Abstract
Opioid use disorders (OUD) is a relapsing condition with high mortality. Opioid maintenance treatment (OMT) reduces heroin use, and overall morbidity and mortality. The prevalence of psychiatric and substance use disorders, potential baseline predictors for psychiatric hospitalization, and psychiatric diagnoses at follow-up were investigated and may give hints about possible preventative strategies. The medical records for 71 patients were reviewed 36 months following referral to OMT from a needle exchange program (NEP). Their psychiatric diagnoses and hospitalizations were identified. Their baseline characteristics were assessed for potential differences between hospitalized versus non-hospitalized patients and between patients with and without psychiatric diagnoses in a longitudinal observational study without controls. A regression analysis was performed to identify predictors for hospitalization when controlling for OMT status. Sixty-five percent of the patients were hospitalized at least once with a psychiatric diagnosis. Substance-related reasons were prevalent, and detoxification occurred among 59% of patients, with sedative- hypnotics (benzodiazepines, zopiclone, zolpidem, and pregabalin) being the substance used by 52% of patients. Baseline use of these drugs and/or buprenorphine predicted for hospitalization when controlling for OMT status. During the follow-up period, 72% of patients met the criteria for a psychiatric diagnosis other than OUD. The prevalence of non-substance use disorders overlapping with SUD was 41%, and that overlapping with anxiety disorder was 27% of all participants. Increased attention to psychiatric co-occurring disorders in the treatment of OUD is required and the importance of addressing sedative-hypnotics use when initiating OMT is highlighted.
1. Introduction
1.1. Opioid Use Disorders
Any opioid use disorder (OUD) puts patients at a lifelong risk for relapses and high mortality [1,2]. Opioid maintenance treatment (OMT) or Medication-Assisted Treatment (MAT), which includes psychiatric treatment and psychosocial intervention [3], is the most common treatment for opioid use disorders and has evidence with respect to reductions in drug use, criminal behavior, HIV risk behavior, and mortality [3,4,5,6,7]. OMT was introduced in the 1960s as a treatment for opioid use disorders [8], and at the turn of the century, buprenorphine was added as an alternative treatment option [4,9].
1.2. Co-Occurring Substance Use Disorders
Co-occurring substance use disorders are prevalent in opioid use disorders and include harmful use of alcohol, cannabis, stimulants, prescription opioids, or sedative-hypnotics (benzodiazepines, z-drugs (zopiclone and zolpidem), and pregabalin) [10,11,12,13]. When starting OMT, most individuals often report concomitant use of benzodiazepines [13,14]. The use of benzodiazepines during OMT has been associated with more psychopathology and a worse clinical course in several studies, with poly-drug use, risky injection practices, and poor psychosocial outcome [14,15,16,17,18,19]. Benzodiazepines may cause somnolence and confusion, contribute to mortality rates, and have been identified in up to 80% of methadone- or buprenorphine-related deaths [2,13,14,19,20]. Opioid users who also use benzodiazepines regularly are nearly three times as likely to have psychiatric hospitalizations during the preceding year [13].
1.3. Co-Occurring Psychiatric Disorders
Less data are available on the impact of co-occurring psychiatric disorders—other than substance-use related disorders—on the clinical course of opioid use disorders. The lifetime co-occurring disorders rates of other psychiatric disorders range between 44 and 86% [11,21,22]. Mood disorders and personality disorders are the most common, with the highest prevalence rates reported for major depressive disorder (4–44%) and antisocial personality disorder (ASPD) (25–39%), [12,23].
Psychiatric co-occurring disorders has a negative impact on drug dependence treatment outcomes in general [24,25,26,27].
Starting treatment with psychiatric disorders, such as major depression or antisocial personality disorder, is indicated to have a worse outcome [24,25]. However, for individuals in OMT, other studies suggest that psychiatric co-occurring disorders do not seem to have any significant impact on opioid use or retention in treatment [12,27,28,29,30]. Ngo et al. reported that, although comorbid heroin users entered treatment with a significantly higher risk of drug-related hospitalization than non-comorbid users, substantial reductions in drug-related hospitalization post-treatment were found [31]. The primary treatments for opiate dependence, such as methadone or buprenorphine maintenance or residential treatment, are associated with substantial improvements in depression [32].
The number of psychiatric beds in many high-income countries have been reported to have been reduced considerably since the late 1980s [33,34]. Some authors have therefore pointed out that being admitted to psychiatric in-patient care could be seen as a marker for a psychiatric condition with a high degree of severity [35].
1.4. The Present Cohort-Needle Exchange Program
We previously described the present cohort of individuals with OUD rapidly transferred from a needle exchange program (NEP) to OMT [36]. This low-threshold treatment aimed to increase accessibility, to avoid waitlists, to personalize treatment options, and to provide treatment design that focused on harm reduction, with a particular focus on the retention of low-adherence patients [37]. Despite reporting a high degree of psychosocial problems and poly-drug use, a majority of the 71 individuals that started OMT were still in treatment after 12 months [38]. The need for in-patient treatment after starting OMT and its relation to co-occurring disorders or baseline characteristics is, however, not thoroughly studied. A low-threshold OMT does not require abstinence and aims to reduce barriers to treatment. Therefore, other concomitant substance abuse may be expected as well as hospital admissions.
1.5. Purpose
The overall purpose of this study is to better outline the prevalence and manifestations of psychiatric co-occurring disorders in patients with opioid use disorders entering OMT after being referred from an NEP. The aims were to investigate the prevalence of psychiatric and substance use disorder-related hospitalization and potential baseline predictors of this among OMT patients with a 36-month follow-up from inclusion. Concurrent psychiatric diagnoses were also investigated. Therefore, possible preventative strategies could be proposed.
2. Materials and Methods
2.1. Study Design, Recruitment, and Inclusion/Exclusion Criteria
The study was performed in the city of Malmö located in the southern part of Sweden. The city has a population of approximately 300,000. The NEP is located in the university hospital area and run by the Department of Infectious Diseases. The outpatient OMT clinic is run by the Addiction Center Malmö. In Sweden, OMT is only permitted at special clinics supervised by a medical doctor and specialist in psychiatry or addiction medicine. The present study is a naturalistic follow-up of psychiatric co-occurring disorders and psychiatric hospitalization of patients receiving OMT.
The individuals were participants in the Malmö Treatment Referral and Intervention Study (MATRIS) [36] and were included between 21 October 2011 and 1 April 2013. Overall, 92% used heroin during the previous month. A treatment choice of either methadone or buprenorphine was made at the study inclusion and on an individual basis. The choice of medication and dosage was outside of the study protocol and based on individual clinical characteristics. The inclusion criteria were (1) primary injection use of heroin as documented in the NEP patient record, (2) having a minimum of two previous visits to the NEP office/treatment center, and (3) living in the region of Skåne, Sweden. Additionally, according to Swedish legislation, patients were required to be at least 20 years of age and to have had a diagnosed opioid use disorder for a minimum of one year [39]. Finally, for treatment initiation, a positive urine toxicology test for opioids (morphine derivatives, methadone, or buprenorphine) was required. The exclusion criteria were pregnancy, severe unstable psychiatric condition at baseline, other ongoing treatments for substance use disorders, and inability to understand information and to provide informed consent. Informed consent was given for 36-month follow-up from the date of inclusion. The study was approved by the Regional Ethics Review Board, Lund, Sweden, and registered at clinicaltrials.gov (No. NCT01457872).
In the primary MATRIS trial, 95% of included subjects were successfully referred to treatment [36], and the one-year retention among treatment initiators was 82% [38], similar to outcomes previously reported from a survey of OMTs in previous Swedish methadone maintenance treatment programs [40]. Out of the original 75 individuals who filled all of the criteria, 71 successfully entered treatment and constituted the analyzed cohort.
2.2. Baseline Data
The interview data included sociodemographic characteristics and self-reported data on substance-specific drug use and overdoses, previous suicide attempts, and history of psychiatric treatment (see Figure 1). A substance use assessment was adopted from the widely known Addiction Severity Index [41].
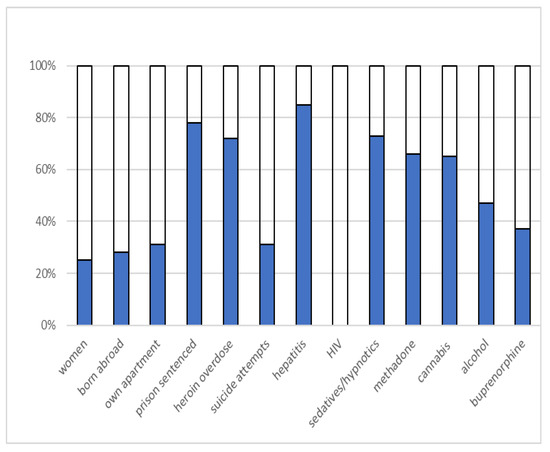
Figure 1.
Sociodemographic factors.
Opioid overdoses were defined as a lifetime history of opioid intake leading to difficulty in breathing, bluish skin, unconsciousness, or difficulty being woken up after intake of heroin. Drug use was specified for common illicit and prescription drugs with addictive potential, such as opioids, cannabis, central stimulating drugs, hallucinogenic drugs, and sedative-hypnotics. Based on their shared GABAergic effects and reports of a similar misuse pattern [42], benzodiazepines, z-drugs (zopiclone and zolpidem), and pregabalin were classified together as sedative-hypnotics. These drugs may cause somnolence and confusion and contribute to the risk of mortality [20,43]. Likewise, buprenorphine and buprenorphine-naloxone were grouped together. These drugs are increasingly used to self-medicate, often in combination with opioids, and are associated with self-harm, hospitalization, and opioid overdose mortality risk [44]. Detoxification from benzodiazepines was required when the medical risk in combination with OMT was high. Sometimes, out-patient treatment was adequate; sometimes, the patients applied for support from Social Security; and sometimes, in-patient detoxification was required.
Age, gender, housing, country of birth, previous suicide attempts, opiate overdose, and substance use at baseline were included as potential baseline predictors for psychiatric hospitalization at follow-up.
2.3. Outcome Measures
Patient records were reviewed in the medical database (software Melior, Siemens AB, Upplands Väsby, Sweden) from the date of inclusion in MATRIS and three years after. The focus of the reviews were psychiatric co-occurring disorders and treatment in two aspects: (1) hospitalization and (2) psychiatric diagnosis in their medical record, both measured over 36 months of follow-up.
Reasons for hospitalization were defined both by the diagnoses [45] and measures taken at the hospital stay from the text of medical records. All psychiatric diagnoses (ICD-10 codes F1-F9) from the case records were included. The number of admissions as well as the duration of time spent in psychiatric in-patient care were recorded.
2.4. Statistical Analysis
Baseline characteristics were assessed for potential differences between hospitalized versus non-hospitalized patients and between patients with and without a psychiatric diagnosis. Comparisons were made using a Chi-square test for binary variables and Fisher’s exact test whenever less than five observations were found in one category. For age, the Mann–Whitney test was used. The level of significance was set at p < 0.05. Finally, a logistic regression analysis (95% CI) was used to adjust potential baseline predictors for one another.
A Cox regression proportional hazard analysis with time-varying co-variates was performed to determine whether discharge from OMT predicted hospitalization while controlling for other variables that were significantly associated with hospitalization.
Both the logistic regression and the Cox regression proportional hazard analysis were carried out as a direct, non-stepwise analysis involving variables that were significantly associated with the outcome in the non-adjusted, bivariate analyses.
SPSS Statistics, version 22 (IBM Corp., Armonk, NY, USA), was used to perform the statistical analysis.
3. Results
3.1. Psychiatric and Substance Use Disorders Related to Hospitalization
During 36 months of follow-up, 65% were hospitalized on at least one occasion with a psychiatric diagnosis. Descriptive information on the reasons for hospitalization and its prevalence are presented in Table 1. The results show that substance-related reasons were the most prevalent and that detoxification occurred for 59% of the study population.

Table 1.
Participant prevalence of psychiatric and substance use disorders and other reasons for hospitalization. (N = 71).
Detoxification from sedative-hypnotics was required by more than half (52%) of the study participants, all of which were for benzodiazepines and z-drugs (zopiclone and zolpidem) and some of which were for additional pregabalin.
3.2. Potential Baseline Predictors of Hospitalization
Use of addictive non-opioid drugs (by self-report) at baseline apart from opioids were common, mainly sedative-hypnotics (73%), alcohol (47%), amphetamine (32.4), and cannabis (19.7%).
Baseline use of sedative-hypnotics, and buprenorphine were both found to be significantly associated with hospitalization (Table 2). When controlling these two variables for one another in a logistic regression, hospitalization was only significantly associated with baseline use of buprenorphine (OR 4.2, CI 1.2–14.5, p = 0.025) and not with baseline use of sedative-hypnotics (OR 3.1, CI 1.0–9.8, p = 0.052).

Table 2.
Participant characteristics of subjects with and without psychiatric hospitalization by self-report at baseline. (N = 71).
When performing a Cox proportional hazard regression with time to discharge as a time-varying covariate, hospitalization was significantly predicted by discharge from OMT (HR 2.0, CI 1.0–3.9, p = 0.039). Likewise, hospitalization was predicted by baseline use of sedative-hypnotics (HR 2.6, CI 1.2–5.7, p = 0.015), or buprenorphine (HR 2.3, CI 1.3–4.3, p = 0.005).
3.3. Comorbid Psychiatric Diagnoses
Psychiatric co-occurring disorders was found to be highly prevalent. Descriptive information on psychiatric diagnoses within three years from inclusion and their prevalence are provided in Appendix A. In the three-year period, 72% of the study participants obtained a psychiatric diagnosis other than opioid dependence. Overall, 63% had some kind of SUD and 32% of the sample were dependent on sedative-hypnotics. When excluding SUDs, less than half of the individuals (41%) were diagnosed; the most prevalent subgroup was anxiety disorders (27% of all participants).
No baseline characteristic was found to predict a non-SUD psychiatric diagnosis (Table 3).

Table 3.
Participant characteristics of subjects with and without psychiatric diagnosis *.
4. Discussion
The results of this study showed that most patients with OUD who had been referred from an NEP to OMT needed psychiatric hospital care during the 36-month follow-up, mainly for substance-related reasons, among which sedative-hypnotics detoxification predominated. Baseline buprenorphine predicted a significant risk of hospitalization. Both buprenorphine, and sedative-hypnotics predicted time to first hospitalization. Psychiatric co-occurring disorders was common, mainly SUD, and among non-SUD patients, anxiety was the most common.
One of the main findings was the predominance of detoxifications from sedative-hypnotics (benzodiazepines and z-drugs (zopiclone and zolpidem)), with 52% of in-patient treatment episodes in this group of patients with primary opioid dependence. Studies that report the rates of detoxification are sparse, to the best of our knowledge. One study showed that 18% of the patients in traditional OMT were admitted for detoxification at least once [15]. However, that study had a shorter follow-up, at most 24 months, and an unknown proportion of patients. Therefore, that study is not quite comparable with this one. One could expect the need for in-patient detoxification to be higher in patients referred from an NEP to OMT due to a high degree of polysubstance use at baseline. Preventative measures are important.
Previous research has shown that patients in treatment for opioid dependence—typically opioid use disorders—often misuse benzodiazepines either for the purpose of self-medication of psychiatric symptoms, for the purpose of becoming ‘high’, or in order to potentiate the effects of the prescribed opioid maintenance medication or an illicit opioid including heroin [44,46,47]. This has been explained as a ‘heroin-like’ effect of benzodiazepines when taken with methadone as well as enabling a prolonged effect of the illicit drugs and a smoother withdrawal [13].
Sedative-hypnotics (benzodiazepines, z-drugs (zopiclone and zolpidem), and pregabalin) contribute to opioid overdose mortality [13,19,48,49,50] and are present in 10–80% of methadone-related deaths and in 80% of buprenorphine-related deaths [13,14]. Concomitant use of benzodiazepines, including prescribed doses, increases sedation and interferes with cognitive function in patients maintained on methadone or buprenorphine and affects physical parameters when taken together with methadone [14]. Furthermore, the misuse of benzodiazepines during OMT is associated with continued use of opioids and illicit drugs, and problems with mental health [17,18,51,52]. Given the benzodiazepine-related harms in opioid-dependent patients, researchers have called for increased focus on the treatment of psychiatric co-occurring disorders in this condition to decrease the reasons for self-medication use of benzodiazepines [44]. Finally, dispensation of sedative-hypnotics, or benzodiazepines to patients in OMT are common and have pros and cons [53]. It has recently been shown that a substantial minority of OMT patients in Sweden were dispensed benzodiazepines (15%) and z-hypnotics (26%) [54]. Other sources of prescription are of course also possible. (Patients at the present unit were never prescribed these drugs.)
Non-prescribed use of buprenorphine prior to treatment start was a significant predictor of hospitalization and remained so after controlling for baseline use of sedative-hypnotics. Whereas this finding is difficult to interpret, it can be hypothesized that patients were using this as a treatment rather than recreationally, as supported by the literature [55]. A recent review also reported results indicating that most people using illicit buprenorphine did so for reasons related to misuse, that is, as efforts to manage opioid withdrawal symptoms or to achieve or maintain abstinence rather than a motive to get high [56]. Intuitively, this non-prescribed use of buprenorphine might not necessarily be associated with a more severe course in treatment.
This result suggests that sedative-hypnotic drugs constitute a significant clinical problem, as shown by the prediction of hospitalization, and—despite not being the patient’s primary drug of abuse—as the most common reason for it during OMT.
Psychiatric co-occurring disorders were common, and SUD predominated. The proportion of patients that received a non-SUD psychiatric diagnosis in this study was 41%. Studies in similar patient populations have reported 44–86%, albeit for lifetime prevalence [11,12,21,57]. However, in a large study of co-occurring disorders among OMT patients using SCID (Structured Clinical Interview for DSM-IV-Axis I Disorders) at 1–8 weeks, as many as 75% had a non-SUD psychiatric disorder [12]. When excluding other substance use disorders, the prevalence of psychiatric disorders in the present study was comparable with earlier reports [11,21,22,48], although lower rates have been reported from China, where only 30% matched criteria for a non-SUD psychiatric [57]. The most common non-SUD diagnosis was anxiety, with 27% of all participants. As mentioned above, researchers’ have called for increased focus on the treatment of co-occurring disorders to decrease the reason for self-medication with benzodiazepines [44]. Anxiety and misuse could be assumed to be correlated. Persons with anxiety may start self-medication, and benzodiazepines could sometimes worsen anxiety. Both problems should be identified and addressed.
Standing out in the literature is the high prevalence of personality disorders, with estimations of 25–40% having antisocial personality disorder (ASPD) [12,57]. Our finding that only 7% fulfilled a personality disorder diagnosis and one single individual had ASPD is probably an underestimation of the actual prevalence. This is probably since we used ICD diagnoses at in-patient or out-patient admissions, where personality diagnoses had lower priority and could not be easily evaluated due to drug use, lack of diagnostic instruments, etc.
The present findings on SUD show a clinically relevant prevalence, particularly as 32% were diagnosed with sedative-hypnotics dependence and 31% diagnosed with poly-substance drug use dependence (notably, these overlap to a large extent). However, this may be compared with past-month misuse estimates ranging from 7–73%, with a majority (67%) of studies reviewed reporting rates greater than 40% for people in treatment for OUD [58]. That is in the upper end and could be expected, as compared with studies that mostly had high threshold admittance. The rate of self-reported misuse at baseline was 73%, which may also be compared with the same estimates.
Recent studies have suggested that the use of benzodiazepines is negatively associated with retention in OMT [59,60,61], whereas other studies failed to demonstrate such an association [16,17].
The importance of socio-demographics for treatment outcome and psychiatric severity including gender differences in co-occurring disorders has been reported [62]. Associations between SUD and specific psychiatric disorders (including other SUDs) have been investigated as underlying factors of co-occurring disorders [10]. The results showed weak but significant associations [10].
The number of psychiatric beds in many high-income countries have been reported to have been reduced considerably since the late 1980s [33,34]. Some authors have therefore pointed out that being admitted to psychiatric in-patient care could be seen as a marker for a psychiatric condition with a high degree of severity [35]. Therefore, the present study could be presumed to deal with high severity, and some less serious cases may have been missed out.
An important limitation of the present study is the final sample size, as it reduces the number of co-variates by as much as possible to include potential baseline predictors of outcome. The small sample size may affect interpretation and generalizability. Information about the clients who chose not to participate in the present study is limited.
No structured diagnostic assessment was made, and therefore, the diagnostic accuracy cannot be regarded as optimal. However, the study design with one single site and diagnostic interviews mainly performed by the same psychiatrist should reduce diagnostic random errors and treatment confounders to some degree. Regardless, as secondary diagnoses were also included—with the purpose of identifying co-occurring disorders—another issue is that diagnoses tend to persist and that diagnoses set before OMT initiation may routinely but wrongfully have been repeated during hospitalization. Although a structured diagnostic instrument such as the (SCID) [63] would have improved specificity, its validity would have been hampered by patients’ current drug use.
Finally, as the present study deals with NEP, the findings may not be sufficiently applicable to general OMT. Populations attending SEPs have been previously reported to have a high degree of drug use severity and a high prevalence of psychiatric co-occurring disorders compared with other opioid users [64]. When referred to maintenance treatment, SEP attendees have also been reported to have a poorer treatment response [65].
5. Conclusions
In conclusion, most patients who entered standard OMT following referral from a needle exchange program (NEP) with psychological and psychosocial treatment needed psychiatric hospital care. Baseline drug use of sedative-hypnotics, and buprenorphine predicted a worse clinical course with respect to psychiatric in-patient care for three years following OMT initiation. The problem of buprenorphine use needs further exploration. This study supports the assumption that psychiatric diagnoses other than SUD are common among patients enrolled with OUD, with anxiety disorders being the most prevalent co-occurring disorders. This requires increased attention toward psychiatric co-occurring disorders in the treatment of OUD and highlights the importance of addressing sedative-hypnotics use when initiating OMT.
Author Contributions
M.B.: methodology, investigation, data curation, writing—original draft 2 and editing, project administration. A.B.: methodology, investigation, data curation, writing—original draft 1. J.F.: methodology, investigation, data curation, writing—review and editing, project administration, supervision. L.B.: methodology, validation, writing—review and editing, supervision. P.I.: methodology, investigation, data curation, writing—review and editing, project administration. S.N.: methodology, investigation, data curation, writing—review and editing, project administration. K.T.: methodology, investigation, data curation, writing—review and editing, project administration. A.H.: methodology, investigation, data curation, writing—review and editing, project administration, formal analysis, supervision (main supervisor). All authors have read and agreed to the published version of the manuscript.
Funding
The National Swedish Research Council for Working Life and Social Sciences and Region Skåne for financial support. Grant No. 2009-1724.
Institutional Review Board Statement
The study was approved by the regional ethics board Lund, Sweden. The institutional body supporting the study (the national Swedish Research Council for Working Life and Social Sciences).
Informed Consent Statement
All participants gave informed consent and inclusion took place between 21 October 2011 and 1 April 2013. Trial Registration: Malmö Treatment referral and intervention study (MATRIS), NCT01457872, https://clinicaltrials.gov/ct2/show/NCT01457872 (accessed on 29 September 2021).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection regulations by the ethics board and by the regional hospital authority, and can be shared in anonymized format only after review by these authorities.
Acknowledgments
The authors thank The national Swedish Research Council for Working Life and Social Sciences and Region Skåne for financial support, the staff at the syringe exchange in Malmö for excellent collaboration, and the participants for their willingness to engage in the study.
Conflicts of Interest
Anders Håkansson is employed at Lund University in a research position that is sponsored by AB Svenska Spel, which is the state-owned gambling operator of Sweden. He also has research funding acquired from the research council of AB Svenska Spel and from the Swedish alcohol retail monopoly Systembolaget AB. None of these organizations were involved in any way in the present research. Martin Bråbäck was invited to speak at the 7th Nordic Opioid Addiction Treatment Conference, Helsinki, Finland. The conference was supported by RB Pharmaceuticals. M.B. received travel funds but no speaker fee, and was also invited speaker at the Scottish Needle Exchange Conference 2014. The conference was supported by Reckitt Benckiser, neo360, Exchange supplies.org, Methameasure Ltd., Pasante and solutions action management. M.B. did not receive any speaker fee but travel and accommodation were covered by the organizers”. and for myself potential conflicting interests”; Louise Brådvik received a scholarship of 50,000 SEK in 2001 and a speaker fee of 12,000 SEK in 2012 from the Swedish Lundbeck Foundation”. The other authors declare no conflict of interest.
Appendix A

Table A1.
Information on psychiatric diagnoses within three years from inclusion and their prevalence.
Table A1.
Information on psychiatric diagnoses within three years from inclusion and their prevalence.
| N (%) | |
|---|---|
| Substance Use Disorder (SUD) * (F1) ** | 45 (63.4) |
| Alcohol | |
| Abuse | 4 (5.6) |
| Dependence | 6 (8.5) |
| Cannabis | |
| Abuse | 3 (4.2) |
| Dependence | 6 (8.5) |
| Sedatives and hypnotics | |
| Abuse | 3 (4.2) |
| Dependence | 23 (32.4) |
| Cocaine | |
| Abuse | 1 (1.4) |
| Dependence | 0 (0.0) |
| Stimulants | |
| Abuse | 2 (2.8) |
| Dependence | 5 (7.0) |
| Volatile solvents | |
| Abuse | 0 (0.0) |
| Dependence | 1 (1.4) |
| Poly-substance drug use | |
| Abuse | 1 (1.4) |
| Dependence | 22 (31.0) |
| Psychotic disorders (F2) | 2 (2.8) |
| Non-organic psychotic disorder | 2 (2.8) |
| Non-organic psychosis | 1 (1.4) |
| Mood disorders (F3) | 8 (11.3) |
| Bipolar disease | 1 (1.4) |
| Depression | 6 (8.5) |
| Unspecified | 1 (1.4) |
| Anxiety disorders (F4) | 19 (26.8) |
| Generalized Anxiety Disorder (GAD) | 1 (1.4) |
| Mixed anxiety/depressive disorder | 12 (16.9) |
| Anxiety, unspecified | 8 (11.3) |
| Personality disorders (F6) | 5 (7.0) |
| Anti-social | 1 (1.4) |
| Emotionally instable | 4 (5.6) |
| Unspecified | 1 (1.4) |
| Mixed type | 1 (1.4) |
| Autism spectrum disorders (F8) | 1 (1.4) |
| Atypical autism | 1 (1.4) |
| Asperger syndrome | 1 (1.4) |
| Attention deficit disorders (F9) | 7 (9.9) |
| Attention deficit disorder (ADD) | 2 (2.8) |
| Attention deficit hyperactivity disorder (ADHD) | 2 (2.8) |
| Unspecified | 3 (4.2) |
| Any psychiatric diagnose | 51 (71.8) |
* Other than opioids, as opioid dependence is a criterion for study-inclusion. ** F1–F9 refers to the ICD-10 classification used in the medical journals. Substance abuse is here defined for those with abuse only. If patients had both an abuse and dependence diagnosis of one and the same substance, only the latter is counted.
References
- Hser, Y.-I.; Hoffman, V.; Grella, C.E.; Anglin, M.D. 33-year follow-up of narcotics addicts. Arch. Gen. Psychiatry 2001, 58, 503–508. [Google Scholar] [CrossRef]
- Degenhardt, L.; Grebely, J.; Stone, J.; Hickman, M.; Vickerman, P.; Marshall, B.D.L.; Bruneau, J.; Altice, F.L.; Henderson, G.; Rahimi-Movaghar, A.; et al. Global patterns of opioid use and dependence: Harms to populations, interventions, and future action. Lancet 2019, 394, 1560–1579. [Google Scholar] [CrossRef]
- Opioid Maintenance Treatment—A Call for a Joint European Quality Care Approach. Available online: https://www.karger.com/article/Abstract/432395 (accessed on 29 September 2021).
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014, 2, CD002207. [Google Scholar] [CrossRef] [PubMed]
- Brugal, M.T.; Domingo-Salvany, A.; Puig, R.; Barrio, G.; De Olalla, P.G.; De la Fuente, L. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction 2005, 100, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Kakko, J.; Svanborg, K.D.; Kreek, M.J.; Heilig, M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: A randomised, placebo-controlled trial. Lancet 2003, 361, 662–668. [Google Scholar] [CrossRef]
- Sordo, L.; Barrio, G.; Bravo, M.J.; Indave, B.I.; Degenhardt, L.; Wiessing, L.; Ferri, M.; Pastor-Barriuso, R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ 2017, 357, j1550. [Google Scholar] [CrossRef]
- Joseph, H.; Stancliff, S.; Langrod, J. Methadone maintenance treatment (MMT): A review of historical and clinical issues. Mt. Sinai J. Med. 2000, 67, 347–364. [Google Scholar]
- Bart, G. Maintenance Medication for Opiate Addiction: The Foundation of Recovery. J. Addict. Dis. 2012, 31, 207–225. [Google Scholar] [CrossRef]
- Compton, W.M.; Thomas, Y.F.; Stinson, F.S.; Grant, B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry 2007, 64, 566–576. [Google Scholar] [CrossRef]
- Rodríguez-Llera, M.; Domingo-Salvany, A.; Brugal, M.T.; Silva, T.; Sanchez-Niubo, A.; Torrens, M. Psychiatric comorbidity in young heroin users. Drug Alcohol Depend. 2006, 84, 48–55. [Google Scholar] [CrossRef]
- Cacciola, J.S.; Alterman, A.I.; Rutherford, M.J.; McKay, J.R.; Mulvaney, F.D. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001, 61, 271–280. [Google Scholar] [CrossRef]
- Jones, J.D.; Mogali, S.; Comer, S.D. Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012, 125, 8–18. [Google Scholar] [CrossRef]
- Lintzeris, N.; Nielsen, S. Benzodiazepines, Methadone and Buprenorphine: Interactions and Clinical Management. Am. J. Addict. 2010, 19, 59–72. [Google Scholar] [CrossRef]
- Specka, M.; Bonnet, U.; Heilmann, M.; Schifano, F.; Scherbaum, N. Longitudinal patterns of benzodiazepine consumption in a German cohort of methadone maintenance treatment patients. Hum. Psychopharmacol. Clin. Exp. 2011, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Votaw, V.R.; Geyer, R.; Rieselbach, M.M.; McHugh, R.K. The epidemiology of benzodiazepine misuse: A systematic review. Drug Alcohol Depend. 2019, 200, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Brands, B.; Blake, J.; Marsh, D.C.; Sproule, B.; Jeyapalan, R.; Li, S. The Impact of Benzodiazepine Use on Methadone Maintenance Treatment Outcomes. J. Addict. Dis. 2008, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bleich, A.; Gelkopf, M.; Weizman, T.; Adelson, M. Benzodiazepine abuse in a methadone maintenance treatment clinic in Israel: Characteristics and a pharmacotherapeutic approach. Isr. J. Psychiatry Relat. Sci. 2002, 39, 104–112. [Google Scholar] [PubMed]
- Darke, S.; Zador, D. Fatal heroin ‘overdose’: A review. Addiction 1996, 91, 1765–1772. [Google Scholar]
- Abrahamsson, T.; Berge, J.; Öjehagen, A.; Håkansson, A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017, 174, 58–64. [Google Scholar] [CrossRef]
- Brooner, R.K.; King, V.L.; Kidorf, M.; Schmidt, C.W., Jr.; Bigelow, G.E. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch. Gen. Psychiatry 1997, 54, 71–80. [Google Scholar] [CrossRef]
- Schäfer, I.; Eiroa-Orosa, F.J.; Verthein, U.; Dilg, C.; Haasen, C.; Reimer, J. Effects of psychiatric comorbidity on treatment outcome in patients undergoing diamorphine or methadone maintenance treatment. Psychopathology 2010, 43, 88–95. [Google Scholar] [CrossRef]
- Jansson, I.; Hesse, M.; Fridell, M. Personality disorder features as predictors of symptoms five years post-treatment. Am. J. Addict. 2008, 17, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Compton, W.M., 3rd; Cottler, L.B.; Jacobs, J.L.; Ben-Abdallah, A.; Spitznagel, E.L. The role of psychiatric disorders in predicting drug dependence treatment outcomes. Am. J. Psychiatry 2003, 160, 890–895. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.; Luborsky, L.; Woody, G.E.; O’Brien, C.P.; Druley, K.A. Predicting response to alcohol and drug abuse treatments: Role of psychiatric severity. Arch. Gen. Psychiatry 1983, 40, 620–625. [Google Scholar] [CrossRef] [PubMed]
- King, V.L.; Kidorf, M.S.; Stoller, K.B.; Carter, J.A.; Brooner, R.K. Influence of antisocial personality subtypes on drug abuse treatment response. J. Nerv. Ment. Dis. 2001, 189, 593–601. [Google Scholar] [CrossRef]
- Pani, P.P.; Maremmani, I.; Pacini, M.; Lamanna, F.; Maremmani, A.G.; Dell’Osso, L. Effect of psychiatric severity on the outcome of methadone maintenance treatment. Eur. Addict. Res. 2011, 17, 80–89. [Google Scholar] [CrossRef]
- Gelkopf, M.; Weizman, T.; Melamed, Y.; Adelson, M.; Bleich, A. Does psychiatric comorbidity affect drug abuse treatment outcome? A prospective assessment of drug abuse, treatment tenure and infectious diseases in an Israeli methadone maintenance clinic. Isr. J. Psychiatry Relat. Sci. 2006, 43, 126–136. [Google Scholar]
- Rosic, T.; Naji, L.; Bawor, M.; Dennis, B.B.; Plater, C.; Marsh, D.C.; Thabane, L.; Samaan, Z. The impact of comorbid psychiatric disorders on methadone maintenance treatment in opioid use disorder: A prospective cohort study. Neuropsychiatr. Dis. Treat. 2017, 13, 1399–1408. [Google Scholar] [CrossRef]
- Astals, M.; Diaz, L.; Domingo-Salvany, A.; Martin-Santos, R.; Bulbena, A.; Torrens, M. Impact of co-occurring psychiatric disorders on retention in a methadone maintenance program: An 18-month follow-up study. Int. J. Environ. Res. Public Health 2009, 6, 2822–2832. [Google Scholar] [CrossRef]
- Ngo, H.T.; Tait, R.J.; Hulse, G.K. Hospital psychiatric comorbidity and its role in heroin dependence treatment outcomes using naltrexone implant or methadone maintenance. J. Psychopharmacol. 2010, 25, 774–782. [Google Scholar] [CrossRef]
- Nunes, E.V.; Sullivan, M.A.; Levin, F.R. Treatment of depression in patients with opiate dependence. Biol. Psychiatry 2004, 15, 793–802. [Google Scholar] [CrossRef]
- Priebe, S.; Badesconyi, A.; Fioritti, A.; Hansson, L.; Kilian, R.; Torres-Gonzales, F.; Turner, T.; Wiersma, D. Reinstitutionalisation in mental health care: Comparison of data on service provision from six European countries. BMJ 2005, 330, 123–126. [Google Scholar] [CrossRef]
- Wahlbeck, K.; Westman, J.; Nordentoft, M.; Gissler, M.; Laursen, T.M. Outcomes of Nordic mental health systems: Life expectancy of patients with mental disorders. Br. J. Psychiatry 2018, 199, 453–458. [Google Scholar] [CrossRef]
- Olsson, M.O.; Öjehagen, A.; Brådvik, L.; Håkansson, A.C. Predictors of Psychiatric Hospitalization in Ex-Prisoners With Substance Use Problems: A Data-Linkage Study. J. Drug Issues 2015, 45, 202–213. [Google Scholar] [CrossRef]
- Bråbäck, M.; Nilsson, S.; Isendahl, P.; Troberg, K.; Brådvik, L.; Hakansson, A. Malmö Treatment Referral and Intervention Study (MATRIS)—effective referral from syringe exchange to treatment for heroin dependence: A pilot randomized controlled trial. Addiction 2016, 111, 866–873. [Google Scholar] [CrossRef]
- Kourounis, G.; Richards, B.D.W.; Kyprianou, E.; Symeonidou, E.; Malliori, M.-M.; Samartzis, L. Opioid substitution therapy: Lowering the treatment thresholds. Drug Alcohol Depend. 2016, 161, 1–8. [Google Scholar] [CrossRef]
- Bråbäck, M.; Ekström, L.; Troberg, K.; Nilsson, S.; Isendahl, P.; Brådvik, L.; Håkansson, A. Malmö Treatment Referral and Intervention Study—High 12-Month Retention Rates in Patients Referred from Syringe Exchange to Methadone or Buprenorphine/Naloxone Treatment. Front. Psychiatry 2017, 8, 161. [Google Scholar] [CrossRef]
- (Socialstyrelsen) TNBoHaW. Socialstyrelsens föreskrifter och allmänna råd om läkemedelsassisterad behandling vid opioidberoende [The Board’s provisions and general guidelines on medication-assisted treatment in opiate dependence]. HSLF-FS 2016, 1, 1–6. [Google Scholar]
- Romelsjö, A.; Engdahl, B.; Stenbacka, M.; Fugelstad, A.; Davstad, I.; Leifman, A.; Thiblin, I. Were the changes to Sweden’s maintenance treatment policy 2000–06 related to changes in opiate-related mortality and morbidity? Addiction 2010, 105, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.; Kushner, H.; Metzger, D.; Peters, R.; Smith, I.; Grissom, G.; Pettinati, H.; Argeriou, M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992, 9, 199–213. [Google Scholar] [CrossRef]
- Carter, C.R.; Kozuska, J.L.; Dunn, S.M. Insights into the structure and pharmacology of GABA(A) receptors. Future Med. Chem. 2010, 2, 859–875. [Google Scholar] [CrossRef]
- Evoy, K.E.; Sadrameli, S.; Contreras, J.; Covvey, J.R.; Peckham, A.M.; Morrison, M.D. Abuse and Misuse of Pregabalin and Gabapentin: A Systematic Review Update. Drugs 2020, 81, 125–156. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Knopfli, B.; Schmid, O.; Prica, M.; Strasser, J.; Prieto, L.; Wiesbeck, G.A.; Dürsteler-MacFarland, K.M. Treatment or “high”: Benzodiazepine use in patients on injectable heroin or oral opioids. Addict. Behav. 2013, 38, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.Y.; Handelsman, L.; Bickel, W.K.; Griffiths, R.R. Benzodiazepine and sedative use/abuse by methadone maintenance clients. Drug Alcohol Depend. 1993, 32, 257–266. [Google Scholar] [CrossRef]
- Spiga, R.; Huang, D.B.; Meisch, R.A.; Grabowski, J. Human methadone self-administration: Effects of diazepam pretreatment. Exp. Clin. Psychopharmacol. 2001, 9, 40–46. [Google Scholar] [CrossRef]
- Warner-Smith, M.; Darke, S.; Lynskey, M.; Hall, W. Heroin overdose: Causes and consequences. Addiction 2001, 96, 1113–1125. [Google Scholar] [CrossRef]
- Lembke, A.; Papac, J.; Humphreys, K. Our Other Prescription Drug Problem. N. Engl. J. Med. 2018, 378, 693–695. [Google Scholar] [CrossRef]
- Lader, M. Benzodiazepines revisited-will we ever learn? Addiction 2011, 106, 2086–2109. [Google Scholar] [CrossRef]
- Fatséas, M.; Lavie, E.; Denis, C.; Auriacombe, M. Self-perceived motivation for benzodiazepine use and behavior related to benzodiazepine use among opiate-dependent patients. J. Subst. Abuse. Treat. 2009, 37, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, D.; Jacobs, S.; Karg, I.; Luedecke, C.; Schneider, U.; Cimander, K.; Baumann, P.; Ruether, E.; Poser, W.; Havemann-Reinecke, U. Psychiatric co-occurring disorders and additional abuse of drugs in maintenance treatment with L- and D,L-methadone. World J. Biol. Psychiatry 2010, 11, 390–399. [Google Scholar] [CrossRef]
- Dahlman, D.; Abrahamsson, T.; Kral, A.H.; Hakansson, A. Nonmedical Use of Antihistaminergic Anxiolytics and Other Prescription Drugs among Persons with Opioid Dependence. J. Addict. 2016, 2016, 9298571. [Google Scholar] [CrossRef]
- Vold, J.H.; Skurtveit, S.; Aas, C.; Chalabianloo, F.; Kloster, P.S.; Johansson, K.A.; Fadnes, L.T. Dispensations of benzodiazepines, z-hypnotics, and gabapentinoids to patients receiving opioid agonist therapy; a prospective cohort study in Norway from 2013 to 2017. BMC Health Serv. Res. 2020, 25, 352. [Google Scholar] [CrossRef]
- Vold, J.H.; Aas, C.; Skurtveit, S.; Odsbu, I.; Chalabianloo, F.; Reutfors, J.; Halmøy, A.; Johansson, K.A.; Fadnes, L.T. Potentially addictive drugs dispensing to patients receiving opioid agonist therapy: A register-based prospective cohort study in Norway and Sweden from 2015 to 2017. BMJ Open 2020, 10, e036860. [Google Scholar] [CrossRef] [PubMed]
- Peles, E.; Schreiber, S.; Naumovsky, Y.; Adelson, M. Depression in methadone maintenance treatment patients: Rate and risk factors. J. Affect. Disord. 2007, 99, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Eiroa-Orosa, F.J.; Haasen, C.; Verthein, U.; Dilg, C.; Schäfer, I.; Reimer, J. Benzodiazepine use among patients in heroin-assisted vs. methadone maintenance treatment: Findings of the German randomized controlled trial. Drug Alcohol Depend. 2010, 112, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Moratti, E.; Kashanpour, H.; Lombardelli, T.; Maisto, M. Intravenous misuse of buprenorphine: Characteristics and extent among patients undergoing drug maintenance therapy. Clin. Drug Investig. 2010, 30 (Suppl. 1), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liao, Y.; Wang, Q.; Chawarski, M.C.; Hao, W. Profiles of psychiatric disorders among heroin dependent individuals in Changsha, China. Drug Alcohol Depend. 2015, 149, 272–279. [Google Scholar] [CrossRef][Green Version]
- Håkansson, A.; Widinghoff, C.; Abrahamsson, T.; Gedeon, C. Correlates of Nine-Month Retention following Interim Buprenorphine-Naloxone Treatment in Opioid Dependence: A Pilot Study. J. Addict. 2016, 2016, 6487217. [Google Scholar] [CrossRef]
- Franklyn, A.M.; Eibl, J.K.; Gauthier, G.; Pellegrini, D.; Lightfoot, N.E.; Marsh, D.C. The impact of benzodiazepine use in patients enrolled in opioid agonist therapy in Northern and rural Ontario. Harm Reduct. J. 2017, 14, 6. [Google Scholar] [CrossRef]
- Peles, E.; Adelson, M.; Schreiber, S. Benzodiazepine usage during 19.5 years in methadone maintenance treatment patients and its relation to long-term outcome. Isr. J. Psychiatry Relat. Sci. 2014, 51, 285–288. [Google Scholar]
- Sinha, R.; Rounsaville, B.J. Sex differences in depressed substance abusers. J. Clin. Psychiatry 2002, 63, 616–627. [Google Scholar] [CrossRef]
- Lobbestael, J.; Leurgans, M.; Arntz, A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II). Clin. Psychol. Psychother. 2011, 18, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kidorf, M.; Disney, E.R.; King, V.L.; Neufeld, K.; Beilenson, P.L.; Brooner, R.K. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend. 2004, 74, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.; King, V.; Peirce, J.; Kolodner, K.; Brooner, R.; Kidorf, M. A comparison of 1-year substance abuse treatment outcomes in community syringe exchange participants versus other referrals. Drug Alcohol Depend. 2008, 97, 122–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).