
- Wilkinson, D.; Finlay, I.; Pollard, A.J.; Forsberg, L.; Skelton, A. Should we delay COVID-19 vaccination in children? Br. Med. J. 2021, 374, n1687. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.; Casillas, A.; Castellon-Lopez, Y.; Mansfield, L.N.; Morris, D.; Barron, J.; Ntekume, E.; Landovitz, R.; Vassar, S.D.; Norris, K.C.; et al. COVID-19 vaccine decision-making factors in racial and ethnic minority communities in Los Angeles, California. J. Am. Med. Assoc. Netw. Open 2021, 4, e2127582. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.; Lopes, L.; Sparks, G.; Kirzinger, A.; Kearney, A.; Stokes, M.; Brodie, M. KFF COVID-19 Vaccine Monitor: October 2021; Kaiser Foundation: Oakland, CA, USA, 2021. [Google Scholar]
- Zimet, G.; Silverman, R.; Fortenberry, J.D. Coronavirus disease in 2019 and vaccination of children and adolescents: Prospects and challenges. J. Pediatrics 2021, 231, 254–258. [Google Scholar] [CrossRef]
- AuYoung, M.; Espinosa, P.R.; Chen, W.-T.; Juturu, P.; Young, M.-E.D.T.; Casillas, A.; Adkins-Jackson, P.; Hopfer, S.; Kissam, E.; Alo, A.K.; et al. Addressing racial/ethnic inequities in vaccine hesitancy and uptake: Lessons learned from the California Alliance against COVID-19. J. Behav. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Skirrow, H.; Bedford, H.; Wighton, K. COVID-19 vaccines for teenagers: Conversations and consent. Br. Med. J. 2021, 374, n2312. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Gee, J.; Johnson, T.; Jazwa, A.; Marquez, P.; Miller, E.; Su, J.; Shimabukuro, T.T.; Shay, D.K. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination—Five US mass vaccination sites, April 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 685–688. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).