Abstract
Persistent organic pollutants (POPs) have adverse effects on the human health and ecosystem functioning. Graphene oxide (GO) has been developed to remove trace levels of POPs from wastewater samples. However, many questions involved in these processes are still unresolved (e.g., the role of π–π interaction, the effect of GO on the degradation of POPs, and so on). Revealing the microscopic interactions between GO and POPs is of benefit to resolve these questions. In the present study, a quantum chemical calculation was used to calculate the molecular doping and adsorption energy between eight representative POPs and GO. The influences of GO on the thermodynamic parameters, such as the Gibbs free energy and the highest occupied molecular orbital (HOMO)-lowest unoccupied molecular orbital (LUMO) gap, were also reported. We found the molecular doping is dependent on the species of POPs. The adsorption energy of the majority of POPs on GO is between 7 and 8 kJ/mol. Consequently, the GO may make degradation of POPs in wastewater more productive and lead to a change of kinetics of the degradation of POPs.
1. Introduction
Persistent organic pollutants (POPs) denote organic chemicals that are resistant to environmental degradation through photolytic, chemical, and biological processes [1]. They have half-lives ranging from decades to centuries in soil, water, and air [2,3]. Besides the persistence in the environment, compounds that make up POPs also have a high order of bioaccumulation and biomagnification. In detail, they can accumulate in the fatty tissue of living organisms including humans, and are found at higher concentrations at higher levels in the food chain [4,5]. They are also toxic to humans and wildlife, and are widely associated with adverse effects on growth, development, reproduction, and survival [6]. For example, polycyclic aromatic hydrocarbon (PAH), which is a kind of common POPs, is classified as possible or probable human carcinogens [7]. As an emerging POP of concern, perfluorooctane sulfonic acid (PFOS) has been found to be associated with fetal birth defects by altering the expression of crucial genes, reducing ATP production, inducing reactive oxygen species (ROS), and stimulating cell apoptosis in the early stages of cardiogenesis [8]. In addition, even the background-level exposure to polychlorinated biphenyls (PCBs) among children in the general population can also negatively influence their metabolic health [9]. The POPs are listed in Table 1, which were obtained from Stockholm Convention on Persistent Organic Pollutants [10]. The majority of these chemicals are currently or were in the past used as pesticides, solvents, and industrial chemicals. Many studies suggested that POPs can also be generated during the processes of burning events, industrial production, and urban garbage incineration [11].

Table 1.
All POPs listed in the Stockholm Convention.
Graphene oxide (GO), the oxidized analogue of graphene, is a two-dimension structured and oxygenated planar molecular material. It has been widely used in multidisciplinary fields, such as nonlinear optical materials [12,13], targeted drug carriers [14,15], and flexible rechargeable battery electrodes [16,17]. There are also many studies introducing GO into the advanced treatment of wastewater. Lee et al., demonstrated that the nanoplatelets of GO can suppress the fouling of membrane bioreactors during wastewater treatment [18]. Wu et al., used rhamnolipid-functionalized GO to adsorb methylene-blue from wastewater [19]. Zhao et al., suggested that few-layered GO nanosheets may be superior sorbents for heavy metals in ion pollution management [20]. Pavagadhi et al., found that GO could effectively adsorb microcystin-LR and microcystin-RR, and could be reused as an adsorbent following ten cycles of adsorption/desorption with no significant loss in its adsorption capacity [21]. Piaskowski et al., revealed that GO materials could affect physicochemical and biological processes of wastewater treatment [22]. Actually, GO naturally have significant pore volume, high conductivity, and large surface area. These physicochemical properties make it have excellent adsorption and catalytic capacity on organic pollutants, which can be used to remove organic pollutants in the aqueous phase [23]. The number of studies involved in GO for the removal of trace levels of POPs from water samples also keeps increasing in the past decade. In Zeng et al.’s study, the synthesized Fe3O4@GO presented a high enrichment of PCBs from environmental water samples [24]. Le et al. introduced GO into the electron-Fenton process to remove dye pollutants, presenting quite high efficiency [25]. Koushik et al. developed a method to perform rapid dehalogenation by using GO–silver nanocomposite [26]. Gupta et al. utilized a superhydrophobic sponge coated with reduced GO to separate oils and organic solvents from water [27]. Uddin et al., demonstrated that reduced GO-NiFe2O4 nanocomposites could be used for the electrochemical oxidation of hydrazine [28]. Wu et al., found that reduced GO-ferrite hybrids were effective adsorbents for sulfonamides in wastewater [29].
Although the processes of removing different kinds of POPs by GO were studied profoundly, many questions involved in these processes are still unresolved. For example, the roles of π–π interaction, the effects of GO on the degradation of POPs, etc. Revealing the interactions between GO and POPs is useful not only to understand the influences of GO on POPs, but also to promote the modification of GO for better treatment of POPs in wastewater. In the present study, a semi-empirical quantum chemistry method, AM1, was used to calculate the molecular doping and adsorption energy between eight selected POPs and GO. The influence of GO on the thermodynamic parameters, such as the Gibbs free energy, was also reported. Furthermore, physical-chemistry models were implemented with the data from quantum chemical calculation to illustrate the potential significance of GO for removal of POPs in wastewater treatment.
2. Methods
2.1. Chemicals
Several typical chemicals, naphthalene, hexachlorobutadiene, dichloro-diphenyl-trichloroethane (DDT), PCB15, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), lindane, 4,4′-dibromodiphenyl ether (BDE15), and PFOS, as the representative of POPs were studied here. The information about these chemicals is listed in Table 2, and the structures are shown in Figure 1A. In the Stockholm Convention on Persistent Organic Pollutants (POPs), POPs are divided into three categories: pesticides, industrial chemicals, and unintentional productions. Eight ones commonly found in the environment were selected to cover the three categories for the present study. For PCDDs, PCBs, and PBDEs, which contain a variety of congeners, we selected the most representative ones, i.e., TCDD, PCB15, and BDE15, respectively. Therefore, the results based on these chemicals are also informative for the studies of other different POPs. The GO supercell (58 carbon atoms, Figure 1B) was selected as the proxy of GO.

Table 2.
The information about studied chemicals.
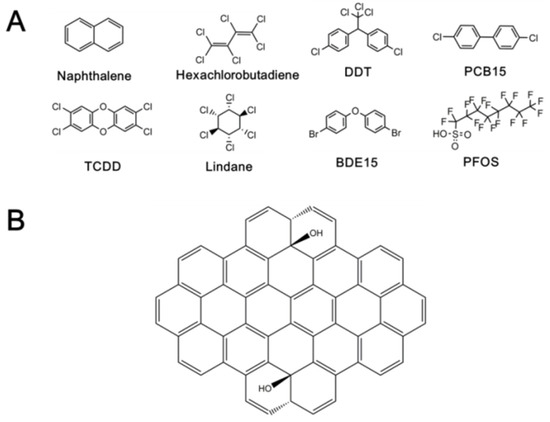
Figure 1.
The structure of studied chemicals (A) and the proxy of GO (B).
2.2. Computational Details
All of the optimization and vibrational frequencies analysis mentioned below were performed using Gaussian 09 (Gaussian Inc., Wallingford, CT, USA) with AM1 method. As a computational physics and chemistry program, Gaussian 09 software typically uses density functional theory (DFT) method for electronic and geometric structure optimization (geometric optimization, transition states, single-point calculation, and reaction path modeling), molecular properties, vibration analysis, electrostatic potential, electron density, and multipole moments. However, given that the systems studied here were a bit large, the AM1 method was used to facilitate the investigation of molecular doping and adsorption energy. The schematic of all the procedures was summarized in Figure 2, and naphthalene was presented as a proxy of all studied POPs. To evaluate the electron transfer between POPs and GO, the structures of complex of each POPs and GO were optimized. The process of finding the minimum energy configuration of molecules through geometric optimization was accomplished by locating the minimum and transition states on the potential plane of molecular orbitals. Gaussian 09 calculated the wave function and energy of the initial geometry and then went on to find new geometries with lower energy. The lowest energy geometry was found by repeating this process. Mulliken atomic charges of each atom were obtained by vibrational frequencies analysis. The charge distribution on atoms indicated the formation of donor and acceptor pairs involved in charge transfer in molecules. Mulliken atomic charge affected dipole moment, molecular polarization, electronic structure and other properties of molecular systems, and played an important role in quantum mechanical calculations [30]. The total number of changes on the atoms of POPs is the number of changes of the corresponding molecules, and then we can identify whether the molecules get or lose electrons. The adsorption energy of different POPs on the surface of GO is calculated by Equation (1),
where is the adsorption energy, which was used to reveal whether GO can eliminate POPs and the related removal mechanisms, is total energy of complex of GO and POPs, is total energy of GO and is total energy of each studied chemicals. GO also affects the Gibbs free energy, HOMO and LUMO. These quantum chemical parameters are informative for the application of GO for eliminating POPs from wastewater.
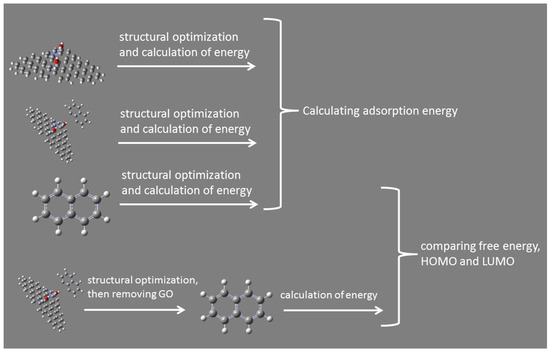
Figure 2.
The flow chart of calculation in this study.
The values of isolated POPs were calculated and compared to the values of POPs undergoing the influence of GO. To calculate values for isolated chemicals, it is needed to optimize the structure of studied chemicals individually and to analyze the respective vibrational frequencies. To get the values influenced by GO, we optimized the structure of studied chemicals with GO first, and then removed GO and performed vibrational frequency analysis on the corresponding studied chemicals.
3. Results and Discussion
3.1. Molecular Doping on GO and Adsorption Energy
Molecular doping, which means that charge transfer between the studied POPs and the surface of GO, is analyzed. As shown in Table 3, naphthalene, DDT, TCDD, and lindane are electron acceptors, and they get 0.00048, 0.00113, 0.000698, and 0.000977 charges from GO, respectively. Hexachlorobutadiene, PCB15, BDE15, and PFOS are electron donors, and they give 0.00134, 0.003182, 0.002278, and 0.002611 charges to GO, respectively. When the organic chemical is the electron donor, the number of electron transfer should be many times higher than when it is the electron acceptor, which may be caused by the fact that electrons on GO have much stronger delocalization potential than those on organic molecules. Therefore, as long as GO can absorb electrons, it tends to maintain the state of absorbing electrons all the time. By contrast, if a small organic molecule absorbs electrons, those electrons are immediately close to “saturation”. Leenaerts et al., have studied molecular doping of small inorganic molecules (e.g., H2O and NH3) adsorbed on the surface of graphene [31]. The number of electron transfer from their study is 1–2 orders of magnitude larger than that obtained here. This difference can be attributed to the difference of adsorbent (graphene vs GO) or adsorbate (small inorganic molecules vs POPs). Further research on HOMO and LUMO values when GO is present or absent with the solvation effect would be helpful in understanding this difference.

Table 3.
Molecular doping and adsorption energy of POPs.
of these 8 POPs were calculated and listed in Table 3. Seven of these values are between 7 and 8 kJ/mol. It implies the majority of POPs have a similar value of adsorption energy on GO, and more experimental research are needed certainly to validate this theoretical inference. However, the one for TCDD is only ~2.8 kJ/mol, which means the interaction between TCDD and GO is much weaker than that for other kinds of POPs. It may be caused by the π–π interaction [32] between TCDD and GO. Xiao et al., found that π–π electron-donor-acceptor interaction (EDA) interaction was not conducive to the delocalization of positively charged alkyl groups and charges into other rings, and the charge was far beyond the correlation with ring insulation amines [33]. The relationship between molecular doping and adsorption energy was also analyzed, and no significant relationship was found. Therefore, the results obtained by the AM1 method indicated that the adsorption of the POPs studied here on GO may be independent on molecular doping. As only eight chemicals were studied here, the robustness should be validated by more cases. However, Yang et al., investigated the adsorption of 1-naphthylamine, 1-naphthol, and naphthalene by GO/iron oxide composites, and they revealed that the adsorption of aromatic compounds was primarily influenced by EDA interaction [34]. The different views on adsorption mechanism may be due to the different adsorbents and compounds used.
3.2. Potential Influence on Degradation Reactions
The effect of GO on the degradations of POPs in wastewater is analyzed. The reaction of degradation can be presented by
The reaction gets equilibrium when Equation (3) occurs.
Here, is Gibbs free energy of POPs as mentioned above. The value is when the chemical is isolated, and the value is when the chemical is adsorbed onto the surface of GO. is sum of Gibbs free energy of all kinds of products, and its value is assumed not to be affected by GO. Before equilibrium, all reactants will experience transition state. By following these basic concepts, a diagram of the Gibbs free energy profile plotted versus the reaction coordinate is made (Figure 3). is the Gibbs free energy of POPs at transition state, (=) and (=) are the values of energy barrier. denotes the difference between and (). and of each chemical were calculated (Table 4). is systematically larger than , demonstrating that GO may affect the thermodynamic equilibrium of the reactions involved in the degradation. of DDT and TCDD are the largest ones, it may be attributed to the interaction between GO and multiple chlorine atoms of these chemicals, which is supported by the finding that GO is capable of adsorbing chlorine atoms [35].
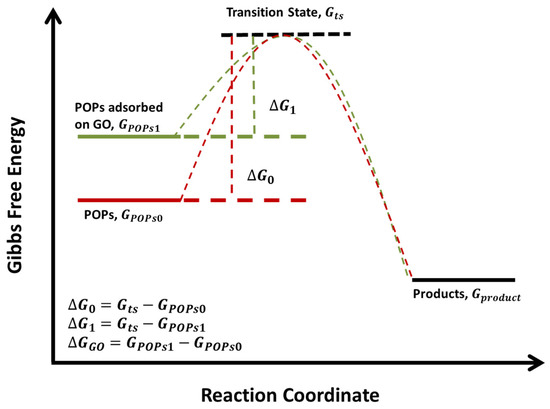
Figure 3.
The conceptual diagram of the Gibbs free energy profile versus the reaction coordinate.

Table 4.
Gibbs free energy of POPs (Hartree).
GO can decrease the energy barrier of reactions involved in degradation. Thus, no matter what is the value of , we can always get . It can be explained by the following equation.
of all chemicals are positive as shown in Table 4. A smaller energy barrier means that reactions can occur more easily. In other words, more POPs can be degraded when GO is present. This point can be further elucidated by analyzing the change of equilibrium constant () which can be written as the following equation.
where [] represents the concentration of . and are the stoichiometry of reactants and products, respectively. From the definition of equilibrium constant from the perspective of statistical physics, can be written as follows:
denotes the difference between the Gibbs free energy of products and reactants, here it is the value of or . is the gas constant, and is the temperature in Kelvin. The exact value of for each chemical cannot be calculated because the final products of these chemicals are unknown. However, no matter what values is, the value of is always greater than because
Therefore, the equilibrium constant without the influence of GO is larger than that when GO is absent.
3.3. Changes of HOMO and LUMO
The effects of GO on the HOMO and LUMO of POPs are studied in this section. In Table 5, all values of HOMO and LUMO when GO is present (HOMO1 and LUMO1) or absent (HOMO0 and LUMO0) are shown. According to the frontier molecular orbital theory [36], HOMO-LUMO gap (), which denotes the energy difference between the HOMO and LUMO, can be used to describe the stability of chemicals. So, this value was also calculated (Table 5). HOMO is the outermost orbital that contains the electron and can act as an electron donor orbital. The LUMO is the innermost orbital that has space to accept electrons which can act as an electron acceptor [30]. The difference in energy between the HOMO and the LUMO is an important indicator of stability. The larger the value of , the most stable the molecule is in chemical reactions.

Table 5.
HOMO, LUMO, and the corresponding gap of POPs (Hartree).
The HOMO-LUMO gap of several compounds with or without GO were compared. The results showed that the order of is lindane > PFOS > hexachlorobutadiene > DDT > BDE15 > PCB15 > naphthalene > TCDD. The addition of GO did not affect the overall ordering of for the compounds studied. But it did cause a change in the HOMO–LUMO energy gap. of naphthalene, DDT, TCDD, and BDE15 are increased by GO, while the values of hexachlorobutadiene, PCB15, lindane, and PFOS are decreased. These changes may affect the kinetics of the degradation of POPs. For example, in sulfate radical-advanced oxidation processes (SR-AOPs) of wastewater [37,38,39], the relationship between the rate constants () of reaction and can be described by Equation (8) [40].
where is the ratio of number of oxygen to carbon atoms. For one chemical, keeps unchanging, so Equation (8) suggests that becomes smaller if increases, and larger if decreases.
On the other hand, the present results also provide ideas for the chemical removal of POPs. GO changes the of POPs, and it may lead to a change of kinetics of the chemical degradation of POPs. In the next study, the effects of GO on the chemical degradation of POPs, the relationships between the degradation kinetics of POPs and the as influenced by GO, etc., should be further investigated by combining experiments and theoretical calculations, such as DFT method and thermal degradation kinetics experiment.
4. Conclusions
In summary, we used a quantum chemical calculation to illustrate the interaction between GO and different kinds of POPs. The molecular doping is dependent on the POPs and no unified law applies to these chemicals. The adsorption energy of POPs on GO is calculated, and the majority of these values are between 7 and 8 kJ/mol. The GO increases Gibbs free energy, and it implies GO can make degradation of POPs in wastewater more productive. GO also changes of POPs, and it may lead to a change in kinetics of the degradation of POPs. However, more sets of POPs are needed to be measured theoretically and experimentally to validate the conclusions. In addition, the present work demonstrates that the innovative method, quantum chemical calculation complemented with thermodynamic and kinetic analyses, introduced in this work can be used to facilitate the study of the interactions between organic pollutants and GO during the process of wastewater treatment.
Author Contributions
Conceptualization, R.Z. and X.W.; methodology, X.W.; supervision, X.W.; validation, Q.W. and X.W.; investigation, Q.W.; resources R.Z.; data curation, Q.W. and Y.L.; writing-original draft preparation, Q.W. and Y.L.; writing-review and editing, R.Z. and X.W.; funding acquisition, R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research is financially supported by the National Natural Science Foundation of China (No. 42177260).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| POPs | Persistent organic pollutants |
| GO | Graphene oxide |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| PAH | Polycyclic aromatic hydrocarbon |
| PFOS | Perfluorooctane sulfonic acid |
| ROS | Reactive oxygen species |
| PCBs | Polychlorinated biphenyls |
| DDT | dichloro-diphenyl-trichloroethane |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| BDE15 | 4,4′-dibromodiphenyl ether |
| EDA | Electron-donor-acceptor |
| SR-AOPs | Sulfate radical-advanced oxidation processes |
References
- Ritter, L.; Solomon, K.R.; Forget, J. A review of persistent organic pollutants. International Programme on Chemical Safety (IPCS). World Health Organ. Tech. Rep. Se 1995, 65, 66. [Google Scholar]
- Weber, R.; Gaus, C.; Tysklind, M.; Johnston, P.; Forter, M.; Hollert, H.; Heinisch, E.; Holoubek, I.; Lloyd-Smith, M.; Masunaga, S.; et al. Dioxin- and POP-contaminated sites−contemporary and future relevance and challenges. Environ. Sci. Pollut. Res. 2008, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Walker, C.H. Organic Pollutants: An Ecotoxicological Perspective; CRC Press: Boca Raton, FL, USA, 2008; pp. 75–79. [Google Scholar]
- Yu, N.; Wang, X.; Zhang, B.; Yang, J.; Li, M.; Li, J.; Shi, W.; Wei, S.; Yu, H. Distribution of perfluorooctane sulfonate isomers and predicted risk of thyroid hormonal perturbation in drinking water. Water Res. 2015, 76, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kirkok, S.K.; Kibet, J.K.; Kinyanjui, T.K.; Okanga, F.I. A review of persistent organic pollutants: Dioxins, furans, and their associated nitrogenated analogues. SN Appl. Sci. 2020, 2, 1729. [Google Scholar] [CrossRef]
- Boström, C.E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110 (Suppl. S3), 451–488. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, Z.; Feng, L.; Wang, Y. Perfluorooctane sulfonate (PFOS) induced embryotoxicity and disruption of cardiogenesis. Toxicol. Vitr. 2013, 27, 1503–1512. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, S.H.; Hong, Y.S.; Ha, E.H.; Park, H. The effect of exposure to persistent organic pollutants on metabolic health among KOREAN children during a 1-year follow-up. Int. J. Environ. Res. Public Health 2016, 13, 270. [Google Scholar] [CrossRef]
- UNEP. Stockholm Convention on Persistent Organic Pollutants. Available online: http://chm.pops.int/ (accessed on 1 August 2022).
- Ruokojärvi, P.; Ettala, M.; Rahkonen, P.; Tarhanen, J.; Ruuskanen, J. Polychlorinated dibenzo-p-dioxins and -furans (PCDDs AND PCDFs) in municipal waste landfill fires. Chemosphere 1995, 30, 1697–1708. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhang, X.; Xu, Y.; Chen, Y.; Tian, J. Nonlinear optical properties of graphene oxide in nanosecond and picosecond regimes. Appl. Phys. Lett. 2009, 94, 021902. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, W.; Zhang, X.; Wang, Y.; Tian, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A graphene hybrid material covalently functionalized with porphyrin: Synthesis and optical limiting property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Hui, D.; Feo, L.; Fraternali, F. Graphene as biomedical sensing element: State of art review and potential engineering applications. Compos. Part B 2018, 134, 193–206. [Google Scholar] [CrossRef]
- David, L.; Singh, G. Reduced graphene oxide paper electrode: Opposing effect of thermal annealing on Li and Na cyclability. J. Phys. Chem. 2014, 118, 28401–28408. [Google Scholar] [CrossRef]
- Lee, J.W.; Lim, S.Y.; Jeong, H.M.; Hwang, T.H.; Kang, J.K.; Choi, J.W. Extremely stable cycling of ultra-thin V2O5 nanowire–graphene electrodes for lithium rechargeable battery cathodes. Energy Environ. Sci. 2012, 5, 9889–9894. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Tang, A.L.L.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R. Removal of microcystin-LR and microcystin-RR by graphene oxide: Adsorption and kinetic experiments. Water Res. 2013, 47, 4621–4629. [Google Scholar] [CrossRef]
- Piaskowski, K.; Zarzycki, P.K. Carbon-based nanomaterials as promising material for wastewater treatment processes. Int. J. Environ. Res. Public Health 2020, 17, 5862. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and graphene oxide-based composites for removal of organic pollutants: A review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Zeng, S.; Gan, N.; Weideman-Mera, R.; Cao, Y.; Li, T.; Sang, W. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem. Eng. J. 2013, 218, 108–115. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Lacour, S.; Oturan, N.; Oturan, M.A.; Cretin, M. High removal efficiency of dye pollutants by electron-fenton process using a graphene based cathode. Carbon 2015, 94, 1003–1011. [Google Scholar] [CrossRef]
- Koushik, D.; Sen Gupta, S.; Maliyekkal, S.M.; Pradeep, T. Rapid dehalogenation of pesticides and organics at the interface of reduced graphene oxide–silver nanocomposite. J. Hazard. Mater. 2016, 308, 192–198. [Google Scholar] [CrossRef]
- Gupta, S.; He, W.-D.; Tai, N.-H. A comparative study on superhydrophobic sponges and their application as fluid channel for continuous separation of oils and organic solvents from water. Compos. Part B 2016, 101, 99–106. [Google Scholar] [CrossRef]
- Uddin, M.E.; Kim, N.H.; Kuila, T.; Lee, S.H.; Hui, D.; Lee, J.H. Preparation of reduced graphene oxide-NiFe2O4 nanocomposites for the electrocatalytic oxidation of hydrazine. Compos. Part B 2015, 79, 649–659. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, H.; Chen, R.; Pham-Huy, C.; Hui, X.; He, H. Adsorptive removal of trace sulfonamide antibiotics by water-dispersible magnetic reduced graphene oxide-ferrite hybrids from wastewater. J. Chromatogr. 2016, 1029–1030, 106–112. [Google Scholar] [CrossRef]
- Gidado, A.S.; Maigari, A.; Galadanci, G.S.M. Geometry optimization and vibrational frequencies of tetracene molecule in gas phase and in methanol based on density funtional theory and restricted hartree-fock. Bayero J. Pure Appl. Sci. 2017, 10, 18–31. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. 2008, 77, 125416. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Tang, S.; Feng, W. Preparation of a graphene oxide–phthalocyanine hybrid through strong π–π interactions. Carbon 2010, 48, 211–216. [Google Scholar] [CrossRef]
- Xiao, F.; Pignatello, J.J. pi(+)-pi interactions between (hetero) aromatic amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ. Sci. Technol. 2015, 49, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Wen, T.; Ren, X.; Huang, Y.; Wang, X. Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloids Surf. A 2013, 422, 118–125. [Google Scholar] [CrossRef]
- Ioni, Y.V.; Ivannikova, A.S.; Shapovalov, S.S.; Gubin, S.P. Study of the interaction of graphene oxide with chlorine. Russ. Chem. Bull. 2022, 71, 675–679. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Costanza, J.; Otaño, G.; Callaghan, J.; Pennell, K.D. PCE oxidation by sodium persulfate in the presence of solids. Environ. Sci. Technol. 2010, 44, 9445–9450. [Google Scholar] [CrossRef]
- Yen, C.-H.; Chen, K.-F.; Kao, C.-M.; Liang, S.-H.; Chen, T.-Y. Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: Feasibility and comparison with common oxidants. J. Hazard. Mater. 2011, 186, 2097–2102. [Google Scholar] [CrossRef]
- Zhou, L.; Sleiman, M.; Ferronato, C.; Chovelon, J.M.; de Sainte-Claire, P.; Richard, C. Sulfate radical induced degradation of beta2-adrenoceptor agonists salbutamol and terbutaline: Phenoxyl radical dependent mechanisms. Water Res. 2017, 123, 715–723. [Google Scholar] [CrossRef]
- Xiao, R.; Ye, T.; Wei, Z.; Luo, S.; Yang, Z.; Spinney, R. Quantitative structure–activity relationship (QSAR) for the oxidation of trace organic contaminants by sulfate radical. Environ. Sci. Technol. 2015, 49, 13394–13402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).