Abstract
(1) Background: The number of breast-cancer patients and survivors is increasing in the last years. Physical activity seems to be a feasible and useful complementary intervention to improve the physical, psychological, and social spheres and decrease some symptoms, especially for survivors. Consequently, the objective of the present umbrella review was to analyze the efficacy of different physical-activity interventions in the physical, mental, and social spheres of breast-cancer survivors. (2) Methods: Systematic reviews and meta-analyses of randomized controlled trials on breast-cancer survivors and physical-activity effects were searched on the electronic databases PubMed, Web of Science, and Scopus till 9 August 2022. The quality of the studies included was evaluated, and the results were narratively analyzed. (3) Results: Physical-activity intervention generally improves the physical, mental, and social spheres of breast-cancer survivors, but the studies included present heterogeneity in the protocols adopted. (4) Conclusions: A well-structured and planned physical-activity intervention is useful for improvements in the physical, mental, and social spheres of breast-cancer survivors, but the studies presented high heterogeneity. Yoga seems to be the most effective physical intervention to complement medical therapy.
1. Introduction
New treatment protocols, intervention techniques, and early diagnosis increase the number of breast-cancer (BC) survivors, as well as the possibility of reaching an older age; however, they also cause BC survivors to present other diseases and comorbidity situations []. Furthermore, in old age, there are profound changes in body composition, which could generate sarcopenia, with decreases in strength and the aerobic and functional capacities, and an increase in the body-fat volume []. A second aspect to consider is that BC patients and survivors frequently experience higher levels of anxiety and depression, poorer quality of life (QoL), higher levels of fatigue, poorer physical functioning, and urinary dysfunction []. A final aspect to consider is the limited understanding of cancer in the social domains, and especially in social functioning []. These coexisting medical conditions could increase the risk of treatment toxicity [] and, consequently, the risk of death.
The World Health Organization defined health as complete physical, mental, and social wellbeing [], highlighting the attention on these three elements. Furthermore, programs adequately designed and adapted to the patient, when compared with medical intervention or treatment, reduce health risks, and limit the healthcare-system costs [,]. Usually, a well-planned intervention brings about positive outcomes from a physical point of view [], decreases cardiovascular disease, hypertension, type 2 diabetes, site-specific cancers, and mental-health problems (such as anxiety and depression), reduces adiposity, and improves cognitive health and sleep quality [].
The regular practice of a physical activity, always alongside medical therapy, is also essential for the prevention and treatment of BC. Physical-activity programs during illness reduce the risk of BC-related death after diagnosis [,]. It seems that being physically active throughout life prevents BC [] and reduces the BC incidence [], mortality, and morbidity [,], and especially if associated with weight loss [] and among postmenopausal women []. It is also fundamental to be physically active before the diagnosis; indeed, there is an inverse relationship between the physical-activity level and all-cause and BC-related deaths and events [,]. These aspects make it interesting to better understand physical activity’s role in BC survivors, and especially because physical activity is a safe, feasible, and efficacious intervention in BC patients who are undergoing different types of treatment []. It is also useful in the reduction in or management of the symptoms []. Physical activity could therefore help reintegrate patients into daily routines, work, and family life []. Exercise training during BC treatment is often associated with a progressive improvement in physical, physiological, and psychological health, as well as functional parameters [,]. It creates a blend directly with the tumor and its microenvironment, which may promote its use as an additional coadjuvant therapy []: a long-term physical-activity intervention reduces the risk of BC through increased global DNA methylation [].
Different physical-exercise interventions at different durations adapted to the physical conditions of the person seem to reduce the risk of BC []. Aerobic training, resistance training [], interventions based on flexibility, yoga, tai chi, qigong, and Pilates [], or exercise in general [], have been proposed, with different outcomes and conclusions. Despite the large number of studies on this topic, heterogeneity in the results and methodological limitations have made the findings of the studies limited []. This confusion on the effects of physical-activity interventions on BC symptoms makes it necessary to deeply investigate the topic [] to also understand the magnitude of the positive effects of physical activity among BC survivors []. Consequently, the objective of the present study was to evaluate previously published systematic reviews and meta-analyses of randomized controlled trials with the same topic []. The question analyzed was the study of the effects of physical activity on BC survivors, and we extrapolated information about exercise-training effects on BC-related symptoms, focalizing the attention on the physical, mental, and social spheres.
2. Materials and Methods
This umbrella review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines []. PRISMA checklist [] and the search strategy is included in Supplementary Materials.
2.1. Search Strategy
The included articles were searched on the electronic databases PubMed, Web of Science, and Scopus. Systematic reviews and meta-analyses were included if they were published up to the 09th of August 2022. Different keywords were adopted: (a) breast cancer and breast neoplasm; (b) exercise and physical activity; (c) review and meta-analysis. The keywords were matched through the Boolean operators AND or OR. A string was adopted: (“breast cancer” OR “breast neoplasm”) AND (exercise OR “physical activity”) AND (review OR meta-analysis), in the three databases.
2.2. Eligibility Criteria
Inclusion and exclusion criteria for population, intervention, comparison, outcomes, and study design (PICO-S) were considered. The population was composed of BC survivors (despite the age). Reviews were excluded if the sample investigated included other types of cancer diagnoses. Studies were excluded if the physical-exercise intervention was not structured. The intervention had to include structured physical exercise. The comparison was with control groups, both sedentary and active, and pre-and postintervention. Outcomes had to be parameters related to the physical, mental, and social characteristics. Other studies that were designed differently from systematic reviews and meta-analyses of randomized controlled trials were excluded. Only English-written studies were included.
2.3. Data Sources, Study Sections, and Data Extraction
In the first step, manuscripts were stored in EndNote X8 (EndNote version X8; Thompson Reuters, New York, NY, USA), and the duplicate selection was performed. In the second phase, two independent investigators screened the reviews against the eligibility criteria based on the title, abstract, and full text. The eventual disagreement between the two investigators was solved by the principal investigator.
Information related to the first author and year of publication, review methodology, databases screened, number of reviews included, objective of the study, risk of bias assessment and score, conclusion of the study, population screened, training characteristics, and main results were stored in the tables. A descriptive and narrative synthesis was adopted to analyze the results. A meta-analysis was not performed due to the possibility that including studies considered in more than one systematic review increased the risk of bias [].
2.4. Quality Assessment
The quality of the included systematic reviews and meta-analyses of randomized controlled trials was assessed with the rating scale: “Assessment of Multiple Systematic Reviews” (AMSTAR) []. It consists of 11 items and presents the reliability and validity []. Studies with a final score between 0 and 4 were considered of poor quality. A score between 5 and 7 was considered of moderate quality. A score above 8 indicated high quality. A score of 0 was adopted if “no sufficient information were available”, and a score of 1 was adopted if “enough information” was collected. All included reviews were scored independently by two investigators, and disagreement was resolved by the principal investigator.
3. Results
A total of 6908 studies (PubMed: 1310; Web of Science: 2835; Scopus: 2763) were found after the search of the electronic databases. After duplicate removal, title, and abstract screening, a total of 253 studies were collected for full-text analysis. A final number of 12 systematic reviews and meta-analyses are included in this umbrella review. The screening process is summarized in Figure 1.
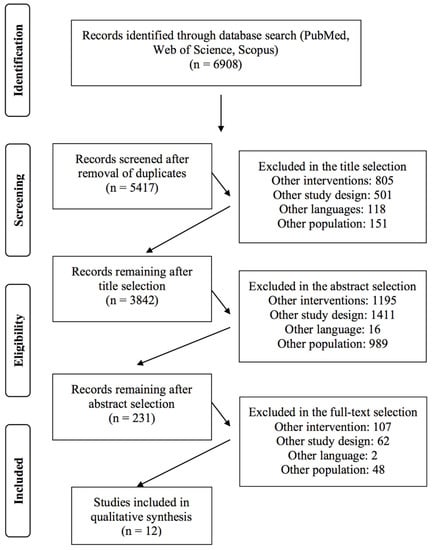
Figure 1.
Flow chart of the selection criteria process of the included studies.
3.1. Characteristics of the Included Studies
Nine studies adopted PRISMA guidelines, and one study also adopted the Cochrane Handbook. MEDLINE (Pubmed), Scopus, Web of Science, PsycINFO, CENTRAL, and EBSCO were mainly adopted to search the articles. The number of included reviews in the studies ranged from eight to fifty-seven. Four studies adopted the Cochrane Handbook to detect the quality of the included studies, while three studies adopted the Physiotherapy Evidence Database (PEDro) scale. More details about the study characteristics are provided in Table 1.

Table 1.
Characteristics of the included studies.
3.2. Physical, Psychological, and Social Sphere Outcomes
The participants in the included interventions were not only BC survivors, but there were also patients at stages 0 to 4 undergoing therapy and surgery. The age ranged from 18 to 83 years, but most of the studies had an age that ranged from 45 to 55 years. Two studies had no information in this regard [,].
The interventions adopted were yoga (n = 3), tai chi (n = 2), aerobic training, and resistance training (n = 4), or a combination of both training methods (n = 2). Water exercise and horseback riding were adopted in one study, such as stretching exercises. The proposed exercise training ranged from 6 weeks to 52 weeks, with the majority of the interventions being 18 weeks long. The weekly frequency ranged from 1 to 6 times (mean: 3 times a week), and the duration of the single session was from 30 to 120 min (mean: 90 min).
Aerobic training statistically significantly reduced cancer-related fatigue, while aerobic-resistance training seemed to result in the greatest benefit in terms of physical functioning []. Another of the included studies detected that walking at a high intensity had the largest effect on the cardiac function []. The study of Ramirez-Velez, 2021 [], detected that exercise training reduced cancer-related fatigue, with a statistical difference. Exercise training decreased the systolic and diastolic blood pressure, triglyceride levels, and body-mass index; with a statistical difference, it increased the peak oxygen uptake, maximal oxygen consumption, and high-density leptin cholesterol, while no statistical differences were detected in the changes in the mean arterial pressure, peak heart rate, and peak respiratory exchange ratio []. Supervised exercise training statistically improves functional and physical wellbeing []. Yoga presented statistically significant effects on cancer-related fatigue []. Tai chi and conventional therapy are more effective for cancer-related fatigue at 3 months []. Tai chi also improved cancer-related fatigue, with no statistical difference in the bone mineral density, body-mass index, or muscle strength []. The positive effects of exercise on cancer-related fatigue are also confirmed by other studies [].
Yoga, if compared with the treatment group, had small short-term effects on the QoL and functional, social, and spiritual wellbeing, but when compared with an active control, large short-term effects on mental wellbeing, anxiety, depression, stress, and psychological distress were detected []. Yoga also presented statistically significant effects on anxiety and depression, with an overall enhancement of QoL []. The study of Liu, 2020 [], found a statistically significant improvement after tai chi in QoL at 3 months. Tai chi and conventional therapy are more effective on QoL at 3 and 6 months. There were no statistical differences from conventional therapy in improving the sleep parameters, depression, or body-mass index. Tai chi also improved emotional wellbeing, with a statistical difference []. The study by Ramirez-Velez, 2021 [], detected that exercise training reduced, with a statistical difference, anxiety and depression, and produced increases in body image, QoL, and emotional function.
Supervised exercise training has nonstatistically significant effects on the social and emotional wellbeing domains []. There is a significant heterogeneity depending on whether the intervention is proposed in groups or not []. More details of the studies are included in Table 2.

Table 2.
Characteristics of the interventions.
3.3. Risk of Bias Assessment
The quality of the included studies ranged from four to ten, with a mean of 4/9. Within the included studies, the overall quality is mainly moderate, and the risk of bias is medium. Two studies had no scores. The results of one review are unclear. A summary is provided in Table 1 and Table 3.

Table 3.
Quality assessment through the “assessment of multiple systematic reviews” (AMSTAR) of the included systematic reviews.
4. Discussion
Systematic reviews and meta-analyses of randomized controlled trials are highly heterogeneous. Despite this important limitation, the findings suggest that exercise training, regardless of its structure and typology, has positive outcomes on the physical, psychological, and social health spheres in BC survivors. Among the activities analyzed, yoga was ideal because its effects are on the three spheres analyzed. It is fundamental to consider that exercise training can only complement medical therapy and not replace it.
Most of the physical-training interventions of the included studies, even if there were differences in the typology (aerobic, resistance training, or combining both), structure (duration, frequency, intensity), or modality (biking, swimming walking, riding a horse), presented improvements in physical functioning [] and cardiovascular-system functions []. These findings suggest the importance of practicing physical activity to reduce the risk of cardiovascular problems and improve the overall QoL. The results of physical activity are also indirect; indeed, one of the included studies [] correlated the reduction in body weight with the reduction in the fasting insulin levels. Our findings are similar to other studies in which similar positive physical outcomes were detected [,].
From a psychological point of view, exercise training improves psychological health [] anxiety and depression, QoL, and emotional function [,]. Mindful physical activities, such as yoga, are suggested for their positive outcomes, and especially on mental health (psychological and social health) [,]. Our findings are in line with other studies in which the positive effects of physical activity on the psychological outcomes and QoL in patients after treatment for BC were detected []. In a few words, exercise training has a positive effect on the health outcomes in cancer survivors []. Another adopted “mind-based” intervention was tai chi, but the results are contradictory, with one study suggesting improvements in cancer-related fatigue [], while another study asserts that there is a lack of a statistically significant improvement in the QoL and other clinical endpoints []. Two studies detected no long-term improvements in mental wellbeing, physical wellbeing, anxiety, depression, psychological distress [], and QoL improvement [], which makes it necessary to consider physical activity as a lifestyle and not a temporary intervention. One deeply investigated aspect was the QoL, and different studies [,,] detected its improvement after exercise training; this trend was also observed in the literature [,]. Different scientific articles detected positive outcomes of exercise training (despite the training-intervention modality) on cancer-related-fatigue symptoms [,,,], and especially when proposed with conventional supportive care interventions []. The cancer-related-fatigue parameters were reduced, and especially with aerobic training [,]. These findings, also supported by the literature [], and especially if the training is supervised [], have to be seriously considered to, first, allow BC patients the practice of physical exercise, and, second, increase the possibility of being more active in daily living.
From a social point of view, the reviews of the literature, again, suggest that yoga [,] and tai chi (with conventional support care) [] have positive outcomes. Another aspect to consider is that, even if no statistically significant effects were detected [], it is fundamental to work in groups to improve the social aspects and interpersonal relationships, and, as the literature suggests, this has also positive outcomes on social issues [].
The strength of this study is that it provides community feedback about the positive outcomes of physical activity on BC survivors in terms of health. Fundamentally, clinicians consider this aspect after the critical phase and during recovery, and especially because, despite the typology of physical exercise, postdiagnosis physical activity is associated with an increase in the survival rate among BC survivors []. It is also important for BC patients to be active to decrease the mortality risk []. Physical activity, whether prediagnosis or postdiagnosis, is associated with a better prognosis for BC [].
The most important limitation of this review is the heterogeneity of the included studies in the methodology (eligibility criteria, outcomes measures) and the sample included (age, cancer trajectory). Heterogeneity was also detected in the intervention characteristics in terms of duration and frequency. This limitation made it difficult to synthesize the data. In our manuscript, we tried to include only moderate- or high-quality systematic reviews and meta-analyses, but the included original articles presented different results in the quality assessment. Furthermore, as we detected heterogeneity between the reviews investigated, heterogeneity was also within the articles included in the reviews. Future studies should focalize attention on the different effects of physical activity on BC patients under therapy or under other conditions.
5. Conclusions
The benefits that arise from this practice of different typologies of exercise training (the studies included were highly heterogeneous) are the physical, psychological, and social health of BC survivors. Among the interventions proposed in the included studies, yoga seems to be an appropriate exercise because it has positive outcomes in the physical, psychological, and social spheres. Systematic reviews and meta-analyses of randomized controlled trials over the last ten years affirm that, despite the cancer history, exercise training is suggested.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph191610391/s1, PRISMA checklist and the search strategy is included.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by M.Z. and L.P. The first draft of the manuscript was written by L.P. and G.M. (Giuseppe Musumeci), and M.Z., G.M. (Grazia Maugeri), and V.D. commented on the previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the University Research Project Grant (PIACERI Found—NATURE-OA—2020–2022), the Department of Biomedical and Biotechnological Sciences (BIOMETEC), the University of Catania, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Casla, S.; Hojman, P.; Márquez-Rodas, I.; López-Tarruella, S.; Jerez, Y.; Barakat, R.; Martín, M. Running away from side effects: Physical exercise as a complementary intervention for breast cancer patients. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2015, 17, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Campbell, W.W. Sarcopenia and age-related changes in body composition and functional capacity. J. Nutr. 1993, 123, 465–468. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, V.; Daudt, H.; Kazanjian, A. Survivorship care plans for breast cancer patients: Understanding the quality of the available evidence. Curr. Oncol. 2017, 24, e446–e465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheville, A.L.; Troxel, A.B.; Basford, J.R.; Kornblith, A.B. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2621. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, M.; Venetis, K.; Sajjadi, E.; Piciotti, R.; de Sire, A.; Fusco, N. Understanding the biology of volumetric muscle loss for an individualized exercise rehabilitation approach in breast cancer patients. Curr. Opin. Pharmacol. 2021, 58, 27–34. [Google Scholar] [CrossRef]
- WHO. Costitution of the World Health Organization. 1946. Available online: https://www.who.int/about/who-we-are/constitution (accessed on 15 March 2022).
- Galloway, R.D. Health promotion: Causes, beliefs and measurements. Clin. Med. Res. 2003, 1, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Fries, J.F.; Harrington, H.; Edwards, R.; Kent, L.A.; Richardson, N. Randomized controlled trial of cost reductions from a health education program: The California Public Employees’ Retirement System (PERS) study. Am. J. Health Promot. 1994, 8, 216–223. [Google Scholar] [CrossRef]
- Brach, J.S.; Simonsick, E.M.; Kritchevsky, S.; Yaffe, K.; Newman, A.B.; Health, A.; Body Composition Study Research, G. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J. Am. Geriatr. Soc. 2004, 52, 502–509. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhang, D.F.; Kang, S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2013, 137, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Pizot, C.; Boniol, M.; Mullie, P.; Koechlin, A.; Boniol, M.; Boyle, P.; Autier, P. Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. J. Clin. Oncol. 2016, 52, 138–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyigor, S.; Kanyilmaz, S. Exercise in patients coping with breast cancer: An overview. World J. Clin. Oncol. 2014, 5, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Fontein, D.B.; de Glas, N.A.; Duijm, M.; Bastiaannet, E.; Portielje, J.E.; Van de Velde, C.J.; Liefers, G.J. Age and the effect of physical activity on breast cancer survival: A systematic review. Cancer Treat. Rev. 2013, 39, 958–965. [Google Scholar] [CrossRef]
- Hardefeldt, P.J.; Penninkilampi, R.; Edirimanne, S.; Eslick, G.D. Physical Activity and Weight Loss Reduce the Risk of Breast Cancer: A Meta-analysis of 139 Prospective and Retrospective Studies. Clin. Breast Cancer 2018, 18, e601–e612. [Google Scholar] [CrossRef]
- Goncalves, A.K.; Florencio, G.L.D.; Silva, M.; Cobucci, R.N.; Giraldo, P.C.; Cote, N.M. Effects of Physical Activity on Breast Cancer Prevention: A Systematic Review. J. Phys. Act. Health 2014, 11, 445–454. [Google Scholar] [CrossRef]
- Fairman, C.M.; Focht, B.C.; Lucas, A.R.; Lustberg, M.B. Effects of exercise interventions during different treatments in breast cancer. J. Community Supportive Oncol. 2016, 14, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Baumann, F.T.; Bloch, W.; Weissen, A.; Brockhaus, M.; Beulertz, J.; Zimmer, P.; Streckmann, F.; Zopf, E.M. Physical Activity in Breast Cancer Patients during Medical Treatment and in the Aftercare—A Review. Breast Care 2013, 8, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Battaglini, C.L.; Mills, R.C.; Phillips, B.L.; Lee, J.T.; Story, C.E.; Nascimento, M.G.B.; Hackney, A.C. Twenty-five years of research on the effects of exercise training in breast cancer survivors: A systematic review of the literature. World J. Clin. Oncol. 2014, 5, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; Garcia-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; Garcia-Honduvilla, N.; Alvarez-Mon, M.; Bujan, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2021, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Boyne, D.J.; O’Sullivan, D.E.; Olij, B.F.; King, W.D.; Friedenreich, C.M.; Brenner, D.R. Physical Activity, Global DNA Methylation, and Breast Cancer Risk: A Systematic Literature Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2018, 27, 1320–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, M.C.; Wörner, E.A.; Verlaan, D.; van Leeuwen, P.A.M. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin. Breast Cancer 2017, 17, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Bekhet, A.H.; Abdallah, A.R.; Ismail, H.M.; Genena, D.M.; Osman, N.A.; El Khatib, A.; Abbas, R.L. Benefits of Aerobic Exercise for Breast Cancer Survivors: A Systematic Review of Randomized Controlled Trials. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Ma, Y.X.; Yun, B.; Wang, Q.; Huang, C.; Han, L. Exercise for fatigue in breast cancer patients: An umbrella review of systematic reviews. Int. J. Nurs. Sci. 2020, 7, 248–254. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Exercise and cancer-related fatigue in adults: A systematic review of previous systematic reviews with meta-analyses. BMC Cancer 2017, 17, 693. [Google Scholar] [CrossRef]
- Reger, M.; Kutschan, S.; Freuding, M.; Schmidt, T.; Josfeld, L.; Huebner, J. Water therapies (hydrotherapy, balneotherapy or aqua therapy) for patients with cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2022, 148, 1277–1297. [Google Scholar] [CrossRef]
- Abdin, S.; Lavall√©e, J.F.; Faulkner, J.; Husted, M. A systematic review of the effectiveness of physical activity interventions in adults with breast cancer by physical activity type and mode of participation. Psychooncology 2019, 28, 1381–1393. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Holtzman, J.; Courneya, K.S.; Mâsse, L.C.; Duval, S.; Kane, R. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1588–1595. [Google Scholar]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Systematic reviews 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.J.; Hamel, C.; Wells, G.A.; Bouter, L.M.; Kristjansson, E.; Grimshaw, J.; Henry, D.A.; Boers, M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009, 62, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Bluethmann, S.M.; Vernon, S.W.; Gabriel, K.P.; Murphy, C.C.; Bartholomew, L.K. Taking the next step: A systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res. Treat. 2015, 149, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Cramer, H.; Lange, S.; Klose, P.; Paul, A.; Dobos, G. Yoga for breast cancer patients and survivors: A systematic review and meta-analysis. BMC Cancer 2012, 12, 412. [Google Scholar] [CrossRef] [Green Version]
- El-Hashimi, D.; Gorey, K.M. Yoga-Specific Enhancement of Quality of Life Among Women With Breast Cancer: Systematic Review and Exploratory Meta-Analysis of Randomized Controlled Trials. J. Evid.-Based Integr. Med. 2019, 24, 2515690x19828325. [Google Scholar] [CrossRef]
- Floyd, A.; Moyer, A. Group vs. individual exercise interventions for women with breast cancer: A meta-analysis. Health Psychol. Rev. 2009, 4, 22–41. [Google Scholar] [CrossRef]
- Juvet, L.K.; Thune, I.; Elvsaas, I.K.O.; Fors, E.A.; Lundgren, S.; Bertheussen, G.; Leivseth, G.; Oldervoll, L.M. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast 2017, 33, 166–177. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, J.; Suh, S.H.; Ligibel, J.; Courneya, K.S.; Jeon, J.Y. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2017, 26, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.P.; Kuo, Y.H.; Tai, W.Y.; Liu, H.E. Exercise effects on fatigue in breast cancer survivors after treatments: A systematic review and meta-analysis. Int. J. Nurs. Pract. 2021, 28, e12989. [Google Scholar] [CrossRef]
- Liu, L.Z.; Tan, H.J.; Yu, S.G.; Yin, H.Y.; Baxter, G.D. The effectiveness of tai chi in breast cancer patients: A systematic review and meta-analysis. Complementary Ther. Clin. Pract. 2020, 38, 101078. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Echavez, J.F.; Gonzalez-Jimenez, E.; Ramirez-Velez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-vélez, R.; Zambom-ferraresi, F.; García-hermoso, A.; Kievisiene, J.; Rauckiene-michealsson, A.; Agostinis-sobrinho, C. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: A systematic review and meta-analysis. Cancers 2021, 13, 264. [Google Scholar] [CrossRef]
- Wang, S.R.; Yang, T.; Qiang, W.M.; Shen, A.M.; Zhao, Z.H.; Chen, X.; Xi, C.X.; Liu, H.; Guo, F.L. Effectiveness of physical exercise on the cardiovascular system in breast cancer patients: A systematic review and meta-analysis of randomized controlled trials. Complementary Ther. Clin. Pract. 2021, 44, 101426. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Pan, L.; Zhang, X.M.; Sun, C.X.; Cui, G.H. Lack of Efficacy of Tai Chi in Improving Quality of Life in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 3715–3720. [Google Scholar] [CrossRef] [Green Version]
- Gebruers, N.; Camberlin, M.; Theunissen, F.; Tjalma, W.; Verbelen, H.; Van Soom, T.; van Breda, E. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: A systematic review. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2019, 27, 109–122. [Google Scholar] [CrossRef]
- McNeely, M.L.; Campbell, K.L.; Rowe, B.H.; Klassen, T.P.; Mackey, J.R.; Courneya, K.S. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ 2006, 175, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Fong, D.Y.; Ho, J.W.; Hui, B.P.; Lee, A.M.; Macfarlane, D.J.; Leung, S.S.; Cerin, E.; Chan, W.Y.; Leung, I.P.; Lam, S.H. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ 2012, 344, e70. [Google Scholar] [CrossRef] [Green Version]
- Speck, R.M.; Courneya, K.S.; Mâsse, L.C.; Duval, S.; Schmitz, K.H. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2010, 4, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, A.; Barrett, S.; Haruna, F.; Mustian, K.; O’Donovan, A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: A systematic review and meta-analysis. Breast 2017, 32, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Piškur, B. Social participation: Redesign of education, research, and practice in occupational therapy* Previously published in Scandinavian Journal of Occupational Therapy 2013; 20: 2–8. Scand. J. Occup. Ther. 2014, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.T.; Ibrahim, J.G.; Khankari, N.; Cleveland, R.J.; Abrahamson, P.E.; Stevens, J.; Satia, J.A.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Post-diagnosis physical activity and survival after breast cancer diagnosis: The Long Island Breast Cancer Study. Breast Cancer Res. Treat. 2014, 145, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, W.J.; Jeong, S.H. The effects of physical activity on breast cancer survivors after diagnosis. J. Cancer Prev. 2013, 18, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.L.; Jiang, T.C.; Ma, T.F.; Zhang, X.H.; Tang, J.H.; Chen, W.X.; Lv, M.M.; Zhao, J.H. Association between physical activity and mortality in breast cancer: A meta-analysis of cohort studies. Eur. J. Epidemiol. 2014, 29, 391–404. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).