Influence of Diagnostic Delay on Survival Rates for Patients with Colorectal Cancer
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Study Variables
2.4. Ethical Considerations
2.5. Statistical Plan
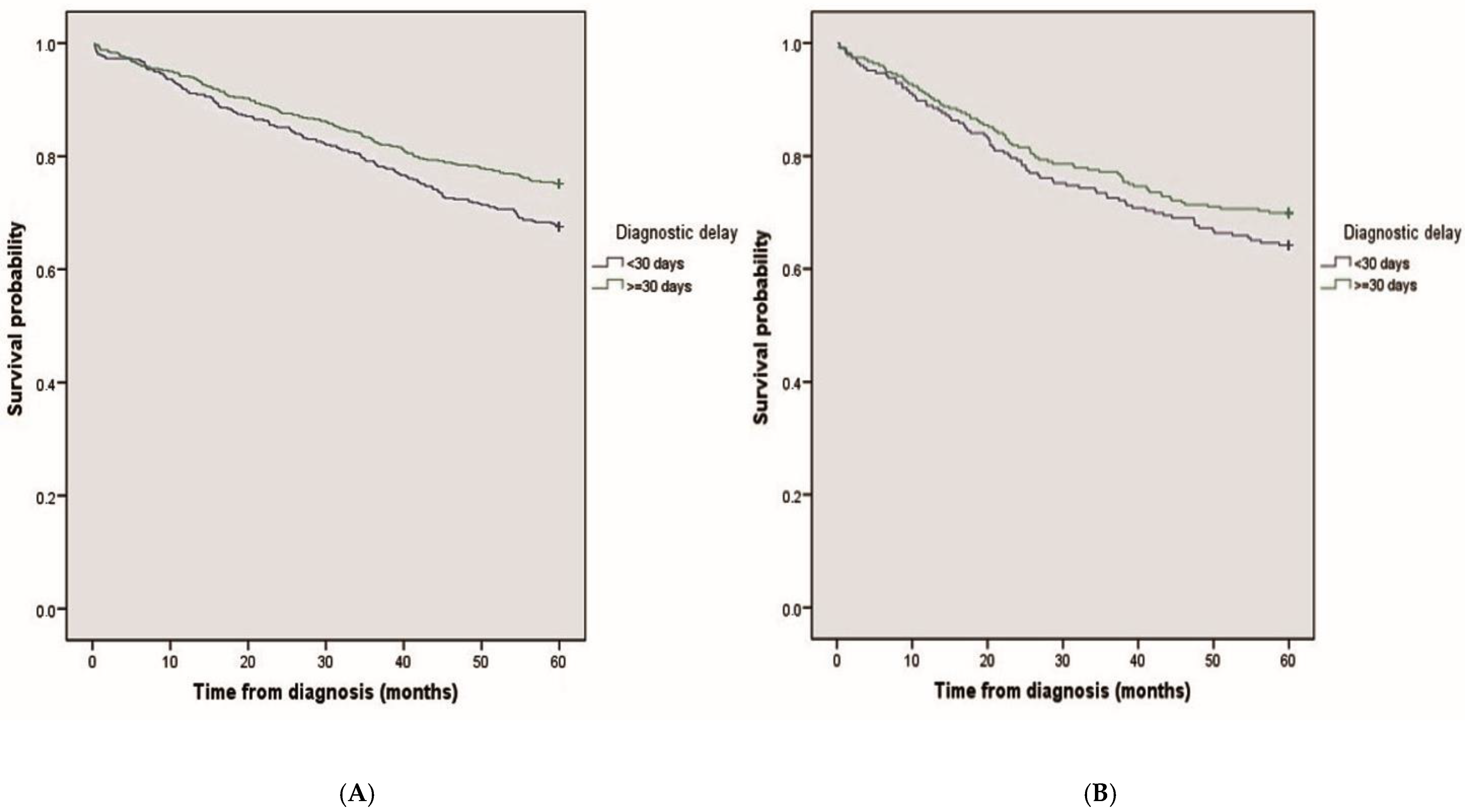
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GLOBOCAN. Colorectal Cancer; Internacional Agency for Research on Cancer: Lyons, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 2 February 2022).
- Seom, S.E. Las Cifras del Cáncer en España 2021; Sociedad Española de Oncología Médica (SEOM): Madrid, Spain, 2021. [Google Scholar]
- Ministerio de Sanidad, C. Programa de Cribado de cáncer Colorrectal. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/Cribado/CribadoCancerColorrectal.htm (accessed on 30 August 2021).
- Koo, M.M.; von Wagner, C.; Abel, G.A.; McPhail, S.; Hamilton, W.; Rubin, G.P.; Lyratzopoulos, G. The nature and frequency of abdominal symptoms in cancer patients and their associations with time to help-seeking: Evidence from a national audit of cancer diagnosis. J. Public Health 2018, 40, e388–e395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, C.C.; Mittal, P.K.; Sullivan, P.S.; Rutherford, R.; Staley, C.A.; Cardona, K.; Hawk, N.N.; Dixon, W.T.; Kitajima, H.D.; Kang, J.; et al. Colorectal Cancer Initial Diagnosis: Screening Colonoscopy, Diagnostic Colonoscopy, or Emergent Surgery, and Tumor Stage and Size at Initial Presentation. Clin. Colorectal Cancer 2016, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de Castro, J.D.; Baiocchi Ureta, F.; Fernández González, R.; Pin Vieito, N.; Cubiella Fernández, J. The effect of diagnostic delay attributable to the healthcare system on the prognosis of colorectal cancer. Gastroenterol. Hepatol. 2019, 42, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Cubiella, J.; Lorenzo, M.; Baiocchi, F.; Tejido, C.; Conde, A.; Sande-Meijide, M.; Castro, M. Impact of a colorectal cancer screening program implantation on delays and prognosis of non-screening detected colorectal cancer. World J. Gastroenterol. 2021, 27, 6689–6700. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.N.; Rai, L.; Samo, K.A.; Saeed, S.; Salam, A.; Khan, H.; Memon, A.S. Factors affecting delay in diagnosis of colorectal cancer: A cross-sectional study from a tertiary care hospital of Karachi, Pakistan. Int. J. Clin. Pract. 2021, 75, e14529. [Google Scholar] [CrossRef] [PubMed]
- Zarcos-Pedrinaci, I.; Fernández-López, A.; Téllez, T.; Rivas-Ruiz, F.; Rueda, A.; Morales Suarez-Varela, M.M.; Briones, E.; Baré, M.; Escobar, A.; Sarasqueta, C.; et al. Factors that influence treatment delay in patients with colorectal cancer. Oncotarget 2017, 8, 36728–36742. [Google Scholar] [CrossRef] [PubMed]
- Zarcos-Pedrinaci, I.; Téllez, T.; Rivas-Ruiz, F.; Padilla-Ruiz, M.D.C.; Alcaide, J.; Rueda, A.; Baré, M.L.; Morales Suárez-Varela, M.M.; Briones, E.; Sarasqueta, C.; et al. Factors Associated with Prolonged Patient-Attributable Delay in the Diagnosis of Colorectal Cancer. Cancer Res. Treat. 2018, 50, 1270–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruitt, S.L.; Harzke, A.J.; Davidson, N.O.; Schootman, M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control 2013, 24, 961–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afifi, A.M.; Elmehrath, A.O.; Ruhban, I.A.; Saad, A.M.; Gad, M.M.; Al-Husseini, M.J.; Bekaii-Saab, T.; Sonbol, M.B. Causes of Death Following Nonmetastatic Colorectal Cancer Diagnosis in the U.S.: A Population-Based Analysis. Oncologist 2021, 26, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Pita-Fernández, S.; González-Sáez, L.; López-Calviño, B.; Seoane-Pillado, T.; Rodríguez-Camacho, E.; Pazos-Sierra, A.; González-Santamaría, P.; Pértega-Díaz, S. Effect of diagnostic delay on survival in patients with colorectal cancer: A retrospective cohort study. BMC Cancer 2016, 16, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, J.M.; Gonzalez, N.; Anton-Ladislao, A.; Redondo, M.; Bare, M.; Fernandez de Larrea, N.; Briones, E.; Escobar, A.; Sarasqueta, C.; Garcia-Gutierrez, S.; et al. Colorectal cancer health services research study protocol: The CCR-CARESS observational prospective cohort project. BMC Cancer 2016, 16, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Factors Related to Adverse Events in Colorectal Cancer-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02488161 (accessed on 9 December 2021).
- Romero Gómez, M.; Bayo Lozano, E.; Conde Herrero, V.; De la Portilla De Juan, F.; Del Nozal Nalda, M.; González Montero, M.C.; Hervás Molina, A.J.; López Hidalgo, J.L.; López Moraleda, I.; Morales Carreño, I.; et al. Cáncer Colorrectal; Proceso Asistencial Integrado: Sevilla, Spain, 2018. [Google Scholar]
- Ramos, M.; Esteva, M.; Cabeza, E.; Llobera, J.; Ruiz, A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur. J. Cancer 2008, 44, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Esteva, M.; Cabeza, E.; Campillo, C.; Llobera, J.; Aguiló, A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: A review. Eur. J. Cancer 2007, 43, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Rupassara, K.S.; Ponnusamy, S.; Withanage, N.; Milewski, P.J. A paradox explained? Patients with delayed diagnosis of symptomatic colorectal cancer have good prognosis. Color. Dis. 2006, 8, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.B.; Rangel, L.E.; Osler, T.M.; Hyman, N.H. Rectal cancer in patients under the age of 50 years: The delayed diagnosis. Am. J. Surg. 2016, 211, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Arhi, C.S.; Burns, E.M.; Bottle, A.; Bouras, G.; Aylin, P.; Ziprin, P.; Darzi, A. Delays in referral from primary care worsen survival for patients with colorectal cancer: A retrospective cohort study. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2020, 70, e463–e471. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.; Rodrigo, I.; Pereda, T.; Funez, R.; Acebal, M.; Perea-Milla, E.; Jimenez, E. Prognostic implications of emergency admission and delays in patients with breast cancer. Support. Care Cancer 2009, 17, 595–599. [Google Scholar] [CrossRef] [PubMed]

| Total | Diagnostic delay | p | |||||
|---|---|---|---|---|---|---|---|
| <30 days | ≥30 days | ||||||
| n: 1688 | % * | n: 744 | % ** | n: 944 | % ** | ||
| Sex | |||||||
| Male | 1073 | 63.6 | 469 | 43.7 | 604 | 56.3 | 0.726 |
| Female | 615 | 36.4 | 275 | 44.7 | 340 | 55.3 | |
| Age 1 | |||||||
| Mean-SD | 68.0 | 10.8 | 68.4 | 10.7 | 67.7 | 10.9 | 0.164 |
| Education 2 | |||||||
| Primary or less | 1082 | 76.3 | 466 | 43.1 | 616 | 56.9 | 0.738 |
| Secondary–university | 337 | 23.7 | 141 | 41.8 | 196 | 58.2 | |
| Habitation status 3 | |||||||
| Living alone | 198 | 14.0 | 86 | 43.4 | 112 | 56.6 | |
| With family | 1180 | 83.5 | 504 | 42.7 | 676 | 57.3 | 0.194 |
| Care home/other situations | 36 | 2.5 | 10 | 27.8 | 26 | 72.2 | |
| BMI 4 | |||||||
| Mean-SD | 27.0 | 6.9 | 26.5 | 7.4 | 27.4 | 6.5 | 0.028 |
| Charlson index | |||||||
| Mean-SD | 2.8 | 1.2 | 2.9 | 1.3 | 2.8 | 1.2 | 0.035 |
| Smoking habit 5 | |||||||
| Never smoked | 759 | 47.2 | 335 | 44.1 | 424 | 55.9 | |
| Current smoker | 213 | 13.2 | 91 | 42.7 | 122 | 57.3 | 0.933 |
| Ex-smoker | 637 | 39.6 | 280 | 44.0 | 357 | 56.0 | |
| Family history of CRC 6 | |||||||
| No | 857 | 84.4 | 347 | 40.5 | 510 | 59.5 | 0.722 |
| Yes | 158 | 15.6 | 61 | 38.6 | 97 | 61.4 | |
| Tumor location | |||||||
| Right colon | 502 | 29.7 | 226 | 45.0 | 276 | 55.0 | |
| Left colon | 726 | 43.0 | 311 | 42.8 | 415 | 57.2 | 0.673 |
| Rectum | 460 | 27.3 | 207 | 45.0 | 253 | 55.0 | |
| TNM stage | |||||||
| I | 374 | 22.2 | 148 | 39.6 | 226 | 60.4 | 0.295 |
| II | 595 | 35.2 | 271 | 45.5 | 324 | 54.5 | |
| III | 531 | 31.5 | 247 | 46.5 | 284 | 53.5 | |
| IV | 188 | 11.1 | 78 | 44.1 | 110 | 58.5 | |
| Degree of differentiation 7 | |||||||
| Low | 1263 | 85.9 | 560 | 44.3 | 703 | 55.7 | 0.212 |
| High | 207 | 14.1 | 102 | 49.3 | 105 | 50.7 | |
| Histologic diagnosis 8 | |||||||
| Adenocarcinoma | 1495 | 91.0 | 650 | 43.5 | 845 | 56.5 | 0.056 |
| Mucinous adenocarcinoma or others | 148 | 9.0 | 77 | 52.0 | 71 | 48.0 | |
| Screening diagnosis 9 | |||||||
| Absent | 1301 | 81.4 | 610 | 46.9 | 691 | 53.1 | <0.001 |
| Present | 297 | 18.6 | 90 | 30.3 | 207 | 69.7 | |
| Mean Survival (Months) 95% CI | p | ||
|---|---|---|---|
| Overall | 49.8 (49.0–50.7) | ||
| diagnostic delay | |||
| <30 days | 48.6 (47.3–50.0) | 0.002 | |
| ≥30 days | 50.8 (49.6–51.9) | ||
| Location | |||
| Right colon | <30 days | 46.8 (44.2–49.4) | 0.002 |
| ≥30 days | 48.6 (46.4–50.9) | ||
| Left colon + rectum | <30 days | 49.4 (47.9–51.0) | |
| ≥30 days | 51.6 (50.4–52.9) | ||
| Crude | Adjusted * | |||
|---|---|---|---|---|
| p | HR 95%CI | p | HR | |
| Diagnostic delay | ||||
| <30 days | 0.002 | 1.00 | 0.034 | 1.00 |
| ≥30 days | 0.76 (0.64–0.90) | 0.80 (0.66–0.98) | ||
| Sex | ||||
| Male | 0.013 | 1.00 | ||
| Female | 0.79 (0.65–0.95) | |||
| Age | ||||
| <0.001 | 1.04 (1.03–1.05) | <0.001 | 1.04 (1.03–1.05) | |
| Education | ||||
| Primary or less | 0.004 | 1.00 | ||
| Secondary–university | 0.69 (0.53–0.88) | |||
| Habitation status | ||||
| Living alone | 1.00 | |||
| With family | 0.043 | 0.78 (0.60–1.02) | ||
| Care home / Other situations | 1.29 (0.73–2.25) | |||
| BMI | ||||
| 0.016 | 1.02 (1.00–1.04) | <0.018 | 1.02 (1.00–1.04) | |
| Charlson index | ||||
| <0.001 | 1.30 (1.23–1.37) | <0.001 | 1.30 (1.22–1.38) | |
| Smoking habit | ||||
| Never smoked | 1.00 | |||
| Current smoker | 0.881 | 1.03 (0.77–1.36) | ||
| Ex-smoker | 1.05 (0.87–1.28) | |||
| Family history of CRC | ||||
| No | 0.017 | 1.00 | ||
| Yes | 0.65 (0.46–0.93) | |||
| Tumor location | ||||
| Right colon | 0.033 | 1.00 | ||
| Left colon + rectum | 0.82 (0.68–0.98) | |||
| TNM stage | ||||
| I | <0.001 | 1.00 | <0.001 | 1.00 |
| II | 1.57 (1.14–2.16) | 1.47 (1.02–2.12) | ||
| III | 2.86 (2.11–3.88) | 3.06 (2.16–4.33) | ||
| IV | 8.38 (6.08–11.5) | 9.38 (6.46–13.6) | ||
| Degree of differentiation | ||||
| Low | <0.001 | 1.00 | ||
| High | 1.63 (1.28–2.07) | |||
| Histologic diagnosis | ||||
| Adenocarcinoma | <0.001 | 1.00 | ||
| Mucinous adenocarcinoma or others | 1.63 (1.24–2.13) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Ruiz, M.; Morales-Suárez-Varela, M.; Rivas-Ruiz, F.; Alcaide, J.; Varela-Moreno, E.; Zarcos-Pedrinaci, I.; Téllez, T.; Fernández-de Larrea-Baz, N.; Baré, M.; Bilbao, A.; et al. Influence of Diagnostic Delay on Survival Rates for Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2022, 19, 3626. https://doi.org/10.3390/ijerph19063626
Padilla-Ruiz M, Morales-Suárez-Varela M, Rivas-Ruiz F, Alcaide J, Varela-Moreno E, Zarcos-Pedrinaci I, Téllez T, Fernández-de Larrea-Baz N, Baré M, Bilbao A, et al. Influence of Diagnostic Delay on Survival Rates for Patients with Colorectal Cancer. International Journal of Environmental Research and Public Health. 2022; 19(6):3626. https://doi.org/10.3390/ijerph19063626
Chicago/Turabian StylePadilla-Ruiz, María, María Morales-Suárez-Varela, Francisco Rivas-Ruiz, Julia Alcaide, Esperanza Varela-Moreno, Irene Zarcos-Pedrinaci, Teresa Téllez, Nerea Fernández-de Larrea-Baz, Marisa Baré, Amaia Bilbao, and et al. 2022. "Influence of Diagnostic Delay on Survival Rates for Patients with Colorectal Cancer" International Journal of Environmental Research and Public Health 19, no. 6: 3626. https://doi.org/10.3390/ijerph19063626
APA StylePadilla-Ruiz, M., Morales-Suárez-Varela, M., Rivas-Ruiz, F., Alcaide, J., Varela-Moreno, E., Zarcos-Pedrinaci, I., Téllez, T., Fernández-de Larrea-Baz, N., Baré, M., Bilbao, A., Sarasqueta, C., Aguirre-Larracoechea, U., Quintana, J. M., Redondo, M., & on behalf of CARESS-CCR Study Group. (2022). Influence of Diagnostic Delay on Survival Rates for Patients with Colorectal Cancer. International Journal of Environmental Research and Public Health, 19(6), 3626. https://doi.org/10.3390/ijerph19063626






